Abstract
The human host is continuously exposed to the egg and the adult worm developmental stages of Schistosoma mansoni during chronic infections with the parasite. To assess the cytokine responses induced by these different costimulating stages and how they are influenced by host age and infection intensity, whole blood samples from a cross-sectional cohort of 226 members of a Ugandan fishing community who had been resident in an area with high transmission of S. mansoni for the previous 10 years or from birth were stimulated with S. mansoni egg antigen (SEA) or worm antigen (SWA). SWA-specific gamma interferon (IFN-γ) production increased with age, and the levels of SWA- and SEA-specific interleukin 3 (IL-3) were weakly correlated with schistosome infection intensity. The production of most cytokines was little affected by age or infection intensity but was either SEA or SWA specific. One hundred thirty-two members of the cohort coproduced IL-5 and IL-13 specifically in response to SWA, whereas only 15 produced these cytokines, and at much lower levels, in response to SEA. IL-10, IL-4, and IFN-γ were also produced in response to SWA, whereas the response to SEA consisted almost exclusively of IL-10. Our results suggest that, in contrast to what has been described for the murine model of S. mansoni and during acute human infections, chronic intense exposure to and infection with S. mansoni in this cohort resulted in very low levels of response to SEA in vitro in the presence of a vigorous and mixed Th1-Th2 response to SWA.
Schistosomiasis is a chronic helminth infection, which is estimated to afflict approximately 200 million people in developing countries. Adult worms live in host blood vessels for years, releasing hundreds of eggs into the bloodstream each day. In areas of high transmission, children become infected when they are old enough to come into contact with contaminated water. In these areas, the combination of the longevity of individual worms (12) and susceptibility to reinfection may result in those exposed to the parasite being infected for most of their lives. However, infection intensity in areas of endemicity rises in early childhood, peaking during the early teenage years and then declining, and adults are more resistant to reinfection than young children (18). In humans living in areas of endemicity for schistosomiasis, parasite-specific antibody (Ab) responses have been shown to be dependent upon host age, (10, 15, 26, 28, 32, 34), infection intensity, (26, 32, 34), and/or the parasite development stage from which target antigens (Ags) were derived (9), but the role played by these factors in the regulation of parasite-specific cytokine responses is less well described.
Cells from individuals chronically infected with schistosomes have been shown to produce cytokines such as interleukin 4 (IL-4) and IL-5 following stimulation with soluble schistosome Ag preparations derived from adult worms (SWA) and eggs (SEA) (1, 3, 13, 31, 35). Recently, a more direct relationship has been demonstrated between the numbers of IL-4-producing cells in the peripheral blood of Schistosoma mansoni-infected Brazilians and their levels of parasite-specific immunoglobulin E (IgE), with the individuals producing the highest levels of IgE having greater numbers of circulating IL-4-positive CD4+ T cells (11).
The SEA-specific responses of cells from acutely infected individuals in vitro are more vigorous than those seen with cells from individuals who are chronically infected (25), and this perhaps mirrors what is seen during experimental murine infection, during which vigorous SEA-specific immune responses predominate after the onset of parasite egg production (at 5 to 6 weeks after infection) but are rapidly down-regulated several weeks later (14, 29). It is difficult to judge to what extent the many detailed immunological studies with rodent schistosomiasis models, particularly in relation to protective immunity and immune-mediated morbidity, are generally applicable to chronically infected and genetically diverse human populations living in areas where schistosomiasis is endemic. Most studies of human schistosome-specific cellular immune responses in areas of endemicity have been carried out with relatively small study cohorts, and the role of factors such as age and intensity or duration of infection have been difficult to assess without running the risk of overparameterization of the statistical analysis. We have previously shown that children from a highly exposed fishing community on the shores of Lake Albert become much more intensely and rapidly reinfected after treatment than adults, despite the fact that adults had more water contact and thus a greater exposure to reinfection (18). Such a community, with a prevalence of infection of almost 100%, a longstanding stable transmission of S. mansoni, and a high degree of exposure across all age groups, provided an opportunity to observe the cross-sectional patterns of human cytokine responses to S. mansoni and the factors that influence these responses. We therefore conducted an immunoepidemiological study in a neighboring fishing village with a similar pattern of infection and transmission. Here we describe the in vitro whole blood cytokine responses of a representative cross-sectional cohort of 226 Ugandans, all of whom had been resident in a area with high S. mansoni transmission for at least 10 years or since birth.
MATERIALS AND METHODS
Study population.
A study cohort was selected from the village of Booma (in the parish of Butiaba), which is adjacent to Lake Albert, Masindi district, in northwestern Uganda. Conditions in the area are largely unsuitable for agriculture, and the entire community is involved in the fishing industry. Two hundred twenty-six individuals balanced for age and sex were selected from the village; each individual fulfilled the criteria of (i) living close to the lake shore, (ii) being between 7 and 50 years old, and (iii) being resident in the village for at least 10 years or since birth. An initial parasitological survey of approximately 10% of the local community had suggested a prevalence of infection with S. mansoni of close to 100%. Before treatment, three further stools were collected on consecutive days and quantitative parasitology was carried out once again for S. mansoni. Two Kato slides (50 mg) were prepared for each stool sample, and these were examined and counted for helminth ova (19). The infection intensity for S. mansoni varied from 0 to 8,100 eggs per gram of stool (epg), and the prevalence across the cohort was 94%. After being bled, individuals were treated twice with 40 mg of praziquantel/kg 2 weeks apart.
Whole blood cultures.
