Abstract
Importance of the field
Devices for the reliable detection of panels of biomarker proteins facilitated by magnetic bead-based technologies have the potential to greatly improve future cancer diagnostics. The reason for this review is to highlight promising research on emerging procedures for protein capture, transport and detection featuring magnetic particles.
Areas covered in this review
The review covers applications of magnetic particles in protein immunoassays in emerging research and commercial methods, and stresses multiplexed protein assays for reliable future cancer diagnostics. Research literature over the past dozen years has been surveyed and specific examples are presented in detail.
Expert Opinion
Magnetic particles are important components of emerging protein detection systems. They need to be integrated into simple inexpensive systems for accurate, sensitive detection of fully validated panels of biomarker proteins to be widely useful in clinical cancer diagnostics.
Keywords: magnetic particles, proteins, cancer biomarkers, off-line protein capture, cancer diagnostics
1. Introduction
The US National Institutes of Health defines biomarkers as “molecules that can be objectively measured and evaluated as indicators of normal or disease processes and pharmacologic responses to therapeutic intervention” [1,2]. Serum protein levels offer the possibility of “snapshots” for health monitoring. Their high potential as measurable biomarkers for cancer detection has created considerable excitement in the biomedical and clinical research communities [3–8]. Certain proteins are overexpressed and secreted into the bloodstream beginning very early in cancer development. These proteins are often specific to one or several types of cancer, and levels of panels of such proteins can give a clear indication of a patient’s status. Thus, serum levels of proteins can serve as biomarkers to detect the onset of cancer, as well as to guide therapy.
Single biomarker proteins, such as prostate specific antigen (PSA) used as a prostate cancer biomarker, typically have insufficient positive predictive value, e. g. about 70% for PSA [9]. There is concern that detection of an indolent form of prostate cancer that is undifferentiated by the PSA test from more serious types leads to unnecessary treatment. Failure to distinguish between indolent and more aggressive forms of cancer is also a common problem with other clinically used single biomarkers, including cancer antigen 125 for ovarian cancer, carbohydrate antigen 199 for pancreatic cancer, and carcinoembryonic antigen for colon cancer [8]. Thus, panels of cancer biomarker proteins will almost certainly be more valuable for reliable cancer detection and therapeutic monitoring, and this has been demonstrated experimentally [3,4,7,10,11].
Currently several biosensor technologies are employed as diagnostic tools for protein detection [12]. Most technologies employ nanomaterials such as gold nanoparticles, quantum dots, carbon nanotubes and magnetic particles to improve detection sensitivity [13]. Low detection limits achieved using nanomaterials can facilitate early cancer detection and accurate prognosis. Virtually any protein detection method that is sensitive enough and can analyze very small samples can be used to measure cancer biomarker proteins in serum, and there is no standard approach at present. For this reason, this article includes any method that could potentially be used for the detection of cancer biomarker proteins.
Clinical or point-of-care (POC) detection of panels of proteins is a formidable bioanalytical challenge. For clinical use, detection must be sensitive, multiplexed, accurate, and reasonably priced. POC requirements are more stringent, requiring fast, automated sample preparation and low cost, technically simple assay devices. These requirements have not been fully met by any specific methodology available to clinicians at present. Ideally, the device should be able to accurately measure both normal and elevated serum levels of proteins. Concentrations that need to be measured may be in sub-pg mL−1 to high ng mL−1 ranges for different biomarker proteins in the same sample. In addition, potential interferants include thousands of proteins present in serum, some well above ng mL−1 levels [4,7]. Accurate devices that can achieve all goals are needed to provide high quality data for biomarker panel validation [14,15] before these protein panels can expected to achieve their full diagnostic potential.
A method for determining multiple proteins in the same sample must be able to selectively fish out a set of low concentration protein analytes from a sea containing thousands of other proteins. It must then be able to selectively differentiate the individual analyte proteins and measure them with accuracy and high sensitivity. Magnetic beads conjugated with antibodies or other protein capture agents provide a simple but effective way to achieve these analytical operations. Typically, labeled magnetic beads with antibodies attached are added to a fluid sample, and the sample is agitated so that specific proteins are captured by antibodies on the relevant bead. Magnetic separation, either manual or automated, is used to remove the protein-laden beads from the sample, and wash away any interfering biomolecules. Labels associated with the magnetic beads are then detected in a selective way, either by using different labels for different proteins in a bar-code like approach, or by first sorting beads with the same labels based on the proteins they have captured, then detecting labels on each type of bead.
Magnetic beads have high surface areas per unit volume, good stability, and enable fast kinetic processes involving solution species compared to bulk solid surfaces [16,17]. A great advantage of magnetic beads, as opposed to non-magnetic nanoparticles, is their ease of manipulation with simple, inexpensive magnets. Very efficient isolation of analyte proteins from biomedical samples can be achieved outside of the detection system, so that detectors need never be exposed to the complex biological sample matrix.
Magnetic beads enable magnetic purification and enrichment of proteins from complex serum samples. For example, magnetic beads coated with antibodies are effectively utilized in quantification of biomarker candidates in plasma at ng mL-1 levels by mass spectrometry (MS) [18]. This approach enabled identification of protein biomarker candidates for head and neck cancer [19], autistic spectrum disorder [20] and other diseases. High throughput lectin coated magnetic particles were used to isolate and analyze glycosylated proteins utilizing LC/MS for biomarker discovery[21].
The present article describes the utility of magnetic beads in a variety of methods developed to achieve sensitive protein detection. Magnetic particle-enabled protein assays constitutes a subset of the larger research area of interfacing nanoparticles or microparticles with protein bioanalyses [22]. In the next section, we discuss sources and properties of magnetic beads. We then present sections addressing uses of magnetic beads interfaced with different types of detectors. Closing sections offer a summary and our opinions.
2. Sources and Types of Magnetic Beads
Many types of magnetic beads are now commercially available. Paramagnetic beads are the most useful for systems requiring magnetic separation and transport as they become magnetic in an applied magnetic field, but have zero magnetization in the absence of a magnetic field. These beads are often called “superparamagnetic” [23]. Ferromagnetic beads feature permanent magnetism.
The most common examples of paramagnetic beads have magnetic iron oxide cores and non-magnetic polymer shells featuring surface chemical functionality for attachment of biomolecules (Figure 1). The magnetic core can also consist of a collection of paramagnetic nanoparticles embedded in a polymer core. Beads with sizes in the range 100 nm to 50 μm in diameter are commercially available with variability in size <±5 %. Suppliers include Solulink, Invitrogen, Bangs Labs, Merck, and others. Bead size determines sedimentation rate and mobility in solution. The outer polymer shell serves to add surface functional groups to the bead and protects the metal oxide core from external media. The outer shell can also consist of agarose, cellulose, porous glass or silica. Surface functional groups include carboxyl, amine, epoxy, hydroxyl, tosyl, and N-hydroxy succinate (NHS)-activated. Beads are also available with surface molecules such as streptavidin, biotin, protein A, protein G, IgG, IgE and IgM (Figure 1). Functional groups can be activated for coupling using the familiar EDC-coupling chemistry for carboxylates or glutaraldehyde for amines to attach to appropriate functional group of biomolecules [7]. Surface tosyl-, NHS-activated and epoxide groups can be used to attach biomolecules directly without cross-linking agents. Particles precoated with streptavidin can capture biotin labeled biomolecules. Protein A coated particles can selectively bind to Fc regions of antibodies for orientated immobilization.
