Abstract
Buruli disease, caused by Mycobacterium ulcerans, is the third most important mycobacterial disease in humans besides tuberculosis and leprosy. We have compared systemic and intralesional cytokine production in patients presenting with a nodular form and a necrotizing, ulcerative form of the disease. Gamma interferon (IFN-γ) levels in response to whole M. ulcerans and Mycobacterium bovis BCG bacilli and in response to purified Ag85 protein from BCG were lower in peripheral blood mononuclear cells (PBMC) cultures from Buruli disease patients than in PBMC from healthy purified protein derivative-positive contacts. Interleukin-4 (IL-4) and IL-13 content was below the detection threshold in these PBMC cultures. IFN-γ production after stimulation with M. ulcerans was significantly lower (P < 0.05) in PBMC cultures from patients with ulcers than in those from patients with nodules. On the other hand, PBMC from Buruli disease patients produced significant levels of IL-10 in response to M. ulcerans (but not to M. bovis BCG) and production was highest in patients with the ulcerative form. Third, semiquantitative reverse transcription-PCR analysis demonstrated a similar difference in the local, intralesional cytokine profile for the two forms of the disease: high IFN-γ but low IL-10 mRNA levels in nodular lesions and high IL-10 but low IFN-γ mRNA levels in ulcerative lesions. Intralesional IL-4 and IL-13 mRNA levels were low and only detected in patients with the ulcerative form. Our results indicate, although they do not formally prove, that production of IL-10 rather than production of IL-4 or IL-13 by Th2-type T cells may be involved in the low M. ulcerans-specific IFN-γ response in Buruli disease patients.
Buruli disease, caused by Mycobacterium ulcerans, is the third most important mycobacterial disease in humans besides tuberculosis and leprosy (28). Manifestations of M. ulcerans disease can range from a painless nodule to an ulcer with undermined edges lined by necrotic tissue, the so-called Buruli ulcer, that can enlarge extensively, eventually lead to osteomyelitis, and necessitate amputation of the infected limb (30).
The precise role of the immune response in M. ulcerans infection is not very clear. The absence of a positive skin test (Burulin test) in Buruli disease patients is often considered an indicator of a weak cellular immune response. However, spontaneous healing can occur and is generally accompanied by a positivation of this Burulin test. In vitro immune analysis has confirmed the notion of a systemic T-cell anergy, as peripheral blood mononuclear cells (PBMC) from patients with active disease or from patients who had recovered from surgical excision of Buruli ulcer showed significantly reduced lymphoproliferation and gamma interferon (IFN-γ) production in response to stimulation with Mycobacterium bovis BCG or Mycobacterium ulcerans (13). Recently, Gooding et al., analyzing the systemic cytokine production of PBMC from patients with Buruli disease and their household contacts, suggested that Th1 responses may prevent the development of the disease (14). Little is known about the local immune responses that develop at the site of infection, that is, in the skin.
In this study we compared systemic and intralesional cytokine expression in patients presenting with the two forms of Buruli disease, nodule and ulcer. First, we confirmed the notion of a systemic T-cell anergy towards mycobacterial antigens in general in patients with Buruli disease. Furthermore, we describe an M. ulcerans-specific, decreased IFN-γ production by PBMC from patients presenting with ulcerative lesions compared to patients with the nodular form. Finally, with semiquantitative reverse transcription-PCR analysis, we show differences in intralesional IFN-γ and interleukin-10 (IL-10) expression profiles in patients suffering from the two forms of the disease. High IFN-γ but low IL-10 mRNA levels were detected in nodular lesions, whereas high IL-10 but low IFN-γ mRNA levels were found in ulcerative lesions.
SUBJECTS AND METHODS
Patients and control subjects.
Fourteen patients 4 to 50 years old were included in this study. Heparinized blood and skin biopsy samples were collected prior to any treatment. The diagnosis for all patients was confirmed by the detection of M. ulcerans in the lesions with the IS2404-specific PCR (24) and/or by M. ulcerans culture at 30 to 32°C (Table 1). None of the patients were seropositive for human immunodeficiency virus and none suffered from active tuberculosis. Informed consent was obtained from the patients, and the human experimentation guidelines of the Centre Hospitalier Andrée Rosemon in Cayenne, French Guyana, were followed in the conduct of this research.
TABLE 1.
