Abstract
Epithelial Na+ Channels (ENaC) are located on alveolar cells and are important in β2-adrenergic receptor-mediated lung fluid clearance through the removal of Na+ from the alveolar airspace. Previous work has demonstrated that genetic variation of the alpha subunit of ENaC at amino acid 663 is important in channel function: cells with the genotype resulting in alanine at amino acid 663 (A663) demonstrate attenuated function when compared to genotypes with at least one allele encoding threonine (T663, AT/TT). We sought to determine the influence of genetic variation at position 663 of ENaC on exhaled Na+ in healthy humans. Exhaled Na+ was measured in 18 AA and 13 AT/TT subjects (age=27±8 vs. 30±10yrs., ht.=174±12 vs. 171±10cm., wt=68±12 vs. 73±14kg., BMI=22±3 vs. 25±4kg/m2, mean±SD, for AA and AT/TT, respectively). Measurements were made at baseline and at 30, 60 and 90 minutes following the administration of a nebulized β2-agonist (albuterol sulfate, 2.5mg diluted in 3ml normal saline). The AA group had a higher baseline level of exhaled Na+ and a greater response to β2-agonist stimulation (baseline= 3.1±1.8 vs. 2.3±1.5mmol/l; 30min-post= 2.1±0.7 vs. 2.2±0.8mmol/l; 60min-post= 2.0±0.5 vs. 2.3±1.0mmol/l; 90min-post= 1.8±0.8 vs. 2.6±1.5mmol/l, mean±SD, for AA and AT/TT, respectively, p<0.05). The results are consistent with the notion that genetic variation of ENaC influences β2-adrenergic receptor stimulated Na+ clearance in the lungs, as there was a significant reduction in exhaled Na+ over time in the AA group.
Keywords: Breath Condensate, Airway Surface Fluid, Beta-agonist, SCNN1A, ADRB2, Gene
1. Introduction
Fluid levels in the alveolar space are normally tightly regulated to optimize gas transfer of oxygen and carbon dioxide between the blood and the airspace within the alveoli (Aarseth et al., 1975; Snyder et al., 2006; Wallin and Leksell, 1994). Conditions such as heart failure and cystic fibrosis disrupt fluid regulation causing either flooding of the alveolar space, or drying of the airways resulting in mucous accumulation. Both conditions result in compromised gas transfer (Boucher, 2004; Chua and Coats, 1995).
Lung fluid balance is normally tightly regulated by the interplay between different ion channels transporting salts and the water, which ultimately follows the flux of these ions. When ions such as sodium (Na+) or chloride (Cl−) are transported from the alveolar space to the interstitium, this causes an osmotic gradient to form, resulting in the translocation of water from the alveoli to the interstitial space (Starling, 1896). One important factor in this ion transport is the epithelial Na+ channel (ENaC, encoded by the gene SCNN1A), a protein found on the luminal surface of many cells including the type I and II epithelia of the alveolar wall (Garty and Palmer, 1997). The primary function of ENaC is to allow cations, in particular Na+, to pass from the apical side (alveolar space) to the basolateral side (interstitial space) of these airway epithelial cells. This results in an overall flux of water out of the alveoli of the lung, a critical element in the regulation of overall fluid balance (Eaton et al., 2004). Over-activity or over-expression of Na+ channels can cause the lungs to be dry, while lower than normal activity can lend to flooding within the alveolar space. Two additional channel proteins critically involved in fluid balance are the cystic fibrosis transmembrane regulator (CFTR) and the calcium activated chloride channels (Fang et al., 2006; Hartzell et al., 2005). These apical membrane mechanisms allow for the outward flux of Cl− into the alveolar space, serving as a balance for the effects of channels such as ENaC. Various stimuli have effects directly and/or indirectly on these channels and transporters, modifying their expression or function. Epithelial Na+ channel function can be modulated by endogenous (such as epinephrine) and exogenous (albuterol) stimuli (Berdiev et al., 2009; Li and Folkesson, 2006; Morris and Schafer, 2002). The β2-adrenergic receptor (ADRB2) stimulates ENaC, and previous research has shown increased ENaC expression with long-term β2-agonist exposure (Berthiaume et al., 1999; Minakata et al., 1998). Studies in rats have shown a strong increase in fluid clearance from the alveolar space after over-expression of ADRB2. Lung fluid clearance has also been found to be attenuated by agents that block Na+ channel function pointing to a role for ENaC in the clearance process (Dumasius et al., 2001; Ma et al., 2000).
Each of the three ENaC subunits (α, β, γ) has been found to exhibit genetic variants in the form of single nucleotide polymorphisms (SNPs). The effects of these alterations in the protein have most commonly been studied in the kidney, where the renin-angiotensin-aldosterone system plays a critical role in Na+ and fluid regulation through ENaC and other channel proteins. Polymorphisms increasing the function of ENaC, primarily those in the β and γ ENaC subunits, have been associated with inheritable forms of hypertension, such as Liddle’s syndrome, a form of salt sensitive hypertension (Azad et al., 2009; Snyder, 2000). The alpha subunit of ENaC shows an especially significant effect on channel function when altered, particularly with respect to the pulmonary epithelium (Tong et al., 2006). Within the alpha subunit, the T663 variant has been associated with an increase in overall ENaC function when compared with the A663 homozygous variant (Samaha et al., 2004; Tong et al., 2006) although others have shown little to no functional effect from the mutation (Ambrosius et al., 1999). One limitation of much of the previous work is that it has been primarily performed in cultured cells. There are very few functional human studies exploring the phenotypic consequences of genetic variation of the alpha subunit of ENaC, beyond those that demonstrate susceptibility to hypertension. Previous investigators have theorized that the increase in function in the T663 variants observed in previous studies are likely due to a higher number of active Na+ channels on the apical membrane because of increased insertion or retention of the channels in the membrane, or by increasing the activity of closed channels (Samaha et al., 2004). The overactive T663 group may have an increased response to exogenous stimuli due to overall individual channel activity, or according to these previous theories a diminished response to stimuli will occur as levels of ENaC expression and activity may be maximally reached by the SNP variant alone.
