Abstract
To determine the role of Toll-like receptor 4 (TLR4) in the immune response to pneumonia, C3H/HeJ mice (which display a mutant nonfunctional TLR4) and C3H/HeN wild-type mice were intranasally infected with either Streptococcus pneumoniae (a common gram-positive respiratory pathogen) or Klebsiella pneumoniae (a common gram-negative respiratory pathogen). In cases of pneumococcal pneumonia, TLR4 mutant mice showed a reduced survival only after infection with low-level bacterial doses, which was associated with a higher bacterial burden in their lungs 48 h postinfection. In Klebsiella pneumonia, TLR4 mutant mice demonstrated a shortened survival after infection with either a low- or a high-level bacterial dose together with an enhanced bacterial outgrowth in their lungs. These data suggest that TLR4 contributes to a protective immune response in both pneumococcal and Klebsiella pneumonia and that its role is more important in respiratory tract infection caused by the latter (gram-negative) pathogen.
Pneumonia is a common and serious illness that is a major cause of morbidity and mortality in humans. Streptococcus pneumoniae and Klebsiella pneumoniae are frequently isolated causative pathogens of pneumonia (1, 7, 8, 34, 53). Because of the high incidence of pneumonia and the increasing resistance of several bacterial strains to antimicrobial agents (8-10), it is vital to gain more insight into the pathogenesis of pneumonia.
The innate immune system is important for the elimination of bacteria from the pulmonary compartment. One of the first steps in activating host defense mechanisms is recognition of pathogens by phagocytic cells. Phagocytes recognize highly conserved motifs (pathogen-associated molecular patterns [PAMPs]) shared by large groups of microorganisms, leading to intracellular signaling and ultimately resulting in the production of cytokines and chemokines and activation of the adaptive immune system (45). One of the best-known PAMPs is endotoxin (lipopolysaccharide [LPS]), which is part of the outer membrane of gram-negative bacteria and responsible for activating innate host defense mechanisms in gram-negative infections (52). Gram-positive bacteria do not contain LPS in their cell walls but express other PAMPs such as lipoteichoic acid (LTA), peptidoglycan (PGN), and lipoproteins.
Recognition of and responses to PAMPs are controlled by several pattern recognition receptors (PRRs). CD14 has been widely accepted as a PRR for a variety of bacterial cell wall components, including LPS (52), LTA (13, 25), and PGN (15). However, CD14 does not contain a cytoplasmic domain and therefore cannot transduce activating signals across the cell membrane. In recent years, Toll-like receptors (TLRs) have been emerging as the key regulators of innate immune responses to infection in mammals (for reviews, see references 6, 30, and 45). By now, 10 different members of the TLR family have been identified; for most of them, one or more PAMPs have been described (2, 45). In complex with CD14 and MD-2, a secreted cell bound protein (32, 40, 43), TLR4 has been shown to mediate LPS responsiveness, implying that TLR4 is the PRR for LPS (12, 14, 21, 22, 43, 47). In contrast, cell wall components of gram-positive bacteria (PGN and LTA) induce inflammatory responses predominantly through TLR2 (27, 31, 42, 46, 54). However, in vitro studies done by Takeuchi et al. (46) show that LTA can also signal via TLR4. Recent investigations studied the role of TLR4 in host defense against respiratory tract infection by gram-negative bacteria in vivo, revealing that this receptor contributes to a protective innate immune response to Haemophilus influenzae (50) and Pasteurella pneumotropica (11, 19) but not to Legionella pneumophila (28). In the present study, we conducted experiments in which gram-positive (S. pneumoniae) and gram-negative (K. pneumoniae) pneumonia was induced in C3H/HeJ mice, which have nonfunctional TLR4, and normal wild-type (WT) C3H/HeN mice. With these experiments, we sought to determine the role of TLR4 in host defense mechanisms in gram-positive and gram-negative pneumonia in mice.
MATERIALS AND METHODS
Animals.
Pathogen-free 8- to 10-week-old, sex-matched, C3H/HeJ (TLR4 mutant) and C3H/HeN (WT) mice were purchased from Charles River (Someren, The Netherlands). C3H/HeJ mice have been demonstrated to have a missense mutation in the third exon of TLR4, resulting in a Pro712→His substitution yielding a nonfunctional TLR4 (22, 35, 36). All experiments were approved by the Animal Care and Use Committee of the University of Amsterdam (Amsterdam, The Netherlands).
Induction of pneumonia.
Pneumonia was induced as described before (26, 37, 41). S. pneumoniae serotype 3 (ATCC 6303; American Type Culture Collection, Rockville, Md.) was used for gram-positive infection. Pneumococci were cultured for 16 h at 37°C in 5% CO2 in Todd-Hewitt broth (Difco, Detroit, Mich.). This suspension was diluted 1:100 in fresh medium and grown for 5 h to midlogarithmic phase. K. pneumoniae serotype 2 (ATCC 43816; American Type Culture Collection) was used for gram-negative infection. Klebsiella bacteria were cultured for 16 h at 37°C in 5% CO2 in tryptic soy broth (Difco). This suspension was diluted 1:100 in fresh medium and grown for 3 h to midlogarithmic phase. S. pneumoniae and K. pneumoniae were harvested by centrifugation at 1,500 × g for 15 min and washed twice in sterile 0.9% saline. Bacteria were resuspended in saline at different concentrations (see Results) determined by plating 10-fold dilutions of the suspensions on blood agar plates. After preparation of the bacterial inocula, mice were lightly anesthetized by being subjected to inhalation of isoflurane (Upjohn, Ede, The Netherlands) and 50 μl of the bacterial suspension was inoculated intranasally. Control mice received 50 μl of saline solution.
