Abstract
Phospholipase C-related inactive protein (PRIP) plays important roles in trafficking to the plasma membrane of GABAA receptor, which is involved in the dominant inhibitory neurotransmission in the spinal cord and plays an important role in nociceptive transmission. However, the role of PRIP in pain sensation remains unknown. In this study, we investigated the phenotypes of pain behaviors in PRIP type 1 knockout (PRIP-1 -/- ) mice. The mutant mice showed hyperalgesic responses in the second phase of the formalin test and the von Frey test as compared with those in wild-type mice. In situ hybridization studies of GABAA receptors revealed significantly decreased expression of γ2 subunit mRNA in the dorsal and ventral horns of the spinal cord in PRIP-1 -/- mice, but no difference in α1 subunit mRNA expression. β2 subunit mRNA expression was significantly higher in PRIP-1 -/- mice than in wild-type mice in all areas of the spinal cord. On the other hand, the slow decay time constant for the spontaneous inhibitory current was significantly increased by treatment with diazepam in wild-type mice, but not in PRIP-1 -/- mice. These results suggest that PRIP-1 -/- mice exhibit the changes of the function and subunits expression of GABAA receptor in the spinal cord, which may be responsible for abnormal pain sensation in these mice.
Findings
Neuropathic pain is a critical disease that is induced by cancer, diabetes, infection or nerve damage [1]. Many studies have suggested that neuropathic pain occurs as a result of functional and/or anatomical changes in the primary sensory ganglia and the dorsal horn of the spinal cord after peripheral nerve injury [2-5]. One of these changes is the activation of microglia in the spinal dorsal horn. These microglia enhance the expression of neurotransmitter receptors and intracellular signaling kinases [6-8]. Changes in the expression of neurotransmitter (e.g., glutamate, ATP and GABA) receptors also occur in the dorsal root ganglion neurons or the spinal neurons [9-12]. GABA is the predominant inhibitory neurotransmitter in the spinal cord and in nociceptive transmission [13-16]. Accordingly, inhibition of GABAA receptors results in hypersensitivity of dorsal horn neurons and allodynia [17-20]. Therefore, reduced GABAergic input to the dorsal horn of the spinal cord is considered to be an important factor in the induction of allodynia.
Phospholipase C (PLC)-related inactive protein (PRIP) was first identified as a novel inositol 1,4,5-trisphospate [Ins(1,4,5)P3] binding protein, which is homologous to PLC-δ1, but is catalytically inactive [21]. The PIRP family consists of at least two types of proteins, PRIP-1 and PRIP-2. PRIP-1 is predominantly expressed in the brain, while PRIP-2 is ubiquitously expressed [22,23]. PRIP-1 has a number of binding partners that include the catalytic subunit of protein phosphatase 1α and GABAA receptor-associated protein [24]. This prompted us to examine the possible involvement of PRIP in GABAA receptor function. One role of PRIP-1 in regulating GABAA receptor functions was suggested based on the changes in GABAA receptor pharmacology and behavior in PRIP-1 knockout (PRIP-1 -/- ) mice [24,25]. It is possible that PRIP-1 -/- mice exhibit abnormal pain sensation because of altered GABAA receptor functions in the central nervous system (CNS). Therefore, in this study, we examined the phenotypes of pain behavior and GABAA receptor subunit mRNA expression in PRIP-1 -/- mice.
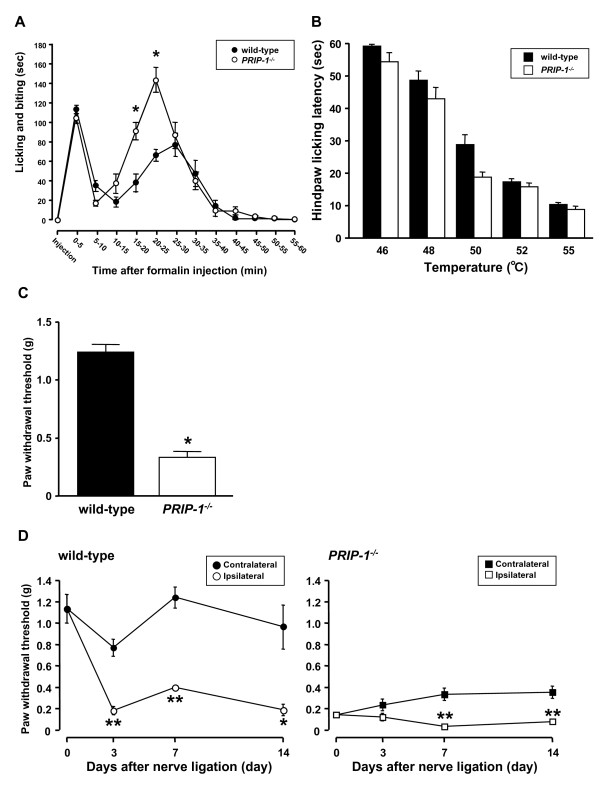
To determine how PRIP-1 affects acute nociceptive hypersensitivity, we first performed the plantar formalin test in PRIP-1 -/- and wild-type mice (Figure 1A). Intraplantar injection of formalin evoked biphasic licking and biting behaviors. The first and second phases lasted 5 min and 60 min, respectively, after formalin injection. PRIP-1 -/- mice failed to show changes in the first phase, but the second phase response was significantly greater and the peak of the second phase occurred earlier in PRIP-1 -/- mice than in wild-type mice.
