1. The paradox
Normally, pain is felt when signals generated by noxious stimuli in thinly myelinated-Aδ or unmyelinated-C nociceptive afferents reach a conscious brain. We propose that the intrinsic innervation of teeth may be a unique exception. Teeth are densely innervated by primary sensory neurons located in the trigeminal ganglion (TRG). There are many Aδ- and C-fibers in the tooth-pulp, with the Aδ-axons ending mostly in the inner third of the dentinal tubules and the C-fibers ending mostly in the pulp itself [5,6,16,21,34,35]. This, coupled with the fact that tooth-pulp stimulation primarily evokes pain, has lead to the doctrine that Aδ- and C-nociceptors dominate intrinsic tooth innervation. Among the few Aβ-fibers present, some might be nociceptive [12], but most are thought to be low-threshold mechanoreceptors (LTMs) that subserve ‘pre-pain’, the non-painful tingling reported when the tooth crown is stimulated electrically at liminal current strength [13,47].
However, as most dental patients can testify, weak mechanical stimuli such as air-puffs and water-spray evoke intense pain when directed at exposed dentin [10]. As these stimuli do not activate nociceptors or evoke pain on skin or gingiva, there must be something different about teeth. Is mechanically evoked dentinal pain indeed due to impulses in afferent nociceptors?
2. A novel solution
It has been proposed that in the presence of tooth pathology such as dental caries, nociceptive endings become sensitized [5,45]. The sensitized nociceptor theory, however is problematic. In the skin, inflammation causes Aδ- and C-nociceptors to become sensitized to heat stimuli, but much less so to weak tactile stimuli [1,3]. Inflamed tooth-pulp afferents also undergo sensitization to thermal stimuli and there is at least some evidence of increased sensitivity to tactile stimuli [5,34]. But even if inflammation does trigger sensitization of tooth-pulp afferents this does not solve the problem. Non-inflamed dentin is frequently exposed when healthy teeth are prepared as anchors for dental bridge work, and it is highly sensitive. Tactile sensitivity is especially prominent when the “dentinal smear layer” left by drilling is removed [8,10].
Another potential solution comes from the “hydrodynamic” theory. This holds that force applied at the top of dentinal tubules is transmitted to the sensory transduction apparatus deep inside by mechanical displacement (flow) of the fluid filling the tubule [4,48]. While this is undoubtedly true, fluid transmission in itself should not amplify the force, making it strong enough to activate nociceptive endings. In principle, amplification could occur as in hydraulic brakes, where modest force applied to the brake pedal (over a long distance) generates a large force on the brake disks (over a short distance). This analogy is conceivable when the dentin is softened by phosphoric acid “etching” [8]. But in freshly exposed dentin, afferent endings reside in tiny tubules in a rigid matrix. Moderate force applied with probes larger than the tubule’s diameter (microns) should not move fluid and hence should not be felt at all. Fluid pressure from air-puffs or water-spray, of course, is freely conveyed to sensory endings within the tubules.
A third proposed solution to the paradox is that odontoblasts, rather than nociceptive sensory endings, respond to weak mechanical displacements and that these secondarily activate the nociceptive endings through a coupling mechanism, perhaps chemical, that does not require force amplification [30].
We offer a radically different explanation. Briefly, we propose that many dentinal afferents are not nociceptors at all, but rather LTMs. Most are probably A-fibers, but low-threshold C-fibers [46] could also contribute. This hypothesis does not require receptor sensitization, force amplification or sui generis forms of stimulus-afferent coupling. Air-puffs and modest water pressure are fully capable of activating normal LTMs. As proof, they evoke a tactile sensation when applied to the gingiva and skin. Our hypothesis is also consistent with the hydrodynamic theory. However, it leaves a key question unsolved. How could activation of LTMs in the dentin evoke pain?
