Abstract
The production of proinflammatory cytokines is likely to play a major pathophysiological role in meningitis and other infections caused by Haemophilus influenzae type b (Hib). Previous studies have shown that Hib porin contributes to signaling of the inflammatory cascade. We examined here the role of Toll-like receptors (TLRs) and the TLR-associated adaptor protein MyD88 in Hib porin-induced production of tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6). Hib porin-induced TNF-α and IL-6 production was virtually eliminated in macrophages from TLR2- or MyD88-deficient mice. In contrast, macrophages from lipopolysaccharide (LPS)-hyporesponsive C3H/HeJ mice, which are defective in TLR4 function, responded normally to Hib porin. Moreover anti-TLR2 antibodies but not anti-TLR4 antibodies significantly reduced Hib porin-stimulated TNF-α and IL-6 release from the human monocytic cell line THP-1. These data indicate that the TLR2/MyD88 pathway plays an essential role in Hib porin-mediated cytokine production. These findings may be useful in the development of alternative therapies aimed at reducing excessive inflammatory responses during Hib infections.
Haemophilus influenzae type b (Hib) is an important cause of respiratory tract infections and meningitis worldwide, especially in young children. Although the advent of vaccination programs has almost eradicated the disease from industrialized countries, this infection is still present in less-developed areas, where it remains the leading cause of childhood meningitis (13). Hib meningitis is associated with increased cerebrospinal fluid levels of proinflammatory cytokines, including tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6), which may have a significant role in the pathophysiology of the disease (5, 10, 33). TNF-α is a key mediator of proinflammatory responses contributing significantly both to host defenses and to the pathophysiology of different infections (4). IL-6, a pleiotropic cytokine, acts in conjunction with other mediators to initiate the early inflammatory response following infection (4, 16).
Although lipopolysaccharide (LPS) has been clearly documented to play a central role in the pathogenesis of gram-negative infections (6), there is significant evidence that other components of gram-negative bacteria, including porins, also exert important roles (12, 45). Porins are trimeric proteins located in the outer membrane and are largely responsible for the molecular sieve properties of this bilayer (21). The major outer membrane protein (P2) of Hib, with an apparent molecular mass of 37,000 to 40,000 Da, has previously been shown to function as a porin and also as a target for protective antibodies in experimental Hib disease (14). It has been demonstrated that Hib porin contributes to signaling of the inflammatory cascade (10), although the Hib lipooligosaccharide is also likely to play an important role (31).
The recognition of microbial products by the host system is mediated by members of the Toll-like receptor (TLR) family (22). TLRs make up a family of evolutionary conserved pattern recognition molecules that are important signal transducers for the induction of mammalian innate immunity responses, including cytokine responses (1, 18, 29, 30, 39). The best-characterized TLRs to date are TLR2 and TLR4. TLR2 is involved in the recognition of a wide array of bacterial products, including peptidoglycan, lipopeptides, zymosan, and bacterial lipoproteins (2, 3, 7, 24, 42, 43), whereas TLR4 is activated by LPS (19). CD14 acts as a broad-specificity coreceptor that can enhance cell activation induced by TLR4 or TLR2 agonists (35). Engagment of TLRs by microbial products results in homodimerization and recruitment of myeloid differentiation factor-88 (MyD88), an adaptor protein essential for transducing activation signals from TLRs and the IL-1 receptor (20, 40).
The present study investigated the role of TLR2, TLR4, and MyD88 in Hib porin-induced cytokine production. Collectively, our data indicate that the proinflammatory effects of Hib porin are mediated by the TLR2/MyD88 pathway.
Cytokine production in macrophages from genetically defective mice.