Figure 1.
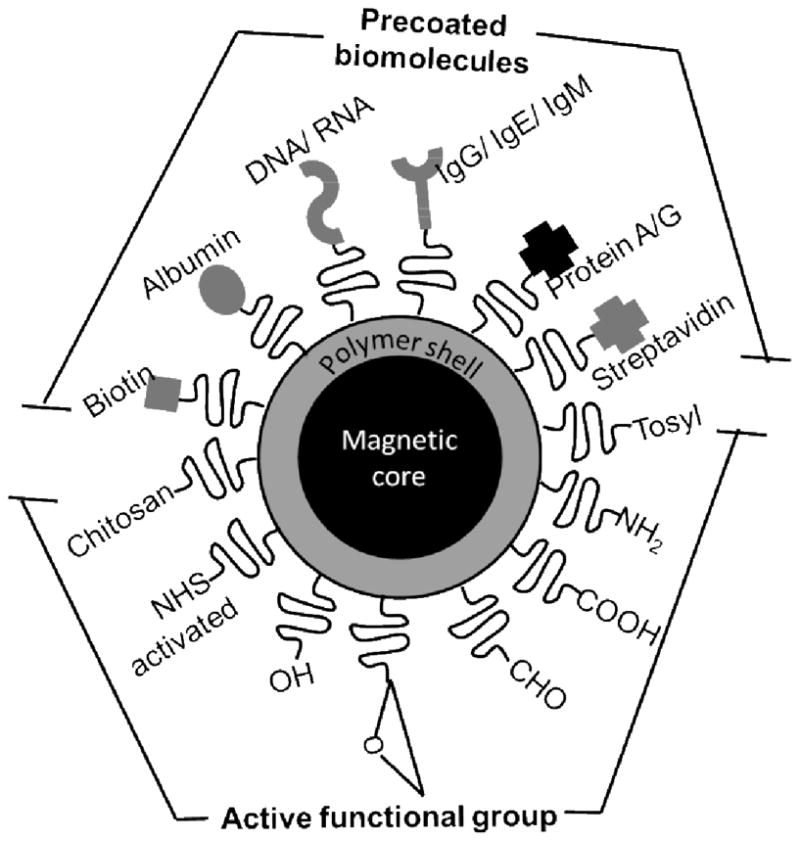
Superparamagnetic beads are commercially available with coatings of either organic functional groups to attach biomolecules like antibodies and enzymes, or precoated with biomolecules that can bind specific partners.
Beads with paramagnetic nanoparticles embedded in a polymer core matrix are often labeled “superparamagnetic”, but may feature multidomain magnetic structures with remnant magnetic moments [23]. This can cause some degree of magnetic clustering due to induced magnetism in neighboring particles. At room temperature, true paramagnetic beads of iron oxide need to have radii in the low nm range. Thus, commonly used beads of 0.1–3 μm diameter featuring polymer-embedded iron oxide nanoparticle cores may show some clustering in dispersions due to magnetic interactions between particles.
In the next section, we present methods utilizing magnetic bead-based assays for enhanced protein detection (Figure 2) that may eventually be suitable for cancer diagnostics. Specifically emphasis is on high sensitivity detection of multiple proteins, and methods include magnetic field sensors, electrochemistry, surface plasmon resonance, electrochemiluminescence (ECL), polymerase chain reaction (PCR) and bio-barcode assays achieved using magnetic particles as platforms or labels. Table 1 summarizes selected results from these methods.
Figure 2.
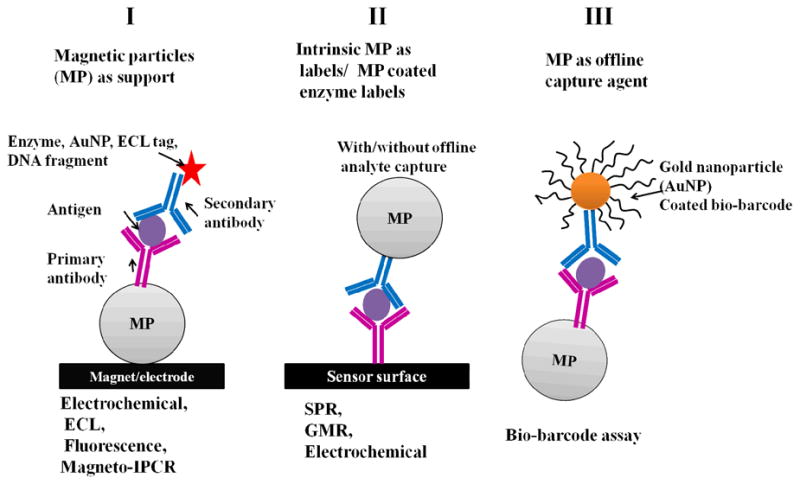
Schematic representation of the use of magnetic particles (MP) as supports (I and III) and labels (II) for protein biomarker measurements using different detectors.
Table 1.