Venous blood was collected into heparin at 10 U/ml (Sigma, Poole, United Kingdom) and immediately diluted 1:4 in RPMI 1640 medium (Sigma) with penicillin (50 U/ml), streptomycin (50 μg/ml), and l-glutamine (2 mM) (Sigma). After thorough mixing, the blood was dispensed into duplicate 48-well flat-bottom plates (Costar, High Wycombe, United Kingdom), and parasite derived Ags were added to a final concentration of 10 μg/ml. The plates were placed in sealed boxes after the addition of a gas-generating kit (Oxoid Ltd., Basingstoke, United Kingdom). The cultures were incubated at 37°C and harvested at 48 and 120 h later, time points which had previously been shown to be optimal for the measurement of particular cytokines of interest (data not shown). Thus, IL-4 and tumor necrosis factor alpha (TNF-α) were measured in 48-h supernatants, and IL-3, IL-5, IL-10, IL-13, and gamma interferon (IFN-γ) were measured in samples from cultures that had been incubated with Ag for 120 h. The supernatants were immediately frozen at −20°C and transported back to the United Kingdom frozen. Supernatants were thawed quickly, virally inactivated by incubation for 2 h in the presence of 0.3% tributyl phosphate (Sigma) and 1% Tween 80 (Sigma), and either assayed for cytokines immediately or stored at −70°C. None of the cytokine assays were affected by the viral inactivation procedure (data not shown). No sample went through more than two freeze-thaw cycles before being assayed for cytokine content. Samples were sometimes diluted to ensure that the values fell on the linear part of the standard curve.
Parasite-derived Ags.
SEA and SWA from S. mansoni were prepared according to established protocols (8). SEA and SWA were prepared from mice infected with the parasite and used at a final concentration of 10 μg/ml in whole blood assays. All Ags were titrated in vitro by using murine splenocytes from infected mice. Endotoxin content was measured by using a Limulus amebocyte lysate kit (QCL-1000; Bio-Whittaker Inc., Walkersville, Md.). The levels of endotoxin in the native Ag used in these studies were 1.44 ng of endotoxin/mg of SEA and 1.23 ng of endotoxin/mg of SWA.
Murine cytokine responses.
Groups of three 10-week-old outbred TO mice were either infected with 250 S. mansoni cercariae or mock infected. Eight weeks later, spleens were removed and single-cell suspensions were cultured at 2.5 × 106 cells/ml in a final volume of 200 μl in RPMI 1640 medium with penicillin (50 U/ml), streptomycin (50 μg/ml), l-glutamine (2 mM), and 10% fetal bovine serum (catalog no. F2442) (all from Sigma). After 72 h, supernatants were harvested and cytokines were measured by enzyme-linked immunosorbent assay (ELISA).
Cytokine assays.
Cytokines were measure by using capture ELISAs and commercially available anticytokine Abs from PharMingen. Briefly, 96-well flat-bottom plates (Immulon 2 HB; Dynex) were coated with 50 μl of capture Ab diluted in bicarbonate buffer (pH 9.6) per well. After incubation overnight at 4°C, the plates were washed three times in washing buffer (phosphate-buffered saline plus 0.03% Tween 20 [Sigma]), and then nonspecific binding was blocked by using incubation buffer (phosphate-buffered saline plus 0.05% Tween 20 [Sigma]) with 1% (wt/vol) low-fat dry milk powder (Marvel; Premier Brands Ltd., Spaling, United Kingdom). After washing three times as before, standards and samples were added in a volume of 50 μl. The cytokine standards were serially diluted in incubation buffer over the ranges of 0 to 1,800 pg/ml for IL-3, IL-4, IL-5, IL-10, and IL-13; 0 to 16,200 pg/ml for TNF-α, and 0 to 600 U/ml for IFN-γ (Genzyme). Samples were added (diluted when appropriate) and were incubated either overnight at 4°C or for 3 h at room temperature (RT) with gentle shaking. The plates were washed again as before, and then the appropriate biotinylated detection Ab was added. After incubation for 1 h with gentle shaking at RT, the plates were washed three times. Finally, poly-horseradish peroxidase (CLB; Mast Group Ltd., Bootle, United Kingdom) was added at the appropriate dilution and incubated for 1 h at RT before final three additional washes and development with 2× o-phenylenediamine dihydrochloride (Sigma). The reaction was stopped at an appropriate point by using 2 M H2SO4, and the plates were read at 490 nm with an EL312 ELISA plate reader (BioTek Instruments). All supernatants (from all individuals and all cultures) were assayed for each cytokine simultaneously. All of the resulting optical density (OD) readings were captured from the plate reader by using Deltasoft II (Biometallics Inc.) and transferred to Microsoft EXCEL, where they were processed by a series of in-house computer macros. These macros fitted calibration curves to 12 standards on each plate, reported poorly fitting curves, interpolated the sample OD values, reported samples with widely differing replicates or OD values greater than 80% of the plateau value for the calibration curve, and, finally, output the interpolated values in a rectangular table of subject by stimulating antigen and bleed. The nonlinear calibration curve took the form ODfitted = a{1 − exp(−b[cytokine])} + c, and a, b, and c were fitted by minimizing Σ(√ODobserved − √ODfitted)2, i.e., by least squares in the square root transformation, since this ensured that approximately equal weight was given to each point. Samples whose OD readings were reported as too high for the calibration curve were diluted and reassayed. Mouse cytokines were measured by using capture ELISAs and commercially available anticytokine Abs from BD-PharMingen as described above. All Abs came from BD Biosciences and were titrated for optimal use in assays prior to use. For the measurement of human cytokines, the following Abs were used: IL-3 capture, BVD8-3G11; IL-3 detection, BVD3-IF9; IL-4 capture, 8D4-8; IL-4 detection, MP4-25D2; IL-5 capture, TRFK5; IL-5 detection, JRS1-5A10; IL-10 capture, JES3-9D7; IL-10 detection, JES3-12G8; IL-13 capture, JES10-5A2; IL-13 detection, B69-2; IFN-γ capture, NIB42; IFN-γ detection, 45B3; TNF-α coating, monoclonal Ab1; and TNF-α detection, monoclonal Ab11. For the measurement of murine cytokines, the following Abs were used: IL-4 capture, BVD4-1D11; IL-4 detection, BVD6-24G2; IL-5 capture, TRFK5; IL-5 detection, TRFK4; IL-10 capture, JES-2A5; IL-10 detection, SXC-1; IFN-γ capture, R4-6A2; and IFN-γ detection, XMG1.2.