No published studies, to date, have investigated the relationship between exhaled Na+ or lung diffusion (DLCO, DM, Vc) and genetic variation of ENaC in humans. β2-adrenergic stimulation is known to cause an increase in expression of the ENaC protein leading to a greater number of available channel units, a more active channel (possibly due to an increase in the time the channel spends in its open position), or both (Berthiaume et al., 1999; Minakata et al., 1998). Here, we sought to determine the influence of genetic variation in the alpha subunit of ENaC at amino acid position 663, on exhaled Na+ in healthy individuals prior to, and following, administration of nebulized albuterol, a common inhaled β2-agonist. It is important to note that the direct measurement of ENaC activity was not part of this study and is outside of the scope of the study. We used a surrogate marker, exhaled sodium in exhaled breath condensate (EBC) fluid, to demonstrate the ultimate effect of increased ENaC activity, which is the clearance of sodium and water from the fluid lining the lower airway. Based on cell work, we predicted that those with at least one allele encoding the T663 variant (more functional in cell work) would show decreased exhaled Na+ in response to a β-agonist as compared to those homozygous for the wild type (A663) variant.
2. Methods
2.1 Subjects
Thirty-one healthy subjects between the ages of 18 and 45 were recruited to participate in the study. The protocol was reviewed and approved by the University of Arizona Institutional Review Board, with all subjects providing written informed consent prior to participation, and all aspects of the study conformed to the Declaration of Helsinki. Following genotyping, subjects were stratified into two groups based on SCNN1A genotype where individuals homozygous for SCNN1A resulting in alanine at amino acid 663 were classified as AA and individuals with at least allele resulting in threonine at amino acid 663 were classified as AT/TT.
2.2 Protocol
The subjects were asked to come to the laboratory in a fasted state, were instructed not to use caffeine, and were not taking any medications that could influence the response to albuterol (β-agonist, β-blocker). Subjects had a 20-gauge venous catheter inserted into a prominent antecubital vein for multiple blood sampling to assess hemoglobin. Prior to the administration of albuterol and 30, 60 and 90 minutes post-nebulization, exhaled breath condensate (EBC) was collected for 25 minutes on the Jaeger EcoScreen (Cardinal Health, Yorba Linda, CA). During each collection, subjects were seated wearing a nose clip while performing calm tidal breathing. A blood sample was taken at the midway point of the EBC collection. Following each EBC collection, measurement of diffusion capacity of the lungs for carbon monoxide and nitric oxide (DLCO/DLNO) was assessed in triplicate. Subjects received albuterol (2.5 mg diluted in 3 ml normal saline) via nebulization using a Power Neb2 nebulizer (Drive Medical, Port Washington, NY). Albuterol administration was conducted with subjects seated and quietly breathing while wearing a nose piece until all liquid had been nebulized, typically ten to twelve minutes. During administration subjects were also instructed to take a deep breath every two minutes to allow for dispersal of the nebulized particles into the lower airways. Once this was completed subjects rested for ten minutes to allow the drug to take effect.
2.3 Determination of ENaC genetic polymorphism
Buccal swabs were collected from the inside of each cheek and placed into a stabilizing solution for storage. Samples were sent to the University of Arizona Genetics Core for genotyping of the ENaC amino acid at position 663 of both alleles using a Taqman SNP assay for rs#2228576 (Applied Biosystems, Carlsbad, California). Initial DNA quantitation and QC was performed using PicoGreen (Life Technologies). Pre-validated primers and probe sets for TaqMan Allelic Discrimination Assay were obtained from Life Technologies. Reactions were run at 10uL, containing TaqMan Universal PCR Master Mix, No AmpEraseR UNG (Life Technologies), 10ng total DNA, and 1X Assay Mix. All samples processed and analyzed on 7900 Real-Time PCR System (Life Technologies) with cycling conditions (95oC for 10 minutes, 50 cycles of 92oC for 15 seconds and 60oC for 1 minute) and Genotyper software (SDS system, version 2.3).
2.4 Quantification of Serum Hemoglobin and Ion Concentrations
From the 12 ml venous blood sample at the midway point of the exhaled breath collection (10–12 min), hemoglobin was measured by a cyanide-free hemoglobin method on an ADVIA 2120 Hematology system. Serum Na+ concentrations were determined from the same venous blood sample using ion-selective electrodes at the University of Arizona Medical Center Pathology Laboratory.
2.5 Collection of Exhaled Breath Condensate
The condensing system used in the present study was the Jaeger Ecoscreen cooling unit described previously (Wheatley et al., 2010). Briefly, sample collection cups were screwed onto the bottom of the condenser which was attached to and inhalation/exhalation valve and then the condenser and cup was inserted into the lumen of the −20°C cooling system which to facilitate condensation of exhaled breath. A new condenser was used for each 25 minute collection within a subject. The potential for contamination of the EBC sample with saliva was avoided by instructing the subjects to swallow accumulated saliva as necessary during the collection. In addition, the Ecoscreen has a saliva trap and the device angles upward away from the mouth so that saliva travels back into the mouth rather than into the condenser so salivary contamination of the sample is avoided.
2.6 Quantification of Exhaled Breath Condensate Ion Concentrations
Exhaled breath condensate samples were stored at −80°C until the time of final batch analysis. Previous testing by our lab and others found no difference in ion concentrations of the same samples tested directly after collection and again after being frozen (Wheatley et al., 2010; Zacharasiewicz et al., 2004). Na+ concentrations were measured using an atomic absorption spectrophotometer (Analyst 100; Perkin-Elmer, Norwalk, CT) with a detection limit of 0.01 μmol/L. emission wavelength of 566.5 nm Na+ as previously described (Mandal et al., 2008; Wheatley et al., 2010). Samples were diluted as needed in order for absorbance to be measured within the prepared standard solution curve with known concentrations of sodium diluted with Milli-Q water (Millipore, Burlington, MA) to concentrations of 0.01, 0.05, 0.10, 0.15, 0.20 mmol/L.
