Abstract
Pavlovian fear conditioning is a useful behavioral paradigm for exploring the molecular mechanisms of learning and memory because a well-defined response to a specific environmental stimulus is produced through associative learning processes. Synaptic plasticity in the lateral nucleus of the amygdala (LA) underlies this form of associative learning. Here we summarize the molecular mechanisms that contribute to this synaptic plasticity in the context of auditory fear conditioning, the form of fear conditioning best understood at the molecular level. We discuss the neurotransmitter systems and signaling cascades that contribute to three phases of auditory fear conditioning: acquisition, consolidation, and reconsolidation. These studies suggest that multiple intracellular signaling pathways, including those triggered by activation of Hebbian processes and neuromodulatory receptors, interact to produce neural plasticity in the LA and behavioral fear conditioning. Together, this research illustrates the power of fear conditioning as a model system for characterizing the mechanisms of learning and memory in mammals, and potentially for understanding fear related disorders, such as PTSD and phobias.
Fear is the emotion that is best understood in terms of brain mechanisms. Because fear plays a prominent role, either directly or indirectly, in a variety of psychiatric conditions, understanding its neural basis is of great importance. The term fear refers to a subjective feeling state and to the behavioral and physiological responses that occur in response to threatening environmental situations. These measurable responses are the subject of scientific investigations of fear in laboratory animals.
Research on fear has been successful in large part because of a behavioral paradigm, which is well-suited for neurobiological analysis: Pavlovian fear conditioning. Fear conditioning is valuable as a neurobiological tool because it involves a specific stimulus, under the control of the experimenter, which reliably elicits a measurable set of behavioral and physiological responses once learning has occurred.
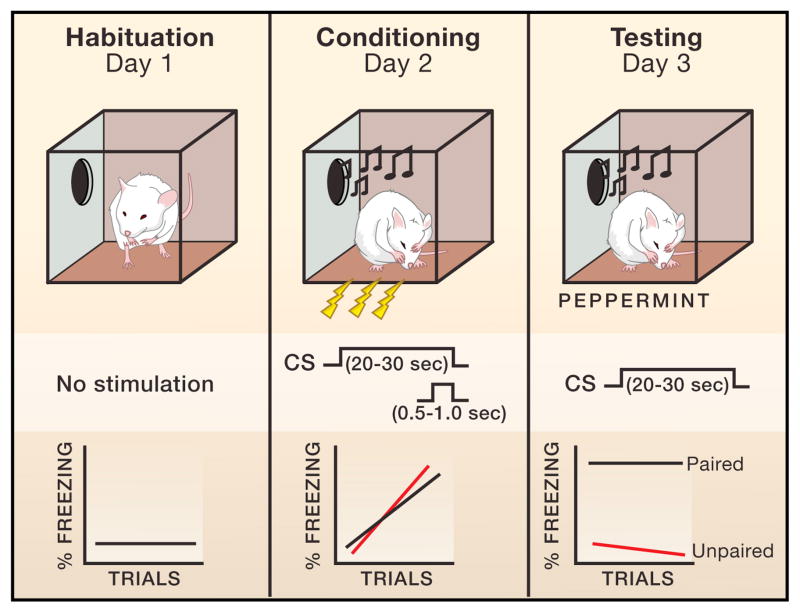
In fear conditioning, an emotionally neutral conditioned stimulus, such as a tone, is paired with an emotionally potent, innately aversive unconditioned stimulus, (e.g., an electric shock) during a conditioning or acquisition phase (Figure 1). This procedure is referred to as auditory fear conditioning. The assessment of conditioning then involves measuring conditioned responses elicited by the auditory conditioned stimulus independent of the unconditioned stimulus during a memory test phase. This somewhat artificial procedure mimics real life experiences, in which the unconditioned stimulus causes pain or other harm and the conditioned stimulus occurs in connection with the harmful one. For example, a rat that is wounded by a cat but escapes may well form a memory of the sound of rustling leaves as the cat was about to attack.
FIGURE 1. Auditory Fear Conditioning in Rats.
In a typical auditory fear conditioning procedure, rats are habituated to the conditioning chamber but given no stimuli. During the conditioning session the electric shock unconditioned stimulus (US) is paired with the auditory conditioned stimulus (CS) several times (usually 1–5). The effects of conditioning are then assessed in a test session during which the conditioned stimulus is presented alone. Most studies measure “freezing” behavior, which is an innate defensive response elicited by the conditioned stimulus after conditioning. An unpaired control group in which the conditioned stimulus and unconditioned stimulus are presented in a non-overlapping manner is often used. The conditioned stimulus elicits little or no freezing prior to conditioning in either the paired or unpaired group (not shown). Both the paired and unpaired group freeze during the training session due to the shock presentation. In the test session, the paired group exhibits considerably more conditioned stimulus-elicited freezing than the unpaired. Differences between the paired and unpaired group reflect the association that is learned as a result of conditioned-unconditioned stimuli pairing.
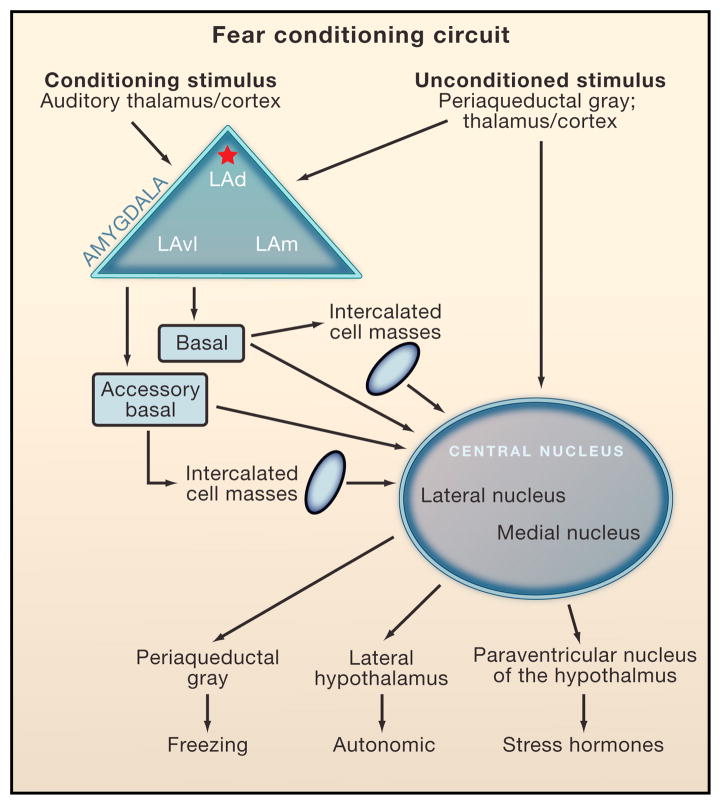
Pavlovian conditioning is believed to take place by the convergence of pathways transmitting the conditioned stimulus and unconditioned stimulus. In fear conditioning, the key circuits involve sensory areas that process the conditioned stimulus and unconditioned stimulus, regions of the amygdala that undergo plasticity during learning, and regions that control the expression of specific conditioned responses (Figure 2 and LeDoux, 2000; Fanselow and Poulos, 2005; Maren 2005; Davis and Whalen, 2001; Sotres-Bayon and Quirk, 2010). These pathways converge in the LA where synaptic plasticity that enhances the response of LA neurons to the conditioned stimulus occurs. As a result, the conditioned stimulus is then able to flow from the LA to the central nucleus of the amygdala (CE). The LA connects with the CE directly and indirectly by way of the basal (B) and intercalated masses of the amygdala. Pathways from CE to downstream areas then control defensive behavior (freezing) and autonomic and endocrine responses. Recent studies implicate the prelimbic cortex in fear expression as well, possibly by way of its connections to B, and from there to CE.
FIGURE 2. Fear Conditioning Circuit.
Convergence of the auditory conditioned stimulus and nociceptive unconditioned stimulus in the amygdala is essential for fear conditioning. Convergence of conditioned and unconditioned stimuli occurs in lateral nucleus of the amygdala (LA), especially in the dorsal subnucleus (LAd), leading to synaptic plasticity in LA. Plasticity may also occur in the central nucleus (CE) and in the auditory thalamus. LA connects with CE directly and indirectly by way of connections in the basal (B), accessory basal (AB), and intercalated (ic) regions. CE connects with hypothalamic and brainstem areas that control the expression of conditioned fear responses, including freezing, autonomic (ANS) and hormonal responses. CeL, lateral nucleus of CE; CeM, medial nucleus of CE; PAG, periaqueductal gray; LH, lateral hypothalamus; PVN, paraventricular nucleus of the hypothalamus.
In this review, we examine recent research on cellular and molecular mechanisms in LA that contribute to auditory fear conditioning. We focus on the LA because molecular changes in this area have been shown to make essential contributions to the formation, storage, and expression of the memory of the experience (see Rodrigues et al, 2004b; Pape and Pare, 2010; Sah et al, 2008). Although molecular changes occur in other areas of the amygdala, and other areas of the brain, the molecular contributions to LA plasticity as it relates to fear learning are understood in the most detail.
We restrict the discussion to molecular mechanisms that have been linked directly to the behavioral expression of conditioning, as opposed to mechanisms that underlie long-term potentiation (LTP), in which synaptic plasticity is induced by electrical or chemical stimulation of LA circuits. LTP has provided a rich array of candidate mechanisms for the plasticity processes that could occur during actual fear learning, but will not be the focus of this review(see Pape and Pare, 2010, Sah et al., 2008, Sigurdsson et al., 2007 and Dityatev and Bolshakov, 2005 for reviews on this topic).
Unique molecular mechanisms are known to underlie different stages of memory formation. Therefore, we have organized the Review by these stages, specifically the acquisition, consolidation, and reconsolidation of memories in the LA. Molecular mechanisms of fear extinction will not be discussed here (for reviews, see (Myers and Davis, 2007; Quirk and Mueller, 2008; Sotres-Bayon et al., 2006; Herry et al., 2010; Pape and Pare, 2010). These are distinguished by measuring the effects of various manipulations, often pharmacological, on fear conditioned responses at different times with respect to training or testing (see Rodrigues et al., 2004b). Table 1 summarizes the phases and how drug manipulations are used to assess effects on a particular phase. We limit our discussion to studies that manipulated molecular processes directly in the LA. Other studies that target multiple brain structures (such as genetic knockout and systemic drug studies) are discussed in (Rodrigues et al, 2004b; Pape and Pare, 2010; Sah et al, 2008; Silva, 2003; Mayford and Kandel, 1999).
TABLE 1.
Relation of Time Course of Drug Administration to Different Aspects of Fear Conditioning
| Acquisition: if a drug given before but not after training affects STM and LTM it is said to disrupt acquisition |
| Consolidation: if drug given before or after training has no effect on STM but impairs LTM it is said to disrupt consolidation. |
| Reconsolidation: if drug given after retrieval of a consolidated memory has no effect within several (2–4) hours after but impairs memory later (usually 24 hours or longer) it is said to disrupt reconsolidation. |
A. Acquisition: Cellular and Molecular Processes in LA that Underlie Learning
Learning is the basis of memory. If there is no learning, there can be no memory later. While this is true in a psychological description of memory, it also appears to be true when looking at the molecules that initiate memory formation. Disruption of molecular mechanisms that mediate memory acquisition invariably affect long term memories as well. We thus start our exploration of the cellular and molecular mechanisms of fear memory formation by considering the acquisition/training phase of fear conditioning during which learning occurs.
1. Hebbian Mechanisms in LA: Contributions to Fear Learning
A common view in neuroscience is that learning involves so-called Hebbian synaptic plasticity. This view is based on Donald Hebb’s (1949) influential proposal, which can be paraphrased as follows: a synaptic input can be strengthened when activity in the presynaptic neuron co-occurs with activity (membrane depolarization, especially depolarizations that produce action potentials) in the postsynaptic neuron (Hebb, 1949; Brown et al., 1990; Sejnowski, 1999). Implicit in Hebb’s original formulation was the idea that associative plasticity can be implemented if a strong presynaptic input depolarizes the postsynaptic neuron at the same time that another presynaptic input weakly stimulates the neuron. As a result, the weak input is strengthened by its temporal relationship with the strong input. The Hebbian hypothesis is especially appealing as an explanation for how simple associative learning, such as that taking place in fear conditioning, might occur. In a Hebbian model of fear conditioning, strong depolarization of LA pyramidal cells evoked by the aversive unconditioned stimulus leads to strengthening of co-active conditioned stimulus inputs onto the same neurons (Blair et al., 2001; LeDoux, 2000; Pare, 2002; Sah et al., 2008).
