Abstract
Obesity is increasing in prevalence in the United States with over 65% of adults considered overweight and 16% of children with BMI > 95 percentile. The heritability of obesity is estimated between 40 and 70%, but the genetics of obesity for most individuals are complex and involve the interaction of multiple genes and environment. There are however several syndromic and non-syndromic forms of obesity that are monogenic and oligogenic that provide insight into the underlying molecular control of food intake and the neural networks that control ingestive behavior and satiety to regulate body weight and which may interact with treatment exposures to produce or exacerbate obesity in childhood cancer survivors.
Keywords: leptin, leptin receceptor, melanocortin 4 receptor, Prader Willi syndrome, Bardet-Biedl syndrome, Alstrom syndrome, 16p11.2 deletion
Introduction
Obesity or increased adiposity is primarily the result of a net imbalance of caloric intake over energy expenditure over time. Even small differences resulting in positive energy balance - when integrated over long periods of time -can produce increased adiposity. In some instances, preferential partitioning of excess calories towards fat can exacerbate the process. With the increasing availability of highly palatable, calorically dense food, as well as increased mechanization and an increasingly sedentary lifestyle, net positive energy balance in many individuals has resulted in alarming increases in obesity worldwide. In the United States, 65% of adults are considered overweight (BMI 25.0–29.9) and more than 30% of the adult population is now considered obese (BMI > 30) [1]. The problem also affects children in whom the percentage with BMI > 95 percentile between the ages of 6–19 is now 16% [2].
Molecular Elements in the Control of Body Weight
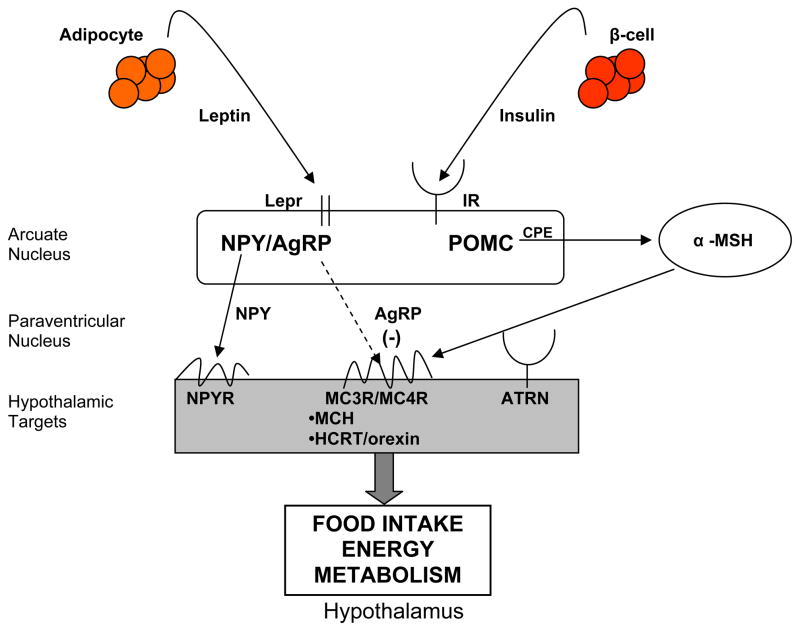
Body weight and fat stores are determined by the net excess or deficit of food intake over energy expenditure. The hypothalamus acts centrally to integrate redundant signaling pathways involving the neuroendocrine and autonomic nervous systems to determine food intake, energy expenditure, and nutrient partitioning. Leptin and insulin are secreted in proportion to peripheral fat mass, and signal the hypothalamus regarding the state of long-term energy stores (Figure 1) [3]. Low concentrations of leptin and insulin generate an anabolic signal to increase food intake and reduce energy expenditure [3]. Leptin and insulin bind to receptors on neurons in the arcuate nucleus, which is partially outside of the blood brain barrier. The arcuate nucleus contains two discrete neuronal populations producing either AgRP and NPY, or pro-opiomelanocortin (POMC) and CART that act reciprocally to increase and decrease food intake, respectively, and to transduce outflow signals regulating body fat stores [3]. Leptin and insulin inhibit the NPY/AgRP neurons and reciprocally stimulate the POMC/CART neurons. AgRP is the naturally occurring inverse agonist of MC3R and MC4R and is expressed in cell bodies in the arcuate that co-express NYP and that project to second order nuclei expressing MC3R and MC4R to stimulate food intake. Energy expenditure is then coordinated through the autonomic nervous system and hypothalamic control of thyroid function.