3. Tooth-pulp afferents
Within the pulp of permanent teeth in experimental animals and humans, 70–90% of axons are devoid of myelin and most of the remainder appear to be Aδ–nociceptors [6,16,21]. But notwithstanding their appearance within the pulp, the parent axons of most pulp afferents are myelinated and have a large diameter and rapid conduct velocity at their point of entry into the tooth [5,7,29,33,36,48]. These observations suggest that in teeth, at least in the types sampled, most dental afferents branch, taper, and lose their myelin soon after they enter the pulp chamber. This is not unlike the terminal part of afferents in other tissues. Fibers that are unmyelinated along their entire length include sympathetic efferents serving the pulpal vasculature [6,21]. The remaining few no doubt include classical C-fiber nociceptors which, along with A-fiber nociceptors, support heat and cold pain sensation.
Our suggestion that many intradental afferents are in fact tapering A-fiber LTMs is consistent with observations on the cell-body-of-origin of these axons within the TRG, identified by applying retrograde tracers to the pulp. Most (70–90%) are large or medium in diameter, have a lucent cytoplasm in electron microscopic images [18,36], and express cytochemical markers common in LTMs (Fig.1,2). These include RT97 (NF200), carbonic anhydrase, calretinin, parvalbumin, calbindin, epithelial Na+ channels (ENaCs), ASIC3, TREK1, and TREK2 [17,18,20,23–27]. Pulp injury triggers upregulation of NPY, another characteristic of LTM afferents [28]. In contrast, most TRG neurons that project to the buccal mucosa or cornea are small and have the dark cytoplasm and molecular markers typical of nociceptors [17,31,32,36,39–41].
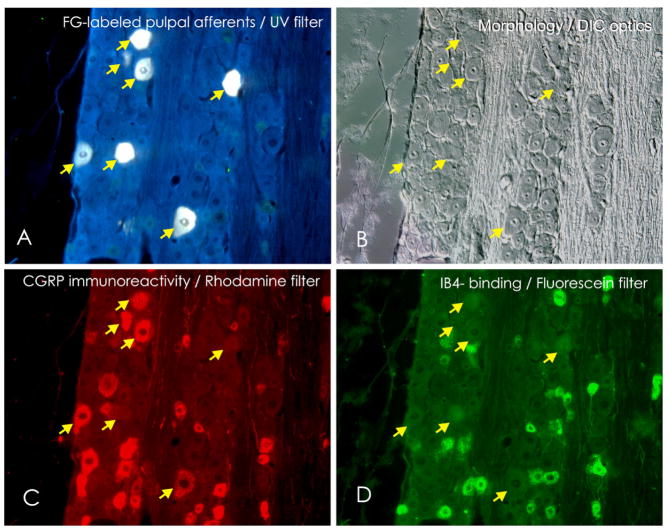
Fig. 1. Many pulpal afferents are of medium and large-diameter, but express neurotransmitters typical of nociceptors.
Images show pulpal afferent neurons in the rat TRG labeled with the tracer fluoro-gold (FG) that was applied to the dentin in the floor of shallow cavities in the mesial and lingual surfaces of the first and second maxillary molars. The cavities were sealed with a light-cured restorative material and after four weeks the TRGs were fixed, removed and processed for immunocytochemistry. A) Arrows show FG-labeled neurons of medium to large sizes (intrinsic fluorescence). B) shows the same field in Nomarski differential interference contrast (DIC) illumination. Many small-diameter neurons are present alongside medium and large neurons. Arrows point to the same neurons indicated in A. C) The same field immunolabeled using an antibody that recognizes CGRP. Many small afferents neurons are labeled, but also some of the medium and large dentinal afferents labeled with FG in panel A (arrows). D) None of the FG-labeled pulpal afferents bind IB4, a lectin associated with a subpopulation of small nociceptive neurons. (Photomicrographs kindly provided by Dr. John Naftel).