Existing data support the idea that processes triggered by unconditioned stimulus depolarization contribute to LA associative plasticity and fear memory formation. Thus, unconditioned stimuli-evoked depolarization is necessary for the enhancement of conditioned stimulus-elicited neural responses in LA after conditioned-unconditioned stimuli pairing (Rosenkranz and Grace, 2002a) and pairing a conditioned stimulus with direct depolarization of LA pyramidal neurons as a unconditioned stimulus supports fear conditioning (Johansen et al., 2010b). While there is evidence that Hebbian plasticity in LA may not entirely explain fear conditioning (see below), it is clear that synaptic plasticity at conditioned stimulus input pathways to the LA does occur with fear conditioning. Supporting this, in-vivo studies demonstrate an enhancement of auditory stimulus-evoked responses in LA neurons after fear conditioning, (see Maren and Quirk, 2004; LeDoux, 2000; Blair et al., 2001, Pape and Pare, 2010 for review). Further, in-vitro experiments find a strengthening of putative auditory thalamo-LA and cortico-LA synapses following the pairing of a conditioned stimulus with an unconditioned stimulus (Clem and Huganir, 2010; McKernan and Shinnick-Gallagher, 1997; Rumpel et al., 2005; Schroeder and Shinnick-Gallagher, 2005). Also supporting the idea that enhancement of synaptic strength in the LA is important for fear learning, LTP, is occluded in amygdala slices from fear conditioned animals (Schroeder and Shinnick-Gallagher, 2004; Schroeder and Shinnick-Gallagher, 2005; Tsvetkov et al., 2002), suggesting that LTP occurs during fear learning.
In the remainder of this section, we will examine the mechanisms mediating possible Hebbian forms of synaptic plasticity in LA during the acquisition of fear conditioning. We will then consider how other mechanisms, specifically monoamine transmitters, could modulate Hebbian plasticity in LA.
a. NMDA type Ionotropic Glutamate Receptors
Hebbian plasticity is believed to involve N-methyl-d-aspartate receptors (NMDARs) located on postsynaptic neurons in LA. NMDARs are known to be coincidence detectors of presynaptic activity (for example in conditioned stimulus input synapses) and postsynaptic depolarization (evoked by the unconditioned stimulus for example) (Malenka and Nicoll, 1999). As a result of correlated pre- and postsynaptic activity NMDARs pass calcium and this is thought to be important for synaptic plasticity and possibly memory formation (Malenka and Nicoll, 1999). Auditory inputs are indeed presynaptic to glutamate receptors, including NMDARs, in LA and use glutamate as a transmitter (see LeDoux, 2000 for review). Further, LA cells that receive auditory inputs also receive unconditioned stimulus inputs (Romanski et al., 1993, Johansen et al. 2010b) and broad spectrum NMDAR antagonists (such as APV) microinjected into the LA and basal amygdala disrupt the acquisition of fear learning (Gewirtz and Davis, 1997; Maren et al., 1996; Miserendino et al., 1990; Rodrigues et al., 2001). APV also disrupts normal synaptic transmission in the LA (Li et al., 1996) and interferes with the expression of previously acquired fear memories (Rodrigues et al., 2001). This finding raises the possibility that APV reduces the acquisition of fear conditioning by inhibiting synaptic transmission instead of blocking second messenger signaling downstream of NMDARs. However, microinjections in LA of an antagonist that targets the GluN2b (formerly called NR2B) subunit of the NMDAR reduces the acquisition of fear conditioning without affecting expression of fear memories or normal synaptic transmission (Rodrigues et al, 2001; Bauer et al., 2002). Thus, in spite of the effects of APV on synaptic transmission and fear expression, NMDARs at conditioned stimulus input synapses in LA may indeed serve as coincidence detectors of presynaptic conditioned-stimulus-evoked activity and postsynaptic depolarization induced by the unconditioned stimulus to initiate associative plasticity during fear conditioning. Further, viral mediated knockdown of the cell adhesion molecule neuroligin-1 in LA attenuates fear memory formation, possibly by reducing NMDAR number (Kim et al, 2008).
Although post-synaptic NMDARs may contribute to Hebbian synaptic plasticity by facilitating an LTP-like processes involving calcium influx, there is a form of NMDAR-dependent LTP that is induced and expressed presynaptically in LA (Fourcaudot et al., 2009; Humeau et al., 2003). This provides a potential alternate mechanism for NMDAR activation during fear learning that is non-Hebbian in nature (i.e. does not require postsynaptic depolarization) and should be taken into consideration when discussing the effects of pharmacological manipulations of the NMDAR in the LA. When a pairing protocol is used, LTP can become independent of NMDAR activation and instead depend on calcium entry through voltage gated calcium channels (Weisskopf et al., 1999) in LA. However, in real learning in whole animals both calcium influx through NMDARs and VGCCs may be required, though VGCCs appear to be involved in consolidation and not acquisition of fear conditioning ((Bauer et al., 2002; McKinney and Murphy, 2006; Shinnick-Gallagher et al., 2003).
b. Ca2+/Calmodulin (Cam) Dependent Protein Kinase II
Calcium elevation in postsynaptic cells is known to lead to autophosphorylation of Ca2+/Calmodulin (Cam) dependent protein kinase II (CaMKII) and this process is integral to memory formation in a variety of learning paradigms (Silva, 2003). CaMKII phosphorylation has been shown to increase in dendritic spines in the LA following fear learning and prevention of CaMKII activation blocks the acquisition of fear (Rodrigues et al., 2004a). CaMKII autophosphorylation can then engage a number of intracellular events in LA neurons which may result directly or indirectly in fear memories (see working model of molecular processes in LA mediating fear conditioning in Figure 3).
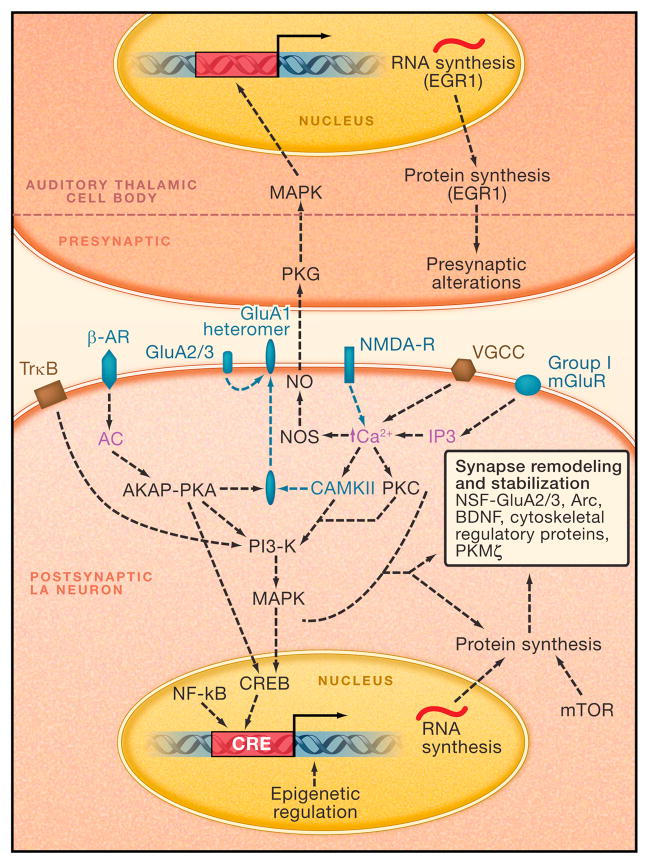
FIGURE 3. Working Model of Molecular Processes in the LA Mediating Acquisition and Consolidation of Fear Memories.
All dotted lines denote hypothetical pathways. Molecules and processes in green are known to be involved in the acquisition of fear conditioning. Molecules and process in black are known to be involved specifically in the consolidation or maintenance of fear conditioning. Purple labels denote molecules or elements whose role is not established for fear conditioning, but are part of an established intracellular signaling pathway. Abbreviations: AC, adenyl cyclase; AKAP, A-kinase anchoring protein; Arc, activity-regulated cytoskeletal-associated protein; β-AR, Beta Adrenergic Receptor; BDNF, Brain Derived Neurotrophic Factor; Ca2+, Calcium; CaMKII, Ca2+/Calmodulin (Cam) Dependent Protein Kinase II; CREB, cAMP Response Element (CRE) binding protein; EGR-1, early growth response gene 1; GluA1, Glutamate AMPA Receptor Subunit 1; GluA2/3, Glutamate AMPA Receptor Subunit 2 and 3 heteromer; IP3, inositol 1,4,5-triphosphate; MAPK, Mitogen Activated Protein Kinase; mGluR, Metabotropic Glutamate Receptor; mTOR, Mammalian Target of Rapamycin; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells NMDA-R, N-methyl-d-aspartate Glutamate Receptor; NO, Nitric Oxide; NOS, Nitric Oxide Synthase; NSF, N-ethylmaleimide-sensitive factor; PI3-K, phosphatidylinositol-3 kinase; PKA, Protein Kinase A; PKC, Protein Kinase C; PKG, cGMP-dependent protein kinase; PKMζ, protein kinase M ζ; RNA, Ribonucleic Acid; TrkB, tyrosine kinase B; VGCC, Voltage Gated Calcium Channel
c. non-NMDA type Ionotropic Glutamate (AMPA) Receptors
Autophosphorylated CaMKII can directly influence STM formation by phosphorylating the serine 831 (ser831) site on the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid type glutamate receptor (AMPAR) GluA1 subunit which produces GluA1 insertion resulting in an increase in synaptic strength at the activated synapse (Malinow and Malenka, 2002). Supporting this as a mechanism for fear learning, GluA1 is increased in the LA following fear conditioning and required for fear memory formation (Humeau et al., 2007; Nedelescu et al., 2010; Rumpel et al., 2005; Yeh et al., 2006). Interestingly, one study found that fear conditioning produces GluA1 insertion at putative thalamic-LA pyramidal cell synapses and that LA GluA1 insertion is necessary for the acquisition of fear learning (Rumpel et al., 2005).
d. Metabotropic Glutamate Receptors
Metabotropic glutamate receptors (mGluR) are also stimulated by glutamate release and group I mGluR (mGluR5) activity in the LA is required for the acquisition, but not consolidation or expression, of fear learning (Rodrigues et al., 2002). Furthermore, activation of group I mGluRs in the LA and basal nucleus enhances the acquisition of fear conditioning (Rudy and Matus-Amat, 2009). In addition, activation of group III mGluRs (mGluR7 and 8) in the LA reduces the acquisition of fear conditioning (Siegl et al., 2008; Schmid and Fendt, 2006). Activation of mGluR5 during conditioning may enhance NMDAR function as well as further increase Ca2+ levels through inositol 1,4,5-triphosphate (IP3) mediated release of Ca2+ from intracellular stores and thereby activate CaMKII and protein kinase C (PKC, which is involved in fear memory consolidation, see below). Although these receptors are activated by glutamate, they may play a more modulatory role in fear learning and not participate directly in Hebbian processes. This is because these receptors do not contribute strongly to depolarization and action potential firing of neurons, they are not downstream of receptors that are involved in Hebbian processes and they do not detect coincident pre and postsynaptic neural activity.
e. Inhibitory GABAergic Neurotransmission
Inhibitory neurotransmission through gamma-aminobutyric acid (GABA) synthesizing interneurons in the LA is important for fear conditioning and potentially for regulating Hebbian processes during fear learning (for review see Pare et al., 2003 and Ehrlich et al., 2009). Local amygdala GABAergic interneurons strongly modulate neural processing in LA projection neurons and LTP induction (Marowsky et al., 2005; Bissiere et al., 2003, Tully et al., 2007; Morozov et al., 2011) and this may serve as a mechanism to gate fear learning and/or memory consolidation. The regulation of LTP induction may be mediated through feedforward inhibition activated by auditory and thalamic inputs onto GABAergic neurons in LA which could control depolarization of LA pyramidal cells undergoing plasticity. In fact, reduction in inhibition by neuromodulators has been shown to enhance LTP (Bissiere et al., 2003, Tully et al., 2007, see below). Supporting a functional role for inhibitory transmission in fear conditioning, GABAA receptor activation reduces the acquisition of fear learning (Muller et al., 1997; Wilensky et al., 1999). In addition, GABA and the GABA synthesizing enzyme GAD65 levels as well as LTP of inhibitory transmission are reduced transiently following fear conditioning (Stork et al., 2002; Szinyei et al., 2007; Bergado-Acosta et al., 2008). Though the specific role of LA GABAergic transmission in fear learning is not entirely clear, one function may be to reduce generalization possibly through actions on the metabotropic GABAB receptor (Shaban et al., 2006; Bergado-Acosta et al., 2008). In addition, blocking the α1 subunit of the GABA receptor in the LA impairs fear learning possibly by increasing ligand initiated GABA receptor conductance in GABA heteromers which do not express this subunit (Wiltgen et al., 2009).
2. Neuromodulatory-Dependent Mechanisms Involved in Fear Learning
While Hebbian plasticity may indeed occur during learning, it does not fully explain learning; especially learning in highly charged emotional situations. It is generally thought that monoamine transmitters, such as norepinepherine (NE) and dopamine (DA), released in emotional situations, regulate glutamatergic transmission and Hebbian plasticity (for review see (Bailey et al., 2000; McGaugh, 2000; Tully and Bolshakov, 2010). The modulation of Hebbian (or activity dependent) plasticity by neuromodulators (such as monoamines) or plasticity that is entirely dependent on neuromodulators and/or postsynaptic activity independent is called heterosynpatic plasticity. This is in contrast with purely activity-dependent Hebbian plasticity, which is referred to as homosynaptic plasticity (Bailey et al., 2000). Indeed, in a variety of model systems it has been shown that monoamines modulate plasticity underlying memory formation (Carew et al, 1984 Bailey et al., 2000 and Glanzman, 2010 for review). Neuromodulators also contribute to fear conditioning.