Figure 1.
Molecular control of energy homeostasis. Peripheral signals including leptin and insulin to bind to receptors on cell bodies in the arcuate nucleus of the hypothalamus. neuropeptide Y (NPY)/agouti-related protein (AgRP) and pro-opiomelanocortin (POMC)/cocaine and amphetamine regulated transcript (CART) neurons in the arcuate nucleus project onto cell bodies in other hypothalamic nuclei to affect energy balance through food intake, energy expenditure, and nutrient partitioning. The melanocortin pathway is an integral part of the control of energy homeostasis. α-MSH (alpha melanotropin) is derived from proteolytic processing of POMC and is an agonist (solid arrow) for melancortin receptor 3 (MC3R)/ (melanocortin receptor 4 (MC4R) centrally producing catabolic effects on energy homeostasis. AgRP is an inverse agonist (dashed arrow) at MC3R/MC4R, producing anabolic effects on energy balance. (BBB: blood brain barrier; LEPR: leptin receptor; IR: insulin receptor; CPE: carboxypeptidase E; ATRN: attractin; MCH: melanin concentrating hormone; HCRT: hypocretin/orexin)
Human adiposity resolves complex interactions among genetic, developmental, behavioral, and environmental influences. Evidence for potent genetic contributions to human obesity is provided by familial clustering of increased adiposity, including a 3–7 fold increased relative risk (λs) among siblings [4] as well as estimates of heritability (the fraction of the total phenotypic variance of a quantitative trait caused by genes in a specified environment) for fat mass between 40 and 70 percent in twin studies [5,6]. Clearly, genetic change cannot account for the recent trends toward increased adiposity. As the environment becomes more, or less, conducive to the development of obesity (ease of access to food, need for physical exertion to obtain it, putative intrauterine and perinatal influences), the median adiposity of the population shifts accordingly. The phenotypic differences among individuals at these extremes of adiposity presumably reflect allelic variation at genes that affect energy intake, expenditure and the chemical form in which excess calories are stored (partitioning).
Genetic factors are currently estimated to account for 40 to 70 percent of the variance in human adiposity [4]. In most individuals, the genetic basis for obesity is complex and likely to involve the interaction of multiple genes as well as gene-by-environment interactions. As with other complex phenotypes, there are rare examples of mono/oligogenic causes for obesity that serve as models for understanding the complex hormonal and neural networks that regulate adiposity, and provide insight to pathways that may account for more common causes of obesity as well as provide targets for therapeutic intervention. In this chapter we review the important genetic and physiological insights provided by the study of these relatively rare forms of obesity.
Non-syndromic Monogenic Obesity
The understanding of body weight regulation in humans has been tremendously aided by the study of monogenic rodent models of obesity. For most of the genes causing obesity in murine models, human counterparts have been identified with generally similar physiology.
Leptin deficiency
Leptin was identified as a cytokine-like hormone secreted almost exclusively by adipocytes and deficient in the obese (Lepob/Lepob) mouse [7]. By screening obese subjects for serum leptin concentrations, Montague et al. were able to identify a single family with two children with undetectable levels of leptin in plasma [8]. Human congenital leptin deficiency is inherited in an autosomal recessive manner and produces extreme, early-onset obesity associated with intense hyperphagia [9]. Deficiency may be the result of a frameshift mutation ΔG133, producing a truncated protein that is not secreted [9] or a missense Arg105Trp mutation that is associated with low levels of circulating leptin [10]. Congenital leptin deficiency in humans is associated with hyperphagia but normal resting and free living energy expenditure, hypogonadotrophic hypogonadism with delayed but spontaneous pubertal development, and abnormalities of T-cell number and function [11]. Injected leptin replacement produced normalization of hyperphagia in three affected children without demonstrable effects on basal metabolic rate or free living energy expenditure even within the setting of weight loss [11], suggesting that the greatest effect of leptin deficiency is on food intake, but that energy expenditure was raised above that expected in a weight-losing subject.