Figure 1.
Chemical, thermal and mechanical-induced pain and PSNL-induced tactile allodynia in PRIP-1 -/- mice and wild-type mice. (A) Duration of licking and biting responses for each 5-min interval after intraplantar injection of formalin in PRIP-1 -/- mice (n = 14) and wild-type mice (n = 10). Each point represents the mean and S.E.M. (*P < 0.05 vs. wild-type). (B) Hindpaw licking latency was measured at five temperatures (46, 48, 50, 52 and 55°C). Each value represents the mean and S.E.M. (n = 6-10). (C) Paw withdrawal threshold in response to tactile stimulation with von Frey filaments in PRIP-1 -/- mice (n = 6) and wild-type mice (n = 6). Each value represents the mean and S.E.M. (*P < 0.05 vs. contralateral side). (D) Paw withdrawal threshold in response to tactile stimulation with von Frey filaments in PRIP-1 -/- mice (n = 5-7) and wild-type mice (n = 8-14) before (0) and 3, 7 and 14 days after PSNL. Each point represents the mean and S.E.M. (*P <0.05, **P <0.01 vs. the contralateral side, n = 6-10).
The hot plate test was performed at five different temperatures (46, 48, 50, 52 and 55°C) (Figure 1B). The latency to hindpaw licking was temperature-dependent and no difference was observed at 46, 48, 52 or 55°C between PRIP-1 -/- and wild-type mice. On the other hand, the mutant mice tended to be hypersensitive at 50°C, although this was not statistically significant.
To determine whether PRIP-1 plays a role in the behavioral responses to mechanical stimuli, the von Frey test was performed in PRIP-1 -/- mice and in wild-type mice (Figure 1C). Mechanical paw withdrawal thresholds were significantly lower in PRIP-1 -/- mice than in wild-type mice. Next, to investigate the influence of PRIP-1 on tactile allodynia during neuropathic pain, we performed partial sciatic nerve ligation (PSNL) in PRIP-1 -/- mice and in wild-type mice (Figure 1D). Wild-type mice showed a significant decrease in ipsilateral paw withdrawal threshold after PSNL, which started at least 3 days after PSNL and lasted for more than 10 days. Although PRIP-1 -/- mice showed greater hypersensitivity than wild-type mice, the PRIP-1 -/- mice also showed a strong decrease in ipsilateral paw withdrawal threshold after PSNL. This effect started at least 7 days after PSNL and lasted for more than 7 days.
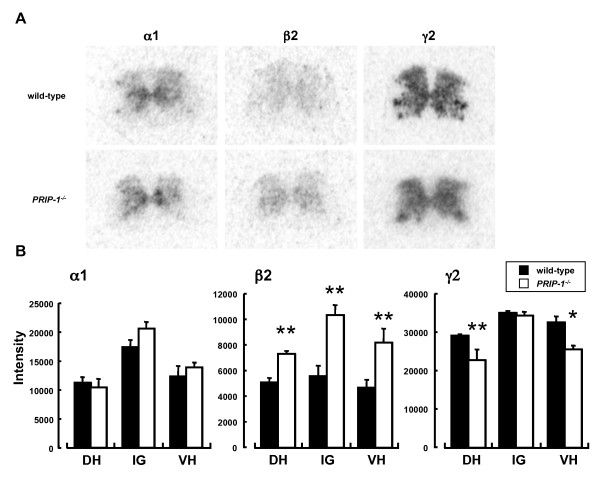
Previous studies showed that transport of the GABAA receptor γ subunit was altered in PRIP-1 -/- mice, thus reducing the sensitivity to benzodiazepine [25,26]. The majority of GABAA receptors in the CNS are believed to consist of α1, β2, and γ2 subunits [27]. Therefore, we investigated the mRNA expression patterns of these GABAA receptor subunits in the spinal cord of PRIP-1 -/- mice and in wild-type mice (Figure 2). α1, β2 and γ2 subunit mRNA expression was observed in the dorsal horn, intermediate gray matter and ventral horn of the spinal cord in these mice. There were no differences in α1 subunit mRNA expression between PRIP-1 -/- mice and wild-type mice. On the other hand, β2 subunit mRNA expression was significantly greater in PRIP-1 -/- mice than in wild-type mice throughout the spinal cord. In contrast, mRNA expression of the γ2 subunit was significantly decreased in the dorsal and ventral horns of the spinal cord in PRIP-1 -/- mice compared with wild-type mice.
