Abstract
Syntrophins are adaptor proteins that link intracellular signaling molecules to the dystrophin based scaffold. In this study, we investigated the function of syntrophins in cell migration, one of the early steps in myogenic differentiation and in regeneration of adult muscle. Hepatocyte growth factor (HGF) stimulates migration and lamellipodia formation in cultured C2 myoblasts. In the migrating cells, syntrophins concentrated in the rear-lateral region of the cell, opposite of the lamellipodia, instead of being diffusely present throughout the cytoplasm of non-migrating cells. When the expression of α-syntrophin, the major syntrophin isoform of skeletal muscle, was reduced by transfection with the α-syntrophin-specific siRNA, HGF stimulation of lamellipodia formation was prevented. Likewise, migration of myoblasts from α-syntrophin knockout mice could not be stimulated by HGF. However, HGF-induced migration was restored in myoblasts isolated from a transgenic mouse expressing α-syntrophin only in muscle cells. Treatment of C2 myoblasts with inhibitors of PI3-kinase not only reduced the rate of cell migration, but also impaired the accumulation of syntrophins in the rear-lateral region of the migrating cells. Phosphorylation of Akt was reduced in the α-syntrophin siRNA-treated C2 cells. These results suggest that α-syntrophin is required for HGF-induced migration of myoblasts and for proper PI3-kinase/Akt signaling.
Keywords: α-syntrophin, myoblast migration, hepatocyte growth factor, α-syntrophin-specific siRNA, α-syntrophin knock-out mouse, PI3-kinase/Akt signaling
Introduction
Cell migration is one of the essential steps in muscle regeneration in adult myofibers as well as in embryonic development. The satellite cells, myogenic stem cells in adult skeletal muscle, can be activated to proliferate in response to various stimuli such as injury and exercise [1, 2]. The activated cells migrate to the injured sites by chemotaxis and start to proliferate. Hepatocyte growth factor (HGF) is one of the chemo-attractants released in response to muscle injury [1, 3]. In addition, HGF facilitates both non-directional and directional migration of cultured C2C12 myoblasts [4]. In response to the stimulus to migrate, the actin-cytoskeleton is reorganized and lamellipodia form in the direction of the leading edge of migrating cells. Other proteins, including PTEN, accumulate at the rear of the migrating cell or trailing edge, opposite of the lamellipodia [5]. Inhibitors of PI3-kinase, LY294002 and Wortmannin, prevent HGF-induced lamellipodial formation and cell migration [4, 6].
The syntrophins are a family of scaffolding proteins that link signaling proteins to the dystrophin protein complex [7]. They interact with multiple signaling proteins via two pleckstrin homology (PH1) domains, a PDZ domain, and a conserved syntrophin unique (SU) domain [8–10]. Both the SU domain and second PH domain are required for binding to dystrophin and other members of the dystrophin protein family (utrophin, dystrobrevins, and DRP2) [10]. The PDZ domain of syntrophins can bind to a variety of signaling proteins, including kinases, various channels, and nitric oxide synthase [11–17]. Syntrophins also have been reported to associate with actin, calmodulin, and phosphatidylinositols [18]. There are five different syntrophin isoforms (α, β1, β2, γ1, and γ2). Four isoforms (all except γ1) are expressed in skeletal muscle, but α-syntrophin is the predominant isoform in this tissue [8].
Studies on syntrophin function have largely focused on mature myofibers and the neuromuscular junction. In the cultured myoblasts, however, α-syntrophin is expressed from the early stages of differentiation when dystrophin, a major binding protein of syntrophin, has not yet been expressed [19]. Little is known about the function of syntrophin in the early stages of muscle development. Recently, we have shown that α-syntrophin is involved in the expression of myogenin, a key myogenic regulatory factor, by interaction with MLL5 in the early stages of myogenic differentiation [20]. In this study, we have focused on the intracellular localization of syntrophin, particularly in the migrating muscle cells. We investigated the changes in intracellular localization of syntrophin following HGF stimulation. By using cultured C2 myoblasts and primary myoblast cultures from α-syntrophin knockout mice, we investigated the function of syntrophin in promoting cell migration in response to HGF. Our results demonstrate that α-syntrophin is required for the HGF-induced migration of cultured myoblasts.
Materials and methods
Materials
HGF was purchased from Peprotech (Rocky Hill, NJ). Anti-PTEN and anti-actin antibodies, control siRNA, α-syntrophin-specific siRNA, and protein A/G were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-p85, anti-Met, anti-pMet (Y1234), and anti-pMet (Y1349) antibodies were from Cell Signaling (Danvers, PA). LY294002, Wortmannin and collagen type I were obtained from Sigma (St. Louis, MO). Lipofectamine 2000, rhodamine-phalloidin, propidium iodide (PI), 4′,6-diamidino-2-phenylindole (DAPI), fluorescein isothiocyanate (FITC)-conjugated, and tetramethyl rhodamine isothiocyanate (TRITC)-conjugated antibodies were from Invitrogen (Carlsbad, CA). pGFPN3 was from Clontech (Mountain View, CA). HindIII and BamHI were from Fermentas (Hanover, MD). Anti-pan-syntrophin and anti-α-syntrophin antibodies were used as previously described [21]. All of the reagents for primary cell culture were also from Invitrogen. Strainers, 24 well plates, and cell culture inserts for three dimensional chamber assay were from BD Falcon (San Jose, CA). The polyvinylidenedifluoride (PVDF) membrane was from Millipore (Billerica, MA). Bradford assay kit was from Bio-Rad (Hercules, CA).
Primary culture of skeletal muscle cells
Primary culture of the skeletal muscle was performed as previously described [20]. In brief, skeletal muscle cells were isolated from the forelimbs and hindlimbs of neonatal mice (0–1 day old) from C57BL6/J wild-type (C57), α-syntrophin-knockout (αKO) [22], α-syntrophin transgenic (FLA) [23], and α/β2-syntrophin-double knockout mice (AB) [24]. Skeletal muscles were dissected free of other tissues including skin, lipid, and bone then minced with forceps. Single muscle cells were isolated with treatment of 0.05 % trypsin EDTA in phosphate buffered saline (PBS) for 45 min and passed through a 70 μm strainer. Cells were pre-plated on a 100 mm culture dish to remove fibroblasts and to enrich for muscle cells twice for 1 h each. Experimental procedures performed on mice were approved by the Institutional Animal Care and Use Committee of the University of Washington.
Three-dimensional migration chamber assay
A three-dimensional migration chamber assay was performed using a tissue culture insert (8.0 μm pore size) in 24-well plates. The upper surface of the membrane was coated with collagen type I and 300 μl of cell suspension (5.0 x 104 cells/ml) was applied to the coated membrane. The lower chamber of the insert contained serum free medium (200 μl) with or without HGF (50 ng/ml). After incubation at 37ºC for 1 h, the cell suspension was removed from the chamber and the surface of the lower chamber was fixed with ice-cold methanol. To recognize moving cells, the lower surface was stained with a hematoxylin solution and cells in the upper chamber were removed with a cotton tip. The hematoxylin-stained cells in the lower surface of the membrane were counted using an inverted microscope. Each experiment was independently carried out four times.
HGF-induced migration assay
C2 myoblasts were seeded on 6-well tissue culture plates with 1.0 x 104 cells/ml. Cells were grown in 10% fetal bovine serum (FBS) containing Dulbecco’s modified Eagle’s medium (DMEM) for 6 h. Cells were serum-starved by incubating in 0.5% FBS containing DMEM for 3 h. Cells were incubated with HGF (50 ng/ml) for 1 h and then fixed with 4% paraformaldehyde. To visualize the cytoskeleton, F-actin was stained with rhodamine-phalloidin. Migrating cells with lamellipodia were quantified by visual analysis on at least 10 randomly selected fields, such that >300 cells were counted per field.
Immunofluorescence assay
Cells were seeded on a cover-slip in 6-well plates (1.0 x 104 cells/ml) and grown in a 5% CO2 humidified incubator for 6 h. Cells treated with or without HGF were fixed with 4% paraformaldehyde in PBS for 10 min and permeabilized with 0.5% Triton X-100 in PBS. Cells were blocked with 10% normal goat serum in PBS for 10 min. All of the primary antibodies were incubated for 1 h and TRITC- or FITC-conjugated secondary antibodies were incubated for 40 min at room temperature. All of the primary antibodies were diluted 1:50 and secondary antibodies were diluted 1:100. Both DAPI and PI were used to stain nuclei. Images were taken with a Zeiss LSM510 confocal laser scanning microscope or Zeiss Axioskop 40 FL microscope.
siRNA transfection
Transfection of α-syntrophin-specific siRNA was performed as described previously [20]. In brief, C2 myoblasts were grown in 24-well plates at a density of 5.0 x 104 cells/ml for 24 h. Cells were transfected with control siRNA or α-syntrophin-specific siRNA by incubation with lipofectamine 2000 for 6 h. The cells were allowed to recover from the transfection by transferring into fresh 10% FBS-containing medium and incubated for 18 h. Subsequently, cells were treated with HGF (50 ng/ml) and incubated for 1 h.