Leptin receptor deficiency
Soon after Lep was identified, the leptin receptor (Lepr) was discovered and loss of function mutations were identified in the diabetes mouse allelic series [12–14] as well as the fatty rat [15,16]. Lepr is a member of the cytokine receptor family and mediates leptin signaling through phosphatidylinositol 3-kinase and signal transducer and activator of transcription-3 (STAT3), predominantly in hypothalamic neurons [17]. STAT3 signaling is crucial for the regulation of food intake, but not critical for the regulation of reproduction and growth. By screening obese human subjects for elevated serum leptin concentrations, a consanguineous family was identified in which three members showed extreme early-onset obesity associated with statural growth retardation caused by impaired growth hormone secretion [18]. All three subjects were homozygous for a splice site mutation in exon 16 that truncates the receptor before the transmembrane domain, rendering all cells incapable of transmitting an intracellular signal. Human leptin receptor deficiency produces extreme obesity in an autosomal recessive manner. Human leptin receptor-deficient subjects have normal basal temperature, resting metabolic rates, spontaneous but delayed puberty, and normal plasma cortisol concentrations [18].
Pro-opiomelancocortin deficiency
Agouti (Yellow) was the first murine gene related to monogenic obesity to be positionally cloned [19]. The autosomal dominant agouti promoter mutation Ay results in ubiquitous ectopic overexpression of agouti signaling protein (ASP) throughout the body, producing the characteristic yellow coat color when it antagonizes the binding of alpha melanocortin stimulating hormone (α MSH) at melancortin 1 receptors (MC1R) in the skin, and producing increased length as well as body mass through antagonism of the melanocortin 3 and 4 receptors (MC3R and MC4R) in the hypothalamus [20]. The natural agonist of the melanocortin receptors, alpha melanocortin stimulating hormone (αMSH), suppresses food intake and increases energy expenditure by actions at MC4R. The physiological antagonist (and inverse agonist) at MC4R was later identified as agouti related protein (AgRP). α MSH and ACTH are both derived from proopiomelanocortin (POMC) by sequential cleavage by prohormone convertases (see PC1 mutation below) and other processing enzymes in the arcuate nucleus of the hypothalamus. Many of the POMC neurons in the arcuate nucleus also express the leptin receptor, and POMC expression is positively correlated with ambient leptin [21]. Targeted disruption of Mc4r and to a lesser extent Mc3r in mice produces obesity and increased linear growth similar to the Ay mice except for the expected lack of effect on coat color [22].
Autosomal recessive POMC deficiency is due to compound heterozygosity or homozygosity for loss-of-function mutations in a small number of human subjects produces severe, early-onset obesity associated with hyperphagia [23–25] due to lack of αMSH acting centrally at MC3R and MC4R. Because of a lack of peripheral α MSH action, the children also demonstrated pale skin color and red hair due to lack of peripheral agonism at MC1R. The five children initially presented with undetectable levels of cortisol and ACTH early in infancy, consistent with the absence of ACTH ligand for the adrenal cortical MC2R. Heterozygous individuals have been found to have intermediate increases in body weight, suggesting a gene dosage effect for POMC [26].
Prohormone convertase 1 deficiency
Like the Pomc knockout mouse, the fat mouse is an example of autosomal recessive obesity of later onset and reduced severity relative to the obese and diabetes mice. Observation of increased levels of circulating proinsulin in these mice led to the identification of the Ser202Pro mutation in the positional candidate gene carboxypeptidase E (Cpe) that is responsible for prohormone cleavage of C-terminal basic residues from prohormones and proneuropeptides such as proinsulin, proneuropeptide Y, progonadotropin, and POMC [27]. Realizing that aberrant prohormone processing could produce obesity, Jackson et al. identified two subjects with compound heterozygous mutations in prohormone convertase 1 (PC1), an enzyme that cleaves prohormones at dibasic amino acids in the step immediately prior to CPE processing [28]. Both subjects have been described as having childhood onset obesity, elevated proinsulin, hypocortisolemia with elevated POMC, reactive hypoglycemia, and hypogonadotropic hypogonadism [28,29]. The subjects’ obesity phenotype is likely due to aberrant POMC and other prohormone processing, and the phenotype of the human subjects recapitulates that of the fat mouse.
Melanocortin 4 receptor deficiency
Perhaps the most common monogenic form of obesity in humans is due to mutations in MC4R. The mutations are generally inherited in a co-dominant manner, and homozygous loss of function mutations have been identified and result in more severe obesity in the homozygous state than the heterozygous state [30–33]. The penetrance of heterozygous MC4R mutations is, however, incomplete for both partially active and inactive MC4R mutations, especially in males [32]. The prevalence of mutations in MC4R appears to vary between <1 to 6 percent of cases of severe obesity [34] depending on the age of onset and severity of obesity in each study population. Notably, most of the mutations identified have been missense mutations and almost all are unique and found in single families [30–33]. There is no evidence to date of common founder mutations that would account for a significant fraction of the variance in obesity. Phenotypically, carriers of MC4R mutations have early onset hyperphagia, increased fat mass, increased lean mass, increased bone mineral density and bone mineral content, increased linear growth, and hyperinsulinemia relative to fat mass [34], findings similar to those of the Mc4r knockout mouse. Unlike subjects with mutations in LEP or LEPR, carriers of MC4R mutations tend to have amelioration of their obesity and hyperinsulinemia over time [34].