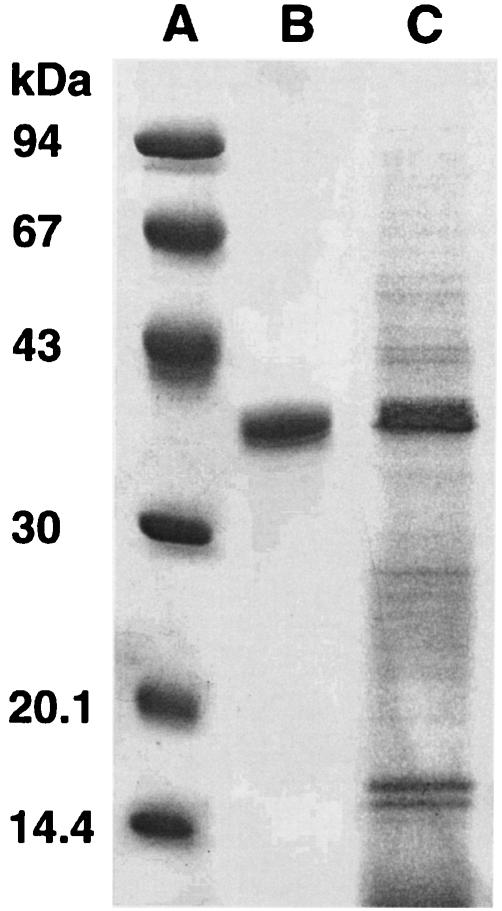
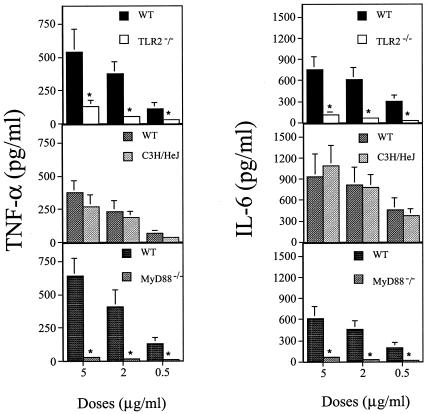
The porin was isolated and purified from bacterial cells of the Hib strain ATTC 9795 using the method described by Nurminen (34). Briefly, the bacterial envelopes were treated with Triton X-100 buffer for 2 h at 37°C in a rotary shaker, dissolved in sodium dodecyl sulfate (SDS; 4% wt/vol in 0.1 M sodium phosphate, pH 7.2) buffer, and applied on an Ultragel ACA34 column equilibrated with 0.25% SDS-sodium azide buffer. Elution flow through the column was 8 ml/h, and 2 ml was collected. The fraction containing proteins, identified by measuring absorption at an optical density of 280 nm, was extensively dialyzed and checked by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) according to the method of Laemmli (23). The protein content of the porin preparation was determined by using the method of Lowry et al. (25). The purity of the porin preparation from Hib was checked by SDS-PAGE (Fig. 1) (10). The purification protocols and methods used to discount the likely contamination by LPS of the porin preparation have been extensively described in previous works (8, 9). The LPS contamination was determined by Limulus amoebocyte lysate assay (Associates of Cape Cod, Inc.; distributed by PBI International, Milan, Italy) as described by Yin et al. (44). The lower detection limit of this assay was 0.1 EU/ml. The LPS concentration in each assay preparation was estimated to be about 0.45 EU/ml compared with that of a standard Hib LPS solution. These contaminating traces of LPS did not induce any biological activity under our experimental conditions (data not shown). In some assays, the LPS activity in the porins was neutralized by adding polymyxin B (PB) at room temperature for 1 h in a ratio of 1:100 (32). To our knowledge, PB interaction with porins has not been described. In all of the experiments performed, the porins plus PB, in a concentration range which is nontoxic for the cells, gave the same results as the porins alone (data not shown), thus further ruling out any possible activity by LPS contamination. In addition, PB alone was unable to induce any cell stimulation (data not shown). In order to ascertain whether TLR2, MyD88, or TLR4 is involved in cytokine responses to Hib porin, peritoneal macrophages from TLR2−/−, MyD88−/−, and LPS-hyporesponsive mice were stimulated with increasing doses of Hib porin. MyD88−/− and TLR2−/− mice, a kind gift of Shizuo Akira (Osaka, Japan), were engineered as previously described (19, 38) on a 129 SV-C57BL/6 background and fivefold backcrossed with C57BL/6 mice. C57BL/6 mice (Charles River Italia, Calco, Italy) served as controls for the TLR2- and MyD88-deficient mice. C3H/HeJ LPS-hyporesponsive mice, which have a loss-of-function mutation in TLR4, and control C3H/HeN mice were also purchased from Charles River. Peritoneal cells were isolated from the peritoneal cavity by washing with ice-cold phosphate buffered saline (PBS; 0.01 M phosphate, 0.15 M NaCl, pH 7.2), pelleted by centrifugation, and resuspended in RPMI 1640 (Invitrogen Life Technologies, San