Figure 2.
mRNA expression of the GABA A receptor α1, β2 and γ2 subunits in the spinal cord of PRIP-1 -/- mice and wild-type mice. (A) Representative film autoradiograms showing the mRNA expression of GABAA receptor α1, β2 and γ2 subunits. (B) Semiquantitative analysis of GABAA receptor α1, β2, and γ2 subunit mRNA expression in the dorsal horn (DH), intermediate gray matter (IG) and ventral horn (VH) of the spinal cord in PRIP-1 -/- mice (black) and wild-type mice (white). Each value represents the mean and S.E.M. (*P < 0.05 , **P < 0.01 vs. wild-type, n = 3).
Spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded as outward currents from 56 substantia geratinosa (SG) neurons, the lamina II of the spinal dorsal horn, with cells held at 0 mV. Exposure to 20 μM bicuculline methiodide (BMI) shifted the baseline holding current (Figure 3A), and this shift tended to be smaller in PRIP-1 -/- mice (3.7 ± 0.6 pA, n = 14) than in wild-type mice (4.7 ± 1.3 pA, n = 23), although it was not statistically significant. After administering 2 μM strychnine to block glycine receptors, the baseline shift was not significantly different between wild-type (0.8 ± 0.7 pA, n = 4) and PRIP-1 -/- mice (-0.4 ± 1.3 pA, n = 5).
Figure 3.
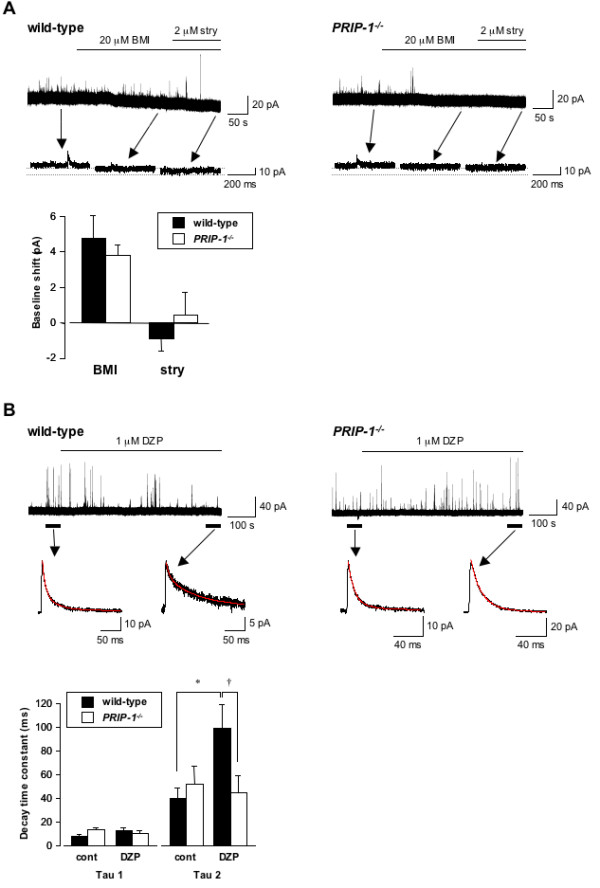
Inhibitory synaptic and extrasynaptic currents in SG neurons of PRIP-1 -/- mice and wild-type mice. (A) Representative traces and expanded traces for wild-type (left) and PRIP-1 -/- (right) showing the shift in baseline holding current elicited by 20 μM BMI and/or 2 μM strychnine (stry). The bar graph shows baseline shift elicited by 20 μM BMI (wild-type, n = 23; PRIP-1 -/- , n = 14) and/or 2 μM strychnine (wild-type, n = 6; PRIP-1 -/- , n = 6). BMI and strychnine were applied for 3-5 min in each experiment. (B) Representative traces and mean sIPSCs for wild-type (left) and PRIP-1 -/- (right) mice showing the sIPSCs elicited by 1 μM DZP. Tau is the decay time constant for the averaged trace of all sIPSCs recorded over 1 min in the absence or presence of DZP. DZP was applied for 8-10 min in each experiment. The red line in the averaged sIPSC trace shows the curve derived from a double-exponential equation. The bar graph shows summary of the tau data. Bars represent means and S.E.M. (*P < 0.05 vs. control, n = 6; †P < 0.05 vs. wild-type, n = 6).