Statistical analysis.
All analysis used SPSS version 10. For each donor, the IL-3, IL-4, IL-5, IL-10, IL-13, IFN-γ, and TNF-α elicited in response to in vitro stimulation with SEA, SWA, and medium alone were assayed in duplicate. Initial analysis was carried out with nonmanipulated data. Exploratory analysis revealed that the data were nonparametrically distributed, and thus appropriate statistical tests were used. For the purposes of analysis, the cohort was divided into seven equal age groups (7 to 9, 10 to 12, 13 to 16, 17 to 23, 24 to 30, 31 to 37, and ≥38 years), and there were equal numbers of males and females in each group. For some analysis the cohort was divided into the following 3 egg count groups: 0 to 160, 161 to 1,200, and >1,200 epg. Correlations between variables were determined by calculating Spearman's rank correlation coefficient for each pair of immune responses. Differences between immune responses were determined by using either the Mann-Whitney test for nonparametric data to compare different responses in the same individuals or the Wilcoxon sign rank test when groups were analyzed pairwise. Detectable cytokine levels were calculated by using the sensitivity of the particular assay as a cutoff and excluding all values below this threshold. For some analysis the specific Ag-induced production of each cytokine was calculated. The spontaneous production was subtracted from that induced by Ag for each individual, and all positive values were included in further analysis.
RESULTS
Total cytokine production with different stimuli.
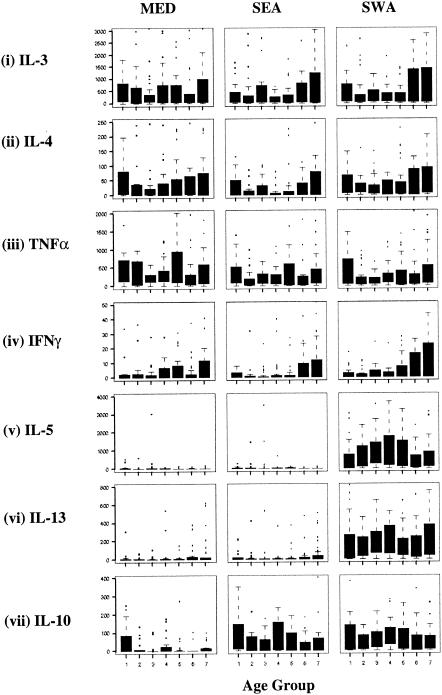
IL-3, IL-4, TNF-α, and IFN-γ were produced by all age groups in the absence of Ag (Fig. 1), and all of these responses, except spontaneous IFN-γ and TNF-α, were highly correlated with one another (Table 1). TNF-α and IL-3 were detectable in approximately 90%, and IL-4, IL-13 and IFN-γ in 39 to 62%, of the cultures in the absence of Ag (Table 2). The levels of spontaneous IL-3, IL-4, and TNF-α were high and were comparable to those seen in the presence of SEA or SWA (Fig. 1i to iii), whereas even though IL-13 was produced by 46% of the cultures in the absence of Ag (Table 2), the levels were very low (Fig. 1vi). Spontaneous IL-5 and IL-10 were produced by a small minority of the cultures, and, when detected, the levels were low (Table 2 and Fig. 1v and vii).
FIG. 1.
Total cytokine responses of the whole cohort plotted by age group. The cohort was divided into seven equal age groups (n = 32) as follows: 1, 7 to 9 years; 2, 10 to 12 years; 3, 13 to 16 years; 4, 17 to 20 years; 5, 21 to 30 years; 6, 31 to 38 years; and 7, >38 years. Cytokines were measured by ELISA after culturing whole blood with either no Ag stimulation (MED), SEA stimulation, or SWA stimulation. Cytokines were measured in picograms per milliliter, except for IFN-γ, which was measured in units per milliliter. The bars indicate 50% of values and are flanked by the upper 75% and the lower 25% of values, and the whiskers encompass the interquartile range. Any observations not covered by the whiskers are plotted separately.
TABLE 1.
Correlations between spontaneously produced cytokines
| Cytokine (n) | Spearman rank correlation coefficient (n)a with:
|
||
|---|---|---|---|
| IL-4 (137) | IFN-γ (89) | TNF-α (187) | |
| IL-3 (205) | +0.705*** (133) | +0.32** (86) | +0.66*** (172) |
| IL-4 (137) | +0.48*** (73) | +0.63*** (128) | |
| IFN-γ (89) | NS (83) | ||
Spearman rank correlation coefficients between different pairs of spontaneously produced (in the absence of Ag stimulation) cytokines (detectable in the absence of Ag stimulation). The number of donor samples included (n) in each correlation is indicated. ***, significant correlations (P ≤ 0.001); **, 0.001 < P < 0.01; *, 0.01 < P < 0.05; NS, not significant.
TABLE 2.
Percentages of SEA- and SWA-stimulated whole blood cultures producing detectable and Ag-specific cytokines in vitro
| Cytokine (n) | Response type | % Responsea with:
|
||
|---|---|---|---|---|
| No antigen | SEA | SWA | ||
| IL-3 (224) | Detectable | 91 | 87 | 95 |
| Antigen specific | 41 | 61 | ||
| IL-4 (220) | Detectable | 62 | 49 | 78 |
| Antigen specific | 18 | 54 | ||
| IL-5 (225) | Detectable | 26 | 28 | 87 |
| Antigen specific | 18 | 55 | ||
| IL-10 (226) | Detectable | 20 | 69 | 80 |
| Antigen specific | 39 | 52 | ||
| IL-13 (226) | Detectable | 46 | 59 | 92 |
| Antigen specific | 42 | 89 | ||
| IFN-γ (226) | Detectable | 39 | 37 | 49 |
| Antigen specific | 17 | 31 | ||
| TNF-α (215) | Detectable | 87 | 81 | 84 |
| Antigen specific | 30 | 32 | ||
The proportion containing detectable levels was calculated by dividing the number which were positive for each cytokine by the total number of blood samples assayed. The proportion containing SEA- or SWA-specific cytokines was calculated after subtracting the levels of cytokine measured in absence of Ag from those measured in the presence of SEA or SWA and dividing this by the total number of blood samples assayed.