Lessons to be learned from non-syndromic obesity
Monogenic forms of non-syndromic obesity have elegantly demonstrated the utility of animal models in identifying key molecular components in the control of food intake and energy expenditure. Most of the recent advances in this field have been based upon the identification of genes and metabolic pathways originally detected and elucidated in rodent models. Furthermore, with the exception of mutations in MC4R, humans with other monogenic forms of non-syndromic obesity were identified only after defining a specific sub-phenotype (high or low leptin, hyperproinsulinemia, hypocortisolemia, unusual skin and hair pigmentation) implicating a defect in a particular molecular pathway. This experience underscores the need, when possible, to collect sub-phenotypic data when attempting to determine the genetic basis for non-syndromic obesity. Additionally, it should be noted that in most cases of non-syndromic obesity in humans, the largest contribution to positive energy balance is apparently excess caloric intake. This characteristic may also be more generally applicable to common forms of obesity and suggests that a significant portion of intervention and prevention strategies should be focused on controlling food intake.
Syndromic obesity
Syndromic obesity is obesity occurring in the clinical context of a distinct set of associated clinical phenotypes. Over 25 syndromic forms of obesity have been identified. Recently, the genetic bases for some of these syndromes have been elucidated, and are beginning to provide insights into the pathogenesis of the derangements of energy homeostasis. Interestingly, although clinically well-defined, there is increasing evidence of genetic heterogeneity for some of these conditions with multiple genes within the same pathway producing identical phenotypes. This finding suggests that for more common polygenetic forms of nonsyndromic obesity, multiple allelic variants within the same molecular pathway may interact in either additive or synergistic ways to produce increasing adiposity. Presented below are a few of the most common syndromic forms of obesity for which the genetic basis has been partially or completely elucidated.
Prader Willi syndrome
Prader Willi syndrome (PWS), the most common syndromic form of obesity, with an incidence of approximately one in 15,000–25,000 live births, is the result of loss of expression of paternal genes on the imprinted region of 15q11–13. PWS is characterized by intrauterine and neonatal hypotonia, poor feeding, and failure to thrive that evolves into extreme hyperphagia and central obesity at one to six years of age if caloric restriction is not imposed [35,36]. Genetically, Prader Willi syndrome can have several etiologies but is always associated with loss of expression of paternally transmitted genes on 15q11–q13. 75% of cases are due to paternal deletions of 15q11–q13, 22% are due to maternal uniparental disomy, less than 3% are due to imprinting errors caused by microdeletions of the imprinting center at the SNURF-SNRPN gene locus, and less than 1% are due to paternal translocations [37]. Regardless of the type of mutation, all patients with PWS share the same basic clinical features. There are no known cases of isolated obesity associated with aberrations in a single gene within the 15q11–q13 critical region [37].
Bardet-Biedl syndrome
Bardet-Biedl syndrome (BBS) is a rare syndromic form of obesity. The genetics of BBS before the discovery of the underlying genes was thought to be classical autosomal recessive. Although the resulting phenotypes are essentially indistinguishable, twelve different genes have now been identified as causing BBS. The disease segregates in families as both a classical autosomal recessive trait as well as a digenetic trait in which three or even four alleles interact to determine the penetrance of BBS or to modify the severity and age of onset of disease manifestations [38]. All of the common forms of BBS have been associated with tri- or tetra-allelic inheritance [39].
Identification of the BBS8 gene encoding a protein involved in pilus formation and twitching mobility that localizes to the basal bodies and centrosome in physical juxtaposition to BBS4 suggests that these proteins play a role in the function of the pericentriolar region of ciliated cells [40]. It is, therefore, possible that the underlying pathogenic mechanism for BBS involves dysfunction of the basal body in ciliated cells [40]. C. elegans orthologs of BBS1, BBS2, BBS7, and BBS8 are also expressed in the ciliated dendritic endings of neurons. The multiple genes causing BBS are components of a molecular complex or act sequentially in the same cellular process(es) to cause progressively more severe dysfunction as mutations are added in genes in the same pathway. This model of multiple mutations within the same pathway may also be more broadly applicable to the much more common polygenic forms of common obesity. The genetic complexity of this very distinct phenotype of BBS may also be a clue to the genetic complexity of polygenic obesity, and to strategies for unraveling it by close attention to gene-gene interactions in putative biochemical, structural and functional pathways.