Part A. Protein detection limits for methods using magnetic particles as labels or platforms
Part B. Protein detection limits
| Biomarker | Off-line capture? | Detection limits | Coating of particles | References |
|---|---|---|---|---|
| Magnetic field sensor | ||||
| S100ββ protein | No | 27 pg mL−1 | 300 nm streptavidin coated | 61 |
| Cancer 63CEA), Eotaxin, granulocyte colony stimulating factor (G-CSF), interleukin-1-alpha (IL-1α), interferon-γ, lactoferrin, tumor necrosis factor alpha (TNF-α) | No | 1 pg mL−1 each biomarker | 50 nm streptavidin coated | 63 |
| CEA, VEGF, lactoferrin, survivin, EPCAM, TNF-α, GCSF, Eotaxin | No | CEA 1pg mL−1, VEGF 10 pg mL−1 | 50 nm streptavidin coated | 64 |
| C-reactive protein (CRP) | No | 25 ng mL−1 in buffer | 0.5–1 μm streptavidin coated | 75 |
| Bio-barcode assay | ||||
| Prostate specific antigen (PSA), human chorionic gonadotropin (HCG),α-fetoprotein (AFP) | Yes | 170 fM in serum | 1 μm tosyl particles | 76 |
| Amyloid-β-derived diffusible ligands(ADDLs) | Yes | 100 aM in CSF | 1 μm polyamine | 76 |
| Interleukin-2 (IL-2) | Yes | 30 aM in serum | 1 μm amine-functionalized | 77 |
| Prostate specific antigen (PSA) | Yes | 3 aM in buffer | 1 μm amine-functionalized | 72 |
| Surface plasmon resonance assay | ||||
| Brain natriuretic peptide (BNP) | No | 25 pg mL−1 | 50 nm streptavidin | 66 |
| Staphylococcal enterotoxin B (SEB) | Yes | 100 pg mL−1 in stool | 50 nm streptavidin | 67 |
| Prostate specific antigen (PSA) | Yes | 10 fg mL−1 in serum | 1 μm tosylated | 68 |
| Electrochemical assay | ||||
| IgG | Yes | 67 aM | 0.83 μm streptavidin | 36 |
| Carcinoembryonic antigen (CEA), cancer antigen 125, cancer antigen 15-3, α-fetoprotein (AFP) | No | <0.5 ng mL−1 | 25 nm silica nickel ferrite | 30 |
| Free (f)-PSA | Yes | < 0.1 ng mL−1 | 3 μm tosylated | 37 |
| PSA | No | 0.5 pg mL−1 in serum | 1 μm carboxyl functionalized | 69 |
| IgG, BSA, β2-microglobulin, C reactive protein | Yes | fM levels | Streptavidin coated | 38 |
| Prostate specific antigen (PSA) | Yes | 1.4 ng mL−1 | Protein G coated | 39 |
| Carcinoembryonic antigen | No | 5 pg mL−1 | 20 nm gold nanosphere | 70 |
| Human IgG | Yes | 260 pg mL−1 | Streptavidin coated | 71 |
| Mouse IgG | Yes | 100 ag mL−1 | 1 μm Tosyl activated | 40 |
| Electrochemical aptamer based assay | ||||
| C-reactive protein, | Yes | 5.4 × 10−2 mg/L | 1.05 μm streptavidin | 41 |
| Thrombin | Yes | 0.5 nM in buffer | 1.05 μm streptavidin | 29 |
| Activated protein C (APC) | Yes | 1 nM | Protein G coated | 42 |
| Magneto-PCR | ||||
| Hepatitis B surface antigen (HBsAg) | Yes | 320 pg mL−1 in serum | Antibody functionalized magnetosome | 51 |
| Platelet-derived growth factor-BB(PDGF-BB) | Yes | 62 fM to 1 nM serum | 1 μm streptavidin | 52 |
| Electrochemiluminescence (ECL) | ||||
| CEA protein | No | 1.6 pg mL−1 | 350 nm streptavidin | 78 |
| Adrenocorticotropic hormone (ACTCH) | Yes | 0.5 ng L−1 | Ru(bpy)32+ ECL label | 44 |
| C-reactive protein (CRP) | Yes | 0.01 μg mL−1 in buffer | 1 μm streptavidin- | 47 |
| Human CRP | Yes | 100 ng mL−1 in buffer | 2.8 μm streptavidin- | 48 |
3. Magnetic particles as platforms
3.1 Electrochemical detection
Electrochemical sensors employing capture antibodies and enzyme-labeled secondary antibodies in sandwich immunoassays can provide high sensitivity, selectivity, low cost, and instrumental simplicity [7,24,25]. Coupling electrochemical sensors with magnetic beads hold significant promise for clinical diagnostic devices. Magnetic beads have been used in enzyme-linked immunosorbent assay (ELISA) formats or as labels for protein detection [67,26–32]. Magnetic beads conjugated with primary antibodies can be held onto a sensor surface by a magnetic field. Target antigens can then be captured onto the magnetic beads followed by adding enzyme-labeled secondary antibodies. A current proportional to protein concentration can be generated by injecting a solution containing a substrate for the enzyme label and using a potential to electrolyze the enzyme product, or by using a mediator that exchanges electrons with the enzyme.
Willner and Katz summarized the use of magnetic beads for controlling magneto-switchable bioelectrocatalytic processes applicable to immunosensors [33]. For example, they reported enhanced bioelectrocatalytic oxidation of glucose by soluble glucose oxidase by using ferrocene-functionalized magnetic particles on a rotating magnetic electrode, where ferrocene acts as the mediator [34]. Wang et al demonstrated ultrasensitive detection using anti-IgG coated magnetic beads that captured IgG followed by addition of DNA/anti-IgG-functionalized polystyrene beads to form a sandwich immunocomplex [35]. Oligonucleotide labels that corresponded to IgG concentration were released in alkaline solution, depurinated by acid, and the free guanine released was measured by stripping potentiometry. Using similar immunoassays, carbon nanotubes coated with thousands of alkaline phosphatase enzymes were employed to achieve an ultralow detection of 67 aM IgG using square wave voltammetry [36]. Tang et al. developed an electrochemical magnetic-controlled microfluidic device for the multiplexed detection of 4 tumor markers, α-fetoprotein (AFP), carcinoembryonic antigen (CEA), cancer antigen 125, cancer antigen 15-3 with DLs < 0.5 ng mL−1 [30]. Sarkar et al reported electrochemical detection of f-PSA using magnetic beads on a screen-printed sensor [37]. Magnetic bead protein capture was done in a cuvette, then, the beads were transferred to the sensor surface. The amperometric response of HRP labels on the bead was developed using hydrogen peroxide to give a DL < 0.1 ng mL−1 f-PSA.
Liu et al. reported a multianalyte electrochemical immunoassay involving dual binding events, based on different semiconductor labels linked to different antibodies and magnetic bead coated antibodies [38]. Zinc sulfide, cadmium sulfide, lead sulfide and copper sulfide nanocrystals were conjugated with anti-β2-microglobulin, anti-IgG, anti-bovine serum albumin, and anti-C-reactive protein antibodies, respectively, to bind with their target proteins on the magnetic bead. The nanocrystals were dissolved in acid and resulting metal ions were detected with stripping voltammetry to achieve fM detection limits (DL). Zani et al demonstrated an electrochemical PSA immunoassay using protein G modified magnetic beads with 8-electrode screen printed arrays [39]. After PSA was captured on magnetic beads equipped with alkaline phosphatase enzyme labeled antibodies, the product of enzyme conversion of 1-naphthyl-phosphate was detected by differential pulse voltammetry for a DL of 1.4 ng mL−1. Selvaraju et al. [40] reported an electrochemical immunoassay for mouse IgG using antibody modified magnetic beads and gold nanocatalysts (AuN) with ultra low detection limit of 100 ag mL−1(0.7 aM) by differential pulse voltammetry. Immunocomplex in which the target protein is captured by both antibody modified magnetic beads and AuN-conjugated antibody was attracted onto ferrocenyl-tethered dendrimer (Fc-D) modified indium tin oxide electrode using an external magnet. AuN in the immunocomplex produces p-aminophenol from the catalytic reduction of p-nitrophenol in the presence of NaBH4. The p-aminophenol is electrochemically oxidized to p-quinone imine via electron mediation by ferrocene, and p-quinone imine is then reduced back to p-aminophenol by NaBH4. The redox cycle amplifies the signal.
Aptamers are short sequences of nucleotides designed to be specific for target proteins, and have also been used as capture agents and labels for sensitive protein detection. Aptamer based assays include magnetic particles as solid supports, and polymerase chain reaction (PCR), electrochemical and fluorescence for aptamer label detection. Electrochemical aptamer based sandwich assays have been developed to detect C-reactive protein (CRP) [41], thrombin and activated protein C (APC) [42].