SWA strongly induced IL-5 and IL-13 in a greater proportion of cultures and at higher levels than were seen in the absence of Ag or in the presence of SEA (Fig. 1v and vi; Table 2). Thus, 87% of cultures produced IL-5 in response to SWA, whereas the proportion which produced the cytokine in response to SEA (28%) was very similar to the proportion that produced IL-5 spontaneously (26%). Similarly, 92% of cultures produced IL-13 in response to SWA, whereas 59% produced the cytokine in response to SEA and 46% produced IL-13 in the absence of Ag.
Total cytokine production, donor age, and infection intensity.
Neither age nor infection intensity was strongly associated with the production of any the cytokines tested, although analysis both within and between different age groups generated a small number of significant relationships. SEA- and SWA-stimulated IL-3, IL-4, and IFN-γ production were highest in those over 31 years old, but only SWA-stimulated IFN-γ had a significant, albeit weak, overall positive correlation with age per se (r = +0.264; P < 0.001). SEA and SWA IL-3 were the only responses that were weakly (positively) correlated with infection intensity (SEA, r = +0.166 and P = 0.013; SWA, r = +0.162 and P = 0.016).
When the study cohort was divided into three numerically balanced categories of infection intensity (0 to 164, 165 to 1,210, and 1,211 to 8,100 epg), those in the highest infection group produced significantly more IL-3 (under all culture conditions) than those in the lowest infection group (SEA, P = 0.015; SWA, P = 0.011; medium with no Ag [MED], P = 0.031 [Mann-Whitney U test]). In those under 12 years of age, SWA-stimulated IL-5 was significantly higher in those who were most heavily infected (1,211 to 8,100 epg) than in the least infected group (P = 0.008). SWA, but not SEA, induced significantly higher levels of IL-3, IL-4, and IFN-γ than were seen in the absence of Ag (for SWA versus MED: IL-3, P = 0.005; IL-4, P < 0.001; IFN-γ, P = 0.028) in those over 16 years old.
The only cytokine which was produced at highest levels in response to SEA was IL-10 (Fig. 1vii and Table 2). When SEA-stimulated IL-10 production within each age group was compared to IL-10 production in response to SWA or without Ag (Mann-Whitney U test), only those under 9 years of age produced significantly higher levels of IL-10 in response to SEA than in response to either SWA (P = 0.003) or in the absence of Ag (P = 0.001). Both SEA and SWA reduced the levels of spontaneously produced TNF-α in cultures from individuals under 38 years old (MED compared with SEA, P < 0.001 [Wilcoxon test]) (Fig. 1iii), and SEA had a similar effect upon spontaneous IL-4 production (Fig. 1ii) but only in those under 16 years old (MED compared with SEA, P = 0.015).
SWA- and SEA-specific cytokine production.
The high levels of cytokines produced in the absence of Ag undoubtedly contributed to the levels seen in the presence of SWA or SEA, and this might have accounted for the fact that there were many strong positive correlations between different pairs of responses, even when the cytokine levels in response to MED, SEA, and SWA varied greatly (results not shown). To more clearly examine the effects of different Ags upon cytokine production, the level of each cytokine produced spontaneously was subtracted from the amount produced in the presence of Ag, and these responses were termed either SEA or SWA specific.
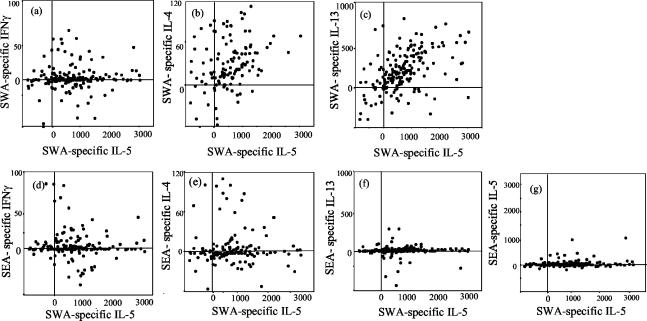
There was marked production of SWA-specific IL-4, IL-5, IL-13, and IFN-γ, with higher levels of these cytokines produced (Fig. 2) in a greater proportion of cultures (Table 2) in response to SWA than in response to SEA. The Spearman rank correlation coefficients between SWA-specific IL-4, IL-5, and IL-13 were all greater than +0.4 and were statistically significant (Table 3). SWA-specific IL-5 and IL-13 positively correlated with SWA-specific IFN-γ, but SWA-specific IL-4 did not (Table 3). SWA-specific IL-5 and IL-13 responses were the most strongly correlated (Table 3), and 58% of the cohort (n = 132) coproduced these two cytokines at similar levels in response to SWA (Fig. 2c).
FIG. 2.
Scatter plots of SWA-specific IL-5 against other SWA- and SEA-specific cytokine responses. Specific cytokines were calculated by subtracting the level of each cytokine produced in the absence of Ag from that produced in the presence of either SEA or SWA for each individual. Cytokines were measured in picograms per milliliter, except for IFN-γ, which was measured in units per milliliter. Values for specific cytokine production are shown for each individual, whether above or below zero.
TABLE 3.
Correlations between SWA-specific cytokines
| Cytokine (n) | Spearman rank correlation coefficient (n)a with:
|
|||
|---|---|---|---|---|
| IL-5 (124) | IL-13 (200) | IFN-γ (68) | IL-10 (115) | |
| IL-4 (122) | +0.47*** (114) | +0.41*** (117) | 0.26 (NS) (44) | +0.41*** (108) |
| IL-5 (124) | +0.68*** (132) | +0.49*** (16) | +0.33*** (109) | |
| IL-13 (200) | +0.28* (64) | +0.30*** (115) | ||
| IFN-γ (68) | 0.25 (NS) (40) | |||
Spearman rank correlation coefficients between pairs of SWA-specific cytokine responses. Specific responses were determined by subtracting the spontaneous response from that induced by Ag for each culture. Only those values above zero were included in the calculation of the correlation coefficient. The number of donor samples included (n) in each calculation is indicated. ***, significant correlations (P ≤ 0.001); **, 0.001 < P < 0.01; *, 0.01 < P < 0.05; NS, not significant.