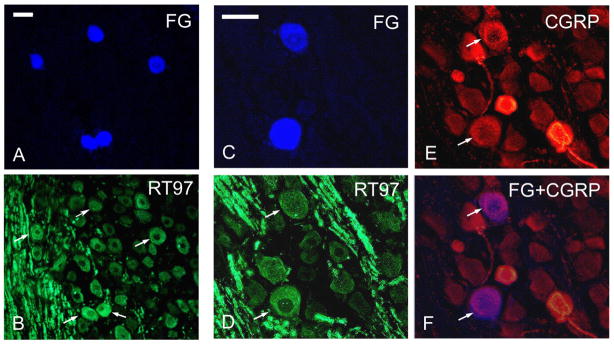
Fig. 2. Individual pulpal neurons in the TRG may express both the nociceptor neurotransmitter CGRP and markers typical of LTMs.
TRG neurons that innervate tooth pulp in rats were labeled by retrograde transport of FG and processed in the same way as described for Fig. 1. A) Five FG-labeled pulpal afferent neurons are shown (intrinsic fluorescence). B) shows the same field immunolabeled using an antibody that recognizes RT97, a marker of medium and large-diameter neuron that are mostly LTMs (Cy2 used as fluorochrome). Arrows indicate the FG-labeled neurons in A. All five express RT97. C) Two large-diameter FG-positive pulpal afferents in the TRG are shown at higher magnification. Both are RT97 immunopositive (arrows in D; Cy3 used as fluorochrome). F) Merging images C and E confirms that these large-diameter RT97 positive pulpal afferents also express CGRP. Scale bars: A,B = 50 μm; C-F = 50 μm.
Evidence that intrinsic tooth afferents are predominantly A-fiber LTMs also comes from observations in rats treated neonatally with capsaicin. In contrast to the massive C-fiber loss seen in hindlimb nerves, pulp afferents resist destruction, and the threshold for eliciting the (nocifensive) jaw-opening reflex remains unchanged [16,43].
Electrophysiological recordings from single tooth-pulp afferents add further support. The same stimuli that cause pain when applied to exposed dentin in humans excite intradental afferents when directed at exposed dentin in animals. These include cold, heat, scraping and strong osmotic stimuli, but also weak mechanical stimuli such as air-puffs and modest hydrostatic pressure. Some fibers are polymodal. Each type of stimulus may activate A-fibers as well as C-fibers [5,8,33,48].
Typical air-puff stimuli are too weak to activate sensitive periodontal afferents and evoke a sensation of (non-painful) tooth loading, but they do evoke pain when directed to exposed dentine [10]. The pain cannot automatically be attributed to LTMs, however, as air-puffs also cause evaporative cooling. We are not aware of attempts to distinguish the tactile from the cooling component, but some clarity may be obtained by considering other tissues. Air-puffs that are non-painful to the gingiva may be painful when directed to dentin. The degree of cooling in the two tissues is similar and likewise the sensitivity of LTMs and cold-sensitive nociceptors. It is therefore reasonable to conclude that the difference in evoked sensation (pain) is largely due to the tactile component of the stimulus. Neutral temperature water-spray applies non-noxious mechanical force without cooling. This has apparently not been tested as a stimulus in electrophysiological experiments, but comparable hydrostatic stimuli applied to exposed dentin do activate intradental afferents [48].
4. Pain following stimulation of A-fiber LTMs
In most tissues, activity in nociceptors normally evokes pain and activity in LTMs normally evokes touch. But there is nothing inherently contradictory about an afferent with low-threshold peripheral characteristics having pain-provoking CNS connectivity. This situation is widely recognized in the context of inflammation and neuropathy [9]. Specifically, due to “central sensitization” A-fiber LTM afferents can evoke tactile allodynia (“Aβ pain”). Our proposal extends this concept by positing that there is constitutive LTM-induced pain in healthy teeth. A number of mechanisms could bring this about.