Next, we examined the effects of diazepam (DZP) on GABAergic sIPSCs in SG neurons (Figure 3B). The mean sIPSCs were best fit into a two-exponential equation. Tau2 was significantly increased by 1 μM DZP in wild-type mice (control: 39.3 ± 8.7 ms, n = 6; DZP: 98.4 ± 20.5 ms, n = 6), but not in PRIP-1 -/- mice (control: 51.6 ± 14.6 ms, n = 6; DZP: 44.2 ± 14.7 ms, n = 6). Tau1 was not affected by DZP in wild-type (control: 7.6 ± 1.3 ms, n = 6; DZP: 11.9 ± 2.9 ms, n = 6) or in PRIP-1 -/- mice (control: 12.0 ± 2.1 ms, n = 6; DZP: 10.0 ± 1.9 ms, n = 6). The 10-90% rise time was not significantly affected by DZP in wild-type (control: 2.9 ± 0.6 ms, n = 6; DZP: 2.7 ± 0.6 ms, n = 6) and PRIP-1 -/- mice (control: 2.8 ± 0.4 ms, n = 6; DZP: 2.9 ± 0.5 ms, n = 6). Peak amplitude was also unaffected by DZP in wild-type (control: 17.1 ± 3.3 pA, n = 6; DZP: 11.4 ± 1.2 pA, n = 6) and PRIP-1 -/- mice (control 19.4 ± 5.1 pA, n = 6; DZP: 22.9 ± 6.3 pA, n = 6). Furthermore, frequency was not significantly affected by DZP in wild-type (control: 0.11 ± 0.02 Hz, n = 6; DZP: 0.09 ± 0.02 Hz, n = 6) and PRIP-1 -/- mice (control: 0.3 ± 0.1 Hz, n = 6; DZP: 0.2 ± 0.1 Hz, n = 6).
PRIP was reported to facilitate the transport of the γ2 subunit-containing GABAA receptor [25,26]. It is thought that the functions of the GABAA receptor are highly influenced by PRIP. GABA plays an important role in nociceptive transmission in the spinal cord as an inhibitory neurotransmitter [13-20]. Thus, we investigated the role of PRIP-1 on acute or chronic pain using PRIP-1 -/- mice. We found that the second phase, but not the first phase, of the licking and biting responses following intraplantar formalin injection was significantly increased in PRIP-1 -/- mice. Intraplantar injection of formalin evokes nociceptive behaviors in rodents such as licking, biting and lifting of the injected paw in a biphasic manner. The first phase of the response is caused by persistent activation and acute sensitization of nociceptors, while the second phase is caused by continual activation of the nociceptors and sensitization of the central synapses via mechanisms triggered by repetitive stimulation during the first phase [28]. Therefore, the hyperalgesic response in PRIP-1 -/- mice induced by intraplantar injection of formalin may occur via central pathways. On the other hand, the thermal sensitivity during the hotplate test at five different temperatures was not significantly different between PRIP-1 -/- mice and wild-type mice. The responses to the hotplate test involve more complex behavior [29,30]. It has been reported that dysfunction of the GABAA receptor can affect heat nociception [31]. However, we found that deficiency in PRIP-1 did not affect responses to noxious heat stimulation. In our in situ hybridization experiment, the mRNA expression of γ2 subunit was decreased in the dorsal horn of the spinal cord in PRIP-1 -/- mice, whereas that of β2 subunit was increased. Our results raise the possibility that the activity of the GABAA receptor containing the γ2 subunit during thermal nociception in PRIP-1 -/- mice may be compensated for by the GABAA receptor composed of the others subunits containing the β2 subunit. Furthermore, although the mechanical paw withdrawal threshold was decreased in PRIP-1 -/- mice, the ipsilateral hypersensitive reaction was more pronounced in PRIP-1 -/- mice after PSNL. These results suggest that tactile allodynia is affected by PRIP-1 deficiency.
The GABAA receptor is a heterooligomeric complex of five subunits and the majority of the receptors in the CNS are thought to consist of α1, β2 and γ2 subunits [27]. We found no differences in α1 subunit mRNA expression between PRIP-1 -/- mice and wild-type mice, whereas β2 subunit mRNA expression was significantly greater in PRIP-1 -/- mice than in wild-type mice throughout the spinal cord. Further studies are required to elucidate the mechanism involved in this change in expression. Interestingly, γ2 subunit mRNA expression in the dorsal and ventral horns of the spinal cord was significantly lower in PRIP-1 -/- mice than in wild-type mice. The γ2 subunit is an important factor that regulates the benzodiazepine sensitivity to GABAA receptors, as well as receptor expression and trafficking [32]. Thus, it is thought that the assembly of GABAA receptors containing the γ2 subunit, as well as the functions of this receptor, are impaired in the spinal cord of PRIP-1 -/- mice. Ataka et al. [33] reported that phasic inhibition is mediated by both GABAergic and glycinergic inhibitory postsynaptic currents, and that the tonic inhibitory currents are mediated by GABAA receptors in the dorsal horn lamina II region of the spinal cord. We thus investigated the tonic inhibitory currents in SG neurons of PRIP-1 -/- mice. These currents were achieved by the application of BMI, but not by strychnine, and tended to be smaller in PRIP-1 -/- mice than wild-type mice, although not statistically significant. This suggests that the function of GABAA receptors containing the γ2 subunit may be compensated for by the GABAA receptor containing β2 subunit in terms of tonic inhibition in PRIP-1 -/- mice. Recently, it is reported that the GABAA receptor δ subunit in the spinal cord mediated tonic inhibition and acute nociception [34]. Tonic inhibitory currents mediated by GABAA receptors in spinal neurons play an important role in the regulation of acute nociception and central sensitization. Therefore, it is possible that PRIP-1 knockout may partially influence of tonic inhibition with some of GABAA receptor subunits. On the other hand, the prolonged Tau2 induced by DZP in wild-type mice was absent in PRIP-1 -/- mice. In previous studies, three types of IPSCs, including glycine receptor-mediated, GABAA receptor-mediated and mixed type, were recorded from lamina II neurons [33,35]. GABAA receptor-mediated IPSCs showed slow decay time constant, while glycine receptor-mediated IPSCs were rapid. Our results suggest that the assembly of GABAA receptor containing γ2 subunit impaired by PRIP-1 knockout may occur the impairment of the function of GABAA receptor in the spinal cord. However, tonic inhibition and kinetics of IPSC were not significantly difference between wild-type and PRIP-1 -/- mice. Therefore, it is thought that the hyperalgesic reactions observed in PRIP-1 -/- mice may involve in other factors in addition to the impairment of GABAergic transmission in the spinal cord of these mice.