Several lines of evidence support the idea that neuromodulatory regulation of Hebbian mechanisms contributes to plasticity in the LA and fear learning. USs and CSs activate neurons in the LC and substantia nigra/VTA, which are thought to provide NE and DA input, respectively, to the LA (Brischoux et al., 2009; Chen and Sara, 2007; Ennis and Aston-Jones, 1988). Further, NE and DA are increased in the amygdala following presentation of aversive stimuli (Galvez et al., 1996; Quirarte et al., 1998; Yokoyama et al., 2005; Young and Rees, 1998). These findings suggest that NE and DA acting through their respective receptors may modulate the acquisition of fear learning possibly by synergizing with Hebbian processes to promote associative neural plasticity in the LA. Below we focus on the modulation of learning related plasticity in LA by NE and DA. While other modulators (serotonin, acetylcholine, endocanabinoids) and various peptides (such as gastrin releasing peptide, NPY, opiates, oxytocin have been studied), the role of these modulators and peptides in the LA during auditory fear conditioning has not been carefully examined.
a. Norepinephrine
Supporting a role for NE involvement in fear memory formation, recent work demonstrates that blockade of NE beta-adrenergic receptors (β-ARs) in the LA interferes with the acquisition of fear learning when given pretraining, but has no effect post-training or before memory recall (Bush et al., 2010). This effect is thus specific to acquisition, as opposed to the post-training consolidation process or to the expression of fear memory (Bush et al., 2010; Lee et al., 2001). Furthermore, preliminary data suggest that activation of β-ARs in the LA synergistically regulates Hebbian processes to trigger LA associative plasticity and fear learning (Johansen et al., Society for Neuroscience Abstract, 2010). While the mechanism of β-AR involvement in the acquisition of fear learning is unclear, one possibility is that they act on GABAergic interneurons to suppress feed-forward inhibition and enhance Hebbian plasticity mechanisms (Tully and Bolshakov, 2010; Tully et al., 2007). While β-ARs are found on GABAergic interneurons, they are also expressed abundantly in LA pyramidal cells (Farb et al., 2010) and might also function synergistically with Hebbian mechanisms (see working model in Figure 3) in these cells to promote plasticity and fear learning (Bailey et al., 2000; Hu et al., 2007; Tully and Bolshakov, 2010; and Johansen et al., Society for Neuroscience Abstract, 2010). β-ARs are coupled to Gs signaling cascades, which activate protein kinase A (PKA). β-AR dependent PKA activation can produce phosphorylation of NMDARs as well as ser845 site on GluA1 which could facilitate AMPAR insertion at the synapse (Hu et al., 2007; Raman et al., 1996). This raises the possibility that β-AR activation could modulate LA Hebbian plasticity mechanisms and the acquisition of fear learning through regulation of glutamate receptor function. In addition, activation of β-AR and PKA reduces calcium activated potassium (SK) channel activity leading to increased excitability of LA neurons and enhanced LTP and this could also occur during learning (Faber et al., 2008). β-AR activation could also regulate Hebbian plasticity induced fear memory consolidation processes through PKA dependent cAMP response element binding-protein (CREB) phosphorylation (which is also phosphorylated by Hebbian processes, see below)(see Alberini, 2009 for review).
In contrast to β-AR effects in LA, blockade of alpha1 adrenergic receptors enhances fear acquisition, but not consolidation (Lazzaro et al., 2010). This effect may be mediated via presynaptic receptors on inhibitory interneurons in LA; since activation of LA alpha1 receptors is known to enhance feed-forward inhibition (Braga et al., 2004) and blockade of these receptors impairs LA IPSCs, enhances EPSCs and facilitates tetanic LTP of field responses in vitro (Lazzaro et al., 2010). However, a role in excitatory pyramidal neurons and amplification of Hebbian processes cannot currently be ruled out. Alpha1 adrenergic receptors are typically coupled to the Gq pathway, stimulating PLC, IP3 and DAG and may result in mobilization of Ca2+ from intracellular stores or influx (Braga et al., 2004; Chen and Minneman, 2005). Thus NE release may also inhibit plasticity in fear circuits via activation of alpha1 adrenoceptors.
b. Dopamine
Other studies suggest that dopamine receptor activation (both D1 and D2 receptor subtypes) in the amygdala contributes to the acquisition of fear conditioning (Greba et al., 2001; Guarraci et al., 2000; Guarraci et al., 1999; Nader and LeDoux, 1999). However, it is not clear from these studies whether DA receptor activity is required specifically in the LA (as the injection sites in most of these studies were centered between LA and CE) or whether activation of these receptors is necessary for acquisition and/or consolidation processes. D1 and D2 receptors are G-protein coupled and traditionally D2 receptors are thought to inhibit adenylate cyclase (Gi-coupled) and D1 receptors to stimulate adenylate cyclase (Gs-coupled). However, in the amygdala it appears that D1 receptors are not Gs coupled, but may instead stimulate phospholipase C (PLC) and IP3 production (Leonard et al., 2003). Like β-ARs, dopamine receptors may modulate Hebbian processes directly by reducing feed-forward inhibition (Bissiere et al., 2003; Marowsky et al., 2005; Rosenkranz and Grace, 2002b). Additionally, again similar to β-ARs, DA receptors are expressed on LA pyramidal neurons (Muly et al., 2009) suggesting that they may also act in a parallel fashion with Hebbian mechanisms to implement LA plasticity and fear learning through their respective signaling pathways.
3. Summary of Acquisition
Current evidence suggests that Hebbian plasticity mechanisms are necessary for associative plasticity in the LA and for the acquisition of fear learning. Supporting this, a number of receptors and intracellular signaling molecules which are known to be important for Hebbian synaptic plasticity are also involved in fear memory formation. In addition, emerging data suggest that neuromodulators, such as NE and DA, may regulate these Hebbian processes during fear learning (see working model in Figure 3). This is an intriguing possibility as it provides another mechanism by which the acquisition of fear memories can be regulated and controlled. It will be important in future work to utilize new optical and genetic tools to more carefully test the plasticity rules (i.e. the temporal, spatial and molecular mechanisms) in the amygdala involved in instantiating fear learning. In addition, it will be vital in future work to better understand how individual receptors and molecules are recruited during fear conditioning and contribute to intracellular processes mediating fear learning, particularly in defined LA cell types.
B. Consolidation: Molecular Processes that Stabilize and Maintain Fear Memory
Consolidation is the process by which temporary STM is stabilized into a persistent LTM. Plasticity important for immediate learning and STM is mediated by covalent modification of existing synaptic proteins (for example, by phosphorylating glutamate receptors) while consolidation of this plasticity is generally thought to occur via activation of second messengers that initiate gene transcription and translation of new proteins (Kandel, 2001; Bailey et al., 2000; Alberini, 2009; Hernandez and Abel, 2008). Hebbian and neuromodulatory mechanisms likely implement the initial intracellular processes during acquisition, but they may also trigger the second messengers that lead to gene transcription and protein translation, processes that stabilize and consolidate LTM. As noted, molecules that are necessary for LTM, but not STM, are said to be involved in memory consolidation (McGaugh, 2000; Rodrigues et al., 2004b).
Most of what we will describe below involves plasticity that is assumed to occur within post-synaptic neurons. Presynaptic plasticity also occurs and will be mentioned as well. As with acquisition, the emphasis will be on the molecules in LA that contribute to memory consolidation.
1. Gene Transcription and Protein Translation
Activation of genes by transcription factors leads to RNA and eventually protein synthesis. Protein synthesis has been implicated in memory consolidation in many systems (Hernandez and Abel, 2008; Davis and Squire, 1984; McGaugh, 2000). Fewer studies have examined RNA synthesis, but it too has been implicated in memory consolidation. Once LA was identified as a key area required for memory formation in fear conditioning, it was natural to ask whether RNA and protein synthesis in LA underlies the consolidation of fear conditioning memories.
Indeed, direct infusion of anisomycin, an inhibitor of protein synthesis, into the LA before or after training has no effect on subsequent STM but impairs the conversion of STM to LTM (Schafe and LeDoux, 2000; Duvarci et al., 2008). Consolidation of STM into LTM is also disrupted by infusion of broad spectrum inhibitors of RNA synthesis or an inhibitor of cap-dependent (a specific form of protein synthesis) RNA synthesis into the LA after fear conditioning (Bailey et al., 1999; Duvarci et al., 2008, Hoeffer et al., 2011). Thus, both gene transcription and protein translation in LA are required for fear memory consolidation. Interestingly, recent work suggests that in addition to new protein synthesis, protein degradation through the ubiquitin-proteasome system may also play a role in fear memory consolidation (Jarome et al., 2011)
New protein synthesis is required for consolidation of fear conditioning, and an overall increase in translation has been observed after training (Hoeffer et al, 2011). Translation occurs in both the soma and dendrites, and dendritic translation appears to have a role in synaptic plasticity (Sutton and Schuman, 2006; Helmstetter et al., 2008). Supporting a role for local dendritic protein synthesis in LA following fear conditioning, a recent study found that the number of polyribosomes increases in LA post-synaptic dendritic spines following fear conditioning (Ostroff et al. 2010). Activity Regulated Cytoskeletal Associated protein (Arc/Arg3.1) is known to be locally translated at synapses following learning (Guzowski et al., 2000) and is upregulated in the LA following fear learning and required for fear memory consolidation (Ploski et al., 2008). Microtubule dependent transport of RNA and proteins from the nucleus to the synapse following fear conditioning may also be important in consolidation of fear memories as one study found that knockout of Stathmin, a regulator of microtubule dynamics, reduces fear memory formation (Shumyatsky et al., 2005). Translational regulation may modulate protein synthesis and activation of an important regulator of protein synthesis, the mammalian target of rapamycin or mTOR, in the LA is required for auditory fear memory consolidation (Parsons et al., 2006b). Thus, spine associated and/or cell body initiated protein synthesis in LA neurons may be important for fear memory consolidation. Molecules such as mTOR could provide dynamic regulation of these processes during fear conditioning.
Transcription, and thus protein synthesis, depends on transcription factors. A major transcription factor implicated in memory formation in a variety of systems is CREB (for review, see Alberini, 2009). CREB’s importance in fear memory consolidation has been demonstrated in studies of genetically altered mice (Bourtchuladze et al., 1994; Kida et al., 2002) and viral overexpression of CREB, specifically in the LA, facilitates LTM for fear (Josselyn et al., 2001, Han et al., 2007; Han et al., 2009; Zhou et al., 2009). CREB also appears to be important for determining which neurons in the LA circuit are recruited into the memory representation (Han et al., 2007; Han et al., 2009; Zhou et al., 2009). This work shows that CREB overexpression in a subset of LA neurons increases the excitability of these neurons and increases the likelihood that they will be incorporated into the fear memory trace.
Once CREB is phosphorylated it can, in combination with several cofactors, promote gene transcription of cAMP response element (CRE) dependent genes (see Alberini, 2009; Deisseroth and Tsien, 2002; Yin and Tully, 1996 for review One of these co-factors is the transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) which is activated following fear conditioning and this activation in the LA is required for fear LTM (though STM was not examined) (Lin et al., 2001).
Supporting the involvement of CRE mediated gene transcription during fear memory consolidation, one study showed that this form of transcription is increased in the LA following associative fear conditioning (though this may also occur non-associatively) (Impey et al., 1998b). In addition, another study found that blocking CRE-mediated transcription of the neurotrophin Brain Derived Neurotrophic Factor (BDNF) reduces LTM for fear (Ou and Gean, 2007).
It is clear that neurochemical and cellular events that occur during fear learning can signal the nucleus to trigger gene transcription through a CREB-dependent mechanism (Figure 5). While there is an increasing amount known about the molecular signaling pathways leading to gene transcription and memory consolidation during fear conditioning, the specific genes that are regulated during fear memory consolidation are relatively unknown. The studies which have examined this question found that a number of genes are upregulated following fear conditioning and some of them contain a CRE site within their promoter region or directly bind CREB (Lamprecht et al., 2009; Ou and Gean, 2007; Ploski et al., 2010; Ploski et al., 2008; Rattiner et al., 2004b; Ressler et al., 2002; Stork et al., 2001, Keeley et al., 2006). While there is inconsistency between the different studies with regard to the specific genes that are upregulated, this work provides a first glimpse into the learning-induced regulation of gene expression following fear conditioning. Another way to study which genes and proteins are important for fear conditioning is to examine the effects of knocking down specific gene products that are regulated by fear conditioning. For example, early growth response gene-1 (Egr-1) and the Neuronal PAS domain protein 4 (Npas4) are upregulated in the LA after fear conditioning and reducing the levels of these proteins in the LA attenuates fear memory consolidation (Maddox et al, 2011; Ploski et al., 2011).
Gene transcription can also be controlled by epigenetic mechanisms in which alterations in chromatin structure and DNA methylation can influence gene expression (Day and Sweatt, 2011). Recent work has implicated learning induced changes in chromatin structure and DNA methylation in fear memory consolidation (Monsey et al. 2011; Bredy and Barad, 2008).