2.7 Measurement of Diffusion of the Lungs for Carbon Monoxide, Alveolar-Capillary Membrane Conductance, and Pulmonary Capillary Volume
At baseline and 30, 60 and 90-minutes post nebulization measures of the diffusion capacity of the lungs for carbon monoxide and nitric oxide (DLCO and DLNO, respectively) were performed simultaneously in triplicate as described previously (Snyder et al., 2007; Snyder et al., 2005; Snyder et al., 2008; Tamhane et al., 2001; Wheatley et al., 2011). DLCO and DLNO were assessed by a rebreathe technique using a 5-liter anesthesia bag containing 0.3% carbon monoxide (C18O), 40 PPM NO (diluted immediately before the test in the bag from an 800 PPM gas mixture), and 35% O2 at a respiratory rate of 32 breaths/minute. A pneumotachograph was connected to a pneumatic switching valve, which allowed for rapid switching from room air to the test gas mixture. Gas concentration was analyzed using a mass spectrometer and NO analyzer (Sievers Instruments, Boulder, CO), which was integrated with custom analysis software for the assessment of DLCO and DLNO. At the end of a normal expiration (end-expiratory lung volume, EELV) the subjects were switched into the rebreathe bag and instructed to nearly empty the bag with each breath for 8–10 consecutive breaths.
The diffusion capacity of the lungs for carbon monoxide is based on the contribution of both the membrane conductance and the hemoglobin binding (Tamhane et al., 2001). The rate of disappearance of the gases with each breath was calculated from the slope of the exponential disappearance for each gas with respect to helium using custom software (Snyder et al., 2005). Unlike DLCO, DLNO is theoretically based solely on membrane conductance as nitric oxide is scavenged 280 times faster by hemoglobin than O2 so its uptake into the blood is instantaneous. For this reason DLNO is considered a direct measure of membrane conductance DMNO as the diffusion resistance of the blood is trivial (Hsia, 2002; Hsia and Raskin, 2005; Roughton and Forster, 1957; Tamhane et al., 2001). DMNO was then used to calculate the DM for carbon monoxide (DMCO) by adjusting for their different diffusion constants based on molecular weight and solubility as described previously (Tamhane et al., 2001). Recent work by Ceridon et al. has demonstrated that a correction factor of 2.11 is most appropriate (Ceridon et al., 2010). Pulmonary-capillary blood volume was then calculated from the DLCO measured by subtracting the resistance to diffusion associated with alveolar-capillary barrier (DMCO) and correcting for differences in the rate of uptake and binding to hemoglobin (1/θ) due to differences in hemoglobin (Hb) concentrations (14.6g/dl being used as the normal hemoglobin concentration) and the alveolar pressure of oxygen.
2.8 Assessment of Airway Function
Subjects performed baseline pulmonary function testing and maximal expiratory flow volume (MEFV) maneuvers (Beck et al., 1999; Johnson et al., 2000). From the MEFV maneuver, forced vital capacity (FVC), forced expiratory volume after one second (FEV1), and forced expiratory flow rate at 25–75% of the forced vital capacity (FEF25–75) were assessed. All subjects were instructed to take a gradual but maximal inspiration prior to the forced exhalation. Flow and volume signals were digitized at a rate of 100 samples/sec and stored for later analysis to determine the FVC, FEV1, and FEF25–75. Percent predicted values for the pulmonary function measures were determined based on predicted equations from NHANES III for FVC, FEV1, and FEF25–75 (Hankinson et al., 1999).
2.9 Statistical Analysis
All statistical comparisons were performed using the SPSS statistical software package (v. 17.0, Chicago, IL). Descriptive statistics were performed to calculate mean, maximum, minimum, and standard deviation for EBC ion concentration, DLCO/DLNO measures and pulmonary function at each collection time point. Independent samples t-tests were used to determine differences between the AA, at amino acid 663, and AT/TT at baseline. Between groups repeated measures analysis of variance was used to evaluate the changes in exhaled Na+, DLCO, DM, Vc and DM/Vc over time in response to albuterol as well as the interaction between time point and genotype group. All data were determined to be normally distributed using a Mauchly’s test prior to the ANOVA. A Huynh-Feldt correction was used to determine statistical significance when data were not normally distributed. To determine if exhaled Na+ was, in part, derived from the airways, correlation analysis was performed between exhaled Na+ and lung diffusion parameters and between exhaled Na+ and pulmonary function. All data are presented as mean±SD, unless otherwise indicated, and an alpha level of 0.05 was selected to indicate significance a priori.
3. Results
Healthy subjects homozygous for a SCNN1A resulting in alanine at amino acid 663, referred to as the AA group, and healthy subjects with at least one SCNN1A allele resulting in threonine at amino acid 663, referred to as the AT/TT group, showed no differences in gender, age, height and weight and BMI. The genotype frequencies were 18 AA, 9 AT, and 4 TT subjects in this study. No differences were observed in any pulmonary function measured between the two genotype groups at baseline (Table 1). As expected, albuterol resulted in an increase in FEV1 and FEF25–75, but there were no differences in this improvement according to SCNN1A genotype. There was a positive, and significant relationship between absolute pulmonary function and exhaled Na+ (r= 0.42, 0.52, and 0.39 for FVC, FEV1, and FEF25–75, respectively, p<0.05 for all); however, this appears to be a function of body size (i.e. those with larger lungs had more exhaled Na+ and greater pulmonary function) because there was no relationship between exhaled Na+ and pulmonary function when expressed as percent predicted.
Table 1.