In conclusion, we have demonstrated that PRIP-1 -/- mice show behavioral abnormalities in response to various noxious stimuli. It is possible that these behaviors in PRIP-1 -/- mice are dependent on changes in the subunits expression and the function of GABAA receptor in the spinal cord. Therefore, defects in PRIP-1, which plays a role in the assembly of the GABAA receptor, are responsible for abnormal behaviors in response to pain.
Methods and Materials
Animals
PRIP-1 -/- mice were used in our experiments. Mice were housed at 24 ± 2°C with a 12/12-h light/dark cycle (lights on at 8:00 am), and were given free access to commercial food and tap water. The experimental procedures were based on the Guidelines of the Committee for Animal Care and Use of Hirosaki University, Hiroshima University and Kyushu University.
Formalin test
Each mouse was placed in a clear plastic cage at least 30 min before the formalin injection to allow it to adapt to the new environment. A solution of 5% formalin in saline (50 μl) was injected into the plantar surface of the right hindpaw. The number of flinches (rapid paw shaking) of the injected paw was recorded as a pain-related behavior. The total number of hind paw flinches was determined during twelve 5-min intervals for 60 min after formalin injection.
Hotplate test
The hotplate tests were performed at five different temperatures (46, 48, 50, 52 and 55°C) (Hot Plate Analgesia Meter MK-350C, Muromachi Kikai Co., Ltd, Tokyo, Japan). The latency to licking of the hindpaw was recorded. To prevent tissue damage, the cut-off time was 60 s for 50°C, 40 s for 52°C, and 30 s for 55°C. Animals not responding within the cutoff time were removed and assigned a score of the cutoff time.
von Frey test
Mechanical sensitivity was determined using von Frey filaments (Semmes-Weinstein monofilaments, Stoelting, IL, USA) with calibrated bending forces (g), as previously described [36]. In brief, the mice were placed individually in a glass cage with a wire-mesh bottom. After the mice had adapted to the testing environment for 60 min, a series of von Frey filaments (0.008, 0.02, 0.04, 0.07, 0.166, 0.407, 0.692, 1.202, 1.479 and 2.0 g) were pressed perpendicularly against the mid-planter surface of the hind paw from below the mesh floor and held for 3-5 s with the filament slightly buckled. Lifting of the paw was recorded as a positive response. The paw withdrawal threshold was defined as the minimum pressure required to elicit a withdrawal reflex of the paw, at least once in five trials.
PSNL
The mice were anesthetized with an intraperitoneal injection of pentobarbital (60 mg/kg). Under a light microscope (SD30; Olympus, Tokyo, Japan), a tight ligature was made using an 8-0 silk suture around approximately 1/3-1/2 of the diameter of the sciatic nerve located on the right-hand side. This method is similar to that described by Seltzer et al. [37]. In sham-operated mice, the nerve was exposed without ligation.
In situ hybridization
Mice were killed by decapitation. The lower lumbar segment (L3-L5) of the spinal cord was immediately resected and frozen in liquid nitrogen and stored at -80°C. Sections (20 μm thick) were cut from each nerve tissue block through L4 using a microtome cryostat (Microm HM500 OM, Walldorf, Germany). The sections were thaw-mounted onto APS-coated slides (Matsunami, Japan) and stored at -30°C until used for in situ hybridization. For in situ hybridization histochemistry, specific oligonucleotides were selected from the following sequences: GABAA receptor α1 subunit GGGGTCACCCCTGGCTAAGTTAGGGGTATAGCTGGTTGCTGTAGG; GABAA receptor β2 subunit TCGTTCCAGGGCGTTGCGGCCAAAACTATGCCTAGGCAACCCAGC; GABAA receptor γ2 [NDS1] subunit CATTTGGATCGTTGCTGATCTGGGACGGATATCAATGGTAGGGGC. The oligonucleotide probes were labeled with [33P]-dATP (2000 Ci/mmol; {DuPont-NEN}[NDS2]) and terminal deoxynucleotidyltransferase (Boehringer Mannheim, Germany). The labeled probes were purified using a QIAquick Nucleotide Removal Kit (Qiagen GmbH, Hilden, Germany). Hybridization was performed as previously described [38], except that the purified probes were diluted to a final concentration of 107 cpm/ml before use. The hybridized sections were exposed to Kodak BioMAX MR films at -80°C and a Kodak MS screen was used to increase the intensity of the hybridization signals. The films were exposed for 7-10 days and then developed. The optical density of the hybridization signals from three sections (the dorsal horn, the intermediate gray matter and the ventral horn of the L4 region) was acquired using an Epson GT-9800 scanner (Epson, Japan). The density was determined using NIH Image (Bethesda, MD, USA) on a Macintosh G5 computer (Apple Computer, Vancouver, WA, USA). The intensity was calculated relative to the background signal.