Determination of bacterial outgrowth.
At 6, 24, and 48 h after infection, mice were anesthetized by the intraperitoneal administration of FFM (fentanyl citrate [0.079 mg/ml], fluanisone [2.5 mg/ml], midazolam [1.25 mg/ml in H2O]) (7.0 ml/kg of body weight) and sacrificed by bleeding out the vena cava inferior. Blood was collected in EDTA-containing tubes. Whole lungs were harvested and homogenized (using a tissue homogenizer [Biospec Products, Bartlesville, Okla.)] at 4°C in 4 volumes of sterile saline solution. Serial 10-fold dilutions were made in sterile saline, and 10-μl volumes were plated on blood agar plates. In addition, 20-μl volumes of blood were plated. Plates were incubated at 37°C in 5% CO2, and CFU were counted after 16 h.
Cell counts in the lungs.
In separate experiments, whole lungs were harvested at 6, 24, and 48 h after the induction of infection. Lungs were crushed and filtered through a 40-μm-pore-size cell strainer (Becton Dickinson, Franklin Lakes, N.J.), and pulmonary cells were suspended in RPMI medium (Bio Whittaker, Verviers, Belgium). Erythrocytes were lysed with ice-cold isotonic NH4Cl solution (155 mM NH4Cl, 10 mM KHCO3, 100 mM EDTA, pH 7.4); the remaining cells were resuspended in RPMI medium. Total cell numbers in each sample were counted using a hemacytometer (Emergo). Differential counts (macrophages, granulocytes, and lymphocytes) in the cell suspensions were assessed using cytospin preparations stained with a modified Giemsa stain (Diff-Quick; Baxter, McGraw Park, Ill.).
Cytokine and chemokine measurements in lung tissue.
For cytokine measurements, lung homogenates were diluted 1:2 in lysis buffer (150 mM NaCl, 15 mM Tris, 1 mM MgCl.H2O, 1 mM CaCl2, 1% Triton X-100, 100 μg of pepstatin A/ml, leupeptin, aprotinin, pH 7.4) and incubated at 4°C for 30 min. Homogenates were centrifuged at 1,500 × g for 15 min, after which the supernatants were stored at −20°C until further use. Cytokine and chemokine levels in lung homogenates were measured by enzyme-linked immunosorbent assay according to the manufacturer's instructions, and tumor necrosis factor (TNF), interleukin-6 (IL-6), macrophage-inflammatory protein 2 (MIP-2), and keratinocyte (KC) assays were all obtained from R&D (Minneapolis, Minn.).
Histologic examination.
Lungs for histologic examination were harvested at 6 and 24 h (K. pneumoniae pneumonia) and at 24 and 48 h (S. pneumoniae pneumonia) after inoculation, fixed in 10% formalin, and embedded in paraffin. Sections (4 μm) were stained with hematoxylin and eosin and analyzed by a pathologist whose investigation was blinded with respect to the identity of the groups.
Statistical analysis.
All data are expressed as means ± standard errors of the means (SEM). Differences between groups were analyzed using a Mann-Whitney U test. Survival studies were analyzed using Kaplan-Meier testing. A P value of <0.05 was considered to represent a statistically significant difference.
RESULTS
Survival.
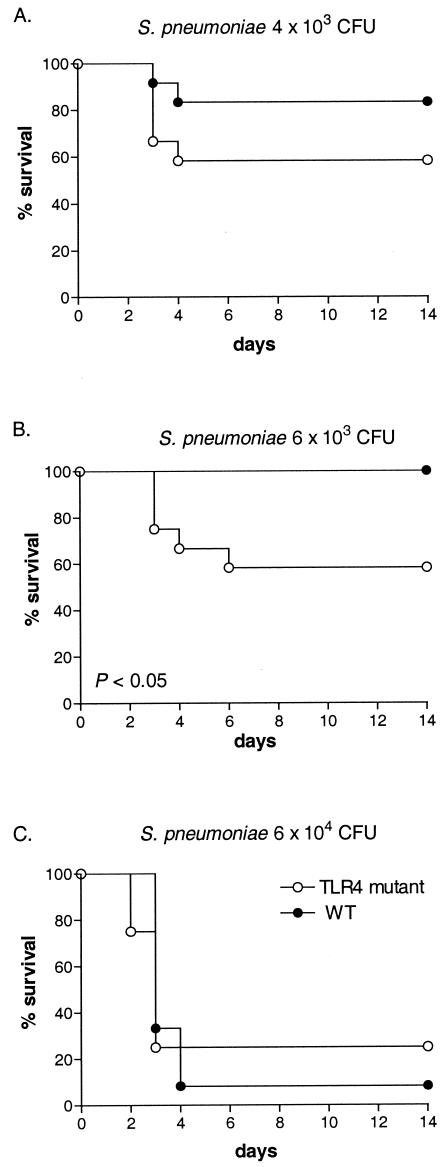
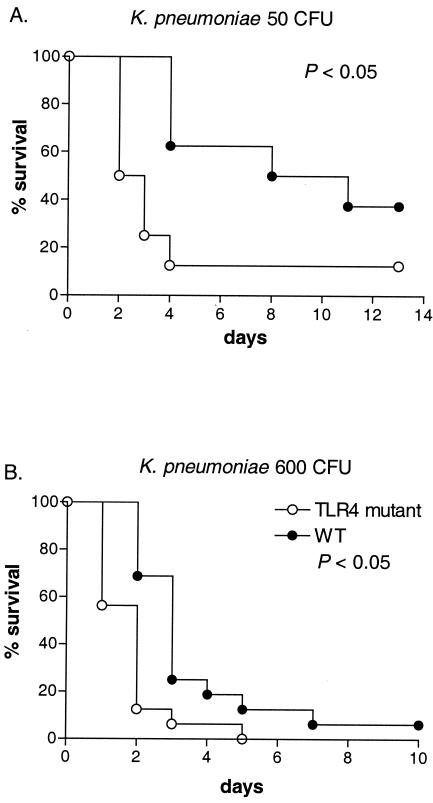
When gram-positive pneumonia was induced by S. pneumoniae, survival rates did not consistently differ between TLR4 mutant and WT mice. At low infectious doses (4 × 103 and 6 × 103 CFU), survival was reduced in TLR4 mutant mice, significantly so after inoculation with 6 × 103 CFU (P < 0.05). However, a higher bacterial dose (6 × 104 CFU) caused similarly high levels of mortality with similar time courses in TLR4 mutant and WT mice (Fig. 1). When gram-negative pneumonia was induced by K. pneumoniae, in contrast, survival was consistently and significantly shortened in TLR4 mutant mice after infection with both a low (50 CFU) and a high (600 CFU) bacterial dose (Fig. 2). Hence, these data suggest that TLR4 might play a modest protective role against mortality during pneumococcal pneumonia induced by relatively low levels of bacterial inocula and that TLR4 has a more important role in the protective immune response to K. pneumoniae pneumonia. Subsequent experiments were done with 104 CFU of S. pneumoniae and 200 CFU of K. pneumoniae.