2. Protein Kinases
Hebbian (and possibly neuromodulatory) mechanisms engaged during learning may activate specific signaling cascades that are dependent on autophosphorylation of CaMKII. As discussed above, CaMKII is required for the acquisition of fear conditioning (Rodrigues et al., 2004a). While CaMKII activation triggers processes that are involved in learning and STM, other kinases such as PKA, MAPK and PKC may also participate (independently or through CaMKII activation) in the consolidation of LTM for different forms of learning (Abel et al., 1997; Adams and Sweatt, 2002). These kinases all phosporylate CREB and convergent activation of CREB by these different pathways could gate the transcription of numerous plasticity-related genes through dimerized binding to CRE during fear memory consolidation (Figure 5, as discussed above).
a. Protein Kinase A (PKA)
PKA is activated following fear learning, but in contrast to CaMKII it is required in the LA for fear memory consolidation (Goosens et al., 2000; Schafe and LeDoux, 2000; Weeber et al., 2000, Figure 3). Although the effect of specific PKA inhibitors on acquisition of fear conditioning has not been tested, one study did find that acquisition was not affected by pre-training disruption of the A-kinase anchoring protein (AKAP150), which localizes PKA to the synapse (Moita et al., 2002). However, like post-training PKA inhibitors, disruption of AKAP after learning reduces fear LTM (Moita et al., 2002). PKA may be activated by NE binding to β-ARs and stimulation of Gs and cAMP during fear conditioning or by increases in intracellular Ca2+ (Impey et al., 1998a; Impey et al., 1994; Wayman et al., 1994). Activation of PKA can then lead to phosphorylation of multiple proteins involved in nuclear signaling and fear memory consolidation (such as CREB and other kinases) and PKA itself can translocate to the nucleus and regulate RNA synthesis.
b. Protein Kinase C (PKC)
PKC activation in the LA is also required for fear memory consolidation (Goosens et al., 2000; Weeber et al., 2000, Figure 3). PKC may be activated by rises in intracellular Ca2+ and subsequent CaMKII phosphorylation (Abel et al., 1997; Adams and Sweatt, 2002) and/or indirectly through mGluRs (as discussed above). PKC may also be activated directly by Ca2+ influx through voltage gated calcium channels (VGCCs), and VGCCs also contribute to fear memory consolidation (Bauer et al., 2002; McKinney and Murphy, 2006; Shinnick-Gallagher et al., 2003). PKC activation can then lead to activation of signaling cascades which regulate gene transcription and could mediate PKC’s role in fear memory consolidation.
c. Mitogen activated protein kinase (MAPK)
CaMKII, PKA and PKC can directly or indirectly phosphorylate mitogen activated protein kinase (MAPK), which is known to play an integral role in memory consolidation in many neural systems (Kandel, 2001; Sweatt, 2004). The MAPK signaling pathway in the LA has also been implicated in auditory fear conditioning, as MAPK is upregulated in the LA following fear learning and blockade of MAPK activation in the LA attenuates fear memory consolidation (Schafe et al., 2000). Furthermore, intra-LA blockade of MAPK phosphorylation also abolishes consolidation of fear conditioned enhancement of auditory conditioned stimulus inputs to the LA (but not auditory inputs to MGm/PIN). This study demonstrates that blocking consolidation of fear conditioning-induced synaptic plasticity in LA reduces fear learning, providing a direct link between synaptic changes occurring in LA and behavior. Specifically, this work shows that MAPK phosphorylation is required in the LA for the consolidation of both fear memories and for associative plasticity of conditioned stimulus inputs to the LA. Further supporting the involvement of MAPK in fear conditioning, phosphatidylinositol-3 kinase (PI3-K) also activates MAPK and is necessary in the LA specifically for fear LTM (Lin et al., 2001; Lin et al., 2003). Finally, protein tyrosine phosphatase, a protein which is known to be convergently activated by G-protein coupled and Hebbian processes in other neural systems (Valjent et al., 2005), may also regulate MAPK signaling in the LA during fear memory consolidation (Paul et al., 2007).
4. Neurotrophin Signaling
Neurotrophin signaling has also been implicated in fear memory consolidation (Cowansage et al. 2010; Rattiner et al., 2005). BDNF mRNA and protein levels are increased after fear conditioning and phosphorylation of the BDNF receptor (tyrosine kinase receptor B, TrkB), which occurs upon ligand binding, is also enhanced following training. Furthermore, blocking activation of LA TrkB receptors, through pharmacological or genetic means, reduces fear memory consolidation (Ou and Gean, 2006; Ou et al., 2010; Rattiner et al., 2004a; Rattiner et al., 2004b). TrkB stimulation by BDNF activates PI3-K and MAPK and the effects of TrkB activation on fear memory consolidation may be mediated through these signaling cascades (Lin et al., 2001; Lin et al., 2003; Schafe et al., 2000). This neurotrophin signaling pathway appears to be specifically involved in fear memory consolidation and not in acquisition. Neutrophin signaling pathways may also converge with parallel Hebbian and neuromodulatory triggered intracellular processes to dynamically regulate experience-dependent alterations in LA synaptic efficacy and fear memory consolidation (Figure 3).
5. Consolidation within Presynaptic Neurons
Much research to date has focused on postsynaptic modifications that occur in LA neurons during fear learning. However, evidence also suggests that presynaptic molecular alterations are important as well for the consolidation of fear learning (Apergis-Schoute et al., 2005; Huang and Kandel, 1998; McKernan and Shinnick-Gallagher, 1997; Ota et al., 2010a; Pan et al., 2008; Tsvetkov et al., 2002).
Recent studies have implicated nitric oxide synthase (NOS) and Nitric Oxide (NO) in fear memory consolidation (Schafe et al., 2005). NO is a soluble gas which is produced in postsynaptic neurons and can retrogradely signal presynaptic terminals. There it can act through guanylyl cyclase and cGMP-dependent protein kinase (PKG) to influence intracellular processes and nuclear signaling in the presynaptic neuron and influence memory consolidation (Brenman and Bredt, 1997, Prast and Philippu, 2001).
Some of the presynaptic terminals that carry auditory information to the LA originate in cell bodies in the MGm/PIN. Fear conditioning produces an increase in the expression of the immediate early gene early growth response gene 1 (EGR-1) in the MGm/PIN neurons as well as an increase in the expression of markers of presynaptic terminals (synaptophysin and synapsin) in the LA (Overeem et al., 2010). Consistent with the idea that NO in LA produces biochemical changes in the auditory thalamus, fear conditioning induced increases in EGR-1 in MGm/PIN neurons requires NMDAR activation, NO signaling and PKG activation in the LA as well as MAPK activation in the MG/PIN (Ota et al., 2010b; Overeem et al., 2010). This suggests that NO acting on MGm/PIN presynaptic terminals in the LA drives changes in gene expression in the thalamus. In addition, the increase in LA synaptophysin and synapsin (both of which are regulated by EGR-1) is dependent on EGR-1 expression in the thalamus as well as NMDAR activation, NO signaling and PKG in LA (Ota et al., 2010a; Overeem et al., 2010). Together these data support the hypothesis that during fear conditioning NO mediates a retrograde signal which activates PKG signaling in presynaptic thalamic input terminals which then activates MAPK-dependent increases in EGR-1 expression in the MGm/PIN cells resulting in an increase in the number of presynaptic terminals in the LA (Figure 3).
6. Maintenance of Fear Memories
Though there has not been much work examining how consolidated memories are maintained over time, evidence suggests that both structural and molecular changes may play a role in this process.
a. Neuronal Structural Alterations Resulting from Fear Learning
As in other systems (Bailey and Kandel, 1993) structural modifications at synapses may be induced by fear learning, and transcriptional and translational processes may serve to stabilize these changes and maintain fear memories (Lamprecht and LeDoux, 2004, Figure 3). These learning-induced synaptic alterations may involve rearrangement of the actin cytoskeleton and/or a change in synapse number.
Some work suggests that fear conditioning produces an increase in synapse number (Ostroff et al., 2010; Radley et al., 2006). In addition, a recent study lends support to the idea that synapse structure is altered following fear conditioning by demonstrating that fear learning produces an increase in synapse size (Ostroff et al., 2010). This may be partly mediated by GluA1 containing AMPA receptor insertion (Humeau et al., 2007; Nedelescu et al., 2010; Rumpel et al., 2005; Yeh et al., 2006) and then replacement by GluA2/3 containing AMPARs (Malinow and Malenka, 2002). This mechanism could function to produce long term increases in spine size (Kasai et al., 2010). Supporting this idea, recent work shows that disrupting N-ethylmaleimide-sensitive factor (NSF) interactions with GluA2, which is necessary to maintain GluA2/3 receptors in the synapse, impairs fear memory consolidation (Joels and Lamprecht, 2010). In addition, fear conditioning drives profilin, a protein that regulates actin dynamics, into spines and profilin positive spines exhibit larger synapses (Lamprecht et al., 2006a). Further supporting a role for cytoskeleton rearrangement during fear memory consolidation, disrupting actin polymerization or the Rho-GAP signaling pathway (which has been implicated in cytoskeletal alterations) disrupts consolidation of fear memories (Lamprecht et al., 2002; Lamprecht et al., 2006b; Mantzur et al., 2009). In addition, myosin light chain kinase, a cytoskeleton regulatory protein, appears to normally inhibit fear learning suggesting that modulation of cytoskeletal dynamics can influence fear memory formation (Lamprecht et al., 2006b). Furthermore, Beta-Catenin, which is involved in structural processes at the synapse and gene transcription, (Takeichi and Abe, 2005) is stabilized in the LA following fear learning and is necessary in the LA for memory consolidation (Maguschak and Ressler, 2008).
b. A Possible Molecular Mechanism Underlying Memory Maintenance
Recent studies demonstrate kinase involvement in memory maintenance (Sacktor, 2008). Specifically, this work indicates that an atypical isoform of PKC called protein kinase Mζ (PKMζ) is particularly important in the maintenance of fear memories after they are formed. Inhibition of PKMζ in the LA following fear learning abolishes fear memories (Kwapis et al., 2009; Migues et al., 2010; Serrano et al., 2008), but see also (Parsons and Davis, 2011). Though the mechanisms for this are not completely understood, it appears that PKMζ maintains fear memories by reducing GluA2 AMPAR subunit removal from synapses and thereby sustaining the synaptic strengthening originally induced by fear learning (Migues et al., 2010). Clearly more work is necessary to elucidate how PKMζ interacts with transcriptional and translational mechanisms to support retention processes over the life of a memory, but these findings open a new area of research that holds great promise.
7. Fear Memory Consolidation Conclusions
Together these data show that multiple interacting intracellular signaling cascades regulate gene transcription in LA neurons to mediate fear memory consolidation (Figure 3 for working model of molecular processes mediating fear conditioning). There are numerous studies examining these interactions in the context of LTP in reduced preparations and this work has been highly beneficial in defining candidate processes which could occur during actual learning. Because of the complexity of these systems, however, it is important to assess these consolidation mechanisms during actual memory formation and the fear memory system is ideal for these types of studies. It will be important in future work to examine how these signaling cascades are regulated by activation of specific receptor subtypes and processes and to define the flow of information in these signaling pathways. This will be important because it appears that external sensory events during fear learning activate multiple processes including Hebbian, neuromodulatory and neurotrophinergic (as discussed above) which together lead to fear memory formation and consolidation. In addition, it will be important in future studies to examine which second messenger pathways are linked to neuromodulatory receptors and how activation of these receptors works synergistically and/or in parallel with Hebbian mechanisms to promote fear learning. To answer these questions it will be necessary to take advantage of molecular genetic techniques to target manipulations of these neuromodulatory receptors and their associated signaling cascades to specific cell types (for example, interneuron vs. pyramidal cells) and to specific synaptic domains (for example, pre vs. postsynaptic).
C. Reconsolidation: Molecular Mechanisms through Which Fear Memory Is Altered after Retrieval
Although fear memories are consolidated and stored following learning, memories can become labile when they are recalled through a process which has been termed “reconsolidation”. During reconsolidation, memories become labile when they are reactivated by presentation of a memory associated environmental cue (such as a fear conditioned CS). Pharmacological or behavioral manipulations following memory reactivation can, like post-training manipulations, transform the newly labile memory (Alberini et al., 2006; Dudai, 2004; Monfils et al., 2009; Nader and Hardt, 2009; Sara, 2000; Schiller et al., 2010; Tronson and Taylor, 2007).
Historically reconsolidation has been studied via systemic pharmacological manipulations in a number of learning systems (see Sara, 2000 for review). The finding that blockade of protein synthesis in the LA disrupts the consolidation of STM into LTM of fear conditioning (Schafe et al., 2001) led the way to the discovery that blockade of protein synthesis in the LA also disrupted reconsolidation after retrieval of a fully consolidated fear memory (Nader et al., 2000). This latter finding sparked a new wave of interest in reconsolidation.
Our understanding of the cellular and molecular mechanisms of memory reconsolidation is at an early stage. Auditory fear conditioning is well-suited for examining these processes because we understand a great deal about fear memory consolidation in LA. Indeed, blockade of reconsolidation reduces auditory conditioned-unconditioned stimulus-evoked neural responses in the LA (Doyere et al., 2007). This suggests that auditory thalamic (and possibly cortical) inputs to LA neurons are depotentiated when reconsolidation is blocked, and physiological evidence supports this idea (Kim et al., 2010). Thus, we will focus specifically on the molecular mechanisms of auditory fear memory reconsolidation in LA neurons.