Electrophysiology
Transverse spinal cord slices were obtained from the L4 and L5 spinal cords of 5-8-week-old mice. Each mouse was deeply anesthetized with halothane, decapitated, and the lumbar spinal cord (L1-L6) was removed. After removing the pia-arachnoid membrane, 450-μm-thick spinal cord slices were cut on a brain slicer (VIBRATOME [NDS3] 3000 SERIES) in ice-cold oxygenated sucrose-substituted artificial cerebrospinal fluid (ACSF). This solution contained (in mM): 250 sucrose, 2.5 KCl, 1 NaH2PO4, 1 MgCl2, 2.5 CaCl2, 25 NaHCO3 and 10 glucose. The solution was bubbled with 95% O2 and 5% CO2. Slices were incubated for at least 1 h in a storage chamber containing ACSF composed of (in mM): 117 NaCl, 3.6 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.5 CaCl2, 25 NaHCO3 and 11 glucose, with 95% O2/5% CO2 at room temperature before recording. A spinal cord slice was transferred to a recording chamber and perfused (5 ml/min) with ACSF at room temperature. SG neurons were viewed on a monitor via a 40 × water-immersion objective lens with an infrared differential interference contrast filter and a CCD camera (ORCA-ER C4742-95, Hamamatsu Photonics, Shizuoka, Japan). Patch pipettes (resistance: 3-5 MΩ), made from borosilicate glass (1.5 mm O.D, TW150-3, World Precision Instruments, {Inc, USA}[NDS4]) was filled with an internal solution containing (in mM): 150 CsCH3SO3, 5 KCl, 3 MgCl2, 0.1 EGTA and 10 HEPES, 3 Mg(ATP)2 and 0.4 NaGTP (pH 7.3 with 1 M CsOH). Membrane currents were recorded with cells held at 0 mV using a Multiclamp 700B (Molecular Devices, Sunnyvale, CA, USA) and digitized at 5-10 kHz with DigiData1322A (Molecular Devices). Data were stored and analyzed using pClamp9.2 software (Molecular Devices), Igor Pro software (WaveMetrics, Inc, Lake Oswego, OR, USA) and {NeuroMatic version 2.00 software}[NDS5]. Series resistance compensation was not applied.
Statistical analysis
Statistical analysis of data was performed using the Mann-Whitney U -test or Student's t -test for comparisons between two groups, or analysis of variance followed by Tukey's test for multiple comparisons. Results are expressed as means and standard error of the mean (S.E.M.). The level of significance was set at P < 0.05 .
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
KM performed the von Frey test, patch clamp experiments, data analysis, and wrote the manuscript. MT performed in situ hybridization experiments. KM, JY and MF participated in data analysis. TK and MH provided PRIP-1 -/- mice and directed the experiments. KM and SU designed and directed the experiments. All authors read and approved the final manuscript.
Contributor Information
Keisuke Migita, Email: migitak@cc.hirosaki-u.ac.jp.
Masahiko Tomiyama, Email: masahiko_tomiyama@med.pref.aomori.jp.
Junko Yamada, Email: jyamada@cc.hirosaki-u.ac.jp.
Masashi Fukuzawa, Email: fukuzawa@cc.hirosaki-u.ac.jp.
Takashi Kanematsu, Email: tkanema2@hiroshima-u.ac.jp.
Masato Hirata, Email: hirata1@dent.kyushu-u.ac.jp.
Shinya Ueno, Email: shinyau@cc.hirosaki-u.ac.jp.
Acknowledgements
We thank Tomoko Moriyama for support with pain behavior experiments. This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (KM: NO. 20700328, SU: NO. 18659455), by a Grant for Hirosaki University Institutional Research and by a Hirosaki University Grant for Exploratory Research by Young Scientists (KM).