Alstrom syndrome
Alstrom syndrome is a rare autosomal recessive, genetically homogeneous disorder characterized by mild truncal obesity that usually begins within the first year of life and continues throughout life unless caloric restriction is imposed [41,42]. The gene for Alstrom syndrome, ALMS1, encodes a gene of unknown function expressed ubiquitously at low levels [43,44]. The mechanism by which loss-of -function mutations in this gene produce obesity or any of the other associated phenotypes is still unknown.
WAGR syndrome
Heterozygous interstitial deletion of 11p13 result in Wilms tumor, aniridia, genitourinary anomalies and mental retardation (WAGR). A subset of WAGR patients are observed to also have hyperphagia and obesity. By molecularly comparing the extent and overlap of the 11p13 deletion in WAGR patients with and without obesity, it became obvious that the patients with obesity encompassed deletions of brain-derived neurotrophic factor (BNDF) [45]. Patient with the BNDF deletion had 50% lower serum BNDF concentrations and significantly higher BMI Z scores throughout childhood than those WAGR patients with deletions that did not encompass BNDF. Obesity was fully penetrant by age 10. BNDF is expressed in the brain and seems to be most directly related to food intake and hyperphagia rather than energy expenditure.
16p11.2 deletions
In screening two cohort of patients with extreme obesity, enriched for patients with birth defects and/or neurocognitive deficiencies using method to detect copy number variations, recurrent, de novo deletions of 16p11.2 were identified in approximately 3% of cases. The obesity phenotype was incompletely penetrant in childhood, and the penetrance increased with age with complete penetrance in the adults [46,47]. Patients were hyperphagic, and energy intake seems to be more affected than energy expenditure in the energy balance. Interestingly, this deletion overlaps with a 593 kb deletion on 16p11.2 associated with autism [48–50]. The deletion includes SH2B1, an adaptor protein involved in leptin and insulin signaling which may be involved in the pathogenesis of the obesity and insulin resistance observed in this deletion [46].
Lessons to be learned from syndromic obesity
Most syndromic forms of obesity are associated with mild to severe cognitive deficits and unusual behaviors. While many other syndromes affecting cognition such as Down syndrome are also associated with a higher incidence of obesity [51], the syndromic forms of obesity described above appear to have specific effects on food intake. These data suggest that there may be specific neuroanatomic or functional deficits, particularly in the hypothalamus, that lead to increased energy intake. The development of new techniques including functional imaging of the brain should permit noninvasive determination of which parts of the brain function aberrantly in the context of food intake in each of the syndromic forms of obesity.
Summary
The human syndromic obesities will probably have most heuristic significance for the new molecules and pathways that they reveal in the complex regulatory system that controls body weight. The relevance of these genes and pathways in the genetics that clearly underlies susceptibility to obesity in humans will require the same sort of large scale analyses that will be needed for other putative molecular players in the relevant processes. Fundamentally, this will require the simultaneous consideration of several alleles of many genes in very large numbers of well phenotyped subjects. Awareness of the relationships among specific candidate molecules gleaned from model organisms, and syndromic or sporadic severe obesities in humans will help to rationalize the selection of genes for any specific analysis, and permit explicit mechanistic hypothesis testing, thereby enhancing statistical power. Further refinement can be achieved by selection of subjects based upon specific subphenotypes.
Table I.