Giuliano Milanese, Italy) supplemented with 2% fetal calf serum (FCS), 100 IU of penicillin/ml, and 100 μg of streptomycin/ml. Peritoneal macrophages were then seeded in 96-well dishes at a density of 2 × 105/well and incubated at 37°C in a 5% humidified CO2 environment. After 24 h, nonadherent cells were removed by washing with medium, and the adherent cells were stimulated for 22 h with different concentrations of purified Hib porin. Results were compared with those observed for cells of the respective control mice suspended in medium alone for spontaneous cytokine release. Murine TNF-α (mouse TNF-α module set; Bender MedSystems, Vienna, Austria) and IL-6 murine (IL-6 reagent set, Euroclone, Wetherby, United Kingdom) concentrations in culture supernatants were determined using commercial enzyme-linked immunosorbent assay (ELISA) kits. The lower detection limit of both these assays was 16 pg/ml of Hib porin-induced dose-dependent TNF-α (Fig. 2, left panels) or IL-6 (Fig. 2, right panels) production in macrophages from wild-type animals. However, these cytokines were almost completely absent in TLR2- or MyD88-deficient mice (Fig. 2). Peritoneal cells derived from C3H/HeJ mice were fully capable of responding to Hib porin stimulation. These data indicate that TLR2 and MyD88 but not TLR4 are involved in Hib porin-induced cytokine production in peritoneal mouse macrophages. Heat treatment (100°C for 1 h) of the porin preparation completely eliminated its ability to induce cytokine production in macrophages. This finding ruled out peptiglycan contamination as a possible cause of TLR2-dependent cell activation by our porin preparation.
FIG. 1.
SDS-PAGE analysis of the outer membrane protein preparation and porin extract from Hib ATCC 9795. The gel was stained with Coomassie blue. Lane A, molecular mass standards (phosphorylase b, 94 kDa; albumin, 67 kDa; ovalbumin, 43 kDa; carbonic anydrase, 30 kDa; trypsin inhibitor, 20 kDa; α-lactalbumin, 14 kDa); lane B, Hib porin (10 μg); lane C, Hib outer membrane protein preparation (10 μg).
FIG. 2.
TNF-α and IL-6 production in peritoneal murine macrophages stimulated with Hib porin. Thioglycolate-elicited peritoneal macrophages from TLR2-deficient (TLR2−/−), LPS-hyporesponsive (C3H/HeJ), and MyD88-defective (MyD88−/−) mice were stimulated with different doses of Hib porin for 22 h. C57BL/6 mice were used as controls for TLR2- and MyD88-deficient mice. C3H/HeN mice were employed as wild-type (WT) controls for C3H/HeJ mice. The columns and bars represent the means ± the standard deviations of the results of three independent observations. The values for IL-6 concentrations in culture supernatants from unstimulated cells were subtracted from data obtained after porin stimulation. These basal expression values never exceeded 60 pg/ml. TNF-α was always undetectable in the supernatants of unstimulated cells. An asterisk (*) indicates results that are significantly different (P < 0.05) from those of the controls, as determined by one-way analysis of variance and the Student-Newman-Keuls test.
Release of IL-8 by HEK 293-transfected cells.