| A663/A663 | T663/A663 or T663/T663 | |
|---|---|---|
| n | 18 | 13 |
| Gender (% female) | 39 | 46 |
| Age (years) | 25±7 | 30±10 |
| Height (cm) | 174±11 | 171±10 |
| Weight (Kg) | 69±12 | 73±14 |
| BMI (kg/m2) | 23±4 | 25±4 |
| FVC (% predicted) | 95±11 | 100±20 |
| FEV1 (% predicted) | 95±11 | 95±18 |
| FEF25–75 (% predicted) | 92±19 | 87±26 |
| Serum Na+ (mmol/L) | 139±2 | 139±2 |
Values are mean ± SD. BMI = Body mass index; VO2PEAK = Maximal oxygen capacity; FVC = Forced vital capacity; FEV1 = Forced expiratory volume in 1 second; FEF25-75 = Forced Expiratory flow volume at 25–75% of the FVC
Baseline measures of exhaled Na+ in EBC were statistically similar between the AA AT/TT groups (p=0.12, Figure 2). For the entire group (both AA and AT/TT) baseline serum Na+ levels were correlated with EBC Na+ levels (r =0.63, p<0.05). Although the Na+ levels in the AT/TT group remained relatively constant throughout the observation period, the concentration of Na+ decreased significantly over the 90 minute time course in the AA group (2.9±1.6 to 1.8±0.7mmol/L for baseline and 90 minutes respectively, p<0.05, Figure 2). The difference in Na+ between the AA and AT/TT groups was determined to be significant at 90 minutes post albuterol (1.8±0.7 vs. 2.5±1.5mmol/L for AA and AT/TT group respectively, p<0.05, Figure 2). The percent change in exhaled Na+ from baseline to 90 minutes was significantly different between genotypes (−25±30 vs. 15±47% for AA and AT/TT group respectively, p<0.05, Figure 3). Measures of DM/Vc and exhaled Na+ were found to be significantly correlated at baseline (r = −0.39, p<0.05) and 90 minutes (r = −0.36, p<0.05, Figure 1). The percent change from baseline to 90 minutes for the measures of exhaled Na+ and DM/Vc were not correlated (r =0.27, p=0.14).
Figure 2. Exhaled Na+ Concentration in Response to Albuterol Administration Based on SCNN1A Genotype.
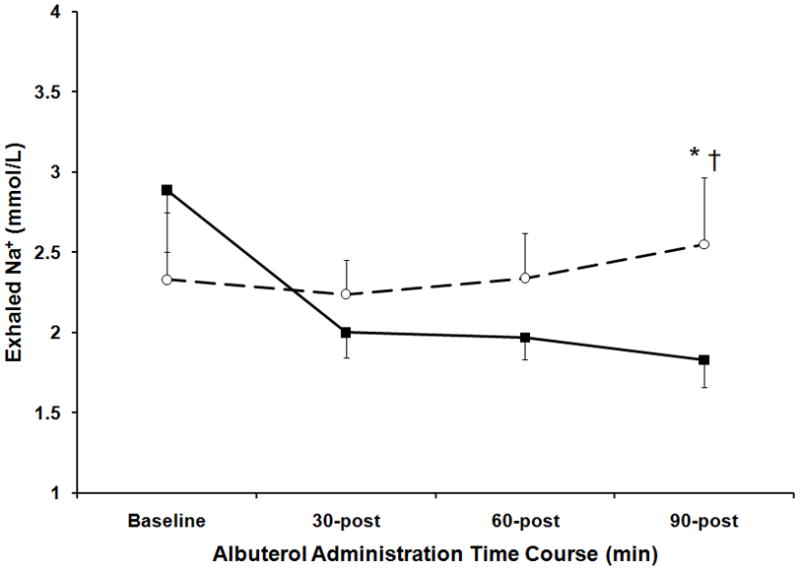
The measured concentration of Na+ in exhaled breath condensate collections at baseline and 30 minutes, 60 minutes and 90 minutes post albuterol administration. Stratification in response to albuterol based on SCNN1A genotype where the closed squares and solid line represent the AA group (homozygous for SCNN1A resulting in alanine at amino acid 663) and the open circle and dashed line represent the AT/TT group (at least one copy of SCNN1A resulting in threonine at amino acid 663). The error bars represent the standard error of the mean. *p<0.05 AA vs. AT/TT, †P<0.05 compared to baseline AT/TT group.
Figure 3. Percent Change in Exhaled Na+ from Baseline to 90 Minutes Post-Albuterol Administration.
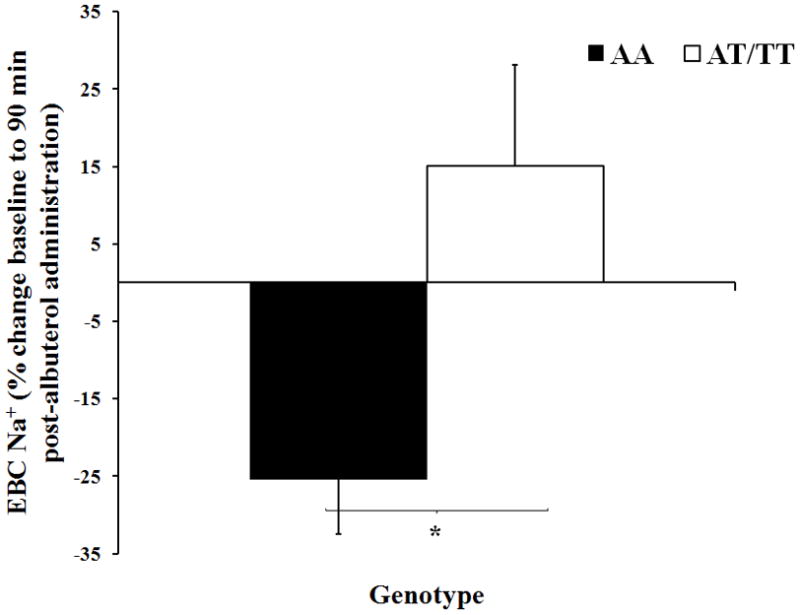
The filled in bars represent the AA group (homozygous for SCNN1A resulting in alanine at amino acid 663) and the open bars represent the AT/TT group (at least one copy of SCNN1A resulting in threonine at amino acid 663). The error bars represent the SE of the mean. *P<0.05 between groups.
Figure 1. Relationship between baseline Na+ in exhaled breath condensate and baseline DM/VC.