The levels of SEA-specific IL-4, IL-5, IL-10, IL-13, and IFN-γ were calculated for each individual, and these responses were compared with each other and with SWA-specific IL-4, IL-5, IL-10, IL-13, and IFN-γ responses for each cytokine by using Spearman rank correlations. There were no significant correlations between any of the SEA-specific responses. Of all of the pairwise comparisons, the only significant correlations were between SEA- and SWA-specific IL-4 (r = +0.71; P < 0.001 [n = 28]) and between SEA- and SWA-specific IL-10 (r = +0.35; P < 0.001 [n = 112]). Figure 2 shows SWA-specific IL-5 plotted against SWA-specific IFN-γ, IL-4, and IL-13 and against SEA-specific IFN-γ, IL-4, IL-13, and IL-5 to illustrate the strong relationship between SWA-specific IL-5 and IL-13 responses and the lack of a relationship between SWA-specific and SEA-specific responses. These plots also illustrate the relatively low numbers of SEA-specific IFN-γ, IL-4, IL-13, and IL-5 responders and, particularly in the case of SEA-specific IL-5 and IL-13, the still smaller number who produced these responses at the high levels induced specifically by SWA.
Responses of murine splenocytes to SEA and SWA.
In order to ensure that the generally higher cytokine responses induced by SWA compared with SEA were not due to the use of a suboptimal batch of SEA, the same batches of SEA and SWA that had been used to stimulate human blood cultures were also used to stimulate the splenocytes from mice infected with S. mansoni. In contrast to the human responses, higher production of IL-4, IL-5, IL-10, and IFN-γ were seen in response to SEA than in response to SWA. The mean levels (± standard deviations) of each cytokine in response to SEA and SWA, respectively, were as follows: IL-4, 88 ± 7 and 43 ± 3 pg/ml; IL-5, 900 ± 380 and 145 ± 20 pg/ml; IL-10, 11,500 ± 1,620 and 5,060 ± 640 pg/ml; and IFN-γ, 1,656 ± 475 and 1,132 ± 323 pg/ml.
DISCUSSION
Schistosomiasis is a chronic condition and people living in high transmission areas can often be continuously exposed to infection and harbor the parasite for most of their lives. Chronically infected individuals are continuously exposed to the egg and the adult worm stages of the schistosome life cycle. In infected populations in Kenya, S. mansoni worms have been estimated to have a mean life span of approximately 9 years (12). Worms secrete some Ags continuously into the bloodstream (7) while it is likely that the host immune system is only exposed to other Ags when worms die. Large numbers of S. mansoni eggs are produced daily. While many are excreted in the feces, others become trapped in host tissues where they secrete soluble Ags for 4 to 5 weeks until they die, exposing their total somatic contents. Thus, throughout the course of repeated and overlapping human schistosome infections, secreted and total Ags from eggs, as well as some worm derived Ags are released into the host bloodstream and tissues.
There is little information about interactions between host age and/or infection intensity and cellular responses to S. mansoni in populations in areas of endemicity. Many interactions between the schistosome and its host are age, duration of infection, or infection intensity dependent. Schistosome-specific Ab isotype responses (34), susceptibility to reinfection (9, 15), and development of severe disease (30) are clearly age dependent in populations in areas of endemicity. SWA-specific IgE, which has been implicated in human resistance, increases with age (9, 26, 34), while humoral responses of some other isotypes decline (33). We have measured cytokines that have previously been described as playing important roles in the interactions between the host and the parasite. IL-4 is central to the orchestration of Th2 responses, to the regulation of IgE production (8), and also to the counterregulation of Th1 responses. IL-13 plays a role in the regulation of IgE (5) and has been shown to be the major profibrogenic cytokine in S. mansoni-infected mice (2). IL-5 is a hallmark cytokine of Th2 responses and is elevated during human and murine schistosome infection (14, 31). This cytokine regulates eosinophils, the levels of which correlate with resistance to reinfection in humans (16). IL-3 is a growth factor for basophils and is elevated in T cells during severe asthma (23). IFN-γ regulates and amplifies Th1 responses and has been associated with hepatosplenomegaly in S. mansoni-infected Kenyan children (27). TNF-α has been associated with schistosomiasis-associated morbidity in humans (21) and mice (17). IL-10 may down-regulate potentially harmful parasite-specific immune responses in humans (22) and mice (36).
We did not find any coherent patterns of association between cytokine production and age or infection intensity. The blood of older individuals made significantly higher levels of SWA-stimulated IFN-γ, and this was the only response that was related to age per se. Roberts and colleagues (31) reported that the level of S. mansoni SEA-specific IL-5 was age related after treatment for S. mansoni and also that there was an association between this response and resistance to subsequent reinfection. Our results might suggest that SWA-specific IL-5 contributes to resistance to infection, because the response was greatest in individuals who had the lowest infection intensity. This was, however, true only in individuals over 38 years old, and in younger individuals the same response was positively correlated with infection intensity. Of all of those measured, the only responses clearly related to infection intensity per se were SWA- and SEA-specific IL-3, suggesting that levels of infection could influence the production of this cytokine, which has, in turn, been shown to play a role in regulation of allergic Th2 responses (23).
Spontaneous IL-3 and TNF-α were produced by almost all individuals; spontaneous IL-4, IL-13, and IFN-γ were produced by many; and the production of IL-5 and IL-10 was highly dependent upon Ag stimulation in vitro. The levels of spontaneous IL-5, IL-10, IL-13, and IFN-γ were relatively low, but the levels of IL-3, IL-4, and TNF-α were higher and generally were not increased by the addition of Ag. The spontaneous production of these cytokines did not show any relationship with S. mansoni infection intensity. Acute S. mansoni infection has, however, been associated with the spontaneous production of IL-6 and IFN-γ (6), while high and variable spontaneous levels of TNF-α from cultured peripheral blood mononuclear cells from individuals from both an epidemic focus of S. mansoni in Senegal (24) and an endemic focus in Kenya (31) have been reported.