Transfection of GFP fusion α-syntrophin
To determine the localization of exogenous α-syntrophin, the full-length α-syntrophin cDNA [8] was cloned into the HindIII and BamHI site of pGFPN3. For transfection of GFP fusion α-syntrophin, C2 cells were grown in 6-well plates (5.0 x 104 cells/ml) for 24 h in an antibiotics-free DMEM and transfected with GFP fusion α-syntrophin by incubation with lipofectamine 2000 for 6 h. Cells were allowed to recover from the transfection by transferring in 10% FBS-containing medium for 18 h. Subsequently, HGF (50 ng/ml) was added into 0.5% FBS-containing medium and incubated for 1 h.
Immunoprecipitation assay
At the indicated culture time, cells were rinsed with cold PBS, harvested with a scraper, and disrupted by ultrasonication in RIPA buffer, pH 7.4, containing 9.1 mM Na2HPO4, 1.7 mM NaH2PO4, 150 mM NaCl, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM PMSF, and 0.1% protease inhibitor cocktail. Cell lysates were centrifuged at 15,000 x g for 3 min to remove cell debris and then protein concentration was determined by Bradford assay. For pre-clearance, cell extracts (500 μg/500 μl) were incubated with 20 μl of protein A/G for 30 min on ice. The proteins were incubated with anti-PTEN or anti-syntrophin antibodies overnight at 4ºC. Protein A/G (20 μl) was added and incubated for 1 h at 4ºC. The immune-complexes were collected by centrifugation and washed with cold PBS for 3 times, then the proteins were separated with SDS-polyacrylamide gel electrophoresis (SDS-PAGE).
Immunoblot assay
To determine protein expression, cells were rinsed twice with cold PBS and mixed with SDS-sample buffer (1.0 M Tris/HCl, pH 6.8, containing 10% glycerol, 2% SDS, 0.025% bromo-phenol blue, and 5% β-mercaptoethanol) and boiled in 100ºC for 5 min. Equal amounts of protein were separated by SDS-PAGE and transferred onto PVDF membrane. The membranes were pre-blocked with 5% bovine serum albumin (BSA) and incubated with the indicated primary antibodies. After incubation with peroxidase-conjugated secondary antibodies, the immunoreactive protein bands were visualized by enhanced chemiluminescence detection with Digital Luminescent Image Analyzer LAS-1000 (Fuji film, Japan). Band intensity was determined by Scion image (Fredrick, MD).
Statistical analysis
Results are presented as mean ± S.E.M. For the statistical analysis of cell migration, two tailed Student’s unpaired t test was performed. A value of p<0.05 was considered to be significant.
Results
Syntrophins specifically concentrates at the trailing edge of migrating C2 myoblasts
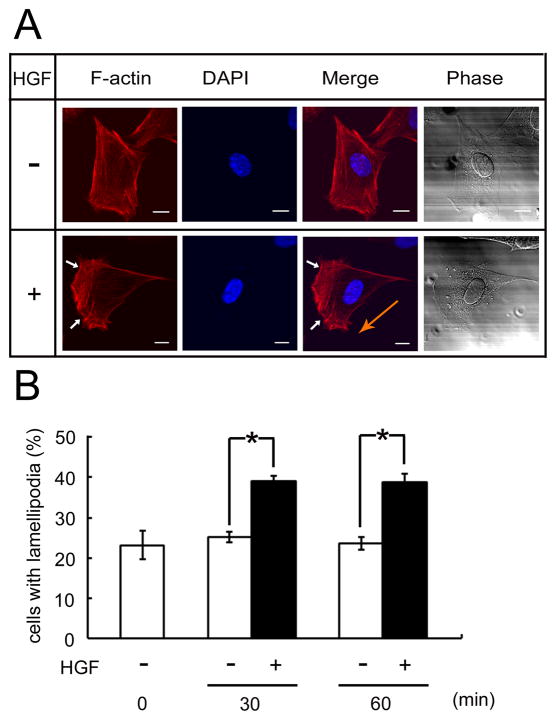
HGF has been reported to regulate cell migration in a variety of cell types [4, 6, 25, 26]. We examined whether HGF can induce migration of C2 cells under our culture conditions. Myoblasts cultured on a cover-slip with HGF were stained with rhodamine-phalloidin which detects actin stress fibers. The cytoskeleton has to rearrange for morphological change in migrating cells [27] and the lamellipodia are formed in the direction of the moving cells [28, 29]. As shown in Fig. 1A, typical morphology of migrating cells with lamellipodia (arrows) were found following HGF incubation. To examine how many cells respond to HGF under the same conditions, cells were exposed to HGF for the indicated periods in a low serum media (Fig. 1B). The cells with lamellipodia were counted in randomly chosen fields. Just before HGF treatment (0 min), approximately 23% of cells in the fields showed lamellipodia. After incubation with HGF for 30 min, however, the percentage of lamellipodia-forming cells increased to 39%, and remained relatively constant at 60 min. In contrast, without HGF, the percentage of cells with lamellipodia did not increase with 60 min incubation.
Fig 1. HGF induces lamellipodia formation of C2 myoblasts.
(A) C2 myoblasts cultured on cover-slips for 6 h in growth media were transferred into serum-starved DMEM, pre-incubated for 1 h, then treated with HGF (50 ng/ml) for 1 h. The formation of lamellipodia (arrows) detected by labeling with rhodamine-phalloidin (red). Orange arrow indicates the direction of cell migration. Nuclei were stained with DAPI (blue). Scale bar is 10 μm. (B) Under the same conditions, cells were incubated with (+) or without (−) HGF (50 ng/ml) for the indicated times (0 min is the time point of HGF treatment). Cells with lamellipodia were counted from 20 randomly selected fields and the formation of lamellipodia was expressed as a ratio to the total nuclei in the fields (*p < 0.001).
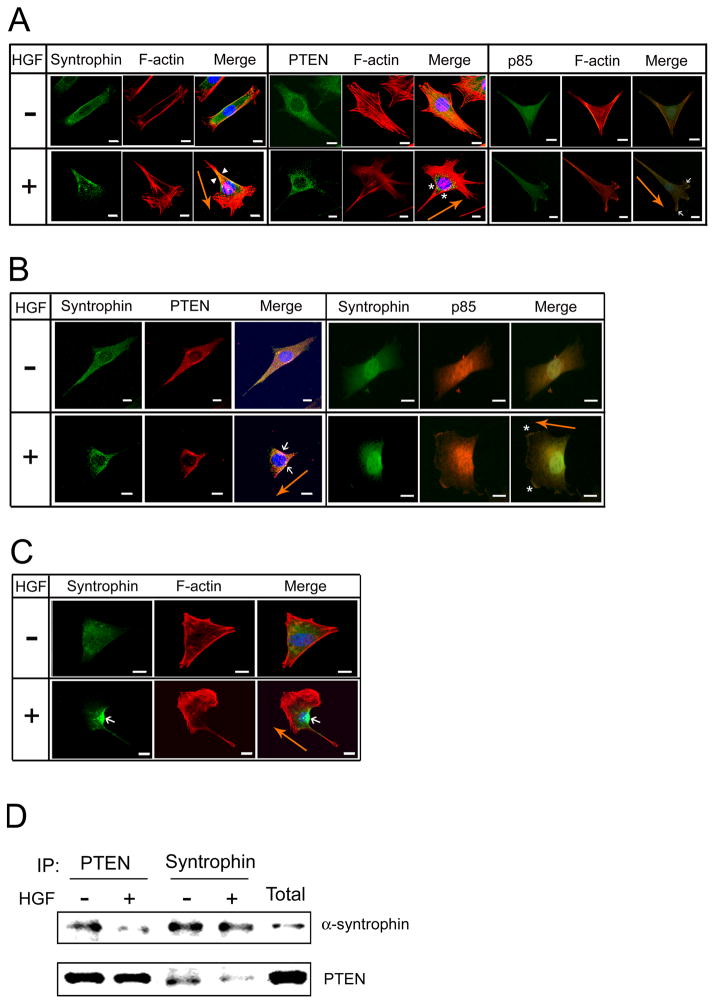
Syntrophins can bind to filamentous actin (F-actin) via its internal domains such as the second PH and the SU domains in cardiac and skeletal muscle [18]. We therefore examined the intracellular localization of syntrophins during actin reorganization in the HGF-induced migrating cells. Without HGF, syntrophins were distributed throughout the cytoplasm of non-migrating cells (Fig. 2A). However, when the cells were incubated with HGF, syntrophins concentrated to the rear and lateral part of the cells, distinctly separate from the lamellipodia (arrowheads in Fig. 2A). Because PTEN is known to accumulate in the rear and lateral part of cells stimulated with chemo-attractant [30, 31], it is widely used as a marker for the rear-lateral part of the migrating cells. In contrast, PI3-kinase localizes at the leading-edge of cells treated with chemo-attractant and stimulates cell migration in various cell types [6, 32–34]. We also found that PTEN is localized in the rear-lateral region of the HGF-induced C2 cells (asterisks in Fig. 2A), while it dispersed in the cytoplasm without HGF. To show the localization of PI3-kinase, cells were stained with anti-p85 antibody, the PI3-kinase regulatory subunit. As expected, p85 was found in the region of lamellipodia in the HGF-induced cells (arrows in Fig. 2A). In co-immunolabeling experiments, PTEN and syntrophins co-localized in the rear-lateral part of the migrating cells (arrows in Fig. 2B). However, syntrophins and p85 separated in the HGF-induced migrating cells (asterisks in Fig. 2B). To confirm the localization of syntrophin in the HGF-induced migrating cells, C2 cells were transfected with GFP fusion α-syntrophin and visualized under confocal laser scanning microscope. The GFP protein can be seen in the rear part of the migrating cells (arrow in Fig. 2C). Then we examined whether syntrophins associate with PTEN by performing an immunoprecipitation assay. α-Syntrophin was precipitated by the anti-PTEN antibody, and conversely, PTEN was precipitated by the anti-pan-syntrophin antibody (Fig. 2D). These results suggest that syntrophins concentrate at the rear-lateral part of the HGF-induced migrating cells.