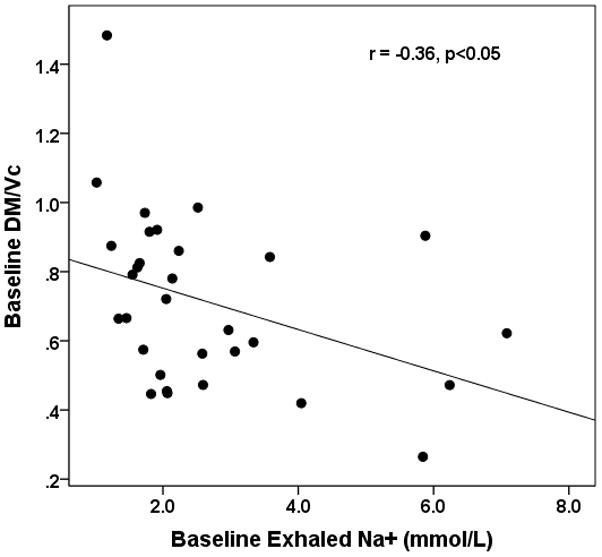
The x-axis represents baseline concentration of Na+ in exhaled breath condensate in mmol/L and the y-axis represents the ratio of the percent change in DM over the percent change in VC. r = −0.36, p<0.05
At baseline there were no observed differences between AA and AT/TT groups in DLCO, DM, Vc, or DM/Vc. Although both groups had an increase in DM/Vc (p<0.05), the groups showed no significant differences in these measures at any point after albuterol administration (Figure 4). There was no significant difference between the groups based on the interaction of genotype and DLCO, DM, Vc, or DM/Vc over time.
Figure 4. Alveolar-Capillary Membrane Conductance Corrected for Pulmonary Capillary Blood volume in Response to Albuterol Based on ENaC Genotype.
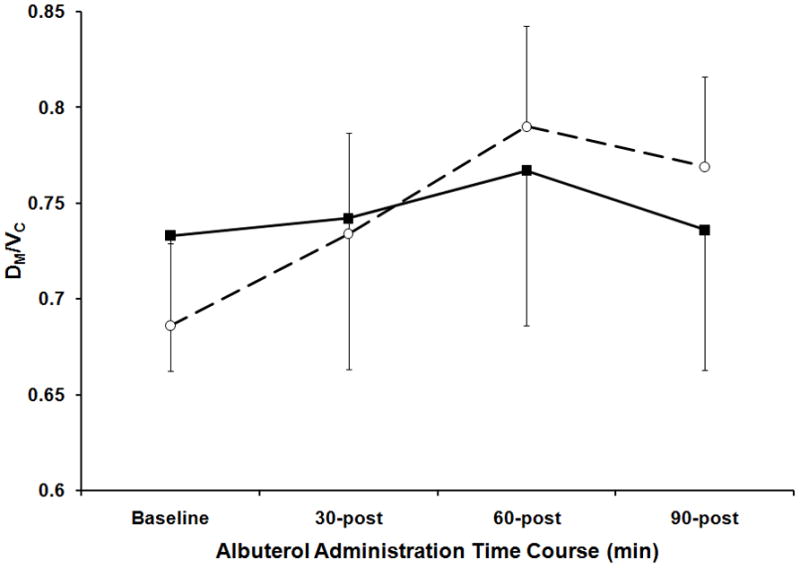
Alveolar-Capillary Membrane Conductance Corrected for Pulmonary Capillary Blood volume (DM/Vc) at baseline and 30 minutes, 60 minutes and 90 minutes post albuterol administration. Stratification in response to albuterol based on SCNN1A genotype where the closed squares and solid line represent the AA group (homozygous for SCNN1A resulting in alanine at amino acid 663) and the open circle and dashed line represent the AT/TT group (at least one copy of SCNN1A resulting in threonine at amino acid 663). The error bars represent the standard error of the mean.
4. Discussion
The results demonstrate a detectable difference in the concentration of exhaled Na+ following albuterol administration according to SCNN1A genotype. Contrary to our prediction, ninety minutes post albuterol the AA group demonstrated a significantly lower exhaled Na+ concentration. Surprisingly, the AT/TT group demonstrated no change in exhaled Na+ following albuterol. Subjects in the AA group demonstrated a greater decrease in Na+ levels in EBC following the administration of albuterol, suggesting a higher rate of Na+ clearance in the airways in this group. The AT/TT group had lower baseline EBC Na+, but an attenuated response with albuterol. These baseline findings are in agreement previous studies which have reported greater basal Na+ channel activity in the T663 variant when examined in animal studies and in vitro models (Tong et al., 2006; Yan et al., 2007).
β2-adrenergic stimulation is known to cause an increase in expression of the ENaC protein leading to a greater number of available channel units, a more active channel (possibly due to an increase in the time the channel spends in its open position), or both (Berthiaume et al., 1999; Minakata et al., 1998). It is important to note that the direct measurement of ENaC activity was not part of this study and is outside of its scope, rather the surrogate marker of exhaled sodium in EBC fluid was used to demonstrate the ultimate effect of increased ENaC activity, the clearance of sodium and water from the fluid lining the lower airway. It is possible that the apparent insensitivity to β2-adrenergic receptor stimulation in the AT/TT group may be the result of the genetic variant driving an increased expression of ENaC channels constitutively. If this was the case, a greater number of channels in the apical membrane of airway epithelia, or a greater number of permanently open channels at the baseline, would attenuate the effect of the interaction between β2-adrenergic receptor stimulation and ENaC upregulation and increased activity (Samaha et al., 2004). It is also possible that a lower basal activity and expression of ENaC in the AA group might lead to a lower rate of reabsorption of Na+ (clearance from the airway) at baseline when compared to the AT/TT group and an enhanced reabsorption of Na+ in response to β2-adrenergic receptor stimulation. On this basis, individuals in the AA group would more effectively clear Na+ from the alveolar space with the help of exogenous β2-adrenergic receptor stimulation.
The functional effects of nebulized albuterol on the lung was examined by measuring the diffusion capacity of the lung for carbon monoxide (DLCO), alveolar-capillary membrane conductance (DM), and pulmonary capillary blood volume (Vc) which can be influenced by changes in lung water. No significant difference in DLCO or its components between genotypes was detected before or after albuterol administration. Interestingly, both genotype groups demonstrated an increase in DM/Vc, suggesting an improvement in gas transfer for a given amount of blood. This observation is likely a direct result of the healthy subject’s lack of an underlying pulmonary dysfunction, therefore there was no detriment in pulmonary function or diffusion characteristics because of adequate compensatory mechanisms to balance ion changes. Examples of compensatory mechanisms that are active in healthy subjects include the CFTR protein and calcium activated chloride channels (Hartzell et al., 2005; Matthay et al., 2002; Tiddens et al., 2010). These proteins work to transport chloride, and subsequently water, into the alveolar space and are likely effectively working to counteract the fluid loss from the airways associated with the AT/TT group’s increased Na+ clearance at baseline. If either of these compensatory mechanisms is not operating normally, such as with CFTR mutations in cystic fibrosis (CF), individuals with an increased Na+ clearance may suffer from pulmonary deficits when fluid balance has been disrupted and the lung becomes too dry.