4.1. Connectivity
Pain on weak tactile stimulation is expected if dentinal LTMs terminate synaptically on trigeminal brainstem neurons that deliver a pain message to higher brain centers. Trigeminal subnucleus caudalis is considered the major nociceptive relay of the trigeminal brainstem complex as it receives massive input from nociceptors that innervate orofacial structures, many of its neurons respond to noxious stimuli, and disrupting the subnucleus may block nocifensive behavior in animals and pain report in humans [37,44]. . Anterograde tracing from the pulp reveals afferent terminations predominantly in the superficial laminae of subnucleus caudalis, but also in its deep laminae [2,31,42]. There is also a substantial termination more rostrally, notably in trigeminal subnuclei interpolaris and oralis. As expected from the fiber tracing, tooth-pulp afferents are activated antidromically by microstimulation in these subnuclei; many (~25%) are Aβ fibers [14,22].
Pulp-evoked responses of postsynaptic neurons in all three subnuclei are consistent with the anatomical data [37]. Some neurons that respond to thermal stimulation of the tooth-pulp have response latencies to electrical stimuli consistent with Aβ fiber input. Weak mechanical dentinal stimuli have apparently not been tried. Interestingly, there is nearly always convergent nociceptive input from non-dental orofacial tissues. Overall, the predominant termination pattern of tooth-pulp afferents resembles that of nociceptors despite the fact that most have anatomical and electrophysiological characteristics of LTMs.
4.2. Neurotransmitter content
Remarkably, in addition to expressing cellular markers of A-fiber LTMs, many large-diameter pulpal afferent somata in the TRG express neurotransmitters, neurotrophin receptors, and other molecules normally associated with synaptic transmission by C-fiber nociceptors (Fig.1,2). These include CGRP, GFRα1 (a GDNF receptor), trkA, TRPA1, TRPV1 and TRPV2, although few express substance P or IB4 [17,18,38, 9]. A significant proportion also expresses Nav1.8 (~75%) and/or Nav1.9 (10%–15%), Na+ channels usually present in small but not large-diameter extra-dental trigeminal afferents [15]. This is reminiscent of “phenotypic switching” in neuropathic pain models where large-diameter afferents begin to express nociceptive neurotransmitters [11,35]. We hypothesize that by virtue of their LTM endings many dentinal afferents respond to weak mechanical stimuli, but by virtue of their central connectivity and neurotransmitter content they activate pain-signaling neurons in the trigeminal brainstem complex and hence evoke pain.
4.3. Constitutive central sensitization
Many mechanisms have been proposed to account for Aβ pain in the context of inflammation and neuropathy [9,11]. Some involve sensitizing changes in spinal and trigeminal brainstem neurons due to the release of pro-inflammatory cytokines and other mediators from neurons, resident glia or invasive immune cells. Others involve changes in network parameters such as the activation of synaptic NMDA receptors, or shifts in excitatory-inhibitory balance due to the loss of inhibitory interneurons or altered descending control. Central sensitizing changes are typically triggered by peripheral nociceptive input. In principle, however, a sensitized state could be the baseline, constitutive status of the central network driven by mechanosensitive dentinal afferents.
5. Low-threshold “algoneurons”?
We stress that we are not referring to nociceptors with fast-conducting myelinated axons that respond selectively to noxious stimuli [12]. By definition, nociceptors have a high activation threshold and encode stimuli in the noxious range. They do not respond to weak innocuous stimuli such as air-puffs and water-spray. Rather, we are referring to a unique type of LTM whose activity signals pain rather than touch. Another term that might sound apt is “low-threshold nociceptors”. This term has been used in relation to pain sensation evoked by highly localized heat and cooling stimuli delivered at non-noxious temperatures [19]. But again low-threshold afferents, by definition, are not nociceptors. In fact, there is no term in current pain taxonomy for afferents that respond to weak stimuli but evoke pain due to idiosyncratic connectivity and/or neurotransmitter content.