Fig 2. Syntrophins are localized at the trailing edge of the migrating cells.
(A) C2 myoblasts on coverslips were prepared as described in Fig 1. Cells were incubated with (+) or without (−) HGF (50 ng/ml) for 1 h and then labeled with anti-pan-syntrophin, anti-PTEN, or anti-p85 antibodies. Arrowheads and asterisks indicate syntrophin and PTEN in rear-lateral part of the cell, respectively. Arrows show p85 in lamellipodia of the cell. PTEN is used as a marker for the trailing edge whereas p85 is used for the lamellipodia at the leading edge of migrating cells. Rhodamine-phalloidin was used to stain F-actin (red). Fluorescence images were taken on a Zeiss LSM 510 confocal laser scanning microscope or Zeiss Axioskop 40 FL microscope. The scale bar indicates 10 μm. (B) Under the same culture conditions, cells were co-labeled with anti-pan-syntrophin and anti-PTEN antibodies, or anti-pan-syntrophin and anti-p85 antibodies. Arrows indicate co-localization of syntrophin and PTEN. Asterisks indicate p85 in lamellipodia. Bar is 10 μm. (C) C2 cells transfected with GFP fusion α-syntrophin were incubated with or without HGF (50 ng/ml). An arrow indicates GFP fusion α-syntrophin in rear-lateral part of the cell. To visualize the cytoskeleton and nuclei, cells were labeled with rhodamine-phalloidin (red) and DAPI, respectively. The scale bar indicates 10 μm. Orange arrow indicates the direction of cell migration. (D) Cells cultured as above were harvested for immunoprecipitation with anti-PTEN or anti-pan-syntrophin antibodies. The immunoprecipitated proteins were separated on SDS-PAGE and subjected to western blot analysis with anti-α-syntrophin or anti-PTEN antibodies. Lane ‘Total’ shows the western blot of the protein extract prior to immunoprecipitation and indicates the size of the expected proteins.
α-Syntrophin is required for HGF-induced migration of myoblasts
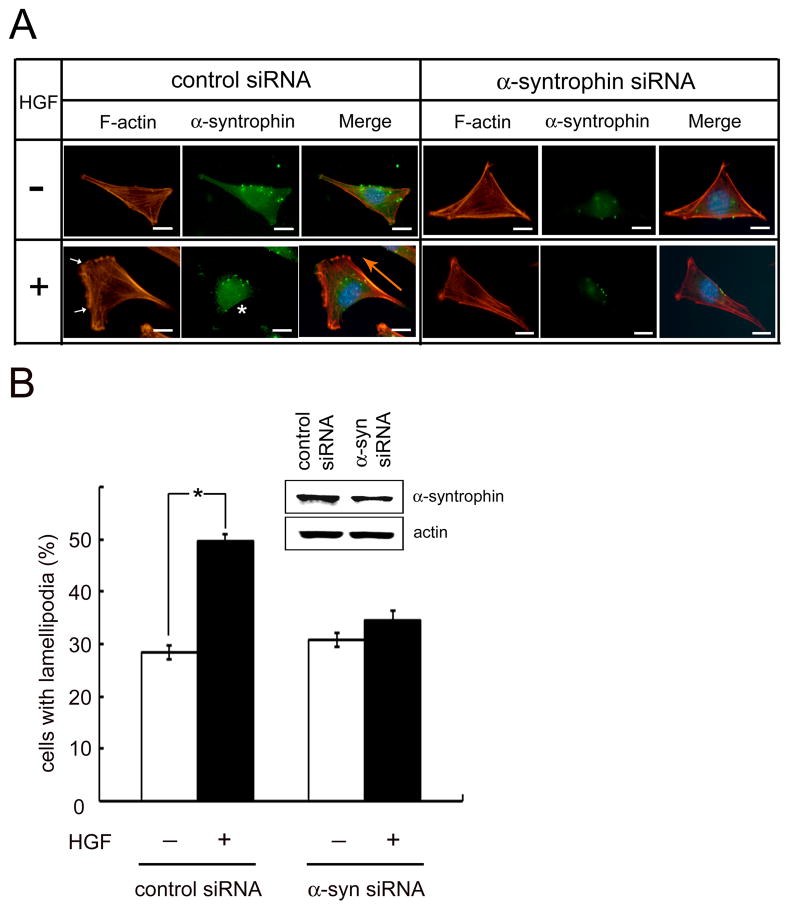
To explore the role of syntrophins in migrating cells, a knockdown experiment using α-syntrophin-specific siRNA was performed. C2 myoblasts grown in 6-well tissue culture plates for 24 h were transfected with control siRNA or α-syntrophin-specific siRNA. The transfected cells were incubated with or without HGF for 1 h. The control siRNA-transfected cells responded to HGF to form lamellipodia (arrows in Fig. 3A) and α-syntrophin was concentrated in the rear-lateral region of the cells (asterisk). In contrast, many of the α-syntrophin-specific siRNA-transfected cells did not form lamellipodia in response to HGF. To gauge the number of cells responding to HGF, cells with lamellipodia were counted from randomly chosen fields. In control siRNA-transfected cells, approximately 28% cells were found to form lamellipodia without HGF. Following treatment with HGF, lamellipodia-forming cells increased to about 49% of the fields. In contrast, the ratio of lamellipodia-forming cells did not significantly increase with HGF treatment in the α-syntrophin-specific siRNA-transfected cells, suggesting that those cells did not respond to HGF (Fig. 3B). The expression of syntrophin in cells transfected with α-syntrophin-specific siRNA is reduced to approximately half the level observed in control siRNA-transfected cells (inset of Fig. 3B).
Fig 3. α-Syntrophin siRNA-treated C2 cells do not respond to HGF stimulation.
(A) Cells were cultured on a 24-well tissue culture plate for 24 h and transfected with control siRNA or α-syntrophin-specific siRNA (100 nM) for 6 h. Cells were then treated with HGF (50 ng/ml) for 1 h and were labeled with rhodamine-phalloidin (red) and anti-α-syntrophin antibody. Arrows indicate lamellipodia and the asterisk shows α-syntrophin in the rear-lateral part of the cell. Big arrow in orange color indicates the direction of migrating cell. Scale bar is 10 μm. (B) Under identical culture conditions, the cells with lamellipodia were observed using a fluorescence microscopy. Cells were counted from 20 randomly selected fields. (*p<0.001). Cell lysates were subjected to western blot to determine protein level of α-syntrophin after siRNA transfection. Actin is used as a loading control.