3.2 Optical detection: Electrochemiluminescence
Electrochemiluminescence (ECL) detection involves electrochemically generated light emission from a luminescent label, and is also promising for multiple protein assays. The most frequently used label is tris (2,2′-bipyridyl) ruthenium(II), [RuBPY], which initiates ECL when its oxidized form reacts with a suitable sacrificial reductant such as tripropylamine [43]. Visible light emission occurs upon electrochemical driving of this process, thus simplifying instrumentation compared to other luminescence approaches. A typical ECL magnetic bead format features antibody-streptavidin-magnetic beads that capture protein analyte. RuBPY-labeled biotinylated secondary antibody is added to bind to the streptavidin, and the beads are washed and magnetically captured onto an electrode for ECL measurement [43].
Magnetic bead methods similar to that describe above are the basis of protein detection instruments such as Roche’s ELECYS, Igen’s Origen and BioVeris’s M-Series. Commercial measurement systems and kits are available for up to 10 proteins. Fluid handling systems assist transport of magnetic bead and ECL labels to the detecting electrodes in 96-well plates. Measured ECL light intensity corresponds to the amount of protein present in the complex medium. Commercial systems have typical DLs of 1–10 pg mL−1 for most proteins, and usually require a non-reusable 96-well plate for each assay. ECL with RuBPY labels has been used for the detection of cancer biomarkers proteins such as adrenocorticotropic hormone [44], cardiac troponin T [45], parathyroid hormone (PTH) [46], C-reactive protein [47,48] and alpha-fetoprotein (AFP) [49]. ECL using 100 nm RuBPY-silica nanoparticle labels has been used for the detection of PSA in cancer patient serum [50].
3.3 Magneto-PCR based assay
Wacker et al. reported a magneto-IPCR (immuno-PCR) utilizing a sandwich immunocomplex formed on magnetic particles with antibody conjugated DNA fragment as labels. The labels are detected using real time PCR to obtain the protein concentration. Magneto-IPCR was used to detect hepatitis B surface antigen (HBsAg) with a detection limit of 320 pg mL-1 [51].
Csordas et al. reported a micromagnetic aptamer PCR (MAP) technology to detect protein biomarkers in serum samples [52]. This technique utilized antibody-coated magnetic particles which capture biomarker proteins in serum followed by binding of a specific aptamer. Then, this complex was magnetically separated and the aptamer was amplified using PCR to provide high sensitivity detection. Platelet-derived growth factor-BB (PDGF-BB) over the range 62 fM to 1 nM was detected using MAP technology in complex serum samples. In a related approach, Tennico et al. used sandwich assays to detect thrombin utilizing aptamer coated magnetic beads, quantum dot labels, and fluorescence microscopy [53].
4. Magnetic particles as labels
4.1 Magnetic Field Sensors
Since biological applications of giant magnetoresistance (GMR) sensors were first reported over a decade ago [54], several magnetic field protein biosensors [55–58] with magnetic bead labels have been described. Detection has been done by magnetic field sensors including GMRs, superconducting quantum interference devices (SQUID) [59] and Hall effect [60]. GMR detects magnetic bead labels by measuring the resistance change of a conducting sensor featuring alternate layers of ferromagnetic and non-magnetic materials in nm thicknesses under applied current and magnetic field. In a typical example, the conducting sensor layer has attached primary antibodies to capture the protein of interest from the sample. After protein capture in this so called “sandwich immunoassay”, the surface is washed and a biotin-labeled secondary antibody is added to bind to the target protein. This is followed by incubation with streptavidin-coated superparamagnetic bead labels that bind strongly to biotin on the secondary antibody (Figure 3). Detection of proteins is achieved by measuring the change in resistance of the conducting layer due to the superparamagnetic bead labels. This method provides high sensitivity at low sensor cost and low background noise.
Figure 3.
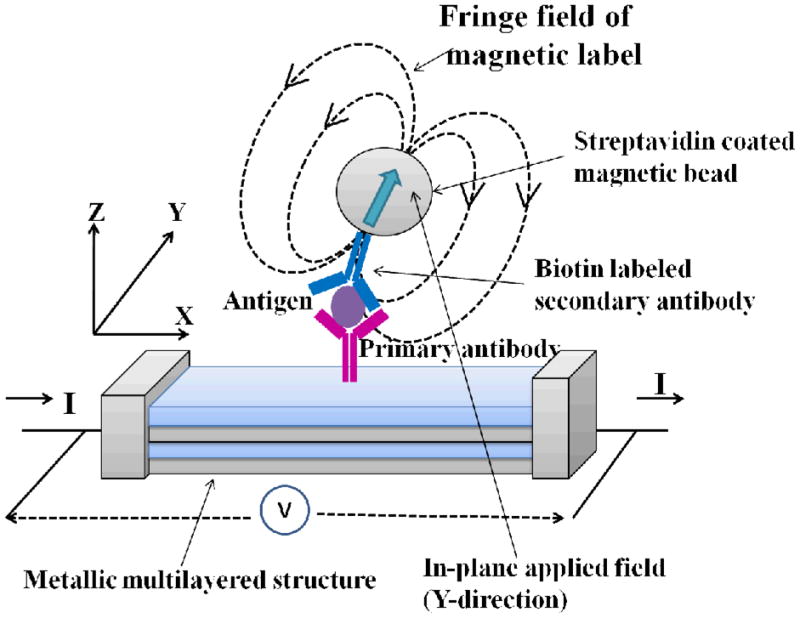
Schematic representation of giant magnetoresistance (GMR) biosensor for the detection of antigen based on the sandwich immunoassay. The sensor detects fringe field of magnetic bead labels under applied in-plane magnetic field. Scheme is adapted from [54].
Palma et al. used streptavidin coated magnetic beads in a magnetic immunosensor to detect stroke and minor head injury marker S100ββ with a detection limit of 27 pg mL−1[61]. The Naval Research Laboratory (NRL) developed a GMR sensor chip array with 64 sensors called the bead ARray Counter (BARC®) measuring biomolecules by detecting superparamagnetic labels [54]. Mulvaney et al. reported magneto-electronic based detection of proteins in complex matrices utilizing magnetic bead labels and fluidic flow discrimination (FFD) [62]. Beads captured on the sensor surface were measured using microscopy and BARC sensors. FFD lowered the non-specific binding and increase the sensitivity of detection for proteins. A 64 sensor-GMR array [63,64] enabled multiplexed detection of proteins biomarkers in clinical samples utilizing magnetic nanoparticle labels with sensitivities in femtomolar and attomolar ranges. Eight protein biomarkers associated with various cancers were detected using this sensor, with insensitivity to matrix effects. The small size and portability of GMR sensor arrays have fueled interest in this approach for protein biosensing at point of care [65].
4.2 Surface Plasmon Resonance
Surface Plasmon resonance (SPR) is an evanescent wave optical reflectance method sensitive to refractive index (RI) differences in surface films up to 300 nm thick above a gold sensor surface. In SPR immunoassays, for example, binding of a protein to a surface antibody changes the RI by increasing the thickness of the surface film. In a typical sandwich immunoassay, the sensor is coated with primary antibody and captures the target protein. If a secondary antibody labeled with a magnetic particle then binds to the captured analyte, a larger RI increase and larger signal will result due to the size and higher RI of the magnetic particle.