To capture this novel concept we propose the term “algoneuron”. We define “algoneurons” as peripheral afferents which, when activated, evoke a sensation of pain. In contrast to “nociceptor”, “algoneuron” focuses on the sensory effect of the afferent’s signal and not its response properties. Under normal circumstances Aδ- and C-fiber nociceptors are algoneurons of high threshold. Aβ nociceptors may also be high-threshold algoneurons, although this remains to be proven. Peripheral sensitization might cause normally high-threshold nociceptors to become low-threshold algoneurons. Likewise, in the presence of central sensitization, A-fiber LTMs may become low-threshold mechano-algoneurons. Many intradental A-fiber LTMs appear to be low-threshold mechano-algoneurons constitutively. Inferring sensory experience exclusively from transduction properties of sensory endings is risky. The term “algoneuron” reminds us that a complex nervous system lies between the afferent ending and conscious perception.
Acknowledgments
We thank John Naftel for permission to use unpublished images and Michael Tal, Margie Byers, Jimmy Hu, Bruce Matthews and Harold Merskey for helpful comments. We acknowledge support from the Swedish Science Council (grant no. 8654 to KF), the Canadian Institutes of Health Research (grant no. MOP4918 to BJS), the US National Institutes of Health (grant no. DE04786 to BJS) and the Hebrew University Center for Research on Pain (to MD).
Footnotes
The authors declare no conflicts of interest with regard to this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andrew D, Greenspan JD. Mechanical and heat sensitization of cutaneous nociceptors after peripheral inflammation in the rat. J Neurophysiol. 1999;82:2649–56. doi: 10.1152/jn.1999.82.5.2649. [DOI] [PubMed] [Google Scholar]
- 2.Arvidsson J, Gobel S. An HRP study of the central projections of primary trigeminal neurons which innervate tooth-pulps in the cat. Brain Res. 1981;210:1–16. doi: 10.1016/0006-8993(81)90880-5. [DOI] [PubMed] [Google Scholar]
- 3.Banik RK, Brennan TJ. Sensitization of primary afferents to mechanical and heat stimuli after incision in a novel in vitro mouse glabrous skin-nerve preparation. Pain. 2008;138:380–91. doi: 10.1016/j.pain.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brannstrom M, Astrom A. The hydrodynamics of the dentin; its possible relationship to dentinal pain. Int Dent J. 1972;22:219–27. [PubMed] [Google Scholar]
- 5.Byers MR, Närhi MV. Dental injury models: experimental tools for understanding neuroinflammatory interactions and polymodal nociceptor functions. Crit Rev Oral Biol Med. 1999;10:4–39. doi: 10.1177/10454411990100010101. [DOI] [PubMed] [Google Scholar]
- 6.Byers MR, Suzuki H, Maeda T. Dental neuroplasticity, neuro-pulpal interactions, and nerve regeneration. Microsc Res Tech. 2003;60:503–15. doi: 10.1002/jemt.10291. [DOI] [PubMed] [Google Scholar]
- 7.Cadden SW, Lisney SJ, Matthews B. Thresholds to electrical stimulation of nerves in cat canine tooth-pulp with A beta-, A delta- and C-fiber conduction velocities. Brain Res. 1983;261:31–41. doi: 10.1016/0006-8993(83)91280-5. [DOI] [PubMed] [Google Scholar]