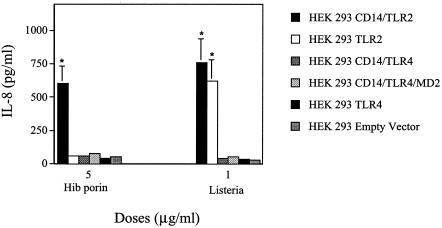
Human embryonic kidney 293 (HEK 293) cells stably transfected with TLR2 (HEK 293-TLR2) and TLR4 (HEK 293-TLR4) or cotransfected with CD14/TLR2 (HEK 293-CD14/TLR2), CD14/TLR4 (HEK 293-CD14/TLR4), or CD14/TLR4/MD2 (HEK 293-CD14/TLR4/MD2) and HEK 293 cells were kindly provided by Terje Espevik (Trondheim, Norway). The cells were maintained in low-glucose Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated FCS, HEPES (10 mM), l-glutamine (2 μg/ml), penicillin (100 IU/ml), streptomycin (100 μg/ml) (all obtained from Invitrogen Life Technologies), and puromycin (1 μg/ml; Sigma Chimica, Milan, Italy). For stimulation experiments, transfected HEK 293 cells were plated in 24-well tissue culture plates (5 × 105/ml) and incubated for 5 h with different concentrations of Hib porin and heat-killed Listeria monocytogenes used as TLR2-dependent positive controls. L. monocytogenes was obtained from a recent clinical isolate. Bacteria were grown to the early stationary phase in a chemically defined medium and were harvested by centrifugation. Killed bacteria were prepared by heat treatment (80°C for 45 min), followed by extensive washing with distilled water and lyophilization. The endotoxin level of the lyophilized bacterial preparation was <0.06 EU/mg, as determined by Limulus assay (26). To investigate whether human TLR2 can also recognize Hib porin, HEK 293 cells transfected with human TLR2 or TLR4 alone or cotransfected with CD14/TLR2, CD14/TLR4, and CD14/TLR4/MD2 were incubated with Hib porin (5 μg/ml) or with heat-killed L. monocytogenes (1 μg/ml). HEK 293 cells were used as a negative control. Since IL-8 production is the most widely used marker of HEK 293 cell activation, IL-8 levels were measured in culture supernatants. IL-8 measurement was performed using a human IL-8 ELISA reagent set (Euroclone). The lower limit of detection for this assay was <25 pg/ml. As shown in Fig. 3, Hib porin induced significant IL-8 production only in CD14/TLR2-transfected cells, suggesting that the coexpression of CD14 with TLR2 is required by these cells to respond to Hib porin stimulation.
FIG. 3.
IL-8 release in stimulated transfected HEK 293 cells. Cells (5 × 105/ml) were stimulated for 5 h with Hib porin (5 μg/ml) or heat-killed L. monocytogenes (1 μg/ml). The columns and bars represent the means ± the standard deviations of the results for three independent observations. IL-8 was undetectable in the supernatants of unstimulated cells. An asterisk (*) indicates a result significantly different (P < 0.05) from that of the controls, as determined by one-way analysis of variance and the Student-Newman-Keuls test.
Effect of MAbs on the release of IL-6 and TNF-α by THP-1 cells stimulated with Hib porin.
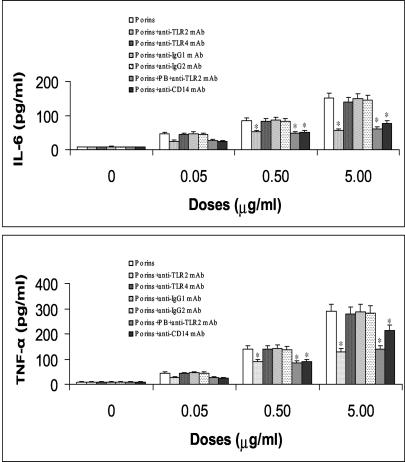
To evaluate the involvement of TLR2, TLR4, or CD14 receptors in Hib porin-induced activation, monoclonal antibodies (MAbs) which recognize either TLR2, TLR4, or CD14 epitopes were used: anti-human TLR2 (immunoglobulin G2a [IgG2a], clone TL2.1; Alexis Biochemicals, Lausen, Switzerland), anti-human TLR4 (IgG1; Alexis Biochemicals), and anti-human CD14 (IgG2b, clone TIB 228; American Type Culture Collection, Manassas, Va.). Purified mouse monoclonal IgG1, IgG2a, or IgG2b (Sigma-Aldrich Srl) used at similar concentrations of the corresponding MAbs served as isotype controls. Cells from THP-1 (ATCC TIB-202), a human promyelomonocytic cell line (41), were cultured at 37°C in an atmosphere of 5% CO2 in complete medium consisting of RPMI 1640 supplemented with 10% FCS, 2 mM l-glutamine, 100 IU of penicillin/ml, and 100 μg of streptomycin/ml. THP-1 cells were subjected to pretreatment with anti-TLR2 MAb (10 μg/ml), anti-TLR4 mAb (10 μg/ml), or anti-CD14 MAb (10 μg/ml) and with appropriate nonimmune isotype controls for 30 min followed by stimulation with Hib porin at a range of concentrations from 0.05 to 5 μg/ml. After 24 h, the supernatants were collected and assayed for the presence of IL-6 and TNF-α. Cell viability