1. Neurotransmitter Systems
Two neurotransmitter systems have been studied most extensively in relation to reconsolidation: glutamate and norepinephrine.
a. Glutamate Receptors and the Triggering of Reconsolidation
Blockade of NMDARs in the LA before memory reactivation blocks the initiation of reconsolidation, as it renders the reactivated memory insensitive to subsequent reduction by intra-LA protein synthesis inhibitors (Ben Mamou et al., 2006). In addition, microinjection of a partial agonist of the NMDAR into the LA before reactivation enhances subsequent fear memories. This same study found that intra-LA microinjection of an AMPAR antagonist had no effect on fear memory reconsolidation. Thus, reconsolidation might be best thought of as a new learning experience, one in which plasticity is initiated via activation of NMDARs. This is somewhat surprising given that AMPAR activation is typically thought of as being necessary to depolarize neurons to the point of allowing calcium to pass through NMDARs. However, in LA NMDARs can contribute to spike generation independent of AMPARs (Li et al., 1995).
b. Norepinephrine Modulates Reconsolidation
Norepinephrine transmission is also involved in reconsolidation, as post-reactivation systemic or intra-LA microinjection of a β-AR antagonist reduces fear memories assayed drug-free at later LTM time points (Debiec and LeDoux, 2004; Muravieva and Alberini, 2010). The effects of β-AR manipulation of consolidation and reconsolidation differ since post-training blockade of β-ARs has no effect on the consolidation of the initial memory (Bush et al., 2010; Lee et al., 2001; Debiec and LeDoux, 2004) but post-reactivation manipulation does. This work suggests that β-AR blockers given after retrieval of traumatic memories may alleviate some anxiety disorders such as PTSD. In fact systemic β-AR blockers disrupts memory reconsolidation in healthy humans (Kindt et al., 2009; Soeter and Kindt, 2010) and a recent study found promising results in PTSD patients (Brunet et al., 2008) but the lack of certain controls limit its conclusions.
2. Intracellular Signaling Cascades: Kinases, Transcription Factors, and Protein Synthesis
A number of studies have examined the intracellular mechanisms mediating fear memory reconsolidation. As with fear memory consolidation, these intracellular processes may be triggered convergently by activation of glutamate and norepinephrine receptors on the cell membrane. Similar to fear memory consolidation, blockade of PKA (Tronson et al., 2006) and MAPK (Duvarci et al., 2005) in the LA following memory reactivation reduces LTM, but not STM. This shows that activation of these molecules, which are downstream of both NMDARs and β-ARs, is required for memory reconsolidation to occur. PKA and MAPK can induce gene transcription through phosphorylation of CREB (as discussed above). Indeed, this pathway may be involved in reconsolidation of fear memories. Thus, CREB is phosphorylated in the amygdala following fear memory reactivation (Hall et al., 2001b) and forebrain expression of a CREB repressor reduces fear memory reconsolidation (Kida et al., 2002). As mentioned previously, CREB phosphorylation can lead to CRE mediated gene transcription and synthesis of new proteins. As noted, protein synthesis is essential to reconsolidation, just as it is to consolidation, since protein synthesis blockade in the LA after reactivation blocks fear reconsolidation (Nader et al., 2000; Parsons et al., 2006a; Parsons et al., 2006b). In addition, as with fear memory consolidation, reducing levels of Arc/Arg3.1, Npas4 or Egr-1 in the LA reduces memory reconsolidation (Maddox and Schafe, 2011a; Maddox et al., 2011; Ploski et al. 2011). Furthermore, mTOR is also required for fear memory reconsolidation suggesting that translational regulation is also important in this process (Parsons et al., 2006a). In addition to translation of new proteins, degradation of existing proteins also appears to be important for fear memory reconsolidation (Jarome et al. 2011). Two proteins that contain CRE sites in their genes are CFOS and Egr-1 and both are upregulated in the amygdala following fear memory reactivation (Hall et al., 2001a; Hall et al., 2001b). Thus CRE mediated gene transcription may be important in restabilizing fear memories following reactivation. In support of this hypothesis, blockade of gene transcription in the LA after memory retrieval blocks auditory fear memory reconsolidation (Duvarci et al., 2008) (but see also: Parsons et al., 2006a). As with fear memory consolidation, gene transcription mediating reconsolidation processes may be controlled by epigenetic mechanisms (Maddox and Schafe, 2011b).
Though this work is just beginning, existing studies reveal that similar (though probably not entirely overlapping: Lee et al., 2004; Tronson and Taylor, 2007) molecular mechanisms are engaged during memory consolidation and reconsolidation. For example, in a contextual fear conditioning paradigm hippocampal BDNF is involved in consolidation but not reconsolidation and the transcription factor zif-268 is required for reconsolidation, but not consolidation (Lee et al., 2004). Future work will surely delineate molecules that are differentially involved in fear memory consolidation and reconsolidation.
3. Combining Extinction and Reconsolidation
While manipulating intracellular processes can interfere with reconsolidation following memory retrieval, behavioral manipulations following reactivation can also alter fear memory. For example, extinction training, which weakens fear memory by repeated presentation of the conditioned stimulus without the US, is usually temporary, as the memory can be revived by exposure to a new context, to the US, or by the simple passage of time (Bouton et al. 2006). However, when extinction training follows a single conditioned stimulus reactivation trial, fear memories do not seem to be recovered by these procedures (Monfils et al., 2009). Thus extinction following a single retrieval trial appears to abolish or alter the previously acquired fear memory. An important question is what the difference is between a reactivation trial and an extinction trial since extinction training is a series of conditioned stimulus presentations. The key factor is that a temporal gap must be placed between the first and second conditioned stimulus presentation in order for the effect to occur. The gap should be between 10 min and 4 hrs in order to be effective. This finding has been replicated (Schiller et al., 2010; Clem and Huganir, 2010) but it does not occur under all conditions (Clem and Huganir, 2010; Chan et al., 2010 Soeter and Kindt, 2011).
The molecular mechanisms by which behavioral manipulations such as this affect memory reconsolidation are only beginning to be studied. Initial work showed that the ser845 PKA site on the AMPAR subunit GluA1 is phosphorylated following memory reactivation (Monfils et al., 2009), suggesting that GluA1 phosphorylation engages reconsolidation processes. Supporting a functional role of GluA1 phosphorylation at the ser845 site in this process, another recent study showed that extinction training after memory reactivation is no longer effective at abolishing or altering the original fear memory in mutant mice with mutations at the ser845 GluA1 phosphorylation site (Clem and Huganir, 2010). Instead, in mutant mice this training procedure produces normal extinction learning, exhibiting both renewal and spontaneous recovery. Thus, reactivation produces phosphorylation at ser845 on GluA1 and this process may be essential in producing lability of LA synapses and triggering reconsolidation rather than extinction. Though only beginning, this is an exciting line of research because it demonstrates the possibility of modulating fear memories with behavioral instead of pharmacological interventions and may have some applicability to treatment of fear related disorders such as PTSD and phobias. However, there are boundary conditions which may limit the effectiveness of this approach (Chan et al., 2010; Clem and Huganir, 2010) and they need to be fully understood before these interventions are used for treatment of anxiety related disorders.
4. Fear Memory Reconsolidation Conclusions
In summary, researchers are beginning to understand the cellular and molecular processes mediating reconsolidation (Figure 4 for working model of molecular processes mediating fear memory reconsolidation). Reconsolidation is an intriguing phenomenon because it provides a mechanism by which previously acquired memories can be altered through subsequent experience; something that is a clear feature of human memory. Understanding the mechanisms of this process is appealing both for understanding how memories are encoded in the brain and because it could provide new treatment avenues for debilitating memory related disorders such as PTSD. Mechanistically, fear memory reconsolidation and fear memory formation and consolidation share some key features in terms of the neurotransmitters that trigger them as well as in the intracellular signaling cascades that are recruited. However, there are also key differences between initial fear memory consolidation and reconsolidation both in terms of the molecules involved and the timing of their involvement. It will be important in future work to parse these differences.
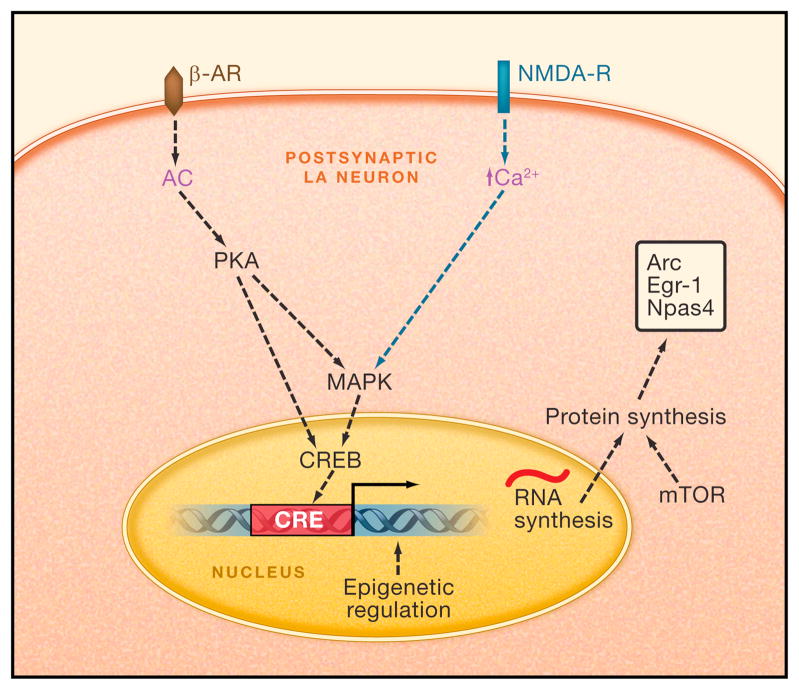
FIGURE 4. Working model of molecular mechanisms mediating fear memory reconsolidation.
All lines are hypothetical. Molecules and processes in green are known to be involved in the initiation of reconsolidation. Molecules and process in black are known to be involved in reconsolidation of fear conditioning. Purple labels denote molecules or elements whose role is not established for fear conditioning, but are part of an established intracellular signaling pathway. AC, adenyl cyclase; AKAP, A-kinase anchoring protein; Arc, activity-regulated cytoskeletal-associated protein; β-AR, Beta Adrenergic Receptor; BDNF, brain derived neurotrophic factor; Ca2+, Calcium; CREB, cAMP Response Element (CRE) binding protein; Egr-1, Early Growth Response Protein 1; MAPK, Mitogen Activated Protein Kinase; mTOR Mammalian Target of Rapamycin; NMDA-R, N-methyl-d-aspartate Glutamate Receptor; Npas4, Neuronal PAS domain protein 4; RNA, Ribonucleic Acid.
D. Conclusions
Research on Pavlovian fear conditioning has revealed in great detail the cellular and molecular mechanisms involved in the acquisition, storage (consolidation), and reconsolidation of memories about harmful events. This paradigm is especially useful for three major reasons. First, it involves specific stimuli that are under the experimenter’s control. The conditioned responses are stereotyped and innate responses that, through learning, are elicited by the conditioned stimulus. Finally, the behavior can be studied similarly in animal models and humans.
By following the conditioned stimulus through the brain, many of the pathways involved in Pavlovian fear conditioning have been mapped. A key brain region for the learning and memory in all mammals, including humans, is the amygdala. Studies in rodents have shown that plasticity occurs in the LA of the amygdala during learning, and this is also where memories are consolidated. Synaptic changes take place in the LA following conditioned- unconditioned stimuli pairing, such that the conditioned stimulus is able to flow through downstream circuitry and elicit fear responses after (but not before) conditioning.