References
- Baron R. Mechanisms of disease: neuropathic pain--a clinical perspective. Nat Clin Pract Neurol. 2006;2:95–106. doi: 10.1038/ncpneuro0113. [DOI] [PubMed] [Google Scholar]
- Ma C, Shu Y, Zheng Z, Chen Y, Yao H, Greenquist KW, White FA, LaMotte RH. Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J Neurophysiol. 2003;89:1588–1602. doi: 10.1152/jn.00855.2002. [DOI] [PubMed] [Google Scholar]
- Li CY, Song YH, Higuera ES, Luo ZD. Spinal dorsal horn calcium channel alpha2delta-1 subunit upregulation contributes to peripheral nerve injury-induced tactile allodynia. J Neurosci. 2004;24:8494–8499. doi: 10.1523/JNEUROSCI.2982-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarpley JW, Kohler MG, Martin WJ. The behavioral and neuroanatomical effects of IB4-saporin treatment in rat models of nociceptive and neuropathic pain. Brain Res. 2004;1029:65–76. doi: 10.1016/j.brainres.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Migita K, Moriyama T, Koguchi M, Honda K, Katsuragi T, Takano Y, Ueno S. Modulation of P2X receptors in dorsal root ganglion neurons of streptozotocin-induced diabetic neuropathy. Neurosci Lett. 2009;452:200–203. doi: 10.1016/j.neulet.2009.01.048. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Ueno H, Kataoka A, Tozaki-Saitoh H, Inoue K. Activation of dorsal horn microglia contributes to diabetes-induced tactile allodynia via extracellular signal-regulated protein kinase signaling. Glia. 2008;56:378–386. doi: 10.1002/glia.20623. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Masuda T, Kitano J, Shimoyama H, Tozaki-Saitoh H, Inoue K. IFN-gamma receptor signaling mediates spinal microglia activation driving neuropathic pain. Proc Natl Acad Sci USA. 2009;106:8032–8037. doi: 10.1073/pnas.0810420106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Sung B, Lim G, Mao J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. J Neurosci. 2003;23:2899–2910. doi: 10.1523/JNEUROSCI.23-07-02899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migita K, Moriyama T, Koguchi M, Honda K, Katsuragi T, Takano Y, Ueno S. Modulation of P2X receptors in dorsal root ganglion neurons of streptozotocin-induced diabetic neuropathy. Neurosci Lett. 2009;452:200–203. doi: 10.1016/j.neulet.2009.01.048. [DOI] [PubMed] [Google Scholar]
- Chen Y, Li GW, Wang C, Gu Y, Huang LY. Mechanisms underlying enhanced P2X receptor-mediated responses in the neuropathic pain state. Pain. 2005;119:38–48. doi: 10.1016/j.pain.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Obata K, Yamanaka H, Fukuoka T, Yi D, Tokunaga A, Hashimoto N, Yoshikawa H, Noguchi K. Contribution of injured and uninjured dorsal root ganglion neurons to pain behavior and the changes in gene expression following chronic constriction injury of the sciatic nerve in rats. Pain. 2003;101:65–77. doi: 10.1016/S0304-3959(02)00296-8. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Nishi S. Primary afferent-evoked glycine- and GABA-mediated IPSPs in substantia gelatinosa neurones in the rat spinal cord in vitro. J Physiol. 1995;482:29–38. doi: 10.1113/jphysiol.1995.sp020497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YZ, Pan HL. Primary afferent stimulation differentially potentiates excitatory and inhibitory inputs to spinal lamina II outer and inner neurons. J Neurophysiol. 2004;91:2413–2421. doi: 10.1152/jn.01242.2003. [DOI] [PubMed] [Google Scholar]
- Polgár E, Hughes DI, Riddell JS, Maxwell DJ, Puskár Z, Todd AJ. Selective loss of spinal GABAergic or glycinergic neurons is not necessary for development of thermal hyperalgesia in the chronic constriction injury model of neuropathic pain. Pain. 2003;104:229–239. doi: 10.1016/S0304-3959(03)00011-3. [DOI] [PubMed] [Google Scholar]
- Drew GM, Siddall PJ, Duggan AW. Mechanical allodynia following contusion injury of the rat spinal cord is associated with loss of GABAergic inhibition in the dorsal horn. Pain. 2004;109:379–388. doi: 10.1016/j.pain.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Puig S, Jones DL. Spinal bicuculline produces hypersensitivity of dorsal horn neurons: effects of excitatory amino acid antagonists. Pain. 1998;77:181–190. doi: 10.1016/S0304-3959(98)00094-3. [DOI] [PubMed] [Google Scholar]
- Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba H, Ji RR, Kohno T, Moore KA, Ataka T, Wakai A, Okamoto M, Woolf CJ. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol Cell Neurosci. 2003;24:818–830. doi: 10.1016/S1044-7431(03)00236-7. [DOI] [PubMed] [Google Scholar]
- Honda K, Horikawa K, Ando S, Koga K, Kawata S, Migita K, Takano Y. The spinal muscarinic M1 receptors and GABAA receptors contribute to the McN-A-343-induced antinociceptive effects during thermal stimulation of mice. J Pharmacol Sci. 2008;108:472–479. doi: 10.1254/jphs.08226FP. [DOI] [PubMed] [Google Scholar]