FIG. 1.
Survival of TLR4 mutant and WT mice after intranasal inoculation with 4 × 103 CFU (A), 6 × 103 CFU (B), or 6 × 104 CFU (C) of S. pneumoniae organisms. A total of 12 mice per group were studied. Survival of TLR4 mutant mice inoculated with 6.5 × 103 CFU was significantly decreased compared to that seen with WT mice (P < 0.05).
FIG. 2.
Survival of TLR4 mutant and WT mice after intranasal inoculation with 50 (A) or 600 (B) CFU of K. pneumoniae organisms. A total of 8 to 16 mice per group were studied. Survival in TLR4 mutant mice was significantly decreased compared to that seen with WT mice in both experiments (P < 0.05).
Bacterial outgrowth.
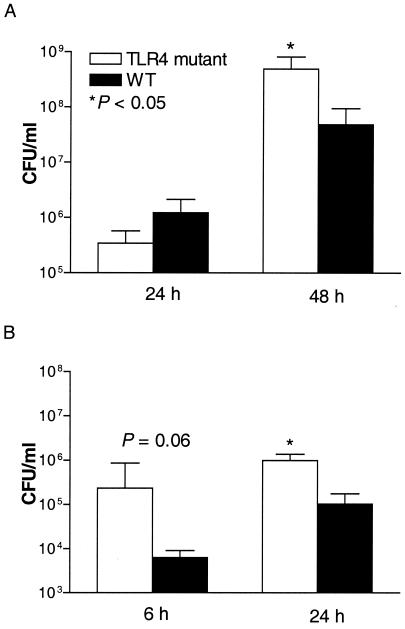
To obtain more insight into the role of TLR4 in early host defense against gram-positive and gram-negative pneumonia, bacterial outgrowth levels in the lungs and blood of TLR4 mutant and WT mice were compared. After infection with 104 CFU of S. pneumoniae, bacteria were counted at 24 and 48 h. Although at 24 h the numbers of CFU were similar in both mouse strains, at 48 h postinfection TLR4 mutant mice had significantly more bacteria in their lungs than WT mice (P < 0.05) (Fig. 3A). The percentages of positive blood cultures in TLR4 mutant and WT mice were similar (at 24 h, 0% of TLR4 mutant cultures and 12.5% of WT mouse cultures; at 48 h, 50% of TLR4 mutant cultures and 37.5% of WT mouse cultures). In gram-negative pneumonia investigations, the bacterial load in the lungs 6 and 24 h after inoculation with 200 CFU of K. pneumoniae was assessed. These time points were chosen earlier than in the pneumococcal model in consideration of the early mortality of TLR4 mutant mice with Klebsiella pneumonia. At both time points, the lungs of TLR4 mutant mice contained more bacteria, although significance was reached only at 24 h (P < 0.05) (Fig. 3B). The percentages of positive blood cultures were equal in both mouse strains (0% at 6 h and 12.5% at 24 h).
FIG. 3.
Bacterial outgrowth in lungs in TLR4 mutant and WT mice at 24 and 48 h after intranasal inoculation with 104 CFU of S. pneumoniae organisms (A) and at 6 and 24 h after intranasal inoculation with 200 CFU of K. pneumoniae organisms (B). Data represent means ± SEM of the results for eight mice. *, P < 0.05 versus results for WT mice.
Granulocyte influx in the lungs.
The influx of granulocytes to the site of inflammation early in infection is an important characteristic of innate host defense mechanisms (55). We therefore determined leukocyte counts and differentials in the lungs at 6 and 24 h after induction of infection. Induction of both gram-positive and gram-negative pneumonia caused an increase of granulocyte numbers in TLR4 mutant and WT mouse lungs compared to the numbers seen with saline controls (data not shown). In cases of gram-positive pneumonia, TLR4 mutant mouse lungs contained numbers of granulocytes similar to those seen with WT mouse lungs at 24 h (Table 1). Similarly, when pneumonia was induced by K. pneumoniae, no differences in granulocyte influx levels in lungs of TLR4 mutant and WT mice were seen after 6 and 24 h (Table 1).
TABLE 1.