was determined by measuring leakage of lactate dehydrogenase activity from cells into the supernatant, using a commercial kit (Roche Diagnostic GmbH; Roche Molecular Biochemicals, Mannheim, Germany). Measurement of human IL-6 and TNF-α was performed, using human IL-6 and human TNF-α ELISA reagent sets (Euroclone), respectively. The lower limits of detection for these assays were 25 and 15 pg/ml, respectively. As shown in Fig. 4, increased release of IL-6 and TNF-α was observed in culture supernatants of THP-1 cells stimulated with Hib porin. IL-6 production was evident with 0.05 μg/ml of porin, and maximal values were achieved with 5 μg/ml. Anti-TLR4 MAb failed to inhibit IL-6 and TNF-α production induced by Hib porin at any of the concentrations tested. The addition of anti-TLR2, however, reduced by 63% and 55%, respectively, IL-6 and TNF-α release from cells stimulated with 5 μg of porins. The control MAbs, which were used as isotype controls, did not affect the porin-induced IL-6 and TNF-α production. Moreover, cytokine production was significantly reduced by the addition of the anti-CD14 MAb (Fig. 4). These data confirm that TLR2 but not the TLR4 pathway is involved in Hib porin stimulation of human monocytic cells.
FIG. 4.
IL-6 and TNF-α release in THP-1 cells treated with anti-TLR2/4 or anti-CD14 MAbs and stimulated with Hib porin. Cells (4 × 106/ml) were preincubated for 30 min with anti-human TLR2 mAb (10 μg/ml), anti-human TLR4 mAb (10 μg/ml), anti-human CD14 (10 μg/ml), IgG1, IgG2a, or IgG2b as an isotype control before the Hib porin (0.05 to 5 μg/ml) was added. The columns and bars represent the means ± the standard deviations of the results of three independent observations (P < 0.05). An asterisk (*) indicates a result that is significantly different (P < 0.05) from the values observed with porins alone by one-way analysis of variance and the Student-Newman-Keuls test.
Discussion.
The host inflammatory response has a major pathophysiological role in bacterial meningitis. Hib meningitis is associated with increased cerebrospinal fluid levels of different mediators, including soluble IL-2 receptor, IL-6, and TNF-α (33). Moreover, systemic treatment with anti-IL-6 antibodies attenuated inflammation in a rat meningitis model (27), implying a detrimental role for IL-6. In a previous study, we observed that the Hib porin may be involved, in conjunction with other bacterial products, in the pathophysiology of Hib meningitis (10). In the present study, we investigated the molecular mechanisms underlying recognition of Hib porin by host cells.
We focused on TLRs and the TLR-associated adaptor protein MyD88, since recent studies have indicated that recognition of bacterial products in innate immunity responses is often mediated by these molecules (15, 24, 40). The main finding of the present study is that TLR2 is involved in Hib porin-induced cytokine production. In fact, TLR2 or MyD88 deficiency was associated with a virtually complete elimination of TNF-α or IL-6 responses to Hib porin in mice. In contrast, no differences in TNF-α or IL-6 release between C3H/HeJ mice and the wild-type controls were observed after Hib porin stimulation (Fig. 2). Therefore, these results indicated that Hib porin-induced cytokine release in mice was strictly TLR2 and MyD88 dependent but completely TLR4 independent.
The role of human TLR2 in response to Hib porin has been investigated in this work. Stable transfection of HEK 293 cells with TLR2, in conjunction with the costimulatory molecule CD14, conferred responsiveness to Hib porin (Fig. 3). In contrast, transfection with TLR2 alone was not sufficient to induce Hib porin responsiveness. These data suggest that CD14 and TLR2 function together in the recognition of Hib-encoded porin. Data obtained with blocking MAbs further supported this conclusion, as shown by the ability of anti-TLR2 and anti-CD14 antibodies but not anti-TLR4 antibodies to significantly reduce IL-6 release and TNF-α in THP1 cells stimulated with Hib porin.