- 8.Charoenlarp P, Wanachantararak S, Vongsavan N, Matthews B. Pain and the rate of dentinal fluid flow produced by hydrostatic pressure stimulation of exposed dentine in man. Arch Oral Biol. 2007;52:625–631. doi: 10.1016/j.archoralbio.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dababneh RH, Khouri AT, Addy M. Dentine hypersensitivity – an enigma ? a review of terminology, epidemiology, mechanisms, aetiology and management. Brit Dent J. 1999;187:606–611. doi: 10.1038/sj.bdj.4800345. [DOI] [PubMed] [Google Scholar]
- 11.Devor M. Ectopic discharge in Aβ afferents as a source of neuropathic pain. Exp Brain Res. 2009;196:115–128. doi: 10.1007/s00221-009-1724-6. [DOI] [PubMed] [Google Scholar]
- 12.Djouhri L, Lawson SN. Aβ-fiber nociceptive primary afferent neurons: a review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res Brain Res Rev. 2004;46:131–45. doi: 10.1016/j.brainresrev.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Dong WK, Shiwaku T, Kawakami Y, Chudler EH. Static and dynamic responses of periodontal ligament mechanoreceptors and intradental mechanoreceptors. J Neurophysiol. 1993;69:1567–82. doi: 10.1152/jn.1993.69.5.1567. [DOI] [PubMed] [Google Scholar]
- 14.Dostrovsky JO, Sessle BJ, Hu JW. Presynaptic excitability changes produced in brain stem endings of tooth pulp afferents by raphe and other central and peripheral influences. Brain Res. 1981;218:141–60. doi: 10.1016/0006-8993(81)91297-x. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson J, Jablonski A, Persson A-K, Hao J-X, Kouya FP, Wiesenfeld-Hallin Z, Xu X-J, Fried K. Behavioral changes and trigeminal ganglion sodium channel regulation in an orofacial neuropathic pain model. Pain. 2005;119:82–94. doi: 10.1016/j.pain.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Fried K, Aldskogius H, Hildebrand C. Proportion of unmyelinated axons in rat molar and incisor tooth pulps following neonatal capsaicin treatment and/or sympathectomy. Brain Res. 1988;463:118–23. doi: 10.1016/0006-8993(88)90533-1. [DOI] [PubMed] [Google Scholar]
- 17.Fried K, Arvidsson J, Robertson B, Brodin E, Theodorsson E. Combined retrograde tracing and enzyme/immunohistochemistry of trigeminal ganglion cell bodies innervating tooth pulps in the rat. Neuroscience. 1989;33:101–9. doi: 10.1016/0306-4522(89)90314-x. [DOI] [PubMed] [Google Scholar]
- 18.Gibbs JL, Melnyk JL, Basbaum AI. Differential TRPV1 and TRPV2 channel expression in dental pulp. J Dent Res. 2011 doi: 10.1177/0022034511402206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green BG, Roman C, Schoen K, Collins H. Nociceptive sensations evoked from ‘spots’ in the skin by mild cooling and heating. Pain. 2008;135:196–208. doi: 10.1016/j.pain.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermanstyne TO, Markowitz K, Fan L, Gold MS. Mechanotransducers in rat pulpal afferents. J Dent Res. 2008;87:834–8. doi: 10.1177/154405910808700910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hildebrand C, Fried K, Tuisku F, Johansson CS. Teeth and tooth nerves. Progr Neurobiol. 1995;145:165–222. doi: 10.1016/0301-0082(94)00045-j. [DOI] [PubMed] [Google Scholar]
- 22.Hu JW, Dostrovsky JO, Sessle BJ. Primary afferent depolarisation of tooth pulp afferents is not affected by naloxone. Nature. 1978;276:283–4. doi: 10.1038/276283a0. [DOI] [PubMed] [Google Scholar]
- 23.Ichikawa H, Sugimoto T. Vanilloid receptor 1-like receptor-immunoreactive primary sensory neurons in the rat trigeminal nervous system. Neuroscience. 2000;101:719–25. doi: 10.1016/s0306-4522(00)00427-9. [DOI] [PubMed] [Google Scholar]