Cellular composition of lungs during pneumonia
| Strain and time point (h) | Resultsa
|
|||
|---|---|---|---|---|
| No. of cells (104) | Granulocytes (%) | Macrophages (%) | Lymphocytes (%) | |
| S. pneumoniae | ||||
| 24 | ||||
| TLR4 mutant | 45.6 ± 6.2 | 31.1 ± 3.7 | 61.3 ± 3.7 | 7.6 ± 1.7 |
| WT | 46.2 ± 6.4 | 34.0 ± 4.9 | 57.3 ± 5.0 | 8.6 ± 1.1 |
| K. pneumoniae | ||||
| 6 | ||||
| TLR4 mutant | 63.5 ± 8.7 | 30.2 ± 3.1 | 44.1 ± 3.1 | 25.6 ± 2.1b |
| WT | 77.6 ± 5.4 | 37.2 ± 5.1 | 55.0 ± 5.8 | 7.8 ± 1.1 |
| 24 | ||||
| TLR4 mutant | 82.3 ± 11.1 | 42.5 ± 5.6 | 35.4 ± 4.8 | 22.1 ± 2.5b |
| WT | 90.5 ± 13.7 | 41.6 ± 3.7 | 23.3 ± 3.3 | 35.1 ± 1.5 |
Results of total leukocyte counts and differential counts (expressed as percentages) in lungs of TLR4 mutant and WT mice infected with S. pneumoniae or K. pneumoniae at 6 and/or 24 h after infection. Data represent means ± SEM for six mice per group at each time point.
P < 0.05 versus WT results at the same time point.
Cytokine and chemokine response to pneumonia.
Local production of cytokines and chemokines plays a role in the protective immune response to respiratory tract infection (44, 55). Therefore, we determined the influence of TLR4 deficiency on pulmonary cytokine concentration with gram-positive and gram-negative pneumonia. Cytokine (TNF-α and IL-6) and chemokine (MIP-2 and KC) levels measured in lung homogenates 24 and 48 h after induction of pneumococcal pneumonia (Table 2) and 6 and 24 h after infection with K. pneumoniae (Table 3) did not differ between TLR4 mutant and WT mice.
TABLE 2.
Cytokine and chemokine concentrations in the lung during S. pneumoniae pneumonia
| Cytokine or chemokine | Results (ng/g of lung) at indicated time (h) postinfectiona
|
|||
|---|---|---|---|---|
| 24
|
48
|
|||
| TLR4 mutant | WT | TLR4 mutant | WT | |
| TNF | 4.8 ± 0.8 | 3.1 ± 0.3 | 8.1 ± 2.6 | 4.6 ± 2.1 |
| IL-6 | 9.6 ± 5.1 | 9.7 ± 4.6 | 66.7 ± 27.1 | 40.1 ± 29.9 |
| KC | 35.8 ± 1.1 | 32.3 ± 0.7 | 110.6 ± 33.5 | 71.2 ± 30.8 |
| MIP-2 | 5.1 ± 1.7 | 6.2 ± 1.7 | 20.5 ± 7.1 | 10.0 ± 5.4 |
Data represent means ± SEM for eight mice per group.
TABLE 3.
Cytokine and chemokine concentrations in the lung during K. pneumoniae pneumonia
| Cytokine or chemokine | Results (ng/g of lung) at indicated time (h) postinfectiona
|
|||
|---|---|---|---|---|
| 6
|
24
|
|||
| TLR4 mutant | WT | TLR4 mutant | WT | |
| TNF | 2.7 ± 0.1 | 2.7 ± 0.1 | 1.9 ± 0.1 | 2.2 ± 0.3 |
| IL-6 | 4.6 ± 1.4 | 2.8 ± 0.2 | 3.3 ± 0.4 | 3.3 ± 0.3 |
| KC | 38.6 ± 1.0 | 38.5 ± 2.5 | 32.3 ± 0.7 | 34.2 ± 0.6 |
| MIP-2 | 33.8 ± 2.7 | 32.1 ± 4.5 | 33.3 ± 2.2 | 36.9 ± 2.3 |
Data represent means ± SEM for eight mice per group.
Histopathology.
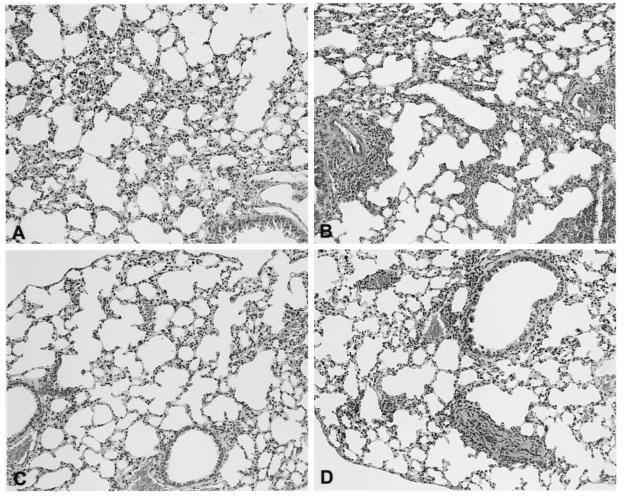
After infection with S. pneumoniae, the lungs of WT mice showed a mild interstitial inflammation composed of monocytes and lymphocytes (Fig. 4A.) The inflammation was slightly more pronounced in TLR4 mutant mice with more perivascular infiltrates (Fig. 4B). After infection with K. pneumoniae, lungs from WT mice had thickened alveolar septae due to mild interstitial inflammation with infiltration of lymphocytes and monocytes (Fig. 4C). In TLR4 mutant mice, the interstitial inflammation was also slightly more pronounced (Fig. 4D).