IL-5, IL-13, IL-4, and IFN-γ were frequently seen in response to SWA, whereas these cytokines were rarely seen specifically in response to SEA. IL-10 was the only cytokine which was seen in response to both SWA and SEA. These results suggest that the response to SWA was not polarized towards Th1 or Th2 but was a mixed Th1-Th2 response, as has been previously suggested from a smaller-cohort study of S. mansoni-infected Brazilians in which cytokine production by peripheral blood mononuclear cells was measured by PCR (35). Parasite-specific T-cell clones from S. mansoni-infected Brazilians produced both IFN-γ and IL-4 in response to parasite Ag in vitro, leading the authors to conclude that they were polarized to neither Th1 nor Th2 but were rather “Th0” (4). In contrast to the vigorous and mixed response of the cohort to SWA, only IL-10 was seen specifically in response to SEA.
When the same batches of SEA and SWA were used to stimulate splenocytes from infected mice, cytokine production was skewed towards the production of IL-4, IL-5, and IL-10, and these cytokines were induced predominantly by SEA, suggesting that the relative lack of responsiveness to SEA demonstrated by the Ugandan cohort was not due to a suboptimal preparation of the Ag. The mice were acutely exposed to a large cercarial challenge, as the aim was only to produce responsive cells in order to test the batches of SEA and SWA used in the human studies. The responses of these infected mice to SEA were, however, typical of those in many other previous studies (14, 29).
Unavoidable differences between experimental murine and natural human infections with S. mansoni may account for the differences in the Ag specificity of responses which we have described. The lightest possible experimental murine infection is relatively much heavier than those typically seen in humans (on a body weight-per-worm pair basis). Most experimental murine infections are acute or subacute and are monitored for several months at most, whereas individuals living in areas of endemicity have often been chronically infected for many years. In a typical murine infection, the immune system would not be exposed to dying adult worms, because the average adult S. mansoni worm would outlive a mouse by several years (12). Typically SEA-specific immune responses in experimentally infected mice coincide with the onset of egg laying, when infected animals are exposed to egg-derived antigens (14). This contrasts sharply with what is seen during experimental infections of rats, during which worms die within 4 weeks of infection before egg laying takes place, but specific Th2 responses still predominate (20). During chronic human infections, immune responses to SEA and SWA may interact and influence each other. It is possible that the vigorous SWA-specific responses that we have observed in vitro (in the absence of SEA) may to some extent be suppressed in vivo by SEA-induced regulatory factors such as IL-10. It is worth noting that in other human studies we have observed vigorous responses to SEA and negligible responses to SWA. In a study of hepatosplenomegaly in Kenyan schoolchildren in an area where transmission of S. mansoni is low to moderate, SEA-specific TNF-α levels were much greater than in this Ugandan cohort, whereas SWA-specific responses were lower (unpublished data). Thus, the duration and high intensity of infection experienced by this Ugandan cohort, in addition to other undefined factors, may well influence human responses to chronic S. mansoni infection.
By selecting a cohort from individuals who had been resident in an area of high S. mansoni transmission for at least 10 years (or since birth) but which was randomized and balanced for age and sex, we were able to examine the influence of these factors on parasite-specific cytokine responses. In contrast to humoral responses and many other aspects of host-parasite interactions in areas of endemicity, S. mansoni-specific cytokine responses were not strongly influenced by host age or intensity of infection but were strongly influenced by the parasite life stage from which stimulating Ags were derived. The responses to SEA were restricted to IL-10, whereas vigorous and mixed responses consisting of IL-4, IL-5, IL-10, IL-13, and IFN-γ were seen in response to SWA. These responses differ from what has been reported in (i) experimental studies in the mouse, (ii) human studies in more recently established foci of endemic or epidemic schistosomiasis, and (iii) our own observations of hepatosplenic children living in areas with less intense transmission. These differences in response to schistosomiasis reinforce the need for human immunological studies to be carried out in a wide variety of epidemiological situations if we are to understand immunity and immunomorbidity in human schistosomiasis and extrapolate from the wealth of detailed immunological knowledge generated from studies in animal models.
Acknowledgments
This study was made possible by funding from the MRC and the Commission of the European Community's Science and Technology for Development program (INCO-DC contract IC18 CT97-0237 and INCO-DEV contract ICA4-CT-1999-10003).
We thank the people of Booma for their participation in this study. We thank Klaudia Walter for helpful discussions and for help with preparation of the figures.
Informed consent was obtained from all who participated in this study, in line with the national guidelines of the Ugandan Ministry of Health, whose ethical review committees approved all of the protocols used.
Editor: W. A. Petri, Jr.
Footnotes
Jovanice Kemijumbi passed away during the preparation of this paper. She made a major contribution to the work presented here and is greatly missed.