Non-syndromic monogenic forms of obesity in rodents and humans
| Gene | Murine Mutation | Murine Phenotype | Human Mutation | Human Phenotype |
|---|---|---|---|---|
| Leptin |
obese Lepob Lepob2j |
Extreme, early-onset obesity, decreased length, hyperphagia, hypogonadotrophic hypogonadism, cold intolerance, hypercosticosteronemia, T-cell abnormalities | ΔG133 Arg105Trp |
Extreme, early-onset obesity, hyperphagia, delayed puberty, T-cell abnormalities |
| Leptin receptor |
diabetes Leprdb |
Extreme, early-onset obesity, decreased length, hyperphagia, hypogonadotrophic hypogonadism, cold intolerance, hypercortisolemia, T-cell abnormalities | Exon 16 splice donor G=>A | Extreme, early-onset obesity, short stature, hyperphagia, delayed puberty |
| Pro-opiomelanocortin | Induced deletion of Pomc | Later onset obesity, slightly increased length, adrenal agenesis, yellow coat color | G7013T, 7133delC, C3804A A6851T 6906delC 6996del 7100insGG 7134delG | Early onset obesity, adrenal insufficiency, red hair |
| Agouti signaling peptide | Ay | Later onset obesity, increased length, yellow coat color | none | |
| Carboxypeptidase E | Cpefat | Late onset obesity, hyperproinsulinemia | none | |
| Proconvertase 1 | Induced deletion of Pc1 | Dwarf with low GHRH, normal weight, elevated proinsulin, normal corticosterone | Gly483Arg, A=>C+4 intron 5 donor splice site, Glu250Stop, Del213Ala | Childhood onset obesity, elevated proinsulin, hypocortisolemia, depressed POMC, reactive hypoglycemia, hypogonadotrophic hypogonadism |
| Melanocortin 4 receptor | Induced deletion of Mc4r | Early onset obesity, hyperphagia, increased fat mass, increased lean mass, increased linear growth, and hyperinsulinemia | Numerous | Early onset obesity, hyperphagia, increased fat mass, increased lean mass, increased bone mineral density and bone mineral content, increased linear growth, and hyperinsulinemia |
Table II.
Syndromic Forms of Human Obesity
| Syndrome | Gene | Mode of inheritance | Phenotype |
|---|---|---|---|
| Prader Willi syndrome | Contiguous gene disorder | Imprinting defect with loss of paternally expressed genes on 15q11–13 | Neonatal hypotonia, poor feeding, evolving into extreme hyperphagia, central obesity, decreased lean body mass, short stature, hypothalamic hypogonadism, mild mental retardation, obsessive compulsive behavior |
| Bardet-Biedl syndrome | At least 12 loci (BBS1- BBS12) 12 genes identified | Oligogenic: either autosomal recessive or tri- tetra allelic | Progressive rod-cone dystrophy, post axial polydactyly, renal cysts, progressive renal disease, dyslexia, learning disabilities, hypogonadism, occasional congenital heart disease, and progressive late childhood obesity |
| Alstrom syndrome | ALMS1 | Autosomal recessive | Mild truncal obesity, short stature, type 2 diabetes, retinopathy, sensorineural hearing loss, nephropathy, dilated cardiomyopathy |
| WAGR syndrome | BNDF | Autosomal dominant | Obesity, Wilms’ tumor, aniridia, genitourinary anomalies, mental retardation |
| 16p11.2 deletion | Autosomal dominant | Progressive obesity, autism/mental retardation |
Acknowledgments
We appreciate the assistance of Josue Martinez with manuscript preparation. This work has been supported in part by NIH DK52431.
References
- 1.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288(14):1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 2.Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291(23):2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 3.Woods SC, Seeley RJ, Porte D, Jr, et al. Signals that regulate food intake and energy homeostasis. Science. 1998;280(5368):1378–1383. doi: 10.1126/science.280.5368.1378. [DOI] [PubMed] [Google Scholar]
- 4.Allison DB, Faith MS, Nathan JS. Risch's lambda values for human obesity. Int J Obes Relat Metab Disord. 1996;20(11):990–999. [PubMed] [Google Scholar]
- 5.Stunkard AJ, Foch TT, Hrubec Z. A twin study of human obesity. Jama. 1986;256(1):51–54. [PubMed] [Google Scholar]
- 6.Stunkard AJ, Harris JR, Pedersen NL, et al. The body-mass index of twins who have been reared apart. N Engl J Med. 1990;322(21):1483–1487. doi: 10.1056/NEJM199005243222102. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 8.Montague CT, Farooqi IS, Whitehead JP, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387(6636):903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 9.Rau H, Reaves BJ, O'Rahilly S, et al. Truncated human leptin (delta133) associated with extreme obesity undergoes proteasomal degradation after defective intracellular transport. Endocrinology. 1999;140(4):1718–1723. doi: 10.1210/endo.140.4.6670. [DOI] [PubMed] [Google Scholar]