FIG. 4.
Representative lung histology of WT (A and C) and TLR4 mutant (B and D) mice 24 h after S. pneumoniae (A and B) and K. pneumoniae (C and D) inoculation. Data are representative of the results for five mice per group after hematoxylin and eosin staining. Magnification, ×10.
DISCUSSION
TLR4 has been implicated as playing an essential role in host defense against gram-negative bacteria by virtue of its capacity to signal LPS-induced inflammatory responses (12, 14, 21, 22, 43, 47). In contrast, components of gram-positive bacteria have been demonstrated to signal predominantly via TLR2 (42, 54), although TLR4 may also play a role herein (27, 29, 31, 42, 46, 54). To determine the relevance of TLR4 in inducing an innate host response to pulmonary infection, we induced pneumonia caused by two common respiratory pathogens (gram-positive S. pneumoniae and gram-negative K. pneumoniae) in TLR4 mutant and WT mice. The outcome of S. pneumoniae pneumonia was modestly influenced by TLR4 deficiency, as reflected by a reduced survival of TLR4 mutant mice after inoculation with a relatively low infectious dose and byan increased bacterial outgrowth in lungs. In K. pneumoniae pneumonia, the protective role of TLR4 was clearer; i.e., TLR4 mutant mice displayed an impaired host response compared to WT mice, as illustrated by consistently and significantly shortened survival times and an increased number of bacteria in their lungs.
Several studies have attributed an important role to TLR2 in activating cells upon stimulation with components of S. pneumoniae. In CD14-expressing Chinese hamster ovary (CHO) cells, heat-killed S. pneumoniae stimulated NF-κB translocation, a response that was greatly enhanced in cells coexpressing TLR2 and CD14, suggesting that the pneumococcus activates both TLR2-dependent and -independent signaling pathways. Activation of the TLR2 pathway by S. pneumoniae can be attributed to several PAMPs expressed within the pneumococcal cell wall, including PGN and LTA (27, 31, 39, 42, 54). The role of TLR2 in pneumococcal infection in vivo was investigated in a model of meningitis, in which S. pneumoniae was injected intracerebrally in TLR2-deficient (TLR2−/−) and WT mice (16, 24). Both studies showed an increased susceptibility of TLR2−/− mice compared to WT mice in the early phase of infection, as expressed by increased bacterial counts in the brain (16, 24) and blood (24), while one study also reported a reduced survival time in TLR2−/− mice (16). In preliminary experiments, however, our laboratory did not observe major differences between TLR2−/− and WT mice with pneumococcal pneumonia in antibacterial defense and survival (S. Knapp, S. Florquin, O. Takeuchi, S. Akira, and T. van der Poll, Abstr. 42nd Intersc. Conf. Antimicrob. Agents Chemother., abstr B-693, 2002). Interestingly, in the previous meningitis studies TLR2−/− mice displayed a virtually unchanged inflammatory response to S. pneumoniae (16, 24). Together, these data suggest that although TLR2 may play a role in the innate immune response to pneumococcal infection in some organs (such as the brain), other PRRs likely are involved.
While our studies were in progress, Malley et al. reported a role for TLR4 in host defense against nasopharyngeal colonization by S. pneumoniae (29). In that study, TLR4 mutant and WT mice were intranasally inoculated with S. pneumoniae without the use of anesthesia (29), which resulted in nasopharyngeal colonization rather than in lower-respiratory-tract infection such as was seen in the present investigation. Using a bioluminescent pneumococcal strain, these authors demonstrated a much higher level of bacterial nasopharyngeal burden (as determined by photon emission assays) in TLR4 mutant mice in the first 3 days after intranasal infection, which was associated with the subsequent development of bacteremia and increased lethality. In a series of elegant experiments it was further shown that pneumolysin, a pore-forming cytolysin toxin secreted by pneumococci (38), interacts with TLR4 and that after nasopharyngeal colonization in either TLR4 mutant or WT mice, pneumolysin-deficient pneumococci are unable to induce invasive disease (29). Together these data suggest that in the nasopharynx, the interaction between pneumolysin and TLR4 is critically involved in the innate immune response to S. pneumoniae. The results of our experiments (using a pneumolysin-producing S. pneumoniae strain) indicate that once pneumococci have reached the lower respiratory tract, TLR4 plays a limited role in pulmonary defense against infection. Although we found that TLR4 mutant mice had increased mortality at low infectious doses and (after 48 h) increased bacterial counts in the lungs compared to WT mice, survival rates were similar at a higher inoculum dose. Malley et al. used only one bacterial dose, which resulted in a 12.5% lethality rate in WT mice without evidence of lower-respiratory-tract infection (29). In that study, the absolute difference in mortality rates between TLR4 mutant and WT mice was similar to the difference between the mortality rates observed for the mouse strains in our present study when low infectious doses causing 17% lethality in WT mice were administered. Together, these findings point to distinct roles for TLR4 in the upper and lower respiratory tracts with respect to innate immunity to pneumococcal infection. Notably, another report showed no difference in outcomes between TLR4 mutant and WT mice in a peritoneal infection model using induction by pneumococci (3).