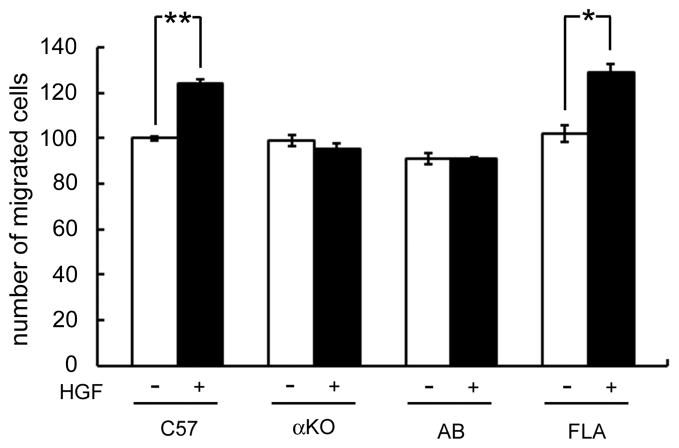
To confirm the role of syntrophin in the HGF-induced migration, we performed the migration assay using primary cultured myoblasts isolated from syntrophin knock-out mice. Skeletal muscle cells of neonatal pups were isolated from wild-type (C57), α-syntrophin knock-out (αKO), α-syntrophin transgenic (FLA), and α/β2-syntrophin double knock-out (AB) mice. To reduce a possible contamination of non-muscle cells including fibroblasts, pre-plating was done twice (see Methods). Isolated myoblasts were seeded on a migration assay chamber with or without HGF, incubated for 1 h and the number of migrating cells was quantified. In the C57 myoblasts, the proportion of migrating cells increased approximately 25% with HGF compared to the non-treated cells (Fig. 4). In myoblasts from αKO, however, the proportion of migrating cells did not increase with treatment of HGF. We then examined migration of the myoblasts from AB to see whether β2-syntrophin contributes to the migration response in the absence of α-syntrophin. The migration ratio was similar to that of αKO and the ratio did not change with treatment of HGF. Interestingly, myoblasts from FLA mice which express α-syntrophin only in muscle cells, showed a response to HGF similar to that of the C57 control myoblasts. The proportion of FLA migrating cells without HGF treatment is similar to that of the other cells, but increases approximately 29% following HGF treatment; this increase corresponds to that of C57 myoblasts. Taken together with the results from α-syntrophin knockdown experiments, these results strongly suggest that α-syntrophin is required for the HGF-induced migration of skeletal muscle myoblasts.
Fig 4. Myoblasts from the α-syntrophin-knockout mice do not respond to HGF stimulation.
Skeletal myoblasts were isolated from C57, α-syntrophin-knockout (αKO), α/β2-syntrophin-double knockout (AB), and α-syntrophin transgenic (FLA) mice and seeded on the insert of a three dimensional chamber assay as described in “Materials and Methods”. After incubation for 1 h, cells were fixed with cold methanol and stained with hematoxylin. The cells migrating to the lower chamber were counted using an inverted microscope. Values are expressed as means ± S.E.M. from four independent experiments (*p<0.01, **p<0.001).
Syntrophin modulates the PI3-kinase/Akt pathway during HGF-induced migration
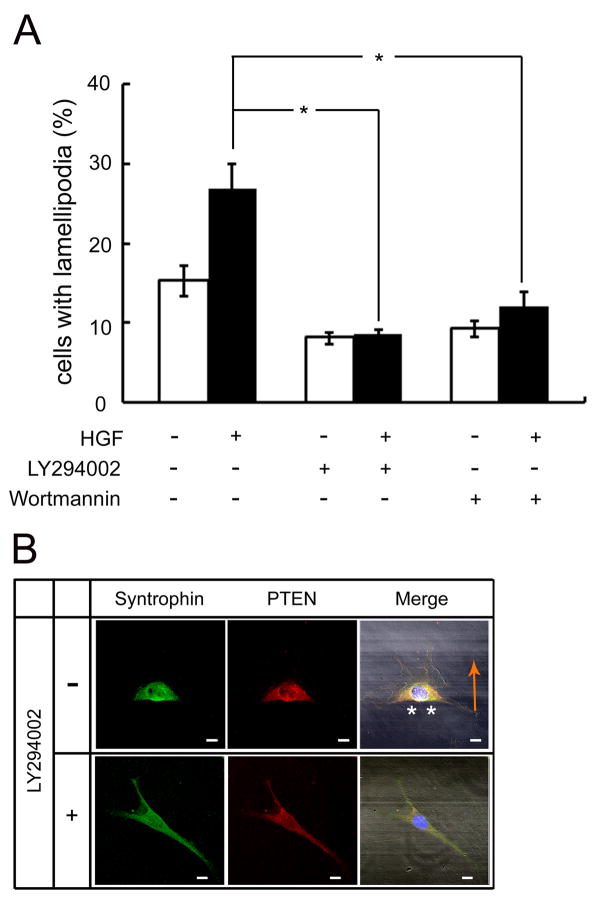
HGF, a known ligand of the c-Met receptor, can induce phosphorylation of c-Met, which then activates the PI3-kinase signaling pathway [35]. To examine whether the effect of syntrophin on the migration of myoblasts is also dependent on PI3-kinase activity, cells were pretreated with LY294002 or Wortmannin, inhibitors of PI3-kinase, and then treated with HGF. When C2 myoblasts were cultured for 3 h under a low serum condition (0.5% FBS), approximately 15% cells of the fields formed lamellipodia. With treatment of HGF, however, 26% of the cells formed actin-enriched lamellipodia (Fig. 5A). However, when cells were pre-incubated with LY294002 or Wortmannin, the ratio of the lamellipodia-forming cells reduced to approximately 8%. The ratio remained near this level even with HGF. To examine the localization of syntrophin in the presence of PI3-kinase inhibitor, cells pre-treated with LY294002 were incubated with HGF for 1 h and the cells were labeled for syntrophins and PTEN. Both proteins localized to the rear-lateral part (asterisks in Fig. 5B) of migrating cells in response to HGF (Fig. 5B Top Panel). In the LY294002-pretreated cells, however, syntrophins and PTEN remained dispersed throughout the cytoplasm with no apparent response to HGF.
Fig 5. Inhibitors of PI3-kinase block the HGF-induced migration of C2 cells.
(A) C2 myoblasts cultured on coverslips were treated with LY294002 (20 μM) or Wortmannin (0.5 μM) for 30 min. Then the cells were incubated with (+) or without (−) HGF for 1 h. Migrating cells were observed by staining with rhodamine-phalloidin. Cells were counted from 20 randomly selected fields (*p<0.001). (B) Under the same culture conditions, cells were labeled with BSA solution containing the anti-pan-syntrophin or anti-PTEN antibodies. Then cells were incubated with fluorescence-conjugated secondary antibodies and DAPI for 40 min at room temperature. The specimens were observed with a Zeiss LSM 510 confocal laser scanning microscope. Asterisks indicate co-localization of the two proteins. Orange arrow indicates the direction of cell migration. Bar indicates 10 μm.