The molecular mechanisms underlying fear learning and memory in the LA involve both Hebbian and neuromodulatory processes. These mechanisms have been implicated in fear conditioning through behavioral studies, in which fear conditioning is chemically or genetically manipulated in LA, and through studies of learning induced synaptic plasticity, using in vivo and in vitro electrophysiological approaches. These studies suggest that a number of molecular mechanisms, including neurotransmitters (e.g., glutamate and monoamines and their receptors) and intracellular signaling events, contribute to these processes. This work demonstrates the power of using the fear conditioning system to decipher molecular processes underlying memory formation and learned changes in neural processing within a specific, behaviorally defined circuit.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- Adams JP, Sweatt JD. Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol. 2002;42:135–163. doi: 10.1146/annurev.pharmtox.42.082701.145401. [DOI] [PubMed] [Google Scholar]
- Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol Rev. 2009;89:121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini CM, Milekic MH, Tronel S. Mechanisms of memory stabilization and destabilization. Cell Mol Life Sci. 2006;63:999–1008. doi: 10.1007/s00018-006-6025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apergis-Schoute AM, Debiec J, Doyere V, LeDoux JE, Schafe GE. Auditory fear conditioning and long-term potentiation in the lateral amygdala require ERK/MAP kinase signaling in the auditory thalamus: a role for presynaptic plasticity in the fear system. J Neurosci. 2005;25:5730–5739. doi: 10.1523/JNEUROSCI.0096-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Giustetto M, Huang YY, Hawkins RD, Kandel ER. Is heterosynaptic modulation essential for stabilizing Hebbian plasticity and memory? Nat Rev Neurosci. 2000;1:11–20. doi: 10.1038/35036191. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Kim JJ, Sun W, Thompson RF, Helmstetter FJ. Acquisition of fear conditioning in rats requires the synthesis of mRNA in the amygdala. Behavioral Neuroscience. 1999;113:276–282. doi: 10.1037//0735-7044.113.2.276. [DOI] [PubMed] [Google Scholar]
- Bauer EP, Schafe GE, LeDoux JE. NMDA receptors and L-type voltage-gated calcium channels contribute to long-term potentiation and different components of fear memory formation in the lateral amygdala. J Neurosci. 2002;22:5239–5249. doi: 10.1523/JNEUROSCI.22-12-05239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Mamou C, Gamache K, Nader K. NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat Neurosci. 2006;9:1237–1239. doi: 10.1038/nn1778. [DOI] [PubMed] [Google Scholar]
- Bergado-Acosta JR, Sangha S, Narayanan RT, Obata K, Pape HC, Stork O. Critical role of the 65-kDa isoform of glutamic acid decarboxylase in consolidation and generalization of Pavlovian fear memory. Learn Mem. 2008;15:163–171. doi: 10.1101/lm.705408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissiere S, Humeau Y, Luthi A. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat Neurosci. 2003;6:587–592. doi: 10.1038/nn1058. [DOI] [PubMed] [Google Scholar]
- Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learn Mem. 2001;8:229–242. doi: 10.1101/lm.30901. [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Braga MF, Aroniadou-Anderjaska V, Manion ST, Hough CJ, Li H. Stress impairs alpha(1A) adrenoceptor-mediated noradrenergic facilitation of GABAergic transmission in the basolateral amygdala. Neuropsychopharmacology. 2004;29:45–58. doi: 10.1038/sj.npp.1300297. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Barad M. The histone deacetylase inhibitor valproic acid enhances acquisition, extinction, and reconsolidation of conditioned fear. Learn Mem. 2008;15:39–45. doi: 10.1101/lm.801108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenman JE, Bredt DS. Synaptic signaling by nitric oxide. Curr Opin Neurobiol. 1997;7:374–378. doi: 10.1016/s0959-4388(97)80065-7. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TH, Kairiss EW, Keenan CL. Hebbian synapses: biophysical mechanisms and algorithms. Annu Rev Neurosci. 1990;13:475–511. doi: 10.1146/annurev.ne.13.030190.002355. [DOI] [PubMed] [Google Scholar]
- Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res. 2008;42:503–506. doi: 10.1016/j.jpsychires.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Bush DE, Caparosa EM, Gekker A, LeDoux J. Beta-adrenergic receptors in the lateral nucleus of the amygdala contribute to the acquisition but not the consolidation of auditory fear conditioning. Front Behav Neurosci. 2010;4:154. doi: 10.3389/fnbeh.2010.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew TJ, Hawkins RD, Abrams TW, Kandel ER. A test of Hebb’s postulate at identified synapses which mediate classical conditioning in Aplysia. J Neurosci. 1984;4:1217–1224. doi: 10.1523/JNEUROSCI.04-05-01217.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WY, Leung HT, Westbrook RF, McNally GP. Effects of recent exposure to a conditioned stimulus on extinction of Pavlovian fear conditioning. Learn Mem. 2010;17:512–521. doi: 10.1101/lm.1912510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FJ, Sara SJ. Locus coeruleus activation by foot shock or electrical stimulation inhibits amygdala neurons. Neuroscience. 2007;144:472–481. doi: 10.1016/j.neuroscience.2006.09.037. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Minneman KP. Recent progress in alpha1-adrenergic receptor research. Acta Pharmacol Sin. 2005;26:1281–1287. doi: 10.1111/j.1745-7254.2005.00224.x. [DOI] [PubMed] [Google Scholar]
- Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330:1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowansage KK, LeDoux JE, Monfils MH. Brain-Derived Neurotrophic Factor: A Dynamic Gatekeeper of Neural Plasticity. Curr Mol Pharmacol. 2010;3:12–29. doi: 10.2174/1874467211003010012. [DOI] [PubMed] [Google Scholar]
- Davis HP, Squire LR. Protein synthesis and memory: a review. Psychol Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Day JJ, Sweatt JD. Epigenetic mechanisms in cognition. Neuron. 2011;70:813–829. doi: 10.1016/j.neuron.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Tsien RW. Dynamic multiphosphorylation passwords for activity-dependent gene expression. Neuron. 2002;34:179–182. doi: 10.1016/s0896-6273(02)00664-5. [DOI] [PubMed] [Google Scholar]
- Dityatev AE, Bolshakov VY. Amygdala, long-term potentiation, and fear conditioning. Neuroscientist. 2005;11:75–88. doi: 10.1177/1073858404270857. [DOI] [PubMed] [Google Scholar]
- Doyere V, Debiec J, Monfils MH, Schafe GE, LeDoux JE. Synapse-specific reconsolidation of distinct fear memories in the lateral amygdala. Nat Neurosci. 2007;10:414–416. doi: 10.1038/nn1871. [DOI] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Nader K, LeDoux JE. Activation of extracellular signal-regulated kinase- mitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. Eur J Neurosci. 2005;21:283–289. doi: 10.1111/j.1460-9568.2004.03824.x. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Nader K, LeDoux JE. De novo mRNA synthesis is required for both consolidation and reconsolidation of fear memories in the amygdala. Learn Mem. 2008;15:747–755. doi: 10.1101/lm.1027208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Ennis M, Aston-Jones G. Activation of Locus Coeruleus from Nucleus Paragigantocellularis: A New Excitatory Amino Acid Pthway in Brain. J Neurosci. 1988;8:3644–3657. doi: 10.1523/JNEUROSCI.08-10-03644.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES, Delaney AJ, Power JM, Sedlak PL, Crane JW, Sah P. Modulation of SK channel trafficking by beta adrenoceptors enhances excitatory synaptic transmission and plasticity in the amygdala. J Neurosci. 2008;28:10803–10813. doi: 10.1523/JNEUROSCI.1796-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annu Rev Psychol. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Farb CR, Chang W, LeDoux JE. Ultrastructural characterization of noradrenergic axons and Beta-adrenergic receptors in the lateral nucleus of the amygdala. Front Behav Neurosci. 2010;4:162. doi: 10.3389/fnbeh.2010.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourcaudot E, Gambino F, Casassus G, Poulain B, Humeau Y, Luthi A. L-type voltage-dependent Ca(2+) channels mediate expression of presynaptic LTP in amygdala. Nat Neurosci. 2009;12:1093–1095. doi: 10.1038/nn.2378. [DOI] [PubMed] [Google Scholar]
- Galvez R, Mesches MH, McGaugh JL. Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiol Learn Mem. 1996;66:253–257. doi: 10.1006/nlme.1996.0067. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Davis M. Second-order fear conditioning prevented by blocking NMDA receptors in amygdala. Nature. 1997;388:471–474. doi: 10.1038/41325. [DOI] [PubMed] [Google Scholar]
- Glanzman DL. Common mechanisms of synaptic plasticity in vertebrates and invertebrates. Curr Biol. 2010;20:R31–36. doi: 10.1016/j.cub.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosens KA, Holt W, Maren S. A role for amygdaloid PKA and PKC in the acquisition of long-term conditional fear memories in rats. Behav Brain Res. 2000;114:145–152. doi: 10.1016/s0166-4328(00)00224-2. [DOI] [PubMed] [Google Scholar]
- Greba Q, Gifkins A, Kokkinidis L. Inhibition of amygdaloid dopamine D2 receptors impairs emotional learning measured with fear-potentiated startle. Brain Res. 2001;899:218–226. doi: 10.1016/s0006-8993(01)02243-0. [DOI] [PubMed] [Google Scholar]
- Guarraci FA, Frohardt RJ, Kapp BS. Amygdaloid D1 dopamine receptor involvement in Pavlovian fear conditioning. Brain Res. 1999;827:28–40. doi: 10.1016/s0006-8993(99)01291-3. [DOI] [PubMed] [Google Scholar]
- Guarraci FA, Frohardt RJ, Falls WA, Kapp BS. The effects of intra-amygdaloid infusions of a D2 dopamine receptor antagonist on Pavlovian fear conditioning. Behav Neurosci. 2000;114:647–651. doi: 10.1037//0735-7044.114.3.647. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippocampal CA1 neurons during the recall of contextual memories. J Neurosci. 2001a;21:2186–2193. doi: 10.1523/JNEUROSCI.21-06-02186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Fear memory retrieval induces CREB phosphorylation and Fos expression within the amygdala. Eur J Neurosci. 2001b;13:1453–1458. doi: 10.1046/j.0953-816x.2001.01531.x. [DOI] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, Neve RL, Guzowski JF, Silva AJ, Josselyn SA. Neuronal competition and selection during memory formation. Science. 2007;316:457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Hsiang HL, Buch T, Waisman A, Bontempi B, Neve RL, Frankland PW, Josselyn SA. Selective erasure of a fear memory. Science. 2009;323:1492–1496. doi: 10.1126/science.1164139. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior. New York: John Wiley and Sons; 1949. [Google Scholar]
- Helmstetter FJ, Parsons RG, Gafford GM. Macromolecular synthesis, distributed synaptic plasticity, and fear conditioning. Neurobiol Learn Mem. 2008;89:324–337. doi: 10.1016/j.nlm.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez PJ, Abel T. The role of protein synthesis in memory consolidation: progress amid decades of debate. Neurobiol Learn Mem. 2008;89:293–311. doi: 10.1016/j.nlm.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luthi A. Neuronal circuits of fear extinction. Eur J Neurosci. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Cowansage KK, Arnold EC, Banko JL, Moerke NJ, Rodriguez R, Schmidt EK, Klosi E, Chorev M, Lloyd RE, Pierre P, Wagner G, LeDoux JE, Klann E. Inhibition of the interactions between eukaryotic initiation factors 4E and 4G impairs long-term associative memory consolidation but not reconsolidation. Proc Natl Acad Sci U S A. 2011;108:3383–3388. doi: 10.1073/pnas.1013063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Real E, Takamiya K, Kang MG, LeDoux J, Huganir RL, Malinow R. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160–173. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Postsynaptic induction and PKA-dependent expression of LTP in the lateral amygdala. Neuron. 1998;21:169–178. doi: 10.1016/s0896-6273(00)80524-3. [DOI] [PubMed] [Google Scholar]
- Humeau Y, Shaban H, Bissiere S, Luthi A. Presynaptic induction of heterosynaptic associative plasticity in the mammalian brain. Nature. 2003;426:841–845. doi: 10.1038/nature02194. [DOI] [PubMed] [Google Scholar]
- Humeau Y, Reisel D, Johnson AW, Borchardt T, Jensen V, Gebhardt C, Bosch V, Gass P, Bannerman DM, Good MA, Hvalby O, Sprengel R, Luthi A. A pathway-specific function for different AMPA receptor subunits in amygdala long-term potentiation and fear conditioning. J Neurosci. 2007;27:10947–10956. doi: 10.1523/JNEUROSCI.2603-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impey S, Wayman G, Wu Z, Storm DR. Type I adenylyl cyclase functions as a coincidence detector for control of cyclic AMP response element-mediated transcription: synergistic regulation of transcription by Ca2+ and isoproterenol. Mol Cell Biol. 1994;14:8272–8281. doi: 10.1128/mcb.14.12.8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impey S, Smith DM, Obrietan K, Donahue R, Wade C, Storm DR. Stimulation of cAMP response element (CRE)-mediated transcription during contextual learning. Nat Neurosci. 1998a;1:595–601. doi: 10.1038/2830. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloulme JC, Chan G, Storm DR. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998b;21:869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Jarome TJ, Werner CT, Kwapis JL, Helmstetter FJ. Activity dependent protein degradation is critical for the formation and stability of fear memory in the amygdala. PLoS One. 2011;6:e24349. doi: 10.1371/journal.pone.0024349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels G, Lamprecht R. Interaction between N-ethylmaleimide-sensitive factor and GluR2 is essential for fear memory formation in lateral amygdala. J Neurosci. 2010;30:15981–15986. doi: 10.1523/JNEUROSCI.1872-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Hamanaka H, Diaz-Mataix L, LeDoux JE. Hebbian and neuromodulatory mechanisms act synergistically to instruct associative memory formation. Soc Neurosci Abst. 2010a:914.15. [Google Scholar]