It should be noted that in the two survival studies using low S. pneumoniae doses of 4 × 103 and 6 × 103 CFU, mortality rates for WT mice were 2/12 and 0/12, respectively (i.e., mortality only after administration of the lowest dose). In this respect it is important to realize that these studies were done several months apart and used different shipments of mice and that some variation is not uncommon when studying the outcome of live infections in this setting. These data suggest that these bacterial challenge levels are at or just over the edge with respect to what can be handled by the normal innate immune system. Importantly, however, all comparisons between WT and TLR4 mutant mice were done in experiments in which all mice were inoculated at the same time with exactly the same inoculum. Hence, comparisons between the two mouse strains were always done in an adequate and valid way. Thus, judging on the basis of the slightly reduced antibacterial defense and the modestly reduced survival of TLR4 mutant mice in our pneumococcal pneumonia model using low doses, together with the recent findings of Malley et al. (29) discussed above, we feel that the conclusion that TLR4 plays a modest role in host defense against respiratory tract infection by S. pneumoniae is justified.
C3H/HeJ mice have long been known to be hyporesponsive to LPS. A mutation in the Tlr4 gene proved responsible for this hyporesponsiveness (35, 36). In vitro studies also identified TLR4 as an essential receptor for LPS (12, 14, 21, 22, 43, 47). In addition to the requirement for TLR4, recognition of LPS requires other molecules such as LPS-binding protein, CD14, and MD-2 (23, 43). Since LPS is an important antigen in gram-negative bacteria and is capable of inducing a strong immune response, it was expected that the lack of functional TLR4 would render mice susceptible to gram-negative infections (5). Indeed, TLR4 seems to be important in host defense against some gram-negative bacteria, as shown by an impaired defense of TLR4 mutant mice during urinary tract infection with Escherichia coli (18) and intraperitoneal infection with Neisseria (51), Klebsiella (49), and Salmonella (4) species. However, host defense in TLR4 mutant mice with E. coli peritonitis was not impaired (17, 20). Our results obtained in the Klebsiella model corroborate those of earlier studies that reported a protective role of TLR4 in gram-negative respiratory-tract infection caused by H. influenzae (50) or P. pneumotropica (11, 19). Of note, in a previous study our laboratory documented an unimpaired host defense in TLR4 mutant mice infected with L. pneumophila; this result may have been caused by the unique structure of Legionella LPS that also fails to interact with CD14 (28, 33).
TLR4 mutant mice displayed an unaltered inflammatory response to pneumococcal and Klebsiella pneumonia. It remains to be established which PRRs play a role in the induction of lung inflammation during infection by the pathogens used here. In this respect the recent description of receptor clusters recognizing LPS is of considerable interest; i.e., accumulating evidence suggests that following LPS stimulation, a signaling complex of receptors is formed which comprises heat shock protein 70 (HSP70), HSP90, CXC chemokine receptor 4, and growth differentiation factor 5 (48). Although it is absolutely clear that TLR4 is important for LPS signaling, it is tempting to speculate that receptor clusters such as those described above can compensate in part for a lack of TLR4 during gram-negative infection in vivo.
We conclude that TLR4 is involved in innate immunity during pneumonia caused by either S. pneumoniae or K. pneumoniae. The role of TLR4 in pneumococcal pneumonia is relatively limited, providing protection only after infection of the lower respiratory tract with low bacterial doses causing little if any mortality in WT mice. In Klebsiella pneumonia, TLR4 is a more important part of an effective immune response in the lungs.
Acknowledgments
We thank J. Daalhuisen, I. Kop, and A. Maas for excellent technical assistance.
Editor: F. C. Fang
REFERENCES
- 1.Bartlett, J. G., and L. M. Mundy. 1995. Community-acquired pneumonia. N. Engl. J. Med. 333:1618-1624. [DOI] [PubMed] [Google Scholar]
- 2.Barton, G. M., and R. Medzhitov. 2002. Toll-like receptors and their ligands. Curr. Top. Microbiol. Immunol. 270:81-92. [DOI] [PubMed] [Google Scholar]
- 3.Benton, K. A., J. C. Paton, and D. E. Briles. 1997. The hemolytic and complement-activating properties of pneumolysin do not contribute individually to virulence in a pneumococcal bacteremia model. Microb. Pathog. 23:201-209. [DOI] [PubMed] [Google Scholar]
- 4.Bernheiden, M., J. M. Heinrich, G. Minigo, C. Schutt, F. Stelter, M. Freeman, D. Golenbock, and R. S. Jack. 2001. LBP, CD14, TLR4 and the murine innate immune response to a peritoneal Salmonella infection. J. Endotoxin Res. 7:447-450. [PubMed] [Google Scholar]
- 5.Beutler, B., and E. T. Rietschel. 2003. Innate immune sensing and its roots: the story of endotoxin. Nat. Rev. Immunol. 3:169-176. [DOI] [PubMed] [Google Scholar]
- 6.Brightbill, H. D., and R. L. Modlin. 2000. Toll-like receptors: molecular mechanisms of the mammalian immune response. Immunology 101:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, P. D., and S. A. Lerner. 1998. Community-acquired pneumonia. Lancet 352:1295-1302. [DOI] [PubMed] [Google Scholar]