This finding differs from results obtained using Salmonella porin, which could induce cytokine production in a CD14-independent and CD11a/CD18-dependent fashion (11). Collectively, these data suggest that porins from different bacteria may engage different coreceptors in cell activation phenomena.
To our knowledge, this is the first study to address the role of TLRs in bacterial porin-mediated cytokine production. A recent report by Massari et al. (28) focused on the role of TLRs in the adjuvant effects of neisserial porin P or B. It was found that such adjuvant activity is mediated by surface expression of B7-2 and class 2 major histocompatibility complex on B cells by TLR2-dependent mechanisms. The presence of the adaptor molecule MyD88 was also required. Collectively, our data and those of Massari et al. indicate that porins from different bacteria may be recognized by TLR2. Studies are in progress to ascertain whether enterobacterial and Pseudomonas aeruginosa porins are also capable of activating TLR2.
Further studies are addressing the possibility that porins present as contaminants in LPS preparations may be at least partially responsible for the TLR2-activating properties of such preparations. Indeed, enterobacterial LPS preparations lose their ability to induce TLR2-dependent responses after removal of contaminant proteins (17), which predominantly consist of porins, protein 2, and lipoprotein (37). Shuto et al. (36) have shown that nontypeable H. influenzae strongly activates NF-κB, a transcriptional activator of multiple-host-response genes, by a TLR2-dependent mechanism. Moreover, P6, a conserved outer membrane lipoprotein of all H. influenzae strains, activated NF-κB by a similar mechanism. Future studies are required to assess the relative contribution of porins, lipoproteins, and lipo-oligosaccharide in the overall ability of H. influenzae to stimulate proinflammatory responses.
In conclusion, our data show that Hib porin-induced cytokine production is mediated by the TLR2/MyD88 pathway. These findings may have implications for the development of anti-inflammatory agents to counteract brain and systemic inflammation in the course of serious Hib infections.
Acknowledgments
We are grateful to Shizuo Akira for providing TLR2- and MyD88-deficient mice and to Terje Espevik for providing TLR-transfected cell lines.
This work was performed with the assistance of a grant from the European Commission (HOSPATH contract no. QLK2-CT-2000-00336) and a grant from MIUR of Italy (PRIN project no. 2001061977_002).
Editor: F. C. Fang
REFERENCES
- 1.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 2.Aliprantis, A. O., R. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 3.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, et al. 1999. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 4.Cerami, A. 1992. Inflammatory cytokines. Clin. Immunol. Immunopathol. 62:3-10. [DOI] [PubMed] [Google Scholar]
- 5.Diab, A., J. Zhu, L. Lindquist, B. Wretlind, H. Link, and M. Bakhiet. 1997. Cytokine mRNA profiles during the course of experimental Haemophilus influenzae bacterial meningitis. Clin. Immunol. Immunopathol. 85:236-452. [DOI] [PubMed] [Google Scholar]
- 6.Evans, T. J., E. Strivens, A. Carpenter, and J. Cohen. 1993. Differences in cytokine response and induction of nitric oxide synthase in endotoxin-resistant and endotoxin-sensitive mice after intravenous gram-negative infection. J. Immunol. 150:5033-5040. [PubMed] [Google Scholar]
- 7.Flo, T. H., O. Halaas, E. Lien, L. Ryan, G. Teti, D. T. Golenbock, A. Sundan, and T. Espevik. 2000. Human Toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J. Immunol. 164:2064-2069. [DOI] [PubMed] [Google Scholar]