This approach has been used in SPR-based immunoassays for high sensitivity protein detection [66–68] Magnetic beads can provide off-line target capture and magnetic separation to decrease non-specific binding of interferants in the sample (Figure 4). Teramura et al. reported SPR immunoassay for proteins using biotin labeled secondary antibodies (Ab2) captured by streptavidin coated magnetic nanobeads to detect brain natriuretic peptide (BNP) with a DL 25 pg mL−1 in buffer [66]. Soelberg et al. used 50 nm streptavidin-coated magnetic nanobeads as labels to detect staphylococcal enterotoxin B (SEB) using SPR at DL 100 pg mL−1 in stool samples [67]. Krishnan et al. recently utilized 1 μm superparamagnetic magnetic bead (MP)-Ab2 bioconjugates for off-line antigen capture of prostate cancer biomarker PSA in serum. These particles were then captured on a primary antibody coated SPR biosensor and an ultralow DL of 10 fg mL−1 (300 aM) [68]. Detection was greatly amplified by utilizing aggregation of the MP-Ab2 on the SPR sensor surface (Figure 5), which provided high sensitivity via a very large RI change per bound protein. Accuracy of SPR detection in this assay was validated using cancer patient serum.
Figure 4.
Illustration of off-line capture of protein analytes using labeled magnetic beads, a technique that can be used with nearly any type of detector. Magnetic beads with bound protein are selectively captured on the sensor surface, while beads without analyte are washed away. Magnetic and SPR methods require only the magnetic bead as a label
Figure 5.
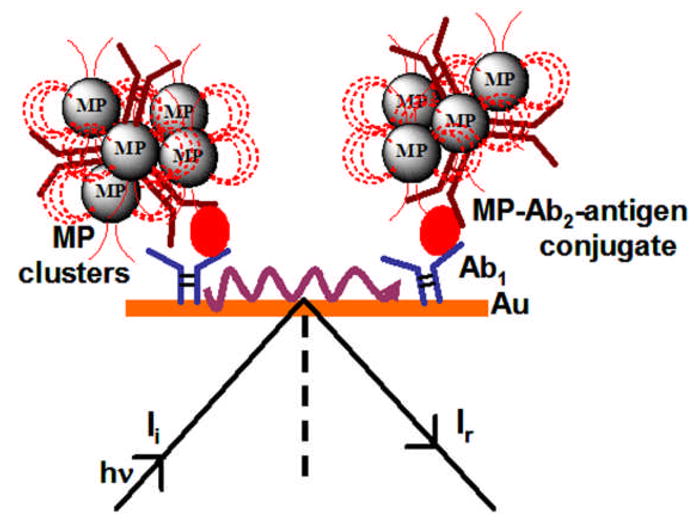
Signal amplification due to aggregation of magnetic particles on SPR sensor surface in immunoassays for the detection of PSA using surface plasmon resonance (SPR) assay.
4.3 Electrochemical detection
Nanostructured amperometric immunosensors were used for PSA in serum employing HRP-labeled magnetic beads with DL of 0.5 pg mL−1 [69]. Tang et al. reported signal amplification using thionine doped magnetic gold nanospheres and HRP to detect carcinoembryonic antigen [70] at 5 pg mL−1. Sandwich immunoassay was fabricated on carbon fiber microelectrode modified with anti-CEA/protein A/nanogold particles. Electrochemical signals were developed by the reduction of H2O2 by labeled gold nanospheres. Ambrosi et al. reported magnetic microbead based electrochemical immunoassay utilizing gold nanoparticle labels for signal enhancement with a detection limit of 260 pg mL−1 of human IgG [71].
4.4 Other detection method: Biobarcode assays
Magnetic beads have been used extensively as solid supports to covalently link biomolecules such as antibodies, DNA and enzymes. Antibody coated magnetic beads provide stronger binding for target proteins by using mechanical agitation. Ultrasensitive detection of several proteins in serum has been achieved using biobarcode-based detection utilizing magnetic beads as solid supports [6]. In this approach, gold or silica nanoparticles act as carriers for polynucleotide strands of known-sequence that serve as biobarcodes. The barcode is the sequence of nucleobases in the polynucleotide label that can be “read” by a complimentary polynucleotide strand. Nam et al. used primary antibody coated magnetic beads to capture target antigen followed by washing and then binding of secondary antibody-gold nanoparticles [72] or silica microparticles [73] with the biobarcodes attached. After magnetic separation of the immunocomplex, the biobarcodes are released by using high temperature and low salt concentration, and detected using a scanometric or colorimetric assay. The polymerase chain reaction (PCR) can be used to enhance sensitivity to very high levels. Biobarcode assays can provide attomolar-level detection of protein biomarkers in serum. Multiplexed detection of prostate specific antigen (PSA), human chorionic gonadotropin (HCG) and α-fetoprotein (AFP) was achieved with this approach utilizing 3 different antibody coated magnetic beads with detection limits of 170 fM in diluted serum [74].
5. Summary
Using magnetic particles for protein capture, manipulation, transport, and labeling has led to methods that detect cancer biomarker proteins at clinically relevant serum levels and below. GMR and SPR detectors benefit from the inherent signal enhancement of the magnetic particle itself, which does not need additional labels. Approaches such as biobarcode assays and nanostructured microfluidic arrays with massively labeled particles can detect proteins well below pg mL−1 levels in serum. Off-line capture of the analyte proteins by magnetic particles ensures that the detectors need never be exposed to the sample, eliminating many possible interferences and greatly lowering non-specific binding (NSB) which is a major source of background in immunoassays.
Despite the above successes, only a small fraction of the methods discussed in this article have been validated using cancer patient samples. However, the few that have been tested with patient samples have demonstrated good accuracy and sensitivity. In addition, cancer diagnostics applications using protein biomarkers will require accurate detection of not a single protein, but panels of 4–10 biomarkers for each cancer. Much less progress has been made on this front, with approaches to multiplexed protein detection systems for clinical or POC use just beginning to appear in research publications. Currently no commercial devices for multiplexed cancer biomarker protein detection are suitable for POC use. Commercial magnetic bead-based methods utilizing ECL seem promising for laboratory-based multiplexed protein determinations, but are relatively expensive and technically demanding.
6. Expert opinion
Clearly, magnetic bead-based technologies are becoming firmly established as important components of the protein detection systems of the future. However, it will be necessary for such systems to detect small panels of proteins and other biomarkers to be most useful for clinical diagnosis. In addition, POC devices will require simple, low cost, technically undemanding methodology. Extensive testing and validation will be necessary on patient samples such as serum and saliva to establish analytical reliability, and clinical sensitivity and selectivity. Interfacing of sensitive, reliable, simple detection protocols with microfluidic or other automated sample handling technologies seems a necessary future step to reach POC. However, the payoff is potentially high for future cancer diagnostics and therapy monitoring. Extensive research and development efforts are underway with the hope of solving these formidable challenges. An exciting dream for the future is a hand-held multiplexed protein detection device about the size of a cell phone that can carry out all sample handling, measurement, and data analysis tasks for panels of biomarker proteins for a given cancer, or better yet, for 100 or more biomarkers for the most prevalent cancers.