- 8.Burwen, D. R., S. N. Banerjee, and R. P. Gaynes. 1994. Ceftazidime resistance among selected nosocomial gram-negative bacilli in the United States. National Nosocomial Infections Surveillance System. J. Infect. Dis. 170:1622-1625. [DOI] [PubMed] [Google Scholar]
- 9.Butler, J. C., J. Hofmann, M. S. Cetron, J. A. Elliott, R. R. Facklam, and R. F. Breiman. 1996. The continued emergence of drug-resistant Streptococcus pneumoniae in the United States: an update from the Centers for Disease Control and Prevention's Pneumococcal Sentinel Surveillance System. J. Infect. Dis. 174:986-993. [DOI] [PubMed] [Google Scholar]
- 10.Campbell, G. D., Jr., and R. Silberman. 1998. Drug-resistant Streptococcus pneumoniae. Clin. Infect. Dis. 26:1188-1195. [DOI] [PubMed] [Google Scholar]
- 11.Chapes, S. K., D. A. Mosier, A. D. Wright, and M. L. Hart. 2001. MHCII, Tlr4 and Nramp1 genes control host pulmonary resistance against the opportunistic bacterium Pasteurella pneumotropica. J. Leukoc. Biol. 69:381-386. [PubMed] [Google Scholar]
- 12.Chow, J. C., D. W. Young, D. T. Golenbock, W. J. Christ, and F. Gusovsky. 1999. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274:10689-10692. [DOI] [PubMed] [Google Scholar]
- 13.Cleveland, M. G., J. D. Gorham, T. L. Murphy, E. Tuomanen, and K. M. Murphy. 1996. Lipoteichoic acid preparations of gram-positive bacteria induce interleukin-12 through a CD14-dependent pathway. Infect. Immun. 64:1906-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du, X., A. Poltorak, M. Silva, and B. Beutler. 1999. Analysis of Tlr4-mediated LPS signal transduction in macrophages by mutational modification of the receptor. Blood Cells Mol. Dis. 25:328-338. [DOI] [PubMed] [Google Scholar]
- 15.Dziarski, R., R. I. Tapping, and P. S. Tobias. 1998. Binding of bacterial peptidoglycan to CD14. J. Biol. Chem. 273:8680-8690. [DOI] [PubMed] [Google Scholar]
- 16.Echchannaoui, H., K. Frei, C. Schnell, S. L. Leib, W. Zimmerli, and R. Landmann. 2002. Toll-like receptor 2-deficient mice are highly susceptible to Streptococcus pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. J. Infect. Dis. 186:798-806. [DOI] [PubMed] [Google Scholar]
- 17.Evans, T. J., E. Strivens, A. Carpenter, and J. Cohen. 1993. Differences in cytokine response and induction of nitric oxide synthase in endotoxin-resistant and endotoxin-sensitive mice after intravenous gram-negative infection. J. Immunol. 150:5033-5040. [PubMed] [Google Scholar]
- 18.Hagberg, L., R. Hull, S. Hull, J. R. McGhee, S. M. Michalek, and C. Svanborg Eden. 1984. Difference in susceptibility to gram-negative urinary tract infection between C3H/HeJ and C3H/HeN mice. Infect. Immun. 46:839-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hart, M. L., D. A. Mosier, and S. K. Chapes. 2003. Toll-like receptor 4-positive macrophages protect mice from Pasteurella pneumotropica-induced pneumonia. Infect. Immun. 71:663-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haziot, A., N. Hijiya, S. C. Gangloff, J. Silver, and S. M. Goyert. 2001. Induction of a novel mechanism of accelerated bacterial clearance by lipopolysaccharide in CD14-deficient and Toll-like receptor 4-deficient mice. J. Immunol. 166:1075-1078. [DOI] [PubMed] [Google Scholar]
- 21.Hirschfeld, M., Y. Ma, J. H. Weis, S. N. Vogel, and J. J. Weis. 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J. Immunol. 165:618-622. [DOI] [PubMed] [Google Scholar]
- 22.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 23.Jiang, Q., S. Akashi, K. Miyake, and H. R. Petty. 2000. Lipopolysaccharide induces physical proximity between CD14 and toll-like receptor 4 (TLR4) prior to nuclear translocation of NF-kappa B. J. Immunol. 165:3541-3544. [DOI] [PubMed] [Google Scholar]
- 24.Koedel, U., B. Angele, T. Rupprecht, H. Wagner, A. Roggenkamp, H. W. Pfister, and C. J. Kirschning. 2003. Toll-like receptor 2 participates in mediation of immune response in experimental pneumococcal meningitis. J. Immunol. 170:438-444. [DOI] [PubMed] [Google Scholar]
- 25.Kusunoki, T., E. Hailman, T. S. Juan, H. S. Lichenstein, and S. D. Wright. 1995. Molecules from Staphylococcus aureus that bind CD14 and stimulate innate immune responses. J. Exp. Med. 182:1673-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lauw, F. N., J. Branger, S. Florquin, P. Speelman, S. J. van Deventer, S. Akira, and T. van der Poll. 2002. IL-18 improves the early antimicrobial host response to pneumococcal pneumonia. J. Immunol. 168:372-378. [DOI] [PubMed] [Google Scholar]
- 27.Lehner, M. D., S. Morath, K. S. Michelsen, R. R. Schumann, and T. Hartung. 2001. Induction of cross-tolerance by lipopolysaccharide and highly purified lipoteichoic acid via different Toll-like receptors independent of paracrine mediators. J. Immunol. 166:5161-5167. [DOI] [PubMed] [Google Scholar]
- 28.Lettinga, K. D., S. Florquin, P. Speelman, R. van Ketel, T. van der Poll, and A. Verbon. 2002. Toll-like receptor 4 is not involved in host defense against pulmonary Legionella pneumophila infection in a mouse model. J. Infect. Dis. 186:570-573. [DOI] [PubMed] [Google Scholar]
- 29.Malley, R., P. Henneke, S. C. Morse, M. J. Cieslewicz, M. Lipsitch, C. M. Thompson, E. Kurt-Jones, J. C. Paton, M. R. Wessels, and D. T. Golenbock. 2003. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. USA 100:1966-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135-145. [DOI] [PubMed] [Google Scholar]