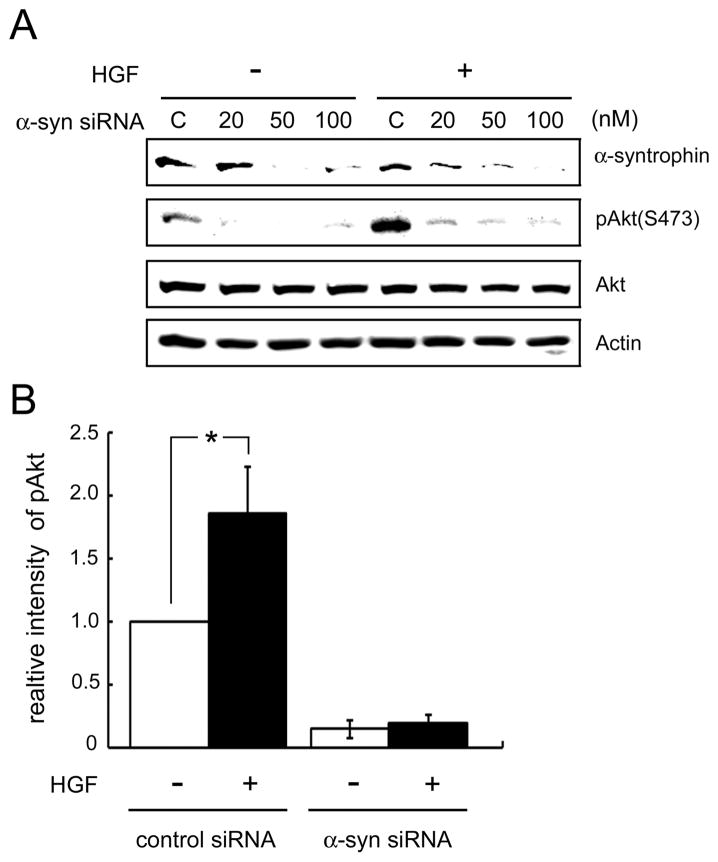
Akt is a key downstream mediator of PI3-kinase, therefore, we next examined whether syntrophin is involved in the phosphorylation of Akt in response to HGF. C2 myoblasts were transfected with control siRNA or α-syntrophin-specific siRNA and then treated with HGF. Transfection of α-syntrophin-specific siRNA reduced the expression of α-syntrophin in a dose-dependent manner (Fig. 6A). Phosphorylated Akt (S473) was detected in control siRNA-transfected cells even without HGF, but it increased more than twice level with treatment of HGF (compare control (C) columns in Fig. 6A). In contrast, the phosphorylation of Akt disappeared in the α-syntrophin-specific siRNA-transfected cells in a dose-dependent manner regardless of HGF treatment. The reduction of phosphorylated Akt occurs even though the total protein level of Akt is unchanged. When the level of the phosphorylated Akt was measured, it raised approximately twice with response to HGF in the control siRNA-transfected cells. In contrast, the level in the cells transfected with α-syntrophin-specific siRNA (100 nM) was less than 10% that of the control siRNA-transfected cells and did not significantly increase with HGF treatment.
Fig 6. Akt phosphorylation is reduced in the α-syntrophin-specific siRNA transfected cells.
(A) C2 cells were transfected with control siRNA (lane C) or increasing concentration of α-syntrophin-specific siRNA and treated with HGF for 1 h. The cells were harvested and the levels of α-syntrophin, Akt, and phosphor-Akt (pAkt) were determined by western blotting. Actin was used as a loading control. (B) Cells transfected with control siRNA or α-syntrophin-specific siRNA (100 nM) were treated with (+) or without HGF (−) for 1 h. Akt phosphorylation (S473 residue) was determined by western blotting. The intensity of phosphor-Akt band was measured with Scion image software. The level of the phosphor-Akt in control siRNA-transfected cells without HGF is expressed as 1.0. Values are expressed as means ± S.E.M. from four independent experiments (*p<0.05).
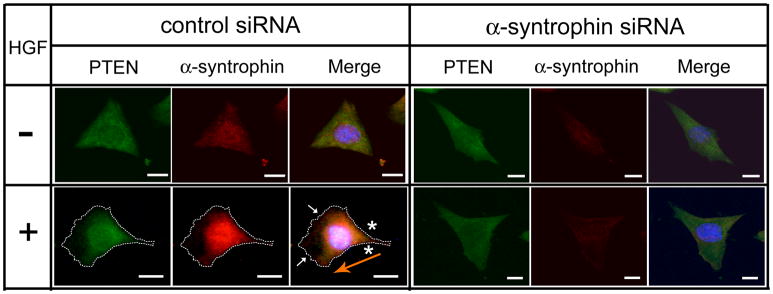
To further establish the relationship between α-syntrophin and Akt signaling, we examined the localization of PTEN in α-syntrophin knock-down cells. In control siRNA-transfected cells without HGF, both α-syntrophin and PTEN were distributed throughout the cytoplasm (Figure 7). With the HGF treatment, control siRNA-transfected cells formed lamellipodia (arrows in Fig. 7) and both α-syntrophin and PTEN concentrated in the rear-lateral part of the cells (asterisks in Fig 7). In contrast, α-syntrophin knock-down cells did not form lamellipodia. Furthermore, PTEN remained distributed throughout the cytoplasm rather than concentrating in the rear-lateral part (similar to control siRNA-transfected cells without HGF). These results indicate that α-syntrophin is intimately involved in the PI3-kinase/Akt pathway in the HGF-induced migration of myoblasts.
Fig 7. Localization of PTEN is disrupted in the α-syntrophin knock-down cells.
Cells grown on a 24-well tissue culture plates for 24 h were transfected with control siRNA or α-syntrophin-specific siRNA (100 nM) for 6 h. Cells were then incubated with (+) or without (−) HGF (50 ng/ml) for 1 h and were labeled with anti-α-syntrophin and anti-PTEN antibodies. Dotted line indicates shape of the cell. Small arrows indicate the lamellipodia of cells and the orange arrow indicates the direction of cell migration. Asterisks indicate co-localization of α-syntrophin and PTEN in the rear-lateral part of the cell. The scale bar is 10 μm.
Discussion
Previous studies by us [20] and others [36] have indicated that in the absence of α-syntrophin muscle regeneration is slowed. We postulated that the slow regeneration could be a result of a defect in myoblast migration, an early step of muscle regeneration. Therefore, we designed a series of experiment to investigate how α-syntrophin affects myoblast migration. Our initial experiments showed that while α-syntrophin is diffusely present in the myoblast cytoplasm, it is specifically targeted to trailing edge of myoblasts induced to migrate by treatment with HGF. This localization of syntrophin is similar to that of PTEN, which is restricted to the sides and the rear of migrating cells [30, 31]. Furthermore, immunoprecipitation experiments demonstrated that a portion of the PTEN and α-syntrophin are in a complex together. This observation suggested that these two proteins are not only co-localized but may also participate in the same signaling pathways.
In the polarization step during initiation of cell migration, the molecular processes at the front and the back of a moving cell are different; Cdc42, Rac, PI3-kinase, PIP3 and microtubules are concentrated in the front of the cells whereas PTEN and myosin II are concentrated in the rear-part of the cells [5]. Activation of PI3-kinase is required for the HGF-induced adherence junction disassembly, lamellipodia formation, and subsequent migration or scattering of MDCK epithelial cells [37, 38]. At the trailing edge of the migrating cells, many proteins function by either modulating tension force or rear retraction by disassembly of focal adhesion assemblies. Myosin light chain kinase (MLCK) phosphorylates myosin light chain, which regulates myosin II activity during regulation of the tractional force at the trailing edge [5]. Recent work identified a novel mechanism of action for PTEN, whereby the C2 domain of PTEN is involved in the inhibition of cell-migration [39]. The binding of syntrophins with PTEN has not been reported before in spite of the PDZ-binding domain of PTEN [40]. In this study, we show that syntrophin can interact with PTEN by co-immunoprecipitation and co-immunostaining results (see Fig. 2). However, the interaction of the two proteins was reduced by HGF treatment in the co-immunoprecipitation assay (see Fig. 2D) (although both proteins moved and concentrated at the trailing edge of the HGF-induced moving cells). Our results do not show whether the binding is direct or indirect. In mature skeletal muscle, α-syntrophin can bind many signaling molecules and kinases including the microtubule-associated serine/threonine kinase (MAST), syntrophin-associated serine/threonine kinase (SAST) [11], stress-activated protein kinase 3 (SAPK3) [12], diacylglycerol kinase δ (DGKδ) [13], neuronal nitric oxide synthase (nNOS) [14], and water channel aquaporin-4 [15–17]. Less is known about the proteins associating with α-syntrophin during muscle development or regeneration. It has been reported that PDZ domains within SAST and MAST can bind to the C-terminal region of PTEN and stabilize its function [40]. Therefore, α-syntrophin may interact with PTEN indirectly via several other binding proteins, or by direct interaction or even a combination of both.
Cell migration is important for various biological functions of muscle cells including differentiation, muscle development, and muscle regeneration. During skeletal muscle regeneration, satellite cells are activated and migrated by chemotaxis to wounded regions of myofibers, from which HGF is released [41, 42]. Wounded muscles require regenerating and/or proliferation of cells. HGF inhibits myogenesis and promotes cell proliferation of cultured C2 myoblasts [43] and chicken skeletal muscle satellite cells [44]. In addition, HGF plays a crucial role in the regulation of limb myogenic cell migration [45, 46]. In this study, we also have shown that HGF stimulated migration of C2 myoblasts and primary cultured myoblasts (see Fig. 1 and Fig. 4). The observation that syntrophins concentrate on the rear-lateral part of those migrating cells imply that it may be involved in the cell polarization preceding cell migration. To investigate the role of α-syntrophin in cell migration, we performed experiments using cells with reduced α-syntrophin expression. In both C2 cells and primary cultured myoblasts from transgenic mice, migration could not be stimulated by HGF. Neither α-syntrophin siRNA-transfected C2 cells nor myoblasts from α-syntrophin knockout mice increased to formation of lamellipodia in response to HGF. Based on these results, we conclude that α-syntrophin is required for the HGF-induced migration of cultured myoblasts.