- 8.Galdiero, F., M. A. Tufano, M. Galdiero, S. Masiello, and M. Di Rosa. 1990. Inflammatory effects of Salmonella typhimurium porins. Infect. Immun. 58:3183-3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galdiero, M., G. Cipollaro de l'Ero, G. Donnarumma, A. Marcatili, and F. Galdiero. 1995. Interleukin-1 and interleukin-6 gene expression in human monocytes stimulated with Salmonella typhimurium porins. Immunology 86:612-619. [PMC free article] [PubMed] [Google Scholar]
- 10.Galdiero, M., M. D'Amico, F. Gorga, C. Di Filippo, M. D'Isanto, M. Vitiello, A. Longanella, and A. Tortora. 2001. Haemophilus influenzae porin contributes to signaling of the inflammatory cascade in rat brain. Infect. Immun. 69:221-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galdiero, M., M. D'Isanto, M. Vitiello, E. Finamore, L. Peluso, and M. Galdiero. 2001. Porins from Salmonella enterica serovar Typhimurium induce TNF-α, IL-6 and IL-8 release by CD14-independent and CD11a/CD18-dependent mechanisms. Microbiology 147:2697-2704. [DOI] [PubMed] [Google Scholar]
- 12.Galdiero, M., M. Vitiello, E. Sanzari, M. D'Isanto, A. Tortora, A. Longanella, and S. Galdiero. 2002. Porins from Salmonella enterica serovar Typhimurium activate the transcription factors activating protein 1 and NF-κB through the Raf-1-mitogen-activated protein kinase cascade. Infect. Immun. 70:558-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gessner, B. D. 2002. Worldwide variation in the incidence of Haemophilus influenzae type B meningitis and its association with ampicillin resistance. Eur. J. Clin. Microbiol. Infect. Dis. 21:79-87. [DOI] [PubMed] [Google Scholar]
- 14.Hansen, E. J., F. R. Gonzales, N. R. Chamberlain, M. V. Norgard, E. E. Miller, L. D. Cope, S. E. Pelzel, B. Gaddy, and A. Clausell. 1988. Cloning of the gene encoding the major outer membrane protein of Haemophilus influenzae type b. Infect. Immun. 56:2709-2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 16.Hirano, T. 1992. Interleukin-6 and its relation to inflammation and disease. Clin. Immunol. Immunopathol. 62:60-65. [DOI] [PubMed] [Google Scholar]
- 17.Hirschfeld, M., Y. Ma, J. H. Weis, S. N. Vogel, and J. J. Weis. 2000. Repurification of lipopolysaccaride eliminates signaling through both human and murine Toll-like receptor 2. J. Immunol. 165:618-622. [DOI] [PubMed] [Google Scholar]
- 18.Horng, T., G. M. Barton, and R. Medzhitov. 2001. TIRAP: an adapter molecule in the Toll signaling pathway. Nat. Immunol. 2:835-841. [DOI] [PubMed] [Google Scholar]
- 19.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 20.Janssens, S., and R. Beyaert. 2002. A universal role for MyD88 in TLR/IL-1R-mediated signaling. Trends Biochem. Sci. 27:474-482. [DOI] [PubMed] [Google Scholar]
- 21.Jeanteur, D., J. H. Lakey, and F. Pattus. 1991. The bacterial porin superfamily: sequence alignment and structure prediction. Mol. Microbiol. 5:2153-2164. [DOI] [PubMed] [Google Scholar]
- 22.Kopp, E. B., and R. Medzhitov. 1999. The Toll-receptor family and control of innate immunity. Curr. Opin. Immunol. 11:13-18. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 277:680-685. [DOI] [PubMed] [Google Scholar]
- 24.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 25.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1985. Protein measurement with Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 26.Mancuso, G., A. Midiri, C. Beninati, G. Piraino, A. Valenti, G. Nicocia, D. Teti, J. Cook, and G. Teti. 2002. Mitogen-activated protein kinases and NF-κB are involved in TNF-α responses to group B streptococci. J. Immunol. 169:1401-1409. [DOI] [PubMed] [Google Scholar]
- 27.Marby D, G. R. Lockhart, R. Raymond, and J. G Linakis. 2001. Anti-interleukin-6 antibodies attenuate inflammation in a rat meningitis model. Acad. Emerg. Med. 8:946-949. [DOI] [PubMed] [Google Scholar]