- 31.Morath, S., A. Stadelmaier, A. Geyer, R. R. Schmidt, and T. Hartung. 2002. Synthetic lipoteichoic acid from Staphylococcus aureus is a potent stimulus of cytokine release. J. Exp. Med. 195:1635-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagai, Y., S. Akashi, M. Nagafuku, M. Ogata, Y. Iwakura, S. Akira, T. Kitamura, A. Kosugi, M. Kimoto, and K. Miyake. 2002. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat. Immunol. 3:667-672. [DOI] [PubMed] [Google Scholar]
- 33.Neumeister, B., M. Faigle, M. Sommer, U. Zahringer, F. Stelter, R. Menzel, C. Schutt, and H. Northoff. 1998. Low endotoxic potential of Legionella pneumophila lipopolysaccharide due to failure of interaction with the monocyte lipopolysaccharide receptor CD14. Infect. Immun. 66:4151-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Podschun, R., and U. Ullmann. 1998. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 11:589-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 36.Qureshi, S. T., L. Lariviere, G. Leveque, S. Clermont, K. J. Moore, P. Gros, and D. Malo. 1999. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J. Exp. Med. 189:615-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rijneveld, A. W., S. Weijer, S. Florquin, C. T. Esmon, J. C. Meijers, P. H. Speelman, P. H. Reitsma, H. ten Cate, and T. van der Poll. Thrombomodulin mutant mice with a strongly reduced capacity to generate activated protein C have an unaltered pulmonary immune response to respiratory pathogens and lipopolysaccharide. Blood, in press. [DOI] [PubMed]
- 38.Rossjohn, J., R. J. Gilbert, D. Crane, P. J. Morgan, T. J. Mitchell, A. J. Rowe, P. W. Andrew, J. C. Paton, R. K. Tweten, and M. W. Parker. 1998. The molecular mechanism of pneumolysin, a virulence factor from Streptococcus pneumoniae. J. Mol. Biol. 284:449-461. [DOI] [PubMed] [Google Scholar]
- 39.Schroder, N. W., S. Morath, C. Alexander, L. Hamann, T. Hartung, U. Zahringer, U. B. Gobel, J. R. Weber, and R. R. Schumann. 2003. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J. Biol. Chem. 278:15587-15594. [DOI] [PubMed] [Google Scholar]
- 40.Schromm, A. B., E. Lien, P. Henneke, J. C. Chow, A. Yoshimura, H. Heine, E. Latz, B. G. Monks, D. A. Schwartz, K. Miyake, and D. T. Golenbock. 2001. Molecular genetic analysis of an endotoxin nonresponder mutant cell line: a point mutation in a conserved region of MD-2 abolishes endotoxin-induced signaling. J. Exp. Med. 194:79-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schultz, M. J., J. Wijnholds, M. P. Peppelenbosch, M. J. Vervoordeldonk, P. Speelman, S. J. van Deventer, P. Borst, and T. van der Poll. 2001. Mice lacking the multidrug resistance protein 1 are resistant to Streptococcus pneumoniae-induced pneumonia. J. Immunol. 166:4059-4064. [DOI] [PubMed] [Google Scholar]
- 42.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J. Biol. Chem. 274:17406-17409. [DOI] [PubMed] [Google Scholar]
- 43.Shimazu, R., S. Akashi, H. Ogata, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189:1777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strieter, R. M., J. A. Belperio, and M. P. Keane. 2002. Cytokines in innate host defense in the lung. J. Clin. Investig. 109:699-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335-376. [DOI] [PubMed] [Google Scholar]
- 46.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 47.Tapping, R. I., S. Akashi, K. Miyake, P. J. Godowski, and P. S. Tobias. 2000. Toll-like receptor 4, but not toll-like receptor 2, is a signaling receptor for Escherichia and Salmonella lipopolysaccharides. J. Immunol. 165:5780-5787. [DOI] [PubMed] [Google Scholar]
- 48.Triantafilou, K., M. Triantafilou, and R. L. Dedrick. 2001. A CD14-independent LPS receptor cluster. Nat. Immunol. 2:338-345. [DOI] [PubMed] [Google Scholar]
- 49.Wang, M., K. C. Jeng, and L. I. Ping. 1999. Exogenous cytokine modulation or neutralization of interleukin-10 enhance survival in lipopolysaccharide-hyporesponsive C3H/HeJ mice with Klebsiella infection. Immunology 98:90-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang, X., C. Moser, J.-P. Louboutin, E. S. Lysenko, D. J. Weiner, J. N. Weiser, and J. M. Wilson. 2002. Toll-like receptor 4 mediates innate immune responses to Haemophilus influenzae infection in mouse lung. J. Immunol. 168:810-815. [DOI] [PubMed] [Google Scholar]
- 51.Woods, J. P., J. A. Frelinger, G. Warrack, and J. G. Cannon. 1988. Mouse genetic locus Lps influences susceptibility to Neisseria meningitis infection. Infect. Immun. 56:1950-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wright, S. D., R. A. Ramos, P. S. Tobias, R. J. Ulevitch, and J. C. Mathison. 1990. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249:1431-1433. [DOI] [PubMed] [Google Scholar]
- 53.Yinnon, A. M., A. Butnaru, D. Raveh, Z. Jerassy, and B. Rudensky. 1996. Klebsiella bacteraemia: community versus nosocomial infection. QJM 89:933-941. [DOI] [PubMed] [Google Scholar]
- 54.Yoshimura, A., E. Lien, R. R. Ingalls, E. Tuomanen, R. Dziarski, and D. Golenbock. 1999. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J. Immunol. 163:1-5. [PubMed] [Google Scholar]
- 55.Zhang, P., W. R. Summer, G. J. Bagby, and S. Nelson. 2000. Innate immunity and pulmonary host defense. Immunol. Rev. 173:39-51. [DOI] [PubMed] [Google Scholar]