The c-Met, receptor tyrosine kinase is the receptor of HGF and it is also called HGF-Receptor. c-Met is a disulfide-linked heterodimer composed of an α subunit (50 kDa) and β subunit (145 kDa) with tyrosine-kinase activity [47]. On binding of HGF, c-Met forms a dimer and is induced to autophosphorylate tyrosine residues generating protein docking sites [37]. Both Ras and PI3-kinase/Akt pathways are important signaling pathways in HGF-induced cell migration [35, 48]. The phosphorylation of tyrosine 1349, 1356, and 1365 in C-terminal on c-Met makes docking sites for interaction with scaffolding and signaling molecules such as PI3-kinase, Ras and PLC-γ [49, 50], while the phosphorylation of tyrosine 1234 and 1235 are related with c-Met kinase activity [51]. We initially examined whether the phosphorylation of c-Met on tyrosine 1234 and 1235 is altered by HGF treatment of C2 myoblasts. Using western blotting with the phosphorylation specific antibody, we could not detect a significant difference on the level of phosphorylation on tyrosine 1234 and 1235 with treatment of HGF (see Supp Fig. 2). We subsequently turned our attention to the effect of α-syntrophin on the PI3-kinase/Akt signaling pathway because the phosphorylation of c-Met on tyrosine 1349 was increased by HGF treatment in C2 myoblasts (see Supp Fig. 2). First, we demonstrated that inhibitors of PI3-kinase blocked the HGF-induced lamellipodium formation (see Fig. 5). The phosphorylation of Akt (Ser 473), a downstream effector of PI3-kinase, increases with HGF incubation (see Fig. 6). Interestingly, when C2 cells are transfected with α-syntrophin-specific siRNA, phosphorylation of Akt was dramatically reduced regardless of HGF stimulation. In the α-syntrophin knock-down C2 cells, the distribution of PTEN in the rear-lateral part of the cells was disrupted even with HGF treatment (see Fig. 7). In this case, it could be that PTEN dephosphorylates PIP3, which inhibits phosphorylation of Akt. These results suggest that syntrophin is involved in the PI3-kinase/Akt pathway in the HGF-induced lamellipodium formation.
α-Syntrophin knockout mice do not have specific symptoms for muscular dystrophy although they do have aberrant neuromuscular junctions [22]. Previously, we have shown that both differentiation of α-syntrophin siRNA C2 cells and the regeneration of muscle in the α-syntrophin knock-out mouse is delayed compared to controls [20]. In this study, we show that both α-syntrophin siRNA C2 cells and the myoblasts from the α-syntrophin knockout mice do not respond to HGF stimulation to initiate cell migration (see Fig. 3 and 4). These results suggest that deficiency of α-syntrophin results in insensitivity to HGF, which may cause delayed differentiation and regeneration.
Based on our observation that syntrophin is concentrated at the trailing edge of migrating myoblasts, we can propose mechanisms for syntrophin function. Intracellular calcium levels are implicated in the disassembly of adhesions at the trailing edge [52]. Important targets of calcium are the calcium-regulated phosphatase, calcineurin [53] and calcium-activated protease, calpain [54], which is involved in adhesion disassembly. Syntrophin can bind to calmodulin via C-terminal 24 amino acids in the SU domain in a Ca2+-dependent manner and α-syntrophin also can bind to plasma membrane Ca2+/calmodulin-dependent ATPase (PMCA) [55, 56]. Both syntrophin and dystrophin are phosphorylated in vivo and in vitro by Ca2+/calmodulin dependent kinase II (CamKII) [57] which is also required for the migration of PDGF-stimulated vascular smooth muscle cell [58]. Together these reports suggest that syntrophins have a role in trailing edge retraction in a calcium-dependent manner. The PH1 domain of α-syntrophin also binds phosphoinositol 4, 5 bisphosphate (PtdIns(4,5)P2) [59, 60] which is formed by phosphatidylinositol phosphate 5 kinase or PTEN. PtdIns(4, 5) P2 is involved in actin organization and focal adhesion formation [61, 62]. In addition, the heterotrimeric Gαβγ is bound by syntrophin in a laminin-dependent manner [63]. It is likely that syntrophins function by linking these diverse signaling molecules to form a functional complex at the trailing edge that can modulate cell migration.
Supplementary Material
Supp Figure 1. Expression of Met in the myoblasts from C57 and α-syntrophin-knockout mice. Primary cultured skeletal muscle cells from C57 or α-syntrophin-knockout (αKO) were incubated with (+) or without (−) HGF (50 ng/ml) for 1 h. Cells were then harvested and the level of Met was determined by western blotting with the specific antibody (Cell Signaling). Actin was used as a loading control.
Supp Figure 2. Expression and phosphorylation of Met in HGF treated myoblasts. C2 myoblasts cultured in growth media for 6 h were transferred into serum-starved DMEM for 1 h. Cells were incubated with (+) or without (−) HGF (50 ng/ml) for 1 h. The level of Met, phosphor-Met (Y1234/1235), phosphor-Met (Y1349), PTEN and α-syntrophin were determined by western blotting with the specific antibodies. Actin was used as a loading control.
Acknowledgments
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2008-531-E00004) to HSK and by NIH grant NS33145 to SCF.
Footnotes
Abreviations
PH-pleckstrin homology, SU-syntrophin unique, HGF-hepatocyte growth factor, C57-C57bl6/J, αKO-α-syntrophin knockout, AB-α, β2-syntrophin double knockout, FLA-transgenic mouse expressing full length α-syntrophin only in muscle cells, DMEM-Dulbecco’s modified Eagle’s medium, DAPI-4′,6-diamidino-2-phenylindole, PI-propidium iodide.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Seale P, Rudnicki MA. A new look at the origin, function, and “stem-cell” status of muscle satellite cells. Dev Biol. 2000;218:115–124. doi: 10.1006/dbio.1999.9565. [DOI] [PubMed] [Google Scholar]
- 2.Schultz E, Jaryszak DL, Valliere CR. Response of satellite cells to focal skeletal muscle injury. Muscle Nerve. 1985;8:217–222. doi: 10.1002/mus.880080307. [DOI] [PubMed] [Google Scholar]
- 3.Sheehan SM, Tatsumi R, Temm-Grove CJ, Allen RE. HGF is an autocrine growth factor for skeletal muscle satellite cells in vitro. Muscle Nerve. 2000;23:239–245. doi: 10.1002/(sici)1097-4598(200002)23:2<239::aid-mus15>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 4.Kawamura K, Takano K, Suetsugu S, Kurisu S, Yamazaki D, Miki H, Takenawa T, Endo T. N-WASP and WAVE2 acting downstream of phosphatidylinositol 3-kinase are required for myogenic cell migration induced by hepatocyte growth factor. J Biol Chem. 2004;279:54862–54871. doi: 10.1074/jbc.M408057200. [DOI] [PubMed] [Google Scholar]
- 5.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 6.Taher TE, Derksen PW, de Boer OJ, Spaargaren M, Teeling P, van der Wal AC, Pals ST. Hepatocyte growth factor triggers signaling cascades mediating vascular smooth muscle cell migration. Biochem Biophys Res Commun. 2002;298:80–86. doi: 10.1016/s0006-291x(02)02397-5. [DOI] [PubMed] [Google Scholar]
- 7.Albrecht DE, Froehner SC. Syntrophins and dystrobrevins: defining the dystrophin scaffold at synapses. Neurosignals. 2002;11:123–129. doi: 10.1159/000065053. [DOI] [PubMed] [Google Scholar]
- 8.Adams ME, Dwyer TM, Dowler LL, White RA, Froehner SC. Mouse alpha 1- and beta 2-syntrophin gene structure, chromosome localization, and homology with a discs large domain. J Biol Chem. 1995;270:25859–25865. doi: 10.1074/jbc.270.43.25859. [DOI] [PubMed] [Google Scholar]