- Kanematsu T, Takeya H, Watanabe Y, Ozaki S, Yoshida M, Koga T, Iwanaga S, Hirata M. Putative inositol 1,4,5-trisphosphate binding proteins in rat brain cytosol. J Biol Chem. 1992;267:6518–6525. [PubMed] [Google Scholar]
- Matsuda M, Kanematsu T, Takeuchi H, Kukita T, Hirata M. Localization of a novel inositol 1,4,5-trisphosphate binding protein, p130 in rat brain. Neurosci Lett. 1998;257:97–100. doi: 10.1016/S0304-3940(98)00810-6. [DOI] [PubMed] [Google Scholar]
- Uji A, Matsuda M, Kukita T, Maeda K, Kanematsu T, Hirata M. Molecules interacting with PRIP-2, a novel Ins(1,4,5)P3 binding protein type 2: Comparison with PRIP-1. Life Sci. 2002;72:443–453. doi: 10.1016/S0024-3205(02)02275-0. [DOI] [PubMed] [Google Scholar]
- Terunuma M, Jang IS, Ha SH, Kittler JT, Kanematsu T, Jovanovic JN, Nakayama KI, Akaike N, Ryu SH, Moss SJ, Hirata M. GABAA receptor phospho-dependent modulation is regulated by phospholipase C-related inactive protein type 1, a novel protein phosphatase 1 anchoring protein. J Neurosci. 2004;24:7074–7084. doi: 10.1523/JNEUROSCI.1323-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanematsu T, Jang IS, Yamaguchi T, Nagahama H, Yoshimura K, Hidaka K, Matsuda M, Takeuchi H, Misumi Y, Nakayama K, Yamamoto T, Akaike N, Hirata M, Nakayama K. Role of the PLC-related, catalytically inactive protein p130 in GABAA receptor function. EMBO J. 2002;21:1004–1011. doi: 10.1093/emboj/21.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizokami A, Kanematsu T, Ishibashi H, Yamaguchi T, Tanida I, Takenaka K, Nakayama KI, Fukami K, Takenawa T, Kominami E, Moss SJ, Yamamoto T, Nabekura J, Hirata M. Phospholipase C-related inactive protein is involved in trafficking of gamma2 subunit-containing GABAA receptors to the cell surface. J Neurosci. 2007;27:1692–1701. doi: 10.1523/JNEUROSCI.3155-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PJ, Bonnert TP, McKernan RM, Farrar S, Le Bourdellès B, Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJ, Thompson SA, Wafford KA. Molecular and functional diversity of the expanding GABAA receptor gene family. Ann N Y Acad Sci. 1999;868:645–653. doi: 10.1111/j.1749-6632.1999.tb11341.x. [DOI] [PubMed] [Google Scholar]
- Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- Pastoriza LN, Morrow TJ, Casey KL. Medial frontal cortex lesions selectively attenuate the hot plate response: possible nocifensive apraxia in the rat. Pain. 1996;64:11–17. doi: 10.1016/0304-3959(95)00070-4. [DOI] [PubMed] [Google Scholar]
- Baliki M, Calvo O, Chialvo DR, Apkarian AV. Spared nerve injury rats exhibit thermal hyperalgesia on an automated operant dynamic thermal escape task. Mol Pain. 2005;1:18. doi: 10.1186/1744-8069-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugarte SD, Homanics GE, Firestone LL, Hammond DL. Sensory thresholds and the antinociceptive effects of GABA receptor agonists in mice lacking the β3 subunit of the GABAA receptor. Neuroscience. 2000;95:795–806. doi: 10.1016/s0306-4522(99)00481-9. [DOI] [PubMed] [Google Scholar]
- Moss SJ, Smart TG. Constructing inhibitory synapses. Nat Rev Neurosci. 2001;2:240–250. doi: 10.1038/35067500. [DOI] [PubMed] [Google Scholar]
- Ataka T, Gu JG. Relationship between tonic inhibitory currents and phasic inhibitory activity in the spinal cord lamina II region of adult mice. Mol Pain. 2006;2:36. doi: 10.1186/1744-8069-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin RP, Labrakakis C, Eng DG, Whissell PD, Koninck YD, Orser BA. Pharmacological enhancement of δ-subunit-containing GABAA receptors that generate a tonic inhibitory conductance in spinal neurons attenuates acute nociception in mice. Pain. 2011;152:1317–1326. doi: 10.1016/j.pain.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Mitchell EA, Gentet LJ, Dempster J, Belelli D. GABAA and glycine receptor-mediated transmission in rat lamina II neurones: relevance to the analgesic actions of neuroactive steroids. J Physiol. 2007;583:1021–1040. doi: 10.1113/jphysiol.2007.134445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Koga K, Moriyama T, Koguchi M, Takano Y, Kamiya HO. Intrathecal α2 adrenoceptor agonist clonidine inhibits mechanical transmission in mouse spinal cord via activation of muscarinic M1 receptors. Neurosci Lett. 2002;322:161–164. doi: 10.1016/S0304-3940(02)00073-3. [DOI] [PubMed] [Google Scholar]
- Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- Tomiyama M, Rodriguez-Puertas R, Cortés R, Christnacher A, Sommer B, Pazos A, Palacios JM, Mengod G. Differential regional distribution of AMPA receptor subunit messenger RNAs in the human spinal cord as visualized by in situ hybridization. Neuroscience. 1996;75:901–915. doi: 10.1016/0306-4522(96)00321-1. [DOI] [PubMed] [Google Scholar]