- 24.Ichikawa H, Sugimoto T. The co-expression of ASIC3 with calcitonin gene-related peptide and parvalbumin in the rat trigeminal ganglion. Brain Res. 2002;943:287–91. doi: 10.1016/s0006-8993(02)02831-7. [DOI] [PubMed] [Google Scholar]
- 25.Ichikawa H, Deguchi T, Fujiyoshi Y, Nakago T, Jacobowitz DM, Sugimoto T. Parvalbumin- and calretinin-immunoreactive trigeminal neurons innervating the rat molar tooth pulp. Brain Research. 1995;679:205–11. doi: 10.1016/0006-8993(95)00234-h. [DOI] [PubMed] [Google Scholar]
- 26.Ichikawa H, Deguchi T, Fujiyoshi Y, Nakago T, Jacobowitz DM, Sugimoto T. Calbindin-D28k-immunoreactivity in the trigeminal ganglion neurons and molar tooth pulp of the rat. Brain Res. 1996;715:71–8. doi: 10.1016/0006-8993(95)01550-7. [DOI] [PubMed] [Google Scholar]
- 27.Ichikawa H, Fukuda T, Terayama R, Yamaai T, Kuboki T, Sugimoto T. Immunohistochemical localization of gamma and beta subunits of epithelial Na+ channel in the rat molar tooth pulp. Brain Res. 2005;1065:138–41. doi: 10.1016/j.brainres.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 28.Itotagawa T, Yamanaka H, Wakisaka S, Sasaki Y, Kato J, Kurisu K, Tsuchitani Y. Appearance of neuropeptide Y-like immunoreactive cells in the rat trigeminal ganglion following dental injuries. Arch Oral Biol. 1993;38:725–28. doi: 10.1016/0003-9969(93)90013-c. [DOI] [PubMed] [Google Scholar]
- 29.Lisney SJW. Some anatomical and electrophysiological properties of tooth-pulp afferents in the cat. J Physiol. 1978;284:19–36. doi: 10.1113/jphysiol.1978.sp012525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magloire H, Maurin JC, Couble ML, Shibukawa Y, Tsumura M, Thivichon-Prince B, Bleicher F. Topical review. Dental pain and odontoblasts: facts and hypotheses. J Orofac Pain. 2010;24:335–49. [PubMed] [Google Scholar]
- 31.Marfurt CF, Turner DF. The central projections of tooth pulp afferent neurons in the rat as determined by the transganglionic transport of horseradish peroxidase. J Comp Neurol. 1984;223:535–47. doi: 10.1002/cne.902230406. [DOI] [PubMed] [Google Scholar]
- 32.Mori H, Ishida-Yamamoto A, Senba E, Ueda Y, Tohyama M. Calcitonin Gene-Related Peptide containing sensory neurons innervating tooth pulp and buccal mucosa of the rat: An immunohistochemical analysis. J Chem Neuroanat. 1990;3:155–63. [PubMed] [Google Scholar]
- 33.Narhi MV, Hirvonen TJ, Hakumaki MO. Responses of intradental nerve fibres to stimulation of dentine and pulp. Acta Physiol Scand. 1982;115:173–78. doi: 10.1111/j.1748-1716.1982.tb07062.x. [DOI] [PubMed] [Google Scholar]
- 34.Narhi MV, Yamamoto H, Ngassapa D, Hirvonen TJ. The neurophysiological basis and the role of inflammatory reactons in dentine hypersensitivity. Arch Oral Biol. 1994;39 (Suppl):23S–30S. doi: 10.1016/0003-9969(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 35.Noguchi K, Obata K, Dai Y. Changes in DRG neurons and spinal excitability in neuropathy. Novartis Found Symp. 2004;261:103–110. [PubMed] [Google Scholar]