Clinical data for patients presenting with lesions due to M. ulcerans infection
| Patient no. | Age (yr) | Sex | Duration of illness (days) | Location of lesions | Lesion forma | Test result
|
||
|---|---|---|---|---|---|---|---|---|
| Ziehl | PCR | Cultureb | ||||||
| 1 | 39 | F | 21 | Lower limb | Nodule | + | + | ND |
| 2 | 38 | F | 90 | Lower limb | Nodule | − | + | + |
| 3 | 32 | F | 180 | Lower limb | Nodule | + | + | + |
| 4 | 4 | F | 180 | Lower limb | Nodule | + | + | − |
| 5 | 50 | M | 360 | Abdomen/thorax | Nodule | − | + | − |
| 6 | 43 | M | 60 | Lower limb | Ulcer | + | + | ND |
| 7 | 38 | F | 60 | Lower limb | Ulcer | + | + | + |
| 8 | 15 | M | 90 | Lower and upper limbs | Ulcer | − | + | + |
| 9 | 48 | F | 120 | Lower limb | Ulcer/plaque | + | + | + |
| 10 | 5 | M | 120 | Lower limb | Ulcer | + | + | − |
| 11 | 50 | F | 180 | Lower limb | Ulcer | + | + | − |
| 12 | 28 | M | 120 | Lower limb | Ulcer | + | + | + |
| 13 | 16 | F | 100 | Lower limb | Ulcer | + | + | − |
| 14 | 41 | F | 120 | Lower Limb | Ulcer | + | + | + |
Clinical data follow the instructions of the Global Buruli Ulcer Initiative group (2).
ND, not determined.
Patients were analyzed according to the instructions of the Global Buruli Ulcer Initiative (2), and their classification is reported in Table 1. Ten control subjects were selected among a group of healthy adults living in the same conditions as the patients but who had no history or clinical signs of M. ulcerans infection. Patients and controls had all received a BCG vaccination. Controls were positive to tuberculin test (induration > 10 mm at 72 h), but the tuberculin sensitivity of the patients was unknown. Blood samples were collected into sterile tubes (Veinoject; Terumo, Leuven, Belgium). Biopsy specimens were taken at the edge of the lesions, at the border of healthy skin (5 mm or more) but including necrotic base and deep tissue, and all parts of the biopsy were processed for cytokine expression.
RNA extraction from biopsy specimens and competitive PCR cytokine analysis.
Total RNA was extracted from biopsies as previously described (4). First-strand cDNA synthesis was performed on total RNA with a first-strand cDNA synthesis kit (Amersham-Pharmacia Biotech). The semiquantitative reverse transcription-PCR analysis was done with the competitors pQA-1 and pQB-3, with the β-actin gene as a housekeeping gene (provided by David Shire, Sanofi Recherche, Labège, France) as previously described (4). Briefly, a constant amount of cDNA was amplified in the presence of fivefold competitor dilutions. After separation of the PCR products for electrophoresis in an agarose gel containing ethidium bromide, we calculated the ratio of the concentration of the cytokine gene to the relative concentration of β-actin. Results are expressed as arbitrary units (A.U.) of this ratio × 100. The detection limit of this assay is 0.0032 pg/μl.
Antigens.
The 30- to 32-kDa Ag85 protein (Ag85A plus Ag85B plus Ag85C) was purified from a 2-week-old culture filtrate of M. bovis BCG (strain GL2) by sequential chromatography on phenyl-Sepharose, DEAE-Sephacel, and Sephadex G75 and used at 10 μg/ml (7). M. ulcerans (type 1 strain 5150, from the Democratic Republic of Congo) was isolated at the Institute of Tropical Medicine of Antwerp. Bacteria were grown on Löwenstein-Jensen at 32 to 33°C for approximately 1 month. Bacteria were resuspended and homogenized in 1 ml of Dubos broth medium (Difco) containing 0.4 g of 2-mm-diameter glass beads (VEL) (27). M. bovis BCG (strain GL2) was grown for 2 weeks as a surface pellicle at 37°C on synthetic Sauton medium and homogenized with metallic beads. Bacterial preparations were killed by heating at 80°C for 2 h. Whole heat-killed mycobacterial suspensions were used at 5 μg/ml (27).
Lymphocyte culture and detection of cytokines.
PBMC were isolated after venipuncture on a Ficoll-Hypaque gradient (d = 1.077) and resuspended in RPMI medium supplemented with 2 mM l-glutamine, 100 U of penicillin per ml, 1 μg of streptomycin per ml (all from Sigma), and 10% human heat-inactivated AB serum. Cultures for cytokine production (106 cells in 1 ml of culture medium) were performed in flat-bottomed 24-well plates with or without antigen. The supernatants were harvested after 2 and 7 days for IFN-γ, IL-4, IL-10, and IL-13 production and stored at −20°C. Cytokine production was quantified with specific IL-4, IL-13, IL-10, and IFN-γ enzyme-linked immunosorbent assays (ELISAs) (Pharmingen, San Diego, Calif.). All ELISAs had a sensitivity of 10 pg/ml.
Statistical analysis.