The findings of this study prompt interesting questions for patients with pulmonary dysfunction resulting from a dry lung (like that observed in patients with CF) or lung fluid accumulation (like that observed in patients with acute respiratory distress syndrome, or heart failure). Individuals homozygous for the A663 variant may benefit from β2-adrenergic receptor stimulation if alveolar flooding is occurring, as Na+ and water clearance may be increased with stimulation. Individuals with CF may respond differently to β2-adrenergic stimulation, as the individuals with the A663 variant can potentially worsen the dry nature of the lungs when treated with β2-agonist therapy, as more ENaC channels are utilized inhibition of ENaC would be the overall therapeutic goal.
The benefits of individualized therapy for many disease states has become clear and pulmonary disorders are no exception. Future innovation is being pursued in this field to limit problems with current CF therapies. In the past researchers have sought to find a way to minimize the alveolar clearance of Na+ in an attempt to benefit CF patients. Sodium channel blockers in inhaled formulations were developed for this purpose, but early attempts with traditional compounds such as amiloride failed, apparently because of the transient binding properties of these compounds (Hirsh et al., 2006). Fortunately, research continues in this field in order to find viable compounds that will be more potent and less reversible than amiloride proved to be on the alveolar epithelium (Mall, 2009). The AA group in our study would seem to benefit from a Na+ channel blocker as their greater clearance of Na+ could be significantly limited with effective therapy, leaving more water in the ASL and a thinner, less viscous mucus in CF. Another unique class of molecules is the P2Y2 receptor agonists. One currently researched member of the P2Y2 receptor agonist class is Denufosol, which offers a favorable alternative to traditional therapies (Hartzell et al., 2005; Nilius and Droogmans, 2003). P2Y2 receptor agonists have multiple mechanisms of action, including inhibition of Na+ channels. Both amiloride and Denufosol present the possibility of decreasing Na+ hyperabsorption over the long term. With appropriate genotyping, the benefits of such therapy may be determined before other therapeutic options are attempted in order to achieve better control over the pathologic problem before excessive damage has been done to the airway.
In a companion paper in this issue of the journal we provide evidence that lung diffusion increases differently according to genetic variation of ENaC at position 663. Because we did not measure exhaled Na+ in the exercise paper these manuscripts are complimentary in that the present study demonstrated that the administration of an exogenous agonist, albuterol, activates ENaC while the exercise paper demonstrates that exercise results in an improvement in lung diffusion (in part due to an increase in alveolar capillary membrane conductance) that is greater in the AA group when compared to the AT/TT group. Interestingly, with exercise, but not with albuterol, we noticed significant differences in lung diffusion which is likely due to the higher “dose” of catecholamines with exercise although further study is certainly warranted.
5. Limitations
Exhaled Na+ levels were used as a surrogate measure of ASL Na+ concentration. Sampling airway surface liquid (ASL) normally presents a challenge due to difficulty involved in obtaining a sample without disrupting the airway with invasive techniques such as broncheoalveolar lavage (BAL). Collecting exhaled breath condensate (EBC) has proven to be a viable method for sampling the non-volatile portion of ASL while avoiding the potential for harming the airway itself (Wheatley et al., 2010; Zacharasiewicz et al., 2004). However, since EBC is not sampled directly from airway surface liquid, it is difficult to determine the exact source of the Na+ in the EBC sample collected. Although Na+ ion quantification in EBC has proven to have significant variability within subjects and between subjects, our previous findings suggest that the source of Na+ is, at least in part, directly supplied from the pulmonary circulation (Wheatley et al., 2010). The present findings highlight that this exhaled Na+ is coming from the airways because there is a negative relationship between exhaled Na+ and DM/Vc (an index of lung water) and a strong positive relationship between absolute pulmonary function and exhaled Na+, suggesting that subjects with large lungs have greater exhaled Na+. In spite of this, differences between the SCNN1A genotype in response to albuterol indicate that independent of the source of EBC these two groups have unique responses to β2-adrenergic receptor stimulation based on genetic factors. A goal for future studies will involve obtaining more direct measures of the fluid lining the lower airways to confirm the patterns observed here, and less invasive studies such as the current one are needed to gather preliminary findings for future studies with such direct sampling methods as broncheoalveolar lavage (BAL). More invasive procedures like BAL may yield a more direct measure of ASL Na+, but introduce a possibility of damaging the airway which may result in sample variation induced by damage to the airway rather than the albuterol which is the sole modulator of interest for these preliminary studies. Also EBC Na+ was found to be related to DM/Vc at baseline and 90 minutes post albuterol administration, indicating that changes in EBC Na+ contribute to changes in the function of the lower airways.
Additionally, this was not a placebo-controlled study and the medium for delivery of albuterol (normal saline) contains Na+. This limitation is diminished, however, because we are comparing the effects of genetic variation of ENaC on our main outcome variables and both genotype groups were administered the same nebulized formula. Future research should include a placebo-controlled arm. Finally, the sample size of this study was relatively small, including only 31 healthy subjects.
6. Conclusion
We have determined differences in exhaled Na+ associated with SCNN1A genotype, which encodes the alpha subunit of ENaC. Although we found that the administration of albuterol resulted in changes in exhaled Na+ that were different according to SCNN1A genotype, there were no differences in lung diffusion according to genotype. This is likely because we studied healthy individuals with multiple active pathways and no evidence for excessive alveolar flooding. Therefore, small changes in exhaled Na+ over a short-term do not seem to influence lung diffusion in healthy subjects. Future research should be performed in disease states such as cystic fibrosis and heart failure where impaired salt and water regulation can lead to dry thick mucus or alveolar flooding, both of which can cause noticeable decrements in pulmonary function and gas diffusion in the lung. Differences in Na+ transport in the lungs due to genetic differences in ENaC could contribute to the phenotypic differences noted in individuals with the same pathological condition.
Highlights.
We explored the influence of genetics of SCNN1A on exhaled Na+ and lung diffusion.