- Johansen JP, Hamanaka H, Monfils MH, Behnia R, Deisseroth K, Blair HT, LeDoux JE. Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proc Natl Acad Sci U S A. 2010b;107:12692–12697. doi: 10.1073/pnas.1002418107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn SA, Shi C, Carlezon WA, Jr, Neve RL, Nestler EJ, Davis M. Long-term memory is facilitated by cAMP response element-binding protein overexpression in the amygdala. J Neurosci. 2001;21:2404–2412. doi: 10.1523/JNEUROSCI.21-07-02404.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 2010;33:121–129. doi: 10.1016/j.tins.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Keeley MB, Wood MA, Isiegas C, Stein J, Hellman K, Hannenhalli S, Abel T. Differential transcriptional response to nonassociative and associative components of classical fear conditioning in the amygdala and hippocampus. Learn Mem. 2006;13:135–142. doi: 10.1101/lm.86906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida S, Josselyn SA, de Ortiz SP, Kogan JH, Chevere I, Masushige S, Silva AJ. CREB required for the stability of new and reactivated fear memories. Nat Neurosci. 2002;5:348–355. doi: 10.1038/nn819. [DOI] [PubMed] [Google Scholar]
- Kim J, Song B, Hong I, Kim J, Lee J, Park S, Eom JY, Lee CJ, Lee S, Choi S. Reactivation of fear memory renders consolidated amygdala synapses labile. J Neurosci. 2010;30:9631–9640. doi: 10.1523/JNEUROSCI.0940-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jung SY, Lee YK, Park S, Choi JS, Lee CJ, Kim HS, Choi YB, Scheiffele P, Bailey CH, Kandel ER, Kim JH. Neuroligin-1 is required for normal expression of LTP and associative fear memory in the amygdala of adult animals. Proc Natl Acad Sci U S A. 2008;105:9087–9092. doi: 10.1073/pnas.0803448105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci. 2009;12:256–258. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lonergan ME, Helmstetter FJ. Protein kinase Mzeta maintains fear memory in the amygdala but not in the hippocampus. Behav Neurosci. 2009;123:844–850. doi: 10.1037/a0016343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R, LeDoux J. Structural plasticity and memory. Nat Rev Neurosci. 2004;5:45–54. doi: 10.1038/nrn1301. [DOI] [PubMed] [Google Scholar]
- Lamprecht R, Farb CR, LeDoux JE. Fear memory formation involves p190 RhoGAP and ROCK proteins through a GRB2-mediated complex. Neuron. 2002;36:727–738. doi: 10.1016/s0896-6273(02)01047-4. [DOI] [PubMed] [Google Scholar]
- Lamprecht R, Farb CR, Rodrigues SM, LeDoux JE. Fear conditioning drives profilin into amygdala dendritic spines. Nat Neurosci. 2006a;9:481–483. doi: 10.1038/nn1672. [DOI] [PubMed] [Google Scholar]
- Lamprecht R, Dracheva S, Assoun S, LeDoux JE. Fear conditioning induces distinct patterns of gene expression in lateral amygdala. Genes Brain Behav. 2009;8:735–743. doi: 10.1111/j.1601-183X.2009.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R, Margulies DS, Farb CR, Hou M, Johnson LR, LeDoux JE. Myosin light chain kinase regulates synaptic plasticity and fear learning in the lateral amygdala. Neuroscience. 2006b;139:821–829. doi: 10.1016/j.neuroscience.2005.12.055. [DOI] [PubMed] [Google Scholar]
- Lazzaro SC, Hou M, Cunha C, LeDoux JE, Cain CK. Antagonism of lateral amygdala alpha1-adrenergic receptors facilitates fear conditioning and long-term potentiation. Learn Mem. 2010;17:489–493. doi: 10.1101/lm.1918210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Berger SY, Stiedl O, Spiess J, Kim JJ. Post-training injections of catecholaminergic drugs do not modulate fear conditioning in rats and mice. Neurosci Lett. 2001;303:123–126. doi: 10.1016/s0304-3940(01)01733-5. [DOI] [PubMed] [Google Scholar]
- Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- Leonard SK, Anderson CM, Lachowicz JE, Schulz DW, Kilts CD, Mailman RB. Amygdaloid D1 receptors are not linked to stimulation of adenylate cyclase. Synapse. 2003;50:320–333. doi: 10.1002/syn.10272. [DOI] [PubMed] [Google Scholar]
- Li X, Phillips RG, LeDoux JE. NMDA and non-NMDA receptors contribute to synaptic transmission between the medial geniculate body and the lateral nucleus of the amygdala. Experimental Brain Research. 1995;105:87–100. doi: 10.1007/BF00242185. [DOI] [PubMed] [Google Scholar]
- Li XF, Stutzmann GE, LeDoux JE. Convergent but temporally separated inputs to lateral amygdala neurons from the auditory thalamus and auditory cortex use different postsynaptic receptors: in vivo intracellular and extracellular recordings in fear conditioning pathways. Learn Mem. 1996;3:229–242. doi: 10.1101/lm.3.2-3.229. [DOI] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Lu HY, Gean PW. The similarities and diversities of signal pathways leading to consolidation of conditioning and consolidation of extinction of fear memory. J Neurosci. 2003;23:8310–8317. doi: 10.1523/JNEUROSCI.23-23-08310.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Lu KT, Leu TH, Chang WC, Gean PW. A role for the PI-3 kinase signaling pathway in fear conditioning and synaptic plasticity in the amygdala. Neuron. 2001;31:841–851. doi: 10.1016/s0896-6273(01)00433-0. [DOI] [PubMed] [Google Scholar]
- Maddox SA, Schafe GE. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for reconsolidation of a Pavlovian fear memory. J Neurosci. 2011a;31:7073–7082. doi: 10.1523/JNEUROSCI.1120-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox SA, Schafe GE. Epigenetic alterations in the lateral amygdala are required for reconsolidation of a Pavlovian fear memory. Learn Mem. 2011b;18:579–593. doi: 10.1101/lm.2243411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox SA, Monsey MS, Schafe GE. Early growth response gene 1 (Egr-1) is required for new and reactivated fear memories in the lateral amygdala. Learn Mem. 2011;18:24–38. doi: 10.1101/lm.1980211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguschak KA, Ressler KJ. Beta-catenin is required for memory consolidation. Nat Neurosci. 2008;11:1319–1326. doi: 10.1038/nn.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Mantzur L, Joels G, Lamprecht R. Actin polymerization in lateral amygdala is essential for fear memory formation. Neurobiol Learn Mem. 2009;91:85–88. doi: 10.1016/j.nlm.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47:783–786. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Stote DL, Fanselow MS. N-methyl-D-aspartate receptors in the basolateral amygdala are required for both acquisition and expression of conditional fear in rats. Behav Neurosci. 1996;110:1365–1374. doi: 10.1037//0735-7044.110.6.1365. [DOI] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, Vogt KE. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron. 2005;48:1025–1037. doi: 10.1016/j.neuron.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Mayford M, Kandel ER. Genetic approaches to memory storage. Trends Genet. 1999;15:463–470. doi: 10.1016/s0168-9525(99)01846-6. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- McKinney BC, Murphy GG. The L-Type voltage-gated calcium channel Cav1.3 mediates consolidation, but not extinction, of contextually conditioned fear in mice. Learn Mem. 2006;13:584–589. doi: 10.1101/lm.279006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT, Nader K. PKMzeta maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat Neurosci. 2010;13:630–634. doi: 10.1038/nn.2531. [DOI] [PubMed] [Google Scholar]
- Miserendino MJ, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature. 1990;345:716–718. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- Moita MA, Lamprecht R, Nader K, LeDoux JE. A-kinase anchoring proteins in amygdala are involved in auditory fear memory. Nat Neurosci. 2002;5:837–838. doi: 10.1038/nn901. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsey MS, Ota KT, Akingbade IF, Hong ES, Schafe GE. Epigenetic alterations are critical for fear memory consolidation and synaptic plasticity in the lateral amygdala. PLoS One. 2011;6:e19958. doi: 10.1371/journal.pone.0019958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozov A, Sukato D, Ito W. Selective suppression of plasticity in amygdala inputs from temporal association cortex by the external capsule. J Neurosci. 2011;31:339–345. doi: 10.1523/JNEUROSCI.5537-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci. 1997;111:683–691. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- Muly EC, Senyuz M, Khan ZU, Guo JD, Hazra R, Rainnie DG. Distribution of D1 and D5 dopamine receptors in the primate and rat basolateral amygdala. Brain Struct Funct. 2009;213:375–393. doi: 10.1007/s00429-009-0214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muravieva EV, Alberini CM. Limited efficacy of propranolol on the reconsolidation of fear memories. Learn Mem. 2010;17:306–313. doi: 10.1101/lm.1794710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Nader K, LeDoux JE. Inhibition of the mesoamygdala dopaminergic pathway impairs the retrieval of conditioned fear associations. Behav Neurosci. 1999;113:891–901. doi: 10.1037//0735-7044.113.5.891. [DOI] [PubMed] [Google Scholar]
- Nader K, Hardt O. A single standard for memory: the case for reconsolidation. Nat Rev Neurosci. 2009;10:224–234. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Nedelescu H, Kelso CM, Lazaro-Munoz G, Purpura M, Cain CK, LeDoux JE, Aoki C. Endogenous GluR1-containing AMPA receptors translocate to asymmetric synapses in the lateral amygdala during the early phase of fear memory formation: an electron microscopic immunocytochemical study. J Comp Neurol. 2010;518:4723–4739. doi: 10.1002/cne.22472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroff LE, Cain CK, Bedont J, Monfils MH, LeDoux JE. Fear and safety learning differentially affect synapse size and dendritic translation in the lateral amygdala. Proc Natl Acad Sci U S A. 2010;107:9418–9423. doi: 10.1073/pnas.0913384107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota KT, Monsey MS, Wu MS, Schafe GE. Synaptic plasticity and NO-cGMP-PKG signaling regulate pre- and postsynaptic alterations at rat lateral amygdala synapses following fear conditioning. PLoS One. 2010a;5:e11236. doi: 10.1371/journal.pone.0011236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota KT, Monsey MS, Wu MS, Young GJ, Schafe GE. Synaptic plasticity and NO-cGMP-PKG signaling coordinately regulate ERK-driven gene expression in the lateral amygdala and in the auditory thalamus following Pavlovian fear conditioning. Learn Mem. 2010b;17:221–235. doi: 10.1101/lm.1592510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou LC, Gean PW. Regulation of amygdala-dependent learning by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol-3-kinase. Neuropsychopharmacology. 2006;31:287–296. doi: 10.1038/sj.npp.1300830. [DOI] [PubMed] [Google Scholar]
- Ou LC, Gean PW. Transcriptional regulation of brain-derived neurotrophic factor in the amygdala during consolidation of fear memory. Mol Pharmacol. 2007;72:350–358. doi: 10.1124/mol.107.034934. [DOI] [PubMed] [Google Scholar]
- Ou LC, Yeh SH, Gean PW. Late expression of brain-derived neurotrophic factor in the amygdala is required for persistence of fear memory. Neurobiol Learn Mem. 2010;93:372–382. doi: 10.1016/j.nlm.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Overeem KA, Ota KT, Monsey MS, Ploski JE, Schafe GE. A role for nitric oxide-driven retrograde signaling in the consolidation of a fear memory. Front Behav Neurosci. 2010;4:2. doi: 10.3389/neuro.08.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan BX, Vautier F, Ito W, Bolshakov VY, Morozov A. Enhanced cortico-amygdala efficacy and suppressed fear in absence of Rap1. J Neurosci. 2008;28:2089–2098. doi: 10.1523/JNEUROSCI.5156-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D. Mechanisms of Pavlovian fear conditioning: has the engram been located? Trends Neurosci. 2002;25:436–437. doi: 10.1016/s0166-2236(02)02243-9. discussion 437–438. [DOI] [PubMed] [Google Scholar]
- Pare D, Royer S, Smith Y, Lang EJ. Contextual inhibitory gating of impulse traffic in the intra-amygdaloid network. Ann N Y Acad Sci. 2003;985:78–91. doi: 10.1111/j.1749-6632.2003.tb07073.x. [DOI] [PubMed] [Google Scholar]
- Parsons RG, Davis M. Temporary disruption of fear-potentiated startle following PKMzeta inhibition in the amygdala. Nat Neurosci. 2011;14:295–296. doi: 10.1038/nn.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Helmstetter FJ. Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. J Neurosci. 2006a;26:12977–12983. doi: 10.1523/JNEUROSCI.4209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Baruch DE, Riedner BA, Helmstetter FJ. Long-term stability of fear memory depends on the synthesis of protein but not mRNA in the amygdala. Eur J Neurosci. 2006b;23:1853–1859. doi: 10.1111/j.1460-9568.2006.04723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Olausson P, Venkitaramani DV, Ruchkina I, Moran TD, Tronson N, Mills E, Hakim S, Salter MW, Taylor JR, Lombroso PJ. The striatal-enriched protein tyrosine phosphatase gates long-term potentiation and fear memory in the lateral amygdala. Biol Psychiatry. 2007;61:1049–1061. doi: 10.1016/j.biopsych.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploski JE, Park KW, Ping J, Monsey MS, Schafe GE. Identification of plasticity-associated genes regulated by Pavlovian fear conditioning in the lateral amygdala. J Neurochem. 2010;112:636–650. doi: 10.1111/j.1471-4159.2009.06491.x. [DOI] [PubMed] [Google Scholar]