- 36.Paik SK, Park KP, Lee SK, Ma SK, Cho YS, Kim YK, Rhyu IJ, Ahn DK, Yoshida A, Bae YC. Light and electron microscopic analysis of the somata and parent axons innervating the rat upper molar and lower incisor pulp. Neuroscience. 2009;162:1279–86. doi: 10.1016/j.neuroscience.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 37.Sessle BJ. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol and Medicine. 2000;11:57–91. doi: 10.1177/10454411000110010401. [DOI] [PubMed] [Google Scholar]
- 38.Silverman JD, Kruger L. An interpretation of dental innervation based upon the pattern of Calcitonin Gene-Related Peptide (CGRP)-immunoreactive thin sensory axons. Somatosens Res. 1987;5:157–75. doi: 10.3109/07367228709144624. [DOI] [PubMed] [Google Scholar]
- 39.Sugimoto T, Takemura M. Tooth pulp primary neurons: cell size analysis, central connection and carbonic anhydrase activity. Brain Res Bull. 1993;30:221–26. doi: 10.1016/0361-9230(93)90247-9. [DOI] [PubMed] [Google Scholar]
- 40.Sugimoto T, Takemura M, Wakisaka S. Cell size analysis of primary neurons innervating the cornea and tooth pulp of the rat. Pain. 1988;32:375–81. doi: 10.1016/0304-3959(88)90050-4. [DOI] [PubMed] [Google Scholar]
- 41.Sugimoto T, Takemura M, Mukai M. Histochemical demonstration of neural carbonic anhydrase activity within the mandibular molar and incisor tooth pulps of the rat. Brain Res. 1990;529:245–54. doi: 10.1016/0006-8993(90)90834-x. [DOI] [PubMed] [Google Scholar]
- 42.Sugimoto T, Fujiyoshi Y, He YF, Xiao C, Ichikawa H. Trigeminal primary projection to the rat brain stem sensory trigeminal nuclear complex and surrounding structures revealed by anterograde transport of cholera toxin B subunit-conjugated and Bandeiraea simplicifolia isolectin B4-conjugated horseradish peroxidase. Neurosci Res. 1997;28:361–71. doi: 10.1016/s0168-0102(97)00064-3. [DOI] [PubMed] [Google Scholar]
- 43.Tal M. The threshold for eliciting the jaw opening reflex in rats is not increased by neonatal capsaicin. Behav Brain Res. 1984;13:197–200. doi: 10.1016/0166-4328(84)90150-5. [DOI] [PubMed] [Google Scholar]
- 44.Tal M, Devor M. Anatomy and neurophysiology of orofacial pain. In: Sharav Y, Benouliel R, editors. Orofacial Pain and Headache. Chapter 2. Blackwell; 2008. pp. 19–44. [Google Scholar]
- 45.Trowbridge HO. Mechanism of pain induction in hypersensitive teeth. In: Rowe NH, editor. Hypersensitive dentin: origin and management. Ann Arbor: University of Michigan; 1985. pp. 1–10. [Google Scholar]
- 46.Vallbo AB, Olausson H, Wessberg J. Unmyelinated afferents constitute a second system coding tactile stimuli of the human hairy skin. J Neurophysiol. 1999;81:2753–63. doi: 10.1152/jn.1999.81.6.2753. [DOI] [PubMed] [Google Scholar]
- 47.Virtanen ASJ, Huopaniemi T, Narhi MVO, Pertovaara A, Wallgren K. The effect of temporal parameters on subjective sensations evoked by electrical tooth stimulation. Pain. 1987;30:361–371. doi: 10.1016/0304-3959(87)90024-8. [DOI] [PubMed] [Google Scholar]
- 48.Vongsavan N, Matthews B. The relationship between the discharge of intradental nerves and the rate of fluid flow through dentine in the cat. Arch Oral Biol. 2007;52:640–647. doi: 10.1016/j.archoralbio.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 49.Yang H, Bernanke JM, Naftel JP. Immunocytochemical evidence that most sensory neurons of the rat molar pulp express receptors for both glial cell line-derived neurotrophic factor and nerve growth factor. Arch Oral Biol. 2006;51:69–78. doi: 10.1016/j.archoralbio.2005.05.002. [DOI] [PubMed] [Google Scholar]