- 9.Piluso G, Mirabella M, Ricci E, Belsito A, Abbondanza C, Servidei S, Puca AA, Tonali P, Puca GA, Nigro V. Gamma1- and gamma2-syntrophins, two novel dystrophin-binding proteins localized in neuronal cells. J Biol Chem. 2000;275:15851–15860. doi: 10.1074/jbc.M000439200. [DOI] [PubMed] [Google Scholar]
- 10.Ahn AH, Freener CA, Gussoni E, Yoshida M, Ozawa E, Kunkel LM. The three human syntrophin genes are expressed in diverse tissues, have distinct chromosomal locations, and each bind to dystrophin and its relatives. J Biol Chem. 1996;271:2724–2730. doi: 10.1074/jbc.271.5.2724. [DOI] [PubMed] [Google Scholar]
- 11.Lumeng C, Phelps S, Crawford GE, Walden PD, Barald K, Chamberlain JS. Interactions between beta 2-syntrophin and a family of microtubule-associated serine/threonine kinases. Nat Neurosci. 1999;2:611–617. doi: 10.1038/10165. [DOI] [PubMed] [Google Scholar]
- 12.Hasegawa M, Cuenda A, Spillantini MG, Thomas GM, Buee-Scherrer V, Cohen P, Goedert M. Stress-activated protein kinase-3 interacts with the PDZ domain of alpha1-syntrophin. A mechanism for specific substrate recognition. J Biol Chem. 1999;274:12626–12631. doi: 10.1074/jbc.274.18.12626. [DOI] [PubMed] [Google Scholar]
- 13.Hogan A, Shepherd L, Chabot J, Quenneville S, Prescott SM, Topham MK, Gee SH. Interaction of gamma 1-syntrophin with diacylglycerol kinase-zeta. Regulation of nuclear localization by PDZ interactions. J Biol Chem. 2001;276:26526–26533. doi: 10.1074/jbc.M104156200. [DOI] [PubMed] [Google Scholar]
- 14.Miyagoe-Suzuki Y, Takeda SI. Association of neuronal nitric oxide synthase (nNOS) with alpha1-syntrophin at the sarcolemma. Microsc Res Tech. 2001;55:164–170. doi: 10.1002/jemt.1167. [DOI] [PubMed] [Google Scholar]
- 15.Neely JD, Amiry-Moghaddam M, Ottersen OP, Froehner SC, Agre P, Adams ME. Syntrophin-dependent expression and localization of Aquaporin-4 water channel protein. Proc Natl Acad Sci U S A. 2001;98:14108–14113. doi: 10.1073/pnas.241508198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue M, Wakayama Y, Liu JW, Murahashi M, Shibuya S, Oniki H. Ultrastructural localization of aquaporin 4 and alpha1-syntrophin in the vascular feet of brain astrocytes. Tohoku J Exp Med. 2002;197:87–93. doi: 10.1620/tjem.197.87. [DOI] [PubMed] [Google Scholar]
- 17.Adams ME, Tesch Y, Percival JM, Albrecht DE, Conhaim JI, Anderson K, Froehner SC. Differential targeting of nNOS and AQP4 to dystrophin-deficient sarcolemma by membrane-directed {alpha}-dystrobrevin. J Cell Sci. 2008;121:48–54. doi: 10.1242/jcs.020701. [DOI] [PubMed] [Google Scholar]
- 18.Iwata Y, Sampaolesi M, Shigekawa M, Wakabayashi S. Syntrophin is an actin-binding protein the cellular localization of which is regulated through cytoskeletal reorganization in skeletal muscle cells. Eur J Cell Biol. 2004;83:555–565. doi: 10.1078/0171-9335-00415. [DOI] [PubMed] [Google Scholar]
- 19.Cooper ST, Maxwell AL, Kizana E, Ghoddusi M, Hardeman EC, Alexander IE, Allen DG, North KN. C2C12 co-culture on a fibroblast substratum enables sustained survival of contractile, highly differentiated myotubes with peripheral nuclei and adult fast myosin expression. Cell Motil Cytoskeleton. 2004;58:200–211. doi: 10.1002/cm.20010. [DOI] [PubMed] [Google Scholar]
- 20.Kim MJ, Hwang SH, Lim JA, Froehner SC, Adams ME, Kim HS. alpha-Syntrophin Modulates Myogenin Expression in Differentiating Myoblasts. PLoS One. 2010;5:e15355. doi: 10.1371/journal.pone.0015355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters MF, Adams ME, Froehner SC. Differential association of syntrophin pairs with the dystrophin complex. J Cell Biol. 1997;138:81–93. doi: 10.1083/jcb.138.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams ME, Kramarcy N, Krall SP, Rossi SG, Rotundo RL, Sealock R, Froehner SC. Absence of alpha-syntrophin leads to structurally aberrant neuromuscular synapses deficient in utrophin. J Cell Biol. 2000;150:1385–1398. doi: 10.1083/jcb.150.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams ME, Mueller HA, Froehner SC. In vivo requirement of the alpha-syntrophin PDZ domain for the sarcolemmal localization of nNOS and aquaporin-4. J Cell Biol. 2001;155:113–122. doi: 10.1083/jcb.200106158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams ME, Kramarcy N, Fukuda T, Engel AG, Sealock R, Froehner SC. Structural abnormalities at neuromuscular synapses lacking multiple syntrophin isoforms. J Neurosci. 2004;24:10302–10309. doi: 10.1523/JNEUROSCI.3408-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bandow K, Ohnishi T, Tamura M, Semba I, Daikuhara Y. Hepatocyte growth factor/scatter factor stimulates migration of muscle precursors in developing mouse tongue. J Cell Physiol. 2004;201:236–243. doi: 10.1002/jcp.20056. [DOI] [PubMed] [Google Scholar]
- 26.Royal I, Lamarche-Vane N, Lamorte L, Kaibuchi K, Park M. Activation of cdc42, rac, PAK, and rho-kinase in response to hepatocyte growth factor differentially regulates epithelial cell colony spreading and dissociation. Mol Biol Cell. 2000;11:1709–1725. doi: 10.1091/mbc.11.5.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 28.Ballestrem C, Wehrle-Haller B, Imhof BA. Actin dynamics in living mammalian cells. J Cell Sci. 1998;111(Pt 12):1649–1658. doi: 10.1242/jcs.111.12.1649. [DOI] [PubMed] [Google Scholar]
- 29.Vicente-Manzanares M, Rey M, Perez-Martinez M, Yanez-Mo M, Sancho D, Cabrero JR, Barreiro O, de la Fuente H, Itoh K, Sanchez-Madrid F. The RhoA effector mDia is induced during T cell activation and regulates actin polymerization and cell migration in T lymphocytes. J Immunol. 2003;171:1023–1034. doi: 10.4049/jimmunol.171.2.1023. [DOI] [PubMed] [Google Scholar]
- 30.Funamoto S, Meili R, Lee S, Parry L, Firtel RA. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 2002;109:611–623. doi: 10.1016/s0092-8674(02)00755-9. [DOI] [PubMed] [Google Scholar]
- 31.Comer FI, Parent CA. PI 3-kinases and PTEN: how opposites chemoattract. Cell. 2002;109:541–544. doi: 10.1016/s0092-8674(02)00765-1. [DOI] [PubMed] [Google Scholar]
- 32.Devreotes P, Janetopoulos C. Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J Biol Chem. 2003;278:20445–20448. doi: 10.1074/jbc.R300010200. [DOI] [PubMed] [Google Scholar]
- 33.Merlot S, Firtel RA. Leading the way: Directional sensing through phosphatidylinositol 3-kinase and other signaling pathways. J Cell Sci. 2003;116:3471–3478. doi: 10.1242/jcs.00703. [DOI] [PubMed] [Google Scholar]
- 34.Goncharova EA, Ammit AJ, Irani C, Carroll RG, Eszterhas AJ, Panettieri RA, Krymskaya VP. PI3K is required for proliferation and migration of human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2002;283:L354–363. doi: 10.1152/ajplung.00010.2002. [DOI] [PubMed] [Google Scholar]
- 35.van der Voort R, Taher TE, Derksen PW, Spaargaren M, van der Neut R, Pals ST. The hepatocyte growth factor/Met pathway in development, tumorigenesis, and B-cell differentiation. Adv Cancer Res. 2000;79:39–90. doi: 10.1016/s0065-230x(00)79002-6. [DOI] [PubMed] [Google Scholar]
- 36.Hosaka Y, Yokota T, Miyagoe-Suzuki Y, Yuasa K, Imamura M, Matsuda R, Ikemoto T, Kameya S, Takeda S. Alpha1-syntrophin-deficient skeletal muscle exhibits hypertrophy and aberrant formation of neuromuscular junctions during regeneration. J Cell Biol. 2002;158:1097–1107. doi: 10.1083/jcb.200204076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Royal I, Park M. Hepatocyte growth factor-induced scatter of Madin-Darby canine kidney cells requires phosphatidylinositol 3-kinase. J Biol Chem. 1995;270:27780–27787. doi: 10.1074/jbc.270.46.27780. [DOI] [PubMed] [Google Scholar]