- 28.Massari, P., P. Henneke, Y. Ho., E. Latz, D. T. Golenbock, and L. M. Wetzler. 2002. Cutting edge: immune stimulation by neisserial porins is Toll-like receptor 2 and MyD88 dependent. J. Immunol. 168:1533-1537. [DOI] [PubMed] [Google Scholar]
- 29.Medzhitov, R., and C. A. Janeway, Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394-397. [DOI] [PubMed] [Google Scholar]
- 30.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135-145. [DOI] [PubMed] [Google Scholar]
- 31.Miyazaki, S., T. Nunoya, T. Matsumoto, N. Furuya, K. Tateda, and K. Yamaguchi. 2001. Lipoolygosaccharide indirectly enhances inflammatory lesions in lungs as a primary infection site by non-encapsulated and type B Haemophilus influenzae through production of cytokines. Cytokine 15:171-174. [DOI] [PubMed] [Google Scholar]
- 32.Morrison, D. C., and D. M. Jacobs. 1976. Binding of polymyxin B to a lipid A portion of bacterial lipopolysaccharides. Immunochemistry 13:813-818. [DOI] [PubMed] [Google Scholar]
- 33.Nishimura, M., H. Tsukahara, M. Hiraoka, Y. Osaka, Y. Ohshima, A. Tanizawa, and M. Mayumi. 2000. Systemic inflammatory response syndrome and acute renal failure associated with Haemophilus influenzae septic meningitis. Am. J. Nephrol. 20:208-211. [DOI] [PubMed] [Google Scholar]
- 34.Nurminen, M. 1985. Isolation of porin trimers. Enterobacterial surface antigen methods for molecular characterization, p. 294. In T. K. Korhonen, E. A. Dawes, and P. H. Makela (ed.), FEMS Symposium 25. Elsevier Science Publishers BV, Amsterdam, The Netherlands.
- 35.Opal, M. S., and C. E. Huber. 2002. Bench-to-bedside review: Toll-like receptors and their role in septic shock. Crit. Care 6:125-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shuto, T., H. Xu, B. Wang, J. Han, H. Kai, X. Gu, T. F. Murphy, D. J. Lim, and J. D. Li. 2001. Activation of NF-κB by nontypeable Haemophilus influenzae is mediated by Toll-like receptor 2-TAK1-dependent NIK-IKKα/β-IκBα and MKK3/6-p38 MAP kinase signaling pathways in epithelial cells. Proc. Natl. Acad. Sci. USA 98:8774-8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sultzer, B. M., and G. W. Goodman. 1976. Endotoxin protein: a B-cell mitogen and polyclonal activator of C3H/HeJ lymphocytes. J. Exp. Med. 144:821-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 39.Takeuchi, O., T. Kawai, H. Sanjo, N. G. Copeland, D. J. Gilbert, N. A. Jenkins, K. Takeda, and S. Akira. 1999. TLR6: a novel member of an expanding Toll-like receptor family. Gene 231:59-65. [DOI] [PubMed] [Google Scholar]
- 40.Takeuchi, O., and S. Akira. 2002. MyD88 as a bottle neck in Toll/IL-1 signaling. Curr. Top. Microbiol. Immunol. 270:155-167. [DOI] [PubMed] [Google Scholar]
- 41.Tsuchiya, S., M. Yamabe, Y. Yamaguchi, Y. Kobayashi, T. Konno, and K. Tada. 1980. Establishment and characterization of a human acute monocytic leukaemia cell line (THP-1). Int. J. Cancer 26:171-175. [DOI] [PubMed] [Google Scholar]
- 42.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401:811-815. [DOI] [PubMed] [Google Scholar]
- 43.Underhill, D. M., A. Ozinsky, K. D. Smith, and A. Aderem. 1999. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc. Natl. Acad. Sci. USA 96:14459-14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yin, E. T., C. Galanos, S. Kinsky, R. A. Bradshow, S. Wessler, O. Luderitz, and M. F. Sarmiento. 1972. Picogram-sensitive assay for endotoxin: gelatin of Limulus polyphemus blood cell lysate induced by purified lipopolysaccharide and lipid A from gram-negative bacteria. Biochim. Biophys. Acta 261:284-289. [DOI] [PubMed] [Google Scholar]
- 45.Zhang, H., D. W. Nielsen, J. W. Peterson, and G. R. Klimpel. 1998. Lipoprotein release by bacteria: potential factor in bacterial pathogenesis. Infect. Immun. 66:5196-5201. [DOI] [PMC free article] [PubMed] [Google Scholar]