Article Highlights.
Magnetic beads facilitate sensitive methodologies for multiple protein detection important for future cancer diagnostics.
Magnetic beads with polymer coatings are commercially available in diameters from 100 nm to 50 μm with surface chemistries amenable to attaching virtually any biomolecule.
Paramagnetic beads are most useful for protein capture, separation, and transport, but may have remnant magnetism leading to some degree of aggregation.
Magnetic beads without surface biomolecules can serve as signal amplifying labels in Surface Plasmon Resonance (SPR) and Giant Magnetoresistance (GMR) sensors.
Use of multiple labels such as enzymes, polynucleotides, or metal complexes either attached to magnetic beads or partner nanoparticles can be used to greatly enhance sensitivity in optical and electrochemical immunoassays.
There is a significant need for validation of magnetic bead-based assays and biomarker panels with sufficiently large numbers of patient samples.
Inexpensive, technically undemanding, automated protein detection devices will be required for future point of care cancer diagnostics and therapy monitoring.
Footnotes
Declaration of Interest: The authors were supported by a PHS grant from NIEHS/NIH and by the NSF.
References
- 1.Atkinson AJ, Colburn WA, DeGruttola VG, et al. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 2.Manne U, Srivastava RG, Srivastava S. Recent advances in biomarkers for cancer diagnosis and treatment. Drug Discov Today. 2005;10:965–976. doi: 10.1016/S1359-6446(05)03487-2. [DOI] [PubMed] [Google Scholar]
- 3.Kulasingam V, Diamandis EP. Strategies for discovering novel cancer biomarkers through utilization of emerging technologies. Nat Clin Pract Oncol. 2008;5:588–599. doi: 10.1038/ncponc1187. [DOI] [PubMed] [Google Scholar]
- 4.Hanash SM, Pitteri SJ, Faca VM. Mining the plasma proteome for cancer biomarkers. Nature. 2008;452:571–579. doi: 10.1038/nature06916. [DOI] [PubMed] [Google Scholar]
- 5.Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nature Rev Cancer. 2005;5:845–856. doi: 10.1038/nrc1739. [DOI] [PubMed] [Google Scholar]
- 6.Giljohan DA, Mirkin CA. Drivers of biodiagnostic development. Nature. 2009;462:461–464. doi: 10.1038/nature08605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7*.Rusling JF, Kumar CV, Gutkind JS, et al. Measurement of biomarker proteins for point-of-care early detection and monitoring of cancer. Analyst. 2010;135:2496–2511. doi: 10.1039/c0an00204f. Comprehensive review of modern methods to measure cancer biomarker proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8**.Hanash SM, Baik CS, Kallioniemi O. Emerging molecular biomarkers—blood-based strategies to detect and monitor cancer. Nat Rev Clin Oncol. 2011;8:142–150. doi: 10.1038/nrclinonc.2010.220. An excellent review describing the current state of use and future possibilities of cancer biomarkers in cancer detection and therapy monitoring. [DOI] [PubMed] [Google Scholar]
- 9.Ward MA, Catto JWF, Hamdy FC. Prostate specific antigen: biology, biochemistry and available commercial assays. Ann Clin Biochem. 2001;38:633–651. doi: 10.1258/0004563011901055. [DOI] [PubMed] [Google Scholar]
- 10.Wagner PD, Verma M, Srivastava S. Challenges for biomarkers in cancer detection. Ann N Y Acad Sci. 2004;1022:9–16. doi: 10.1196/annals.1318.003. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Zhang Z, Rosenzweig J, et al. Proteomics and bioinformatics approaches for identification of serum biomarkers to detect breast cancer. Clin Chem. 2002;48:1296–1304. [PubMed] [Google Scholar]
- 12.Tothill IE. Biosensors for cancer markers diagnosis. Semin Cell Dev Biol. 2009;20:55–62. doi: 10.1016/j.semcdb.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Choi Y-E, Kwak J-W, Park JW. Nanotechnology for early cancer detection. Sensors. 2010;10:428–455. doi: 10.3390/s100100428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mischak H, Allmaier G, Apweiler R, et al. Recommendations for biomarker identification and qualification in clinical proteomics. Sci Transl Med. 2010;2:46ps42. doi: 10.1126/scitranslmed.3001249. [DOI] [PubMed] [Google Scholar]
- 15.Goodsaid FM, Mendrick DL. Translational medicine and the value of biomarker qualification. Sci Transl Med. 2010;2:47ps44. doi: 10.1126/scitranslmed.3001040. [DOI] [PubMed] [Google Scholar]
- 16.Franzreb M, Siemann-Herzberg M, Hobley TJ, et al. Protein purification using magnetic adsorbent particles. Appl Microbiol Biotechnol. 2006;70:505–516. doi: 10.1007/s00253-006-0344-3. [DOI] [PubMed] [Google Scholar]
- 17.Verpoorte E. Beads and chips: new recipes for analysis. Lab Chip. 2003;3:60N–68N. doi: 10.1039/b313217j. [DOI] [PubMed] [Google Scholar]
- 18.Whiteaker JR, Zhao L, Zhang HY, et al. Antibody-based enrichment of peptides on magnetic beads for mass spectrometry-based quantification of serum biomarkers. Anal Biochem. 2007;362:44–54. doi: 10.1016/j.ab.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freed GL, Cazares LH, Fichandler CE, et al. Differential capture of serum proteins for expression profiling and biomarker discovery in pre- and post treatment head and neck cancer samples. The Laryngoscope. 2008;118:61–68. doi: 10.1097/MLG.0b013e31814cf389. [DOI] [PubMed] [Google Scholar]
- 20.Taurines R, Dudley E, Conner AC, et al. Serum protein profiling and proteomics in autistic spectrum disorder using magnetic bead-assisted mass spectrometry. Eur Arch Psychiatry Clin Neurosci. 2010;260:249–255. doi: 10.1007/s00406-009-0066-5. [DOI] [PubMed] [Google Scholar]
- 21.Loo D, Jones A, Hill MM. Lectin magnetic bead array for biomarker discovery. J Proteome Res. 2010;9:5496–5500. doi: 10.1021/pr100472z. [DOI] [PubMed] [Google Scholar]
- 22.de la Escosura-Muniz A, Merkoci A. Electrochemical detection of proteins using nanoparticles: applications to diagnostics. Expert Opin Med Diagn. 2010;4:21–37. doi: 10.1517/17530050903386661. [DOI] [PubMed] [Google Scholar]
- 23*.Gijs MAM, Lacharme F, Lehmann U. Microfluidic applications of magnetic particles for biological analysis and catalysis. Chem Rev. 2010;110:1518–1563. doi: 10.1021/cr9001929. An excellent review discussing fundamental properties of magnetic particles and their applications in microfluidic systems. [DOI] [PubMed] [Google Scholar]