REFERENCES
- 1.Araujo, M. I., A. R. de Jesus, O. Bacellar, E. Sabin, E. Pearce, and E. M. Carvalho. 1996. Evidence of a T helper type 2 activation in human schistosomiasis. Eur. J. Immunol. 26:1399-1403. [DOI] [PubMed] [Google Scholar]
- 2.Chiaramonte, M. G., L. R. Schopf, T. Y. Neben, A. W. Cheever, D. D. Donaldson, and T. A. Wynn. 1999. IL-13 is a key regulatory cytokine for Th2 cell-mediated pulmonary granuloma formation and IgE responses induced by Schistosoma mansoni eggs. J. Immunol. 162:920-930. [PubMed] [Google Scholar]
- 3.Correa-Oliveira, R., L. C. C. Malaquais, P. L. Falcao, I. R. C. Viana, L. M. G. Bahia-Oliveiria, A. M. S. Silveira, L. A. O. Fraga, A. Prata, R. L. Coffmann, J. R. Lambertucci, J. R. Cunha-Melo, O. A. Martins-Filho, R. A. Wilson, and G. Gazzinelli. 1998. Cytokines as determinants of resistance and pathology in human Schistosoma mansoni infection. Brazilian J. Med. Biol. Res. 31:171-177. [DOI] [PubMed] [Google Scholar]
- 4.Couissinier-Paris, P., and A. J. Dessein. 1995. Schistosoma-specific helper T cell clones from subjects resistant to infection by Schistosoma mansoni are Th0/2. Eur. J. Immunol. 25:2295-2302. [DOI] [PubMed] [Google Scholar]
- 5.De France, T., P. Carayon, G. Billian, J. C. Guillemot, A. Minty, D. Caput, and P. Ferrara. 1994. Interleukin 13 is a B cell stimulating factor. J. Exp. Med. 179:135-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Jesus, A. R., A. Silva, L. B. Santana, A. Magalhaes, A. A. de Jesus, R. P. de Almeida, M. A. Rego, M. N. Burattini, E. J. Pearce, and E. M. Carvalho. 2002. Clinical and immunologic evaluation of 31 patients with acute schistosomiasis mansoni. J. Infect. Dis. 185:98-105. [DOI] [PubMed] [Google Scholar]
- 7.De Jonge, N., P. Caluwe, G. W. Hilberath, T. W. Krijger, A. M. Polderman, and A. M. Deelder. 1989. Circulating aniodic antigen levels in serum before and after chemotherapy with Praziquantel in Schistosomiasis mansoni. Trans. R. Soc. Trop. Med. Hyg. 83:368-372. [DOI] [PubMed] [Google Scholar]
- 8.De Vries, J. E., J. M. Carbadillo, and G. Aversa. 1999. Receptors and cytokines involved in allergic Th2 cell responses. J. Allergy Clin. Immunol. 103:S492-496. [DOI] [PubMed] [Google Scholar]
- 9.Dunne, D. W., A. E. Butterworth, A. J. Fulford, H. C. Kariuki, J. G. Langley, J. H. Ouma, A. Capron, R. J. Pierce, and R. F. Sturrock. 1992. Immunity after treatment of human schistosomiasis: association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur. J. Immunol. 22:1483-1494. [DOI] [PubMed] [Google Scholar]
- 10.Dunne, D. W., A. E. Butterworth, A. J. Fulford, J. H. Ouma, and R. F. Sturrock. 1992. Human IgE responses to Schistosoma mansoni and resistance to reinfection. Mem. Inst. Oswaldo Cruz 87:99-103. [DOI] [PubMed] [Google Scholar]
- 11.Dutra, W. O., R. Correa-Oliveira, D. Dunne, L. Cecchini, L. Fraga, M. Roberts, A. Soares-Silveira, M. Webster, H. Yssel, and K. J. Gollob. 2002. Polarized Th2 like cells, in the absence of Th0 cells, are responsible for lymphocyte produced IL-4 in high IgE-producer schistosomiasis patients. BMC Immunol. 3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fulford, A. J., A. E. Butterworth, J. H. Ouma, and R. F. Sturrock. 1995. A statistical approach to schistosome population dynamics and estimation of the lifespan of Schistosoma mansoni in man. Parasitology 110:307-316. [DOI] [PubMed] [Google Scholar]
- 13.Grogan, J. L., P. G. Kremsner, A. M. Deelder, and M. Yazdanbakhsh. 1998. Antigen-specific proliferation and interferon-gamma and interleukin-5 production are down-regulated during Schistosoma haematobium infection. J. Infect. Dis. 177:1433-1437. [DOI] [PubMed] [Google Scholar]
- 14.Grzych, J. M., E. Pearce, A. Cheever, Z. A. Caulada, P. Caspar, S. Heiny, F. Lewis, and A. Sher. 1991. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J. Immunol. 146:1322-1327. [PubMed] [Google Scholar]
- 15.Hagan, P., U. J. Blumenthal, D. Dunn, A. J. Simpson, and H. A. Wilkins. 1991. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature 349:243-245. [DOI] [PubMed] [Google Scholar]
- 16.Hagan, P., H. A. Wilkins, U. J. Blumenthal, R. J. Hayes, and B. M. Greenwood. 1985. Eosinophilia and resistance to Schistosoma haematobium in man. Parasite Immunol. 7:625-632. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann, K. F., P. Caspar, A. W. Cheever, and T. A. Wynn. 1998. IFN-gamma, IL12 and TNF-alpha are required to maintain reduced liver pathology in mice vaccinated with Schistosoma mansoni eggs and IL-12. J. Immunol. 161:4201-4210. [PubMed] [Google Scholar]
- 18.Kabatereine, N. B., B. J. Vennervald, J. H. Ouma, J. Kemijumbi, A. E. Butterworth, D. W. Dunne, and A. J. Fulford. 1999. Adult resistance to schistosomiasis mansoni: age-dependence of reinfection remains constant in communities with diverse exposure patterns. Parasitology 118:101-105. [DOI] [PubMed] [Google Scholar]
- 19.Katz, N., A. Chaves, and J. Pelligrino. 1972. Simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev. Inst. Med. Trop. Sao Paulo 14:397-400. [PubMed] [Google Scholar]
- 20.Khalife, J., C. Cetre, C. Pierot, and M. Capron. 2000. Mechanisms of resistance to S. mansoni infection: the rat model. Parasitol. Int. 49:339-345. [DOI] [PubMed] [Google Scholar]