Wilcoxon rank sum test was used to compare the levels of cytokines. A P value of <0.05 was considered statistically significant.
RESULTS
Systemic T-cell anergy of patients with Buruli disease compared to healthy tuberculin-positive controls.
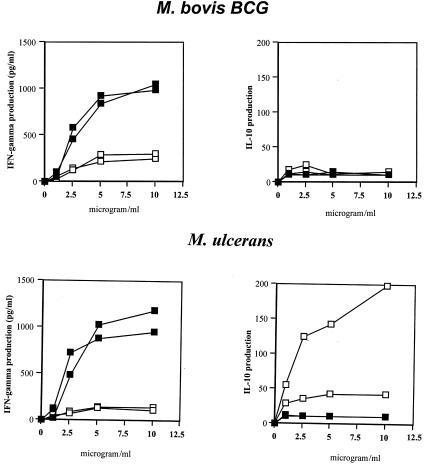
As shown in the preliminary dose-response curve in Fig. 1, the optimal dose of whole killed M. bovis BCG or M. ulcerans bacilli for the induction of IFN-γ and IL-10 in PBMC cultures from two patients and two healthy controls was found to be 5 μg/ml. This antigen concentration was used throughout the entire study. PBMC from patients and healthy purified protein derivative-positive controls were stimulated with whole M. bovis BCG and M. ulcerans bacilli or with purified Ag85 protein from M. bovis BCG culture filtrate. IFN-γ, IL-4, IL-13, and IL-10 production was measured by ELISA in culture supernatants after 2 and 7 days of culture. Production of either of the four cytokines was below the detection limit (10 pg/ml) in the 2-day culture supernatant, and therefore only results after 7 days of stimulation were analyzed further. IL-4 and IL-13 production in response to the different antigens in either patients or in the healthy control subjects was also below the detection level in the 7-day culture supernatant (data not shown).
FIG. 1.
Dose-response curve of IFN-γ and IL-10 production by PBMC from two patients with Buruli disease (open squares) and two purified protein derivative-positive controls (closed squares) after 7 days of stimulation with whole killed M. bovis BCG and M. ulcerans bacteria.
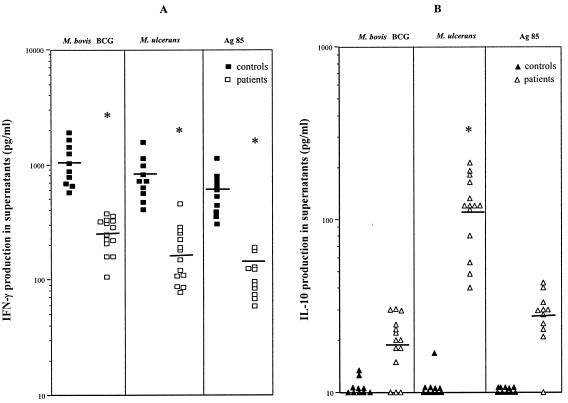
IFN-γ production in response to M. bovis BCG and M. ulcerans was lower in patients with Buruli disease than in tuberculin-positive controls that shared the same living conditions (Fig. 2A). Interestingly, production of IFN-γ in response to purified Ag85 from M. bovis BCG was also significantly lower in patients than in controls (Fig. 2A). Since IFN-γ responses to phytohemagglutinin were identical in the control (1,266 ± 148 pg/ml) and the patient groups (1,058 ± 105 pg/ml), these results are strongly indicative of a lower systemic responsiveness to mycobacterial antigens in patients suffering from Buruli disease. IL-10 production in response to either M. bovis BCG, M. ulcerans, or Ag85 was below threshold levels in healthy controls, but it was detectable in all patients with Buruli disease in response to stimulation with M. ulcerans (but not with M. bovis BCG or Ag85), suggesting that some specific antigen from M. ulcerans could be responsible for this IL-10 production (Fig. 2B)
FIG. 2.
Individual IFN-γ (A) and IL-10 (B) production by PBMC from 14 patients with Buruli disease and 10 purified protein derivative-positive controls after 7 days of stimulation with whole killed M. bovis BCG and M. ulcerans bacteria and Ag85 protein from M. bovis BCG culture filtrate. Horizontal lines indicate the mean for each group. *, P < 0.05, Wilcoxon sum rank test, compared to production in healthy controls.
Differential cytokine production by PBMC from patients presenting with nodular and ulcerative forms of Buruli disease.