- 24.Wang J. Nanomaterial-based electrochemical biosensors. Analyst. 2005;130:421–426. doi: 10.1039/b414248a. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Katz E, Willner I. Biomaterial-nanoparticle hybrid systems for sensing and electronic devices. In: Katz E, Willner I, editors. Bioelectronics: From theory to applications. Wiley-VCH; 2005. pp. 231–264. [Google Scholar]
- 26.Zhang H, Meyerhoff ME. Gold-coated magnetic particles for solid-phase immunoassays: enhancing immobilized antibody binding efficiency and analytical performance. Anal Chem. 2006;78:609–616. doi: 10.1021/ac051720x. [DOI] [PubMed] [Google Scholar]
- 27.Tsai HY, Hsu CF, Chiu IW, et al. Detection of c-reactive protein based on immunoassay using antibody-conjugated magnetic nanoparticles. Anal Chem. 2007;79:8416–8419. doi: 10.1021/ac071262n. [DOI] [PubMed] [Google Scholar]
- 28.Choi J-W, Oh KW, Thomas JH, et al. An integrated microfluidic biochemical detection system for protein analysis with magnetic bead-based sampling capabilities. Lab Chip. 2002;2:27–30. doi: 10.1039/b107540n. [DOI] [PubMed] [Google Scholar]
- 29.Centi S, Tombelli S, Minunni M, et al. Apatmer-based detection of plasma protein by an electrochemical assay coupled to magnetic beads. Anal Chem. 2007;79:1466–1473. doi: 10.1021/ac061879p. [DOI] [PubMed] [Google Scholar]
- 30.Tang D, Yuan R, Chai Y. Magnetic control of an electrochemical microfluidic device with an arrayed immunosensor for simultaneous multiple immunoassays. Clin Chem. 2007;53:1323–1329. doi: 10.1373/clinchem.2006.085126. [DOI] [PubMed] [Google Scholar]
- 31.Thomas JH, Kim SK, Hesketh PJ, et al. Microbead-based electrochemical immunoassay with interdigitated array electrodes. Anal Biochem. 2004;328:113–122. doi: 10.1016/j.ab.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Liu G, Jan MR. Ultrasensitive electrical biosensing of protein and DNA: carbon–nanotube derived amplification of the recognition and transduction events. J Am Chem Soc. 2004;126:3010–3011. doi: 10.1021/ja031723w. [DOI] [PubMed] [Google Scholar]
- 33.Willner I, Katz E. Magnetic control of electrocatalytic and bioelectrocatalytic processes. Angew Chem Int Ed. 2003;42:4576–4588. doi: 10.1002/anie.200201602. [DOI] [PubMed] [Google Scholar]
- 34.Katz E, Willner I. Enhancement of bioelectrocatalytic processes by the rotation of mediator-functionalized magnetic particles on electrode surfaces: comparison with rotating disk electrode. Electroanalysis. 2005;17:1616–1626. [Google Scholar]
- 35.Wang J, Liu G, Munge B, et al. DNA based amplified bioelectronic detection and coding of proteins. Angew Chem Int Ed. 2004;43:2158–2161. doi: 10.1002/anie.200453832. [DOI] [PubMed] [Google Scholar]
- 36.Munge B, Liu G, Collins G, et al. Multiple enzyme layers on carbon nanotubes for electrochemical detection down to 80 DNA copies. Anal Chem. 2005;77:4662–4666. doi: 10.1021/ac050132g. [DOI] [PubMed] [Google Scholar]
- 37.Sarkar P, Ghosh D, Bhattacharyay D, et al. Electrochemical immunoassay for free prostate specific antigen (f-PSA) using magnetic beads. Electroanalysis. 2008;20:1414–1420. [Google Scholar]
- 38.Liu G, Wang J, Kim J, et al. Electrochemical coding for multiplexed immunoassays of proteins. Anal Chem. 2004;76:7126–7130. doi: 10.1021/ac049107l. [DOI] [PubMed] [Google Scholar]
- 39.Zani A, laschi S, Mascini M, et al. A new electrochemical multiplexed assay for PSA cancer marker detection. Electroanalysis. 2011;23:91–99. [Google Scholar]
- 40.Selvaraju T, Das J, Han SW, et al. Ultrasensitive electrochemical immunosensing using magnetic beads and gold nanocatalysts. Biosens Bioelectron. 2008;23:932–938. doi: 10.1016/j.bios.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Centi S, Sanmartin LB, Tombelli S, et al. Detection of C reactive protein (CRP) in serum by an electrochemical aptamer-based sandwich assay. Electroanalysis. 2009;21:1309–1315. [Google Scholar]
- 42.Noori A, Centi S, Tombelli S, et al. Detection of activated protein C by an electrochemical aptamer-based sandwich assay. Anal Bioanal Electrochem. 2010;2:178–188. [Google Scholar]
- 43.Debad JB, Glezer EN, Leland JK, et al. In: Electrogenerated Chemiluminescence. Bard AJ, editor. Marcel Dekker; NY: 2004. pp. 359–396. [Google Scholar]
- 44.Verschraegan I, Anckaert E, Schiettecatte J, et al. Multicenter evaluation of a rapid electrochemiluminescent adrenocorticotropic hormone (ACTH) immunoassay. Clin Chim Acta. 2007;380:75–80. doi: 10.1016/j.cca.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 45.Dominici R, Infusino I, Valente C, et al. Plasma or serum samples: measurements of cardiac troponin T and of other analytes compared. Clin Chem Lab Med. 2004;42:945–951. doi: 10.1515/CCLM.2004.154. [DOI] [PubMed] [Google Scholar]
- 46.Santini SA, Carrozza C, Vulpio C, et al. Assessment of parathyroid function in clinical practice: which parathyroid hormone assay is better? Clin Chem. 2004;50:1247–1250. doi: 10.1373/clinchem.2003.030759. [DOI] [PubMed] [Google Scholar]
- 47.Miao W, Bard AJ. Electrogenerated chemiluminescence. 80. c-reactive protein determination at high amplification with [Ru(bpy)3]2+- containing microspheres. Anal Chem. 2004;76:7109–7113. doi: 10.1021/ac048782s. [DOI] [PubMed] [Google Scholar]
- 48.Zhan W, Bard AJ. Electrogenerated chemiluminescence. 83. immunoassay of human c-reactive protein by Ru(byp)32+- encapsulated liposomes as labels. Anal Chem. 2007;79:459–463. doi: 10.1021/ac061336f. [DOI] [PubMed] [Google Scholar]
- 49.Namba Y, Usami M, Suzuki O. Highly sensitive electrochemiluminescence immunoassay using the ruthenium chelate-labeled antibody bound to the magnetic micro beads. Anal Sci. 1999;15:1087–1093. [Google Scholar]
- 50.Sardesai N, Pan S, Rusling JF. Electrochemiluminescent immunosensor for detection of protein cancer biomarkers using carbon nanotube forests and [Ru-(bpy)3]2+-doped silica nanoparticles. Chem Commun. 2009:4968–4970. doi: 10.1039/b909220j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wacker R, Ceyhan B, Alhorn P, et al. Magneto immuno-PCR: a novel immunoassay based on biogenic magnetosome nanoparticles. Biochem Biophys Res Commun. 2007;357:391–396. doi: 10.1016/j.bbrc.2007.03.156. [DOI] [PubMed] [Google Scholar]