- 21.King, C. L., I. Malhotra, P. Mungai, A. Wamachi, J. Kioko, E. Muchiri, and J. H. Ouma. 2001. Schistosoma haematobium-induced urinary tract morbidity correlates with increased tumor necrosis factor-alpha and diminished interleukin-10 production. J. Infect. Dis. 184:1176-1182. [DOI] [PubMed] [Google Scholar]
- 22.King, C. L., A. Medhat, I. Malhotra, M. Nafeh, A. Helmy, J. Khaudary, S. Ibrahim, M. El-Sherbiny, S. Zaky, R. J. Stupi, K. Brustoski, M. Shehata, and M. T. Shata. 1996. Cytokine control of parasite-specific anergy in human urinary schistosomiasis. IL-10 modulates lymphocyte reactivity. J. Immunol. 156:4715-4721. [PubMed] [Google Scholar]
- 23.Lai, C. K., S. S. Ho, C. H. Chan, R. Leung, and K. N. Lai. 1996. Gene expression of interleukin-3 and granulocyte macrophage colony-stimulating factor in circulating CD4+ T cells in acute severe asthma. Clin. Exp. Allergy 26:138-146. [DOI] [PubMed] [Google Scholar]
- 24.Marguerite, M., M. C. Gallissot, M. Diagne, C. Moreau, M. M. Diakkhate, M. Roberts, F. Remoue, A. Thiam, C. Decam, F. Rogerie, F. Cottrez, J. L. Neyrinck, A. E. Butterworth, R. F. Sturrock, J. P. Piau, B. Daff, M. Niang, I. Wolowczuk, G. Riveau, C. Auriault, and A. Capron. 1999. Cellular immune responses of a Senegalese community recently exposed to Schistosoma mansoni: correlations of infection level with age and inflammatory cytokine production by soluble egg antigen-specific cells. Trop. Med. Int. Health 4:530-543. [DOI] [PubMed] [Google Scholar]
- 25.Montenegro, S. M., P. Miranda, S. Mahanty, F. G. Abath, K. M. Teixeira, E. M. Coutinho, J. Brinkman, I. Goncalves, L. A. Domingues, A. L. Domingues, A. Sher, and T. A. Wynn. 1999. Cytokine production in acute versus chronic human Schistosomiasis mansoni: the cross-regulatory role of interferon-gamma and interleukin-10 in the responses of peripheral blood mononuclear cells and splenocytes to parasite antigens. J. Infect. Dis. 179:1502-1514. [DOI] [PubMed] [Google Scholar]
- 26.Mutapi, F., P. D. Ndhlovu, P. Hagan, and M. E. Woolhouse. 1997. A comparison of humoral responses to Schistosoma haematobium in areas with low and high levels of infection. Parasite Immunol. 19:255-263. [DOI] [PubMed] [Google Scholar]
- 27.Mwatha, J. K., G. Kimani, T. Kamau, G. G. Mbugua, J. H. Ouma, J. Mumo, A. J. Fulford, F. M. Jones, A. E. Butterworth, M. B. Roberts, and D. W. Dunne. 1998. High levels of TNF, soluble TNF receptors, soluble ICAM-1, and IFN-gamma, but low levels of IL-5, are associated with hepatosplenic disease in human schistosomiasis mansoni. J. Immunol. 160:1992-1999. [PubMed] [Google Scholar]
- 28.Ndhlovu, P., H. Cadman, B. J. Vennervald, N. O. Christensen, M. Chidimu, and S. K. Chandiwana. 1996. Age-related antibody profiles in Schistosoma haematobium infections in a rural community in Zimbabwe. Parasite Immunol. 18:181-191. [DOI] [PubMed] [Google Scholar]
- 29.Pearce, E. J., and A. S. MacDonald. 2002. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2:499-511. [DOI] [PubMed] [Google Scholar]
- 30.Qurashi, M. A., N. E. M. A. Elwali, A. A. Abdelhameed, A. Mergani, S. Rahoud, K. E. Elagib, O. K. Saeed, M. M. A. Magzoub, and A. J. Dessein. 1999. Susceptibility to periportal (Symmers) fibrosis in human Schistosoma mansoni infections: evidence that intensity and duration of infection, gender, and inherited factors are critical in disease progression. J. Infect. Dis. 180:1298-1306. [DOI] [PubMed] [Google Scholar]
- 31.Roberts, M., A. E. Butterworth, G. Kimani, T. Kamau, A. J. Fulford, D. W. Dunne, J. H. Ouma, and R. F. Sturrock. 1993. Immunity after treatment of human schistosomiasis: association between cellular responses and resistance to reinfection. Infect. Immun. 61:4984-4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Dam, G. J., F. F. Stelma, B. Gryseels, S. T. Falcao Ferreira, I. Talla, M. Niang, J. P. Rotmans, and A. M. Deelder. 1996. Antibody response patterns against Schistosoma mansoni in a recently exposed community in Senegal. J. Infect. Dis. 173:1232-1241. [DOI] [PubMed] [Google Scholar]
- 33.Webster, M., P. G. Fallon, A. J. Fulford, A. E. Butterworth, J. H. Ouma, G. Kimani, and D. W. Dunne. 1997. IgG4 and IgE responses to Schistosoma mansoni adult worms after treatment. J. Infect. Dis. 175:493-494. [DOI] [PubMed] [Google Scholar]
- 34.Webster, M., B. D. Libranda-Ramirez, G. D. Aligui, R. M. Olveda, J. H. Ouma, H. C. Kariuki, G. Kimani, G. R. Olds, A. J. Fulford, A. E. Butterworth, and D. W. Dunne. 1997. The influence of sex and age on antibody isotype responses to Schistosoma mansoni and Schistosoma japonicum in human populations in Kenya and the Philippines. Parasitology 114:383-393. [DOI] [PubMed] [Google Scholar]
- 35.Williams, M. E., S. Montenegro, A. L. Domingues, T. A. Wynn, K. Teixeira, S. Mahanty, A. Coutinho, and A. Sher. 1994. Leukocytes of patients with Schistosoma mansoni respond with a Th2 pattern of cytokine production to mitogen or egg antigens but with a Th0 pattern to worm antigens. J. Infect. Dis. 170:946-954. [DOI] [PubMed] [Google Scholar]
- 36.Wynn, T. A., and A. W. Cheever. 1995. Cytokine regulation of granuloma formation in schistosomiasis. Curr. Opin. Immunol. 7:505-511. [DOI] [PubMed] [Google Scholar]