We found that the AA genotype had greater decrease in exhaled Na+ with albuterol.
Both groups had a similar increase in lung conductance with albuterol.
Acknowledgments
Funding for this work was provided by HL108962-01 and the University of Arizona Clinical Scholars program. We are grateful for the subjects who graciously donated their time and effort to participate in this study.
Footnotes
8. Conflict of Interest
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarseth P, Karlsen J, Bo G. Effects of catecholamine-infusions and hypoxia on pulmonary blood volume and extravascular lung water content in cats. Acta Physiol Scand. 1975;95:34–40. doi: 10.1111/j.1748-1716.1975.tb10021.x. [DOI] [PubMed] [Google Scholar]
- Ambrosius WT, Bloem LJ, Zhou L, Rebhun JF, Snyder PM, Wagner MA, Guo C, Pratt JH. Genetic variants in the epithelial sodium channel in relation to aldosterone and potassium excretion and risk for hypertension. Hypertension. 1999;34:631–637. doi: 10.1161/01.hyp.34.4.631. [DOI] [PubMed] [Google Scholar]
- Azad AK, Rauh R, Vermeulen F, Jaspers M, Korbmacher J, Boissier B, Bassinet L, Fichou Y, des Georges M, Stanke F, De Boeck K, Dupont L, Balascakova M, Hjelte L, Lebecque P, Radojkovic D, Castellani C, Schwartz M, Stuhrmann M, Schwarz M, Skalicka V, de Monestrol I, Girodon E, Ferec C, Claustres M, Tummler B, Cassiman JJ, Korbmacher C, Cuppens H. Mutations in the amiloride-sensitive epithelial sodium channel in patients with cystic fibrosis-like disease. Hum Mutat. 2009;30:1093–1103. doi: 10.1002/humu.21011. [DOI] [PubMed] [Google Scholar]
- Beck KC, Hyatt RE, Mpougas P, Scanlon PD. Evaluation of pulmonary resistance and maximal expiratory flow measurements during exercise in humans. J Appl Physiol. 1999;86:1388–1395. doi: 10.1152/jappl.1999.86.4.1388. [DOI] [PubMed] [Google Scholar]
- Berdiev BK, Qadri YJ, Benos DJ. Assessment of the CFTR and ENaC association. Mol Biosyst. 2009;5:123–127. doi: 10.1039/b810471a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthiaume Y, Folkesson HG, Matthay MA. Indomethacin does not influence alveolar liquid clearance in anesthetized sheep or rats. Exp Lung Res. 1999;25:517–530. doi: 10.1080/019021499270105. [DOI] [PubMed] [Google Scholar]
- Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004;23:146–158. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- Ceridon ML, Beck KC, Olson TP, Bilezikian JA, Johnson BD. Calculating alveolar capillary conductance and pulmonary capillary blood volume: comparing the multiple- and single-inspired oxygen tension methods. J Appl Physiol. 2010;109:643–653. doi: 10.1152/japplphysiol.01411.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua TP, Coats AJ. The lungs in chronic heart failure. Eur Heart J. 1995;16:882–887. doi: 10.1093/oxfordjournals.eurheartj.a061019. [DOI] [PubMed] [Google Scholar]
- Dumasius V, Sznajder JI, Azzam ZS, Boja J, Mutlu GM, Maron MB, Factor P. {beta}2-Adrenergic Receptor Overexpression Increases Alveolar Fluid Clearance and Responsiveness to Endogenous Catecholamines in Rats. Circ Res. 2001;89:907–914. doi: 10.1161/hh2201.100204. [DOI] [PubMed] [Google Scholar]
- Eaton DC, Chen J, Ramosevac S, Matalon S, Jain L. Regulation of Na+ Channels in Lung Alveolar Type II Epithelial Cells. Proc Am Thorac Soc. 2004;1:10–16. doi: 10.1513/pats.2306008. [DOI] [PubMed] [Google Scholar]
- Fang X, Song Y, Hirsch J, Galietta LJ, Pedemonte N, Zemans RL, Dolganov G, Verkman AS, Matthay MA. Contribution of CFTR to apical-basolateral fluid transport in cultured human alveolar epithelial type II cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L242–249. doi: 10.1152/ajplung.00178.2005. [DOI] [PubMed] [Google Scholar]
- Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- Hartzell C, Putzier I, Arreola J. Calcium-activated chloride channels. Annu Rev Physiol. 2005;67:719–758. doi: 10.1146/annurev.physiol.67.032003.154341. [DOI] [PubMed] [Google Scholar]
- Hirsh AJ, Molino BF, Zhang J, Astakhova N, Geiss WB, Sargent BJ, Swenson BD, Usyatinsky A, Wyle MJ, Boucher RC, Smith RT, Zamurs A, Johnson MR. Design, synthesis, and structure-activity relationships of novel 2-substituted pyrazinoylguanidine epithelial sodium channel blockers: drugs for cystic fibrosis and chronic bronchitis. J Med Chem. 2006;49:4098–4115. doi: 10.1021/jm051134w. [DOI] [PubMed] [Google Scholar]
- Hsia CC. Recruitment of lung diffusing capacity: update of concept and application. Chest. 2002;122:1774–1783. doi: 10.1378/chest.122.5.1774. [DOI] [PubMed] [Google Scholar]
- Hsia CC, Raskin P. The diabetic lung: relevance of alveolar microangiopathy for the use of inhaled insulin. Am J Med. 2005;118:205–211. doi: 10.1016/j.amjmed.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Beck KC, Proctor DN, Miller J, Dietz NM, Joyner MJ. Cardiac output during exercise by the open circuit acetylene washin method: comparison with direct Fick. J Appl Physiol. 2000;88:1650–1658. doi: 10.1152/jappl.2000.88.5.1650. [DOI] [PubMed] [Google Scholar]
- Li T, Folkesson HG. RNA interference for alpha-ENaC inhibits rat lung fluid absorption in vivo. Am J Physiol Lung Cell Mol Physiol. 2006;290:L649–L660. doi: 10.1152/ajplung.00205.2005. [DOI] [PubMed] [Google Scholar]
- Ma T, Fukuda N, Song Y, Matthay MA, Verkman AS. Lung fluid transport in aquaporin-5 knockout mice. J Clin Invest. 2000;105:93–100. doi: 10.1172/JCI8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mall MA. Role of the amiloride-sensitive epithelial Na+ channel in the pathogenesis and as a therapeutic target for cystic fibrosis lung disease. Exp Physiol. 2009;94:171–174. doi: 10.1113/expphysiol.2008.042994. [DOI] [PubMed] [Google Scholar]