- 52.Csordas A, Gerdon AE, Adams JD, et al. Detection of proteins in serum by micromagnetic aptamer PCR (MAP) technology. Angew Chem Int Ed. 2010;49:355–358. doi: 10.1002/anie.200904846. [DOI] [PubMed] [Google Scholar]
- 53.Tennico YH, Hutanu D, Koesdjojo MT, et al. On-chip aptamer-based sandwich assay for thrombin detection employing magnetic beads and quantum dots. Anal Chem. 2010;82:5591–5597. doi: 10.1021/ac101269u. [DOI] [PubMed] [Google Scholar]
- 54.Baselt DR, Lee GU, Natesan M, et al. A biosensor based on magnetoresistance technology. Biosens Bioelectron. 1998;13:731–739. doi: 10.1016/s0956-5663(98)00037-2. [DOI] [PubMed] [Google Scholar]
- 55.Graham DL, Ferreira HA, Freitas PP. Magnetoresistive-based biosensors and biochips. Trends Biotechnol. 2004;22:455–462. doi: 10.1016/j.tibtech.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 56.Tamanaha CR, Mulvaney SP, Rife JC, et al. Magnetic labeling, detection, and system integration. Biosens Bioelectron. 2008;24:1–13. doi: 10.1016/j.bios.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 57.Sandhu A. Biosensing: New probes offer much faster results. Nat Nanotechnol. 2007;2:746–748. doi: 10.1038/nnano.2007.398. [DOI] [PubMed] [Google Scholar]
- 58.Llandro J, Palfreyman JJ, Ionescu A, et al. Magnetic biosensor technologies for medical applications. Med Biol Eng Comput. 2010;48:977–998. doi: 10.1007/s11517-010-0649-3. [DOI] [PubMed] [Google Scholar]
- 59.Enpuku K, Soejima K, Nishimoto T, et al. Biological immunoassays without bound/free separation utilizing magnetic marker and HTS SQUID. IEEE Trans Appl Supercond. 2007;17:816–819. [Google Scholar]
- 60.Aytur T, Foley J, Anwar M, et al. A novel magnetic bead bioassay platform using a microchip-based sensor for infectious disease diagnosis. J Immunol Methods. 2006;314:21–29. doi: 10.1016/j.jim.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 61.Palma RD, Reekmans G, Liu C, et al. Magnetic bead sensing platform for the detection of proteins. Anal Chem. 2007;79:8669–8677. doi: 10.1021/ac070821n. [DOI] [PubMed] [Google Scholar]
- 62.Mulvaney SP, Cole CL, Kniller MD, et al. Rapid, femtomolar bioassays in complex matrices combining microfluidics and magnetoelectronics. Biosens Bioelectron. 2007;23:191–200. doi: 10.1016/j.bios.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 63.Osterfeld SJ, Yu H, Gaster RS, et al. Multiplex protein assays on real-time magnetic nanotag sensing. Proc Natl Acad Sci. 2008;105:20637–20640. doi: 10.1073/pnas.0810822105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gaster RS, Hall DA, Nielsen CH, et al. Matrix-insensitive protein assays push the limits of biosensors in medicine. Nat Med. 2009;15:1327–1333. doi: 10.1038/nm.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kricka LJ, Park JY. Magnetism and magnetoresistance: attractive prospects for point-of-care testing? Clin Chem. 2009;55:1058–1060. doi: 10.1373/clinchem.2009.123927. [DOI] [PubMed] [Google Scholar]
- 66.Teramura Y, Arima Y, Iwata H. Surface plasmon resonance-based highly sensitive immunosensing for brain natriuretic peptide using nanobeads for signal amplification. Anal Biochem. 2006;357:208–215. doi: 10.1016/j.ab.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 67.Soelberg SD, Stevens RC, Limaye AP, et al. Surface plasmon resonance detection using antibody-linked magnetic nanoparticles for analyte capture, purification, concentration, and signal amplification. Anal Chem. 2009;81:2357–2363. doi: 10.1021/ac900007c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krishnan S, Mani V, Wasalathanthri D, et al. Attomolar detection of a cancer biomarker protein in serum by surface plasmon resonance using superparamagnetic particle labels. Angew Chem Int Ed. 2011;50:1175–1178. doi: 10.1002/anie.201005607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mani V, Chikkaveeraiah BV, Patel V, et al. Ultrasensitive immunosensor for cancer biomarker proteins using gold nanoparticle film electrodes and multienzyme-particle amplification. ACS Nano. 2009;3:585–594. doi: 10.1021/nn800863w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang D, Yuan R, Chai Y. Ultrasensitive electrochemical immunosensor for clinical immunoassay using thionine-doped magnetic gold nanospheres as labels and horseradish peroxidase as enhancer. Anal Chem. 2008;80:1582–1588. doi: 10.1021/ac702217m. [DOI] [PubMed] [Google Scholar]
- 71.Ambrosi A, Castaneda MT, Killard AJ, et al. Double-codified gold nanolabels for enhanced immunoanalysis. Anal Chem. 2007;79:5232–5240. doi: 10.1021/ac070357m. [DOI] [PubMed] [Google Scholar]
- 72.Nam J-M, Thaxton CS, Mirkin CA. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science. 2003;301:1884–1886. doi: 10.1126/science.1088755. [DOI] [PubMed] [Google Scholar]
- 73.Nam J-M, Jang K-J, Groves JT. Detection of proteins using colorimetric bio-barcode assay. Nat Protoc. 2007;2:1438–1444. doi: 10.1038/nprot.2007.201. [DOI] [PubMed] [Google Scholar]
- 74.Stoeva SI, Lee JS, Smith JE, et al. Multiplexed detection of protein cancer markers with biobarcoded nanoparticle probes. J Am Chem Soc. 2006;128:8378–8379. doi: 10.1021/ja0613106. [DOI] [PubMed] [Google Scholar]
- 75.Meyer MHF, Hartmann M, Krause H-J, et al. CRP determination based on a novel magnetic biosensor. Biosens Bioelectron. 2007;22:973–979. doi: 10.1016/j.bios.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 76.Georganopoulou DG, Chang L, Nam J-M, et al. Nanoparticle-based detection in cerebral spinal fluid of a soluble pathogenic biomarker for Alzheimers’s disease. Proc Natl Acad Sci. 2005;102:2273–2276. doi: 10.1073/pnas.0409336102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nam J-M, Wise AR, Groves JT. Colorimetric bio-barcode amplification assay for cytokines. Anal Chem. 2005;77:6985–6988. doi: 10.1021/ac0513764. [DOI] [PubMed] [Google Scholar]
- 78.Li M, Sun Y, Li Chen, et al. Ultrasensitive electrogenerated chemiluminescence immunoassay by magnetic nanobead amplification. Electroanalysis. 2010;22:333–337. [Google Scholar]