- 10.Strobel A, Issad T, Camoin L, et al. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet. 1998;18(3):213–215. doi: 10.1038/ng0398-213. [DOI] [PubMed] [Google Scholar]
- 11.Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110(8):1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chua SC, Jr, Chung WK, Wu-Peng XS, et al. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271(5251):994–996. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- 13.Lee GH, Proenca R, Montez JM, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379(6566):632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 14.Tartaglia LA, Dembski M, Weng X, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83(7):1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 15.Phillips MS, Liu Q, Hammond HA, et al. Leptin receptor missense mutation in the fatty Zucker rat. Nat Genet. 1996;13(1):18–19. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- 16.Wu-Peng XS, Chua SC, Jr, Okada N, et al. Phenotype of the obese Koletsky (f) rat due to Tyr763Stop mutation in the extracellular domain of the leptin receptor (Lepr): evidence for deficient plasma-to-CSF transport of leptin in both the Zucker and Koletsky obese rat. Diabetes. 1997;46(3):513–518. doi: 10.2337/diab.46.3.513. [DOI] [PubMed] [Google Scholar]
- 17.Bates SH, Myers MG., Jr The role of leptin receptor signaling in feeding and neuroendocrine function. Trends Endocrinol Metab. 2003;14(10):447–452. doi: 10.1016/j.tem.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Clement K, Ferre P. Genetics and the pathophysiology of obesity. Pediatr Res. 2003;53(5):721–725. doi: 10.1203/01.PDR.0000059753.61905.58. [DOI] [PubMed] [Google Scholar]
- 19.Bultman SJ, Michaud EJ, Woychik RP. Molecular characterization of the mouse agouti locus. Cell. 1992;71(7):1195–1204. doi: 10.1016/s0092-8674(05)80067-4. [DOI] [PubMed] [Google Scholar]
- 20.Rossi M, Kim MS, Morgan DG, et al. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139(10):4428–4431. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- 21.Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997;138(10):4489–4492. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- 22.Huszar D, Lynch CA, Fairchild-Huntress V, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88(1):131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 23.Krude H, Biebermann H, Luck W, et al. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19(2):155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 24.Krude H, Biebermann H, Schnabel D, et al. Obesity due to proopiomelanocortin deficiency: three new cases and treatment trials with thyroid hormone and ACTH4-10. J Clin Endocrinol Metab. 2003;88(10):4633–4640. doi: 10.1210/jc.2003-030502. [DOI] [PubMed] [Google Scholar]
- 25.Buono P, Pasanisi F, Nardelli C, et al. Six novel mutations in the proopiomelanocortin and melanocortin receptor 4 genes in severely obese adults living in southern Italy. Clin Chem. 2005;51(8):1358–1364. doi: 10.1373/clinchem.2005.047886. [DOI] [PubMed] [Google Scholar]
- 26.Farooqi IS, Drop S, Clements A, et al. Heterozygosity for a POMC-null mutation and increased obesity risk in humans. Diabetes. 2006;55(9):2549–2553. doi: 10.2337/db06-0214. [DOI] [PubMed] [Google Scholar]
- 27.Naggert JK, Fricker LD, Varlamov O, et al. Hyperproinsulinaemia in obese fat/fat mice associated with a carboxypeptidase E mutation which reduces enzyme activity. Nat Genet. 1995;10(2):135–142. doi: 10.1038/ng0695-135. [DOI] [PubMed] [Google Scholar]
- 28.Jackson RS, Creemers JW, Ohagi S, et al. Obesity and impaired proohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet. 1997;16(3):303–306. doi: 10.1038/ng0797-303. [DOI] [PubMed] [Google Scholar]
- 29.Jackson RS, Creemers JW, Farooqi IS, et al. Small-intestinal dysfunction accompanies the complex endocrinopathy of human proprotein convertase 1 deficiency. J Clin Invest. 2003;112(10):1550–1560. doi: 10.1172/JCI18784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farooqi IS, Keogh JM, Yeo GS, et al. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348(12):1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 31.Vaisse C, Clement K, Durand E, et al. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Invest. 2000;106(2):253–262. doi: 10.1172/JCI9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farooqi IS, Yeo GS, Keogh JM, et al. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest. 2000;106(2):271–279. doi: 10.1172/JCI9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinney A, Schmidt A, Nottebom K, et al. Several mutations in the melanocortin-4 receptor gene including a nonsense and a frameshift mutation associated with dominantly inherited obesity in humans. J Clin Endocrinol Metab. 1999;84(4):1483–1486. doi: 10.1210/jcem.84.4.5728. [DOI] [PubMed] [Google Scholar]