- 38.Khwaja A, Lehmann K, Marte BM, Downward J. Phosphoinositide 3-kinase induces scattering and tubulogenesis in epithelial cells through a novel pathway. J Biol Chem. 1998;273:18793–18801. doi: 10.1074/jbc.273.30.18793. [DOI] [PubMed] [Google Scholar]
- 39.Raftopoulou M, Etienne-Manneville S, Self A, Nicholls S, Hall A. Regulation of cell migration by the C2 domain of the tumor suppressor PTEN. Science. 2004;303:1179–1181. doi: 10.1126/science.1092089. [DOI] [PubMed] [Google Scholar]
- 40.Valiente M, Andres-Pons A, Gomar B, Torres J, Gil A, Tapparel C, Antonarakis SE, Pulido R. Binding of PTEN to specific PDZ domains contributes to PTEN protein stability and phosphorylation by microtubule-associated serine/threonine kinases. J Biol Chem. 2005;280:28936–28943. doi: 10.1074/jbc.M504761200. [DOI] [PubMed] [Google Scholar]
- 41.Bischoff R. Chemotaxis of skeletal muscle satellite cells. Dev Dyn. 1997;208:505–515. doi: 10.1002/(SICI)1097-0177(199704)208:4<505::AID-AJA6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 42.Miller KJ, Thaloor D, Matteson S, Pavlath GK. Hepatocyte growth factor affects satellite cell activation and differentiation in regenerating skeletal muscle. Am J Physiol Cell Physiol. 2000;278:C174–181. doi: 10.1152/ajpcell.2000.278.1.C174. [DOI] [PubMed] [Google Scholar]
- 43.Anastasi S, Giordano S, Sthandier O, Gambarotta G, Maione R, Comoglio P, Amati P. A natural hepatocyte growth factor/scatter factor autocrine loop in myoblast cells and the effect of the constitutive Met kinase activation on myogenic differentiation. J Cell Biol. 1997;137:1057–1068. doi: 10.1083/jcb.137.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leshem Y, Spicer DB, Gal-Levi R, Halevy O. Hepatocyte growth factor (HGF) inhibits skeletal muscle cell differentiation: a role for the bHLH protein twist and the cdk inhibitor p27. J Cell Physiol. 2000;184:101–109. doi: 10.1002/(SICI)1097-4652(200007)184:1<101::AID-JCP11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 45.Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- 46.Gal-Levi R, Leshem Y, Aoki S, Nakamura T, Halevy O. Hepatocyte growth factor plays a dual role in regulating skeletal muscle satellite cell proliferation and differentiation. Biochim Biophys Acta. 1998;1402:39–51. doi: 10.1016/s0167-4889(97)00124-9. [DOI] [PubMed] [Google Scholar]
- 47.Beviglia L, Matsumoto K, Lin CS, Ziober BL, Kramer RH. Expression of the c-Met/HGF receptor in human breast carcinoma: correlation with tumor progression. Int J Cancer. 1997;74:301–309. doi: 10.1002/(sici)1097-0215(19970620)74:3<301::aid-ijc12>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 48.Ueoka Y, Kato K, Kuriaki Y, Horiuchi S, Terao Y, Nishida J, Ueno H, Wake N. Hepatocyte growth factor modulates motility and invasiveness of ovarian carcinomas via Ras-mediated pathway. Br J Cancer. 2000;82:891–899. doi: 10.1054/bjoc.1999.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 50.Benvenuti S, Comoglio PM. The MET receptor tyrosine kinase in invasion and metastasis. J Cell Physiol. 2007;213:316–325. doi: 10.1002/jcp.21183. [DOI] [PubMed] [Google Scholar]
- 51.Hecht M, Papoutsi M, Tran HD, Wilting J, Schweigerer L. Hepatocyte growth factor/c-Met signaling promotes the progression of experimental human neuroblastomas. Cancer Res. 2004;64:6109–6118. doi: 10.1158/0008-5472.CAN-04-1014. [DOI] [PubMed] [Google Scholar]
- 52.Lee J, Ishihara A, Oxford G, Johnson B, Jacobson K. Regulation of cell movement is mediated by stretch-activated calcium channels. Nature. 1999;400:382–386. doi: 10.1038/22578. [DOI] [PubMed] [Google Scholar]
- 53.Hendey B, Klee CB, Maxfield FR. Inhibition of neutrophil chemokinesis on vitronectin by inhibitors of calcineurin. Science. 1992;258:296–299. doi: 10.1126/science.1384129. [DOI] [PubMed] [Google Scholar]
- 54.Glading A, Lauffenburger DA, Wells A. Cutting to the chase: calpain proteases in cell motility. Trends Cell Biol. 2002;12:46–54. doi: 10.1016/s0962-8924(01)02179-1. [DOI] [PubMed] [Google Scholar]
- 55.Newbell BJ, Anderson JT, Jarrett HW. Ca2+-calmodulin binding to mouse alpha1 syntrophin: syntrophin is also a Ca2+-binding protein. Biochemistry. 1997;36:1295–1305. doi: 10.1021/bi962452n. [DOI] [PubMed] [Google Scholar]
- 56.Williams JC, Armesilla AL, Mohamed TM, Hagarty CL, McIntyre FH, Schomburg S, Zaki AO, Oceandy D, Cartwright EJ, Buch MH, Emerson M, Neyses L. The sarcolemmal calcium pump, alpha-1 syntrophin, and neuronal nitric-oxide synthase are parts of a macromolecular protein complex. J Biol Chem. 2006;281:23341–23348. doi: 10.1074/jbc.M513341200. [DOI] [PubMed] [Google Scholar]
- 57.Madhavan R, Jarrett HW. Calmodulin-activated phosphorylation of dystrophin. Biochemistry. 1994;33:5797–5804. doi: 10.1021/bi00185a018. [DOI] [PubMed] [Google Scholar]
- 58.Pfleiderer PJ, Lu KK, Crow MT, Keller RS, Singer HA. Modulation of vascular smooth muscle cell migration by calcium/ calmodulin-dependent protein kinase II-delta 2. Am J Physiol Cell Physiol. 2004;286:C1238–1245. doi: 10.1152/ajpcell.00536.2003. [DOI] [PubMed] [Google Scholar]
- 59.Chockalingam PS, Gee SH, Jarrett HW. Pleckstrin homology domain 1 of mouse alpha 1-syntrophin binds phosphatidylinositol 4,5-bisphosphate. Biochemistry. 1999;38:5596–5602. doi: 10.1021/bi982564+. [DOI] [PubMed] [Google Scholar]
- 60.Yan J, Wen W, Xu W, Long JF, Adams ME, Froehner SC, Zhang M. Structure of the split PH domain and distinct lipid-binding properties of the PH-PDZ supramodule of alpha-syntrophin. EMBO J. 2005;24:3985–3995. doi: 10.1038/sj.emboj.7600858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van den Bout I, Divecha N. PIP5K-driven PtdIns(4,5)P2 synthesis: regulation and cellular functions. J Cell Sci. 2009;122:3837–3850. doi: 10.1242/jcs.056127. [DOI] [PubMed] [Google Scholar]
- 62.Ling K, Schill NJ, Wagoner MP, Sun Y, Anderson RA. Movin’ on up: the role of PtdIns(4,5)P(2) in cell migration. Trends Cell Biol. 2006;16:276–284. doi: 10.1016/j.tcb.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 63.Zhou YW, Oak SA, Senogles SE, Jarrett HW. Laminin-alpha1 globular domains 3 and 4 induce heterotrimeric G protein binding to alpha-syntrophin's PDZ domain and alter intracellular Ca2+ in muscle. Am J Physiol Cell Physiol. 2005;288:C377–388. doi: 10.1152/ajpcell.00279.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp Figure 1. Expression of Met in the myoblasts from C57 and α-syntrophin-knockout mice. Primary cultured skeletal muscle cells from C57 or α-syntrophin-knockout (αKO) were incubated with (+) or without (−) HGF (50 ng/ml) for 1 h. Cells were then harvested and the level of Met was determined by western blotting with the specific antibody (Cell Signaling). Actin was used as a loading control.
Supp Figure 2. Expression and phosphorylation of Met in HGF treated myoblasts. C2 myoblasts cultured in growth media for 6 h were transferred into serum-starved DMEM for 1 h. Cells were incubated with (+) or without (−) HGF (50 ng/ml) for 1 h. The level of Met, phosphor-Met (Y1234/1235), phosphor-Met (Y1349), PTEN and α-syntrophin were determined by western blotting with the specific antibodies. Actin was used as a loading control.