- Mandal A, Delamere NA, Shahidullah M. Ouabain-induced stimulation of sodium-hydrogen exchange in rat optic nerve astrocytes. Am J Physiol Cell Physiol. 2008;295:C100–110. doi: 10.1152/ajpcell.90636.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev. 2002;82:569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- Minakata Y, Suzuki S, Grygorczyk C, Dagenais A, Berthiaume Y. Impact of beta-adrenergic agonist on Na+ channel and Na+-K+-ATPase expression in alveolar type II cells. Am J Physiol. 1998;275:L414–422. doi: 10.1152/ajplung.1998.275.2.L414. [DOI] [PubMed] [Google Scholar]
- Morris RG, Schafer JA. cAMP Increases Density of ENaC Subunits in the Apical Membrane of MDCK Cells in Direct Proportion to Amiloride-sensitive Na+ Transport. J Gen Physiol. 2002;120:71–85. doi: 10.1085/jgp.20018547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Droogmans G. Amazing chloride channels: an overview. Acta Physiol Scand. 2003;177:119–147. doi: 10.1046/j.1365-201X.2003.01060.x. [DOI] [PubMed] [Google Scholar]
- Roughton FJ, Forster RE. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol. 1957;11:290–302. doi: 10.1152/jappl.1957.11.2.290. [DOI] [PubMed] [Google Scholar]
- Samaha FF, Rubenstein RC, Yan W, Ramkumar M, Levy DI, Ahn YJ, Sheng S, Kleyman TR. Functional polymorphism in the carboxyl terminus of the alpha-subunit of the human epithelial sodium channel. J Biol Chem. 2004;279:23900–23907. doi: 10.1074/jbc.M401941200. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Beck KC, Hulsebus ML, Breen JF, Hoffman EA, Johnson BD. Short-term hypoxic exposure at rest and during exercise reduces lung water in healthy humans. J Appl Physiol. 2006;101:1623–1632. doi: 10.1152/japplphysiol.00481.2006. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Beck KC, Turner ST, Hoffman EA, Joyner MJ, Johnson BD. Genetic Variation Of The {beta}2 Adrenergic Receptor Is Associated With Differences In Lung Fluid Accumulation In Humans. J Appl Physiol. 2007 doi: 10.1152/japplphysiol.01300.2006. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Johnson BD, Beck KC. An open-circuit method for determining lung diffusing capacity during exercise: comparison to rebreathe. J Appl Physiol. 2005;99:1985–1991. doi: 10.1152/japplphysiol.00348.2005. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Olson TP, Johnson BD, Frantz RP. Influence of sildenafil on lung diffusion during exposure to acute hypoxia at rest and during exercise in healthy humans. Eur J Appl Physiol. 2008;103:421–430. doi: 10.1007/S00421-008-0735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder PM. Liddle’s syndrome mutations disrupt cAMP-mediated translocation of the epithelial Na+ channel to the cell surface. J Clin Invest. 2000;105:45–53. doi: 10.1172/JCI7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starling E. On the absorption of fluids from the convective tissue spaces. Journal of Physiology (London) 1896;19:312–326. doi: 10.1113/jphysiol.1896.sp000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamhane RM, Johnson RL, Jr, Hsia CC. Pulmonary membrane diffusing capacity and capillary blood volume measured during exercise from nitric oxide uptake. Chest. 2001;120:1850–1856. doi: 10.1378/chest.120.6.1850. [DOI] [PubMed] [Google Scholar]
- Tiddens HA, Donaldson SH, Rosenfeld M, Pare PD. Cystic fibrosis lung disease starts in the small airways: can we treat it more effectively? Pediatr Pulmonol. 2010;45:107–117. doi: 10.1002/ppul.21154. [DOI] [PubMed] [Google Scholar]
- Tong Q, Menon AG, Stockand JD. Functional polymorphisms in the alpha-subunit of the human epithelial Na+ channel increase activity. Am J Physiol Renal Physiol. 2006;290:F821–827. doi: 10.1152/ajprenal.00312.2005. [DOI] [PubMed] [Google Scholar]
- Wallin CJ, Leksell LG. Estimation of extravascular lung water in humans with use of 2H2O: effect of blood flow and central blood volume. J Appl Physiol. 1994;76:1868–1875. doi: 10.1152/jappl.1994.76.5.1868. [DOI] [PubMed] [Google Scholar]
- Wheatley CM, Baldi JC, Cassuto NA, Foxx-Lupo WT, Snyder EM. Glycemic control influences lung membrane diffusion and oxygen saturation in exercise-trained subjects with type 1 diabetes: alveolar-capillary membrane conductance in type 1 diabetes. European Journal of Applied Physiology. 2011;111:567–578. doi: 10.1007/s00421-010-1663-8. [DOI] [PubMed] [Google Scholar]
- Wheatley CM, Cassuto NA, Foxx-Lupo WT, Snyder EM. Variability in measures of exhaled breath na, influence of pulmonary blood flow and salivary na. Clin Med Insights Circ Respir Pulm Med. 2010;4:25–34. doi: 10.4137/ccrpm.s4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Spruce L, Rosenblatt MM, Kleyman TR, Rubenstein RC. Intracellular trafficking of a polymorphism in the COOH terminus of the alpha-subunit of the human epithelial sodium channel is modulated by casein kinase 1. Am J Physiol Renal Physiol. 2007;293:F868–876. doi: 10.1152/ajprenal.00194.2007. [DOI] [PubMed] [Google Scholar]
- Zacharasiewicz A, Wilson N, Lex C, Li A, Kemp M, Donovan J, Hooper J, Kharitonov SA, Bush A. Repeatability of sodium and chloride in exhaled breath condensates. Pediatr Pulmonol. 2004;37:273–275. doi: 10.1002/ppul.10431. [DOI] [PubMed] [Google Scholar]