- 34.Lubrano-Berthelier C, Cavazos M, Dubern B, et al. Molecular genetics of human obesity-associated MC4R mutations. Ann N Y Acad Sci. 2003;994:49–57. doi: 10.1111/j.1749-6632.2003.tb03161.x. [DOI] [PubMed] [Google Scholar]
- 35.Schulze A, Mogensen H, Hamborg-Petersen B, et al. Fertility in Prader-Willi syndrome: a case report with Angelman syndrome in the offspring. Acta Paediatr. 2001;90(4):455–459. [PubMed] [Google Scholar]
- 36.Boer H, Holland A, Whittington J, et al. Psychotic illness in people with Prader Willi syndrome due to chromosome 15 maternal uniparental disomy. Lancet. 2002;359(9301):135–136. doi: 10.1016/S0140-6736(02)07340-3. [DOI] [PubMed] [Google Scholar]
- 37.Nicholls RD, Knepper JL. Genome organization, function, and imprinting in Prader-Willi and Angelman syndromes. Annu Rev Genomics Hum Genet. 2001;2:153–175. doi: 10.1146/annurev.genom.2.1.153. [DOI] [PubMed] [Google Scholar]
- 38.Badano JL, Kim JC, Hoskins BE, et al. Heterozygous mutations in BBS1, BBS2 and BBS6 have a potential epistatic effect on Bardet-Biedl patients with two mutations at a second BBS locus. Hum Mol Genet. 2003;12(14):1651–1659. doi: 10.1093/hmg/ddg188. [DOI] [PubMed] [Google Scholar]
- 39.Beales PL, Badano JL, Ross AJ, et al. Genetic interaction of BBS1 mutations with alleles at other BBS loci can result in non-Mendelian Bardet-Biedl syndrome. Am J Hum Genet. 2003;72(5):1187–1199. doi: 10.1086/375178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ansley SJ, Badano JL, Blacque OE, et al. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003;425(6958):628–633. doi: 10.1038/nature02030. [DOI] [PubMed] [Google Scholar]
- 41.Alstrom CH, Hallgren B, Nilsson LB, et al. Retinal degeneration combined with obesity, diabetes mellitus and neurogenous deafness: a specific syndrome (not hitherto described) distinct from the Laurence-Moon-Bardet-Biedl syndrome: a clinical, endocrinological and genetic examination based on a large pedigree. Acta Psychiatr Neurol Scand Suppl. 1959;129:1–35. [PubMed] [Google Scholar]
- 42.Marshall JD, Ludman MD, Shea SE, et al. Genealogy, natural history, and phenotype of Alstrom syndrome in a large Acadian kindred and three additional families. Am J Med Genet. 1997;73(2):150–161. doi: 10.1002/(sici)1096-8628(19971212)73:2<150::aid-ajmg9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 43.Hearn T, Renforth GL, Spalluto C, et al. Mutation of ALMS1, a large gene with a tandem repeat encoding 47 amino acids, causes Alstrom syndrome. Nat Genet. 2002;31(1):79–83. doi: 10.1038/ng874. [DOI] [PubMed] [Google Scholar]
- 44.Collin GB, Marshall JD, Ikeda A, et al. Mutations in ALMS1 cause obesity, type 2 diabetes and neurosensory degeneration in Alstrom syndrome. Nat Genet. 2002;31(1):74–78. doi: 10.1038/ng867. [DOI] [PubMed] [Google Scholar]
- 45.Han JC, Liu QR, Jones M, et al. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. N Engl J Med. 2008;359(9):918–927. doi: 10.1056/NEJMoa0801119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bochukova EG, Huang N, Keogh J, et al. Large, rare chromosomal deletions associated with severe early-onset obesity. Nature. 2010;463(7281):666–670. doi: 10.1038/nature08689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walters RG, Jacquemont S, Valsesia A, et al. A new highly penetrant form of obesity due to deletions on chromosome 16p11.2. Nature. 2010;463(7281):671–675. doi: 10.1038/nature08727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar RA, KaraMohamed S, Sudi J, et al. Recurrent 16p11.2 microdeletions in autism. Hum Mol Genet. 2008;17(4):628–638. doi: 10.1093/hmg/ddm376. [DOI] [PubMed] [Google Scholar]
- 49.Marshall CR, Noor A, Vincent JB, et al. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet. 2008;82(2):477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiss LA, Shen Y, Korn JM, et al. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med. 2008;358(7):667–675. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- 51.Bell AJ, Bhate MS. Prevalence of overweight and obesity in Down's syndrome and other mentally handicapped adults living in the community. J Intellect Disabil Res. 1992;36 ( Pt 4):359–364. doi: 10.1111/j.1365-2788.1992.tb00534.x. [DOI] [PubMed] [Google Scholar]