- Ploski JE, Monsey MS, Nguyen T, Dileone RJ, Schafe GE. The Neuronal PAS Domain Protein 4 (Npas4) Is Required for New and Reactivated Fear Memories. PLoS One. 2011;6:e23760. doi: 10.1371/journal.pone.0023760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploski JE, Pierre VJ, Smucny J, Park K, Monsey MS, Overeem KA, Schafe GE. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of pavlovian fear conditioning in the lateral amygdala. J Neurosci. 2008;28:12383–12395. doi: 10.1523/JNEUROSCI.1662-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prast H, Philippu A. Nitric oxide as modulator of neuronal function. Prog Neurobiol. 2001;64:51–68. doi: 10.1016/s0301-0082(00)00044-7. [DOI] [PubMed] [Google Scholar]
- Quirarte GL, Galvez R, Roozendaal B, McGaugh JL. Norepinephrine release in the amygdala in response to footshock and opioid peptidergic drugs. Brain Res. 1998;808:134–140. doi: 10.1016/s0006-8993(98)00795-1. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Johnson LR, Janssen WG, Martino J, Lamprecht R, Hof PR, LeDoux JE, Morrison JH. Associative Pavlovian conditioning leads to an increase in spinophilin-immunoreactive dendritic spines in the lateral amygdala. Eur J Neurosci. 2006;24:876–884. doi: 10.1111/j.1460-9568.2006.04962.x. [DOI] [PubMed] [Google Scholar]
- Raman IM, Tong G, Jahr CE. Beta-adrenergic regulation of synaptic NMDA receptors by cAMP-dependent protein kinase. Neuron. 1996;16:415–421. doi: 10.1016/s0896-6273(00)80059-8. [DOI] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, Ressler KJ. Differential regulation of brain-derived neurotrophic factor transcripts during the consolidation of fear learning. Learn Mem. 2004a;11:727–731. doi: 10.1101/lm.83304. [DOI] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, Ressler KJ. Brain-derived neurotrophic factor in amygdala-dependent learning. Neuroscientist. 2005;11:323–333. doi: 10.1177/1073858404272255. [DOI] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci. 2004b;24:4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Paschall G, Zhou XL, Davis M. Regulation of synaptic plasticity genes during consolidation of fear conditioning. J Neurosci. 2002;22:7892–7902. doi: 10.1523/JNEUROSCI.22-18-07892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. Intra-amygdala blockade of the NR2B subunit of the NMDA receptor disrupts the acquisition but not the expression of fear conditioning. J Neurosci. 2001;21:6889–6896. doi: 10.1523/JNEUROSCI.21-17-06889.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004a;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Bauer EP, Farb CR, Schafe GE, LeDoux JE. The group I metabotropic glutamate receptor mGluR5 is required for fear memory formation and long-term potentiation in the lateral amygdala. J Neurosci. 2002;22:5219–5229. doi: 10.1523/JNEUROSCI.22-12-05219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Farb CR, Bauer EP, LeDoux JE, Schafe GE. Pavlovian fear conditioning regulates Thr286 autophosphorylation of Ca2+/calmodulin-dependent protein kinase II at lateral amygdala synapses. J Neurosci. 2004b;24:3281–3288. doi: 10.1523/JNEUROSCI.5303-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, LeDoux JE, Clugnet MC, Bordi F. Somatosensory and auditory convergence in the lateral nucleus of the amygdala. Behavioral Neuroscience. 1993;107:444–450. doi: 10.1037//0735-7044.107.3.444. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning. Nature. 2002a;417:282–287. doi: 10.1038/417282a. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J Neurosci. 2002b;22:324–337. doi: 10.1523/JNEUROSCI.22-01-00324.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Matus-Amat P. DHPG activation of group 1 mGluRs in BLA enhances fear conditioning. Learn Mem. 2009;16:421–425. doi: 10.1101/lm.1444909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- Sacktor TC. PKMzeta, LTP maintenance, and the dynamic molecular biology of memory storage. Prog Brain Res. 2008;169:27–40. doi: 10.1016/S0079-6123(07)00002-7. [DOI] [PubMed] [Google Scholar]
- Sah P, Westbrook RF, Luthi A. Fear conditioning and long-term potentiation in the amygdala: what really is the connection? Ann N Y Acad Sci. 2008;1129:88–95. doi: 10.1196/annals.1417.020. [DOI] [PubMed] [Google Scholar]
- Sara SJ. Retrieval and reconsolidation: toward a neurobiology of remembering. Learn Mem. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- Schafe GE, LeDoux JE. Memory Consolidation of Auditory Pavlovian Fear Conditioning Requires Protein Synthesis and Protein Kinase A in the Amygdala. J Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Nader K, Blair HT, LeDoux JE. Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci. 2001;24:540–546. doi: 10.1016/s0166-2236(00)01969-x. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J Neurosci. 2000;20:8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Bauer EP, Rosis S, Farb CR, Rodrigues SM, LeDoux JE. Memory consolidation of Pavlovian fear conditioning requires nitric oxide signaling in the lateral amygdala. Eur J Neurosci. 2005;22:201–211. doi: 10.1111/j.1460-9568.2005.04209.x. [DOI] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, LeDoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S, Fendt M. Effects of the mGluR8 agonist (S)-3,4-DCPG in the lateral amygdala on acquisition/expression of fear-potentiated startle, synaptic transmission, and plasticity. Neuropharmacology. 2006;50:154–164. doi: 10.1016/j.neuropharm.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Schroeder BW, Shinnick-Gallagher P. Fear memories induce a switch in stimulus response and signaling mechanisms for long-term potentiation in the lateral amygdala. Eur J Neurosci. 2004;20:549–556. doi: 10.1111/j.1460-9568.2004.03517.x. [DOI] [PubMed] [Google Scholar]
- Schroeder BW, Shinnick-Gallagher P. Fear learning induces persistent facilitation of amygdala synaptic transmission. Eur J Neurosci. 2005;22:1775–1783. doi: 10.1111/j.1460-9568.2005.04343.x. [DOI] [PubMed] [Google Scholar]
- Sejnowski TJ. The book of hebb. Neron. 1999;24:773–776. doi: 10.1016/s0896-6273(00)81025-9. [DOI] [PubMed] [Google Scholar]
- Serrano P, Friedman EL, Kenney J, Taubenfeld SM, Zimmerman JM, Hanna J, Alberini C, Kelley AE, Maren S, Rudy JW, Yin JC, Sacktor TC, Fenton AA. PKMzeta maintains spatial, instrumental, and classically conditioned long-term memories. PLoS Biol. 2008;6:2698–2706. doi: 10.1371/journal.pbio.0060318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaban H, Humeau Y, Herry C, Cassasus G, Shigemoto R, Ciocchi S, Barbieri S, van der Putten H, Kaupmann K, Bettler B, Luthi A. Generalization of amygdala LTP and conditioned fear in the absence of presynaptic inhibition. Nat Neurosci. 2006;9:1028–1035. doi: 10.1038/nn1732. [DOI] [PubMed] [Google Scholar]
- Shinnick-Gallagher P, McKernan MG, Xie J, Zinebi F. L-type voltage-gated calcium channels are involved in the in vivo and in vitro expression of fear conditioning. Ann N Y Acad Sci. 2003;985:135–149. doi: 10.1111/j.1749-6632.2003.tb07078.x. [DOI] [PubMed] [Google Scholar]
- Shumyatsky GP, Malleret G, Shin RM, Takizawa S, Tully K, Tsvetkov E, Zakharenko SS, Joseph J, Vronskaya S, Yin D, Schubart UK, Kandel ER, Bolshakov VY. stathmin, a gene enriched in the amygdala, controls both learned and innate fear. Cell. 2005;123:697–709. doi: 10.1016/j.cell.2005.08.038. [DOI] [PubMed] [Google Scholar]
- Siegl S, Flor PJ, Fendt M. Amygdaloid metabotropic glutamate receptor subtype 7 is involved in the acquisition of conditioned fear. Neuroreport. 2008;19:1147–1150. doi: 10.1097/WNR.0b013e328307f295. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Doyere V, Cain CK, LeDoux JE. Long-term potentiation in the amygdala: A cellular mechanism of fear learning and memory. Neuropharmacology. 2007;52:215–227. doi: 10.1016/j.neuropharm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Silva AJ. Molecular and cellular cognitive studies of the role of synaptic plasticity in memory. J Neurobiol. 2003;54:224–237. doi: 10.1002/neu.10169. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Dissociating response systems: erasing fear from memory. Neurobiol Learn Mem. 2010;94:30–41. doi: 10.1016/j.nlm.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Disrupting reconsolidation: pharmacological and behavioral manipulations. Learn Mem. 2011;18:357–366. doi: 10.1101/lm.2148511. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Cain CK, LeDoux JE. Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biol Psychiatry. 2006;60:329–336. doi: 10.1016/j.biopsych.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Stork O, Ji FY, Obata K. Reduction of extracellular GABA in the mouse amygdala during and following confrontation with a conditioned fear stimulus. Neurosci Lett. 2002;327:138–142. doi: 10.1016/s0304-3940(02)00387-7. [DOI] [PubMed] [Google Scholar]
- Stork O, Stork S, Pape HC, Obata K. Identification of genes expressed in the amygdala during the formation of fear memory. Learn Mem. 2001;8:209–219. doi: 10.1101/lm.39401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Szinyei C, Narayanan RT, Pape HC. Plasticity of inhibitory synaptic network interactions in the lateral amygdala upon fear conditioning in mice. Eur J Neurosci. 2007;25:1205–1211. doi: 10.1111/j.1460-9568.2007.05349.x. [DOI] [PubMed] [Google Scholar]
- Takeichi M, Abe K. Synaptic contact dynamics controlled by cadherin and catenins. Trends Cell Biol. 2005;15:216–221. doi: 10.1016/j.tcb.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Wiseman SL, Olausson P, Taylor JR. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat Neurosci. 2006;9:167–169. doi: 10.1038/nn1628. [DOI] [PubMed] [Google Scholar]
- Tsvetkov E, Carlezon WA, Benes FM, Kandel ER, Bolshakov VY. Fear conditioning occludes LTP-induced presynaptic enhancement of synaptic transmission in the cortical pathway to the lateral amygdala. Neuron. 2002;34:289–300. doi: 10.1016/s0896-6273(02)00645-1. [DOI] [PubMed] [Google Scholar]
- Tully K, Bolshakov VY. Emotional enhancement of memory: how norepinephrine enables synaptic plasticity. Mol Brain. 2010;3:15. doi: 10.1186/1756-6606-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully K, Li Y, Tsvetkov E, Bolshakov VY. Norepinephrine enables the induction of associative long-term potentiation at thalamo-amygdala synapses. Proc Natl Acad Sci U S A. 2007;104:14146–14150. doi: 10.1073/pnas.0704621104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci U S A. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, Impey S, Wu Z, Kindsvogel W, Prichard L, Storm DR. Synergistic activation of the type I adenylyl cyclase by Ca2+ and Gs-coupled receptors in vivo. J Biol Chem. 1994;269:25400–25405. [PubMed] [Google Scholar]
- Weeber EJ, Atkins CM, Selcher JC, Varga AW, Mirnikjoo B, Paylor R, Leitges M, Sweatt JD. A role for the beta isoform of protein kinase C in fear conditioning. J Neurosci. 2000;20:5906–5914. doi: 10.1523/JNEUROSCI.20-16-05906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Bauer EP, LeDoux JE. L-type voltage-gated calcium channels mediate NMDA-independent associative long-term potentiation at thalamic input synapses to the amygdala. J Neurosci. 1999;19:10512–10519. doi: 10.1523/JNEUROSCI.19-23-10512.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, LeDoux JE. Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. J Neurosci. 1999;19:RC48. doi: 10.1523/JNEUROSCI.19-24-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Godsil BP, Peng Z, Saab F, June HL, Linn ML, Cook JM, Houser CR, O’Dell TJ, Homanics GE, Fanselow MS. The alpha1 subunit of the GABA(A) receptor modulates fear learning and plasticity in the lateral amygdala. Front Behav Neurosci. 2009;3:37. doi: 10.3389/neuro.08.037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh SH, Mao SC, Lin HC, Gean PW. Synaptic expression of glutamate receptor after encoding of fear memory in the rat amygdala. Mol Pharmacol. 2006;69:299–308. doi: 10.1124/mol.105.017194. [DOI] [PubMed] [Google Scholar]
- Yin JC, Tully T. CREB and the formation of long-term memory. Curr Opin Neurobiol. 1996;6:264–268. doi: 10.1016/s0959-4388(96)80082-1. [DOI] [PubMed] [Google Scholar]
- Yokoyama M, Suzuki E, Sato T, Maruta S, Watanabe S, Miyaoka H. Amygdalic levels of dopamine and serotonin rise upon exposure to conditioned fear stress without elevation of glutamate. Neurosci Lett. 2005;379:37–41. doi: 10.1016/j.neulet.2004.12.047. [DOI] [PubMed] [Google Scholar]
- Young AM, Rees KR. Dopamine release in the amygdaloid complex of the rat, studied by brain microdialysis. Neurosci Lett. 1998;249:49–52. doi: 10.1016/s0304-3940(98)00390-5. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Won J, Karlsson MG, Zhou M, Rogerson T, Balaji J, Neve R, Poirazi P, Silva AJ. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat Neurosci. 2009;12:1438–1443. doi: 10.1038/nn.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]