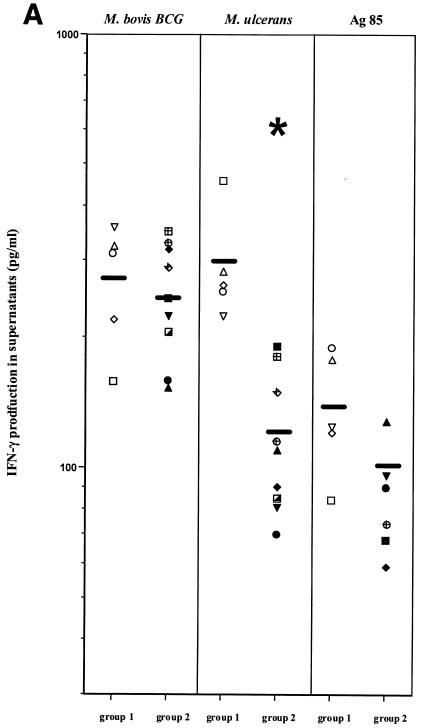
IFN-γ and IL-10 production was compared in PBMC cultures from five patients presenting with nodular lesions and from nine patients with ulcerative lesions. IFN-γ production was identical in response to M. bovis BCG in both patient groups (Fig. 3A). Levels of IFN-γ secretion in response to M. ulcerans were however significantly lower in patients suffering from ulcerative lesions than in patients with nodular lesions (P < 0.05, Fig. 3A). IFN-γ production in response to purified Ag85 from BCG was also lower in patients suffering from ulcerative lesions than in patients with nodular lesions, but this difference was not statistically different.
FIG. 3.
IFN-γ (A) and IL-10 (B) production in PBMC from Buruli disease patients with nodular and ulcerative forms of the disease. Cytokine levels in 7-day culture supernatants of PBMC stimulated with M. bovis BCG, M. ulcerans, and Ag85 from M. bovis BCG were measured by ELISA as described in the Materials and Methods section. Data are represented as individual values obtained for five subjects with nodules (group 1) and nine with ulcers (group 2). *, P < 0.05, Wilcoxon sum rank test, compared to production in patients with nodules.
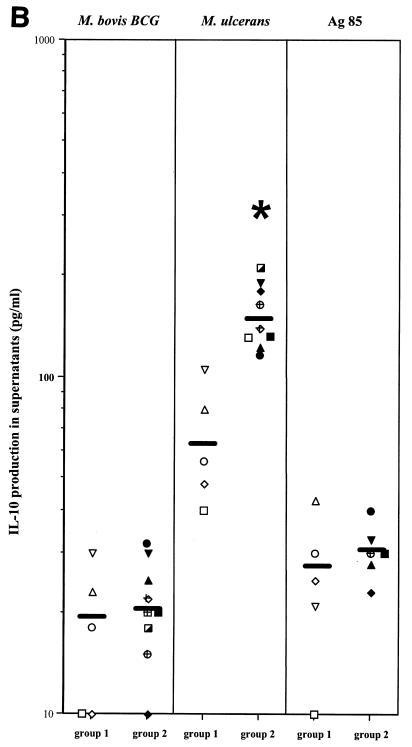
Significant levels of IL-10 were detected in response to M. ulcerans in PBMC cultures from Buruli disease patients, and these levels were higher in PBMC cultures from patients with ulcerative than from patients with nodular forms of disease (P < 0.05, Fig. 3B). IL-10 levels were close to the detection limit (10 to 20 pg/ml) after in vitro stimulation with M. bovis BCG or purified Ag85 in the same Buruli disease patients.
Differential expression of IL-10 and IFN-γ in skin biopsies from patients with Buruli disease.
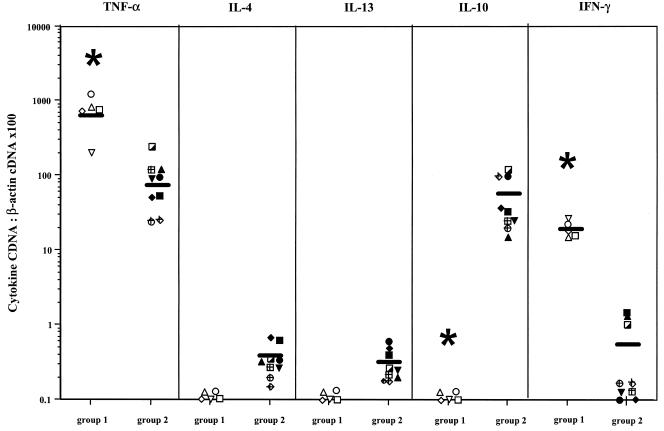
Intralesional cytokine expression was examined by semiquantitative reverse transcription-PCR in patients with Buruli disease presenting either with nodules or with necrotizing ulcers (Fig. 4). Tumor necrosis factor alpha (TNF-α) transcripts were expressed in lesions from both patient groups, and levels of TNF-α expression were higher in nodular (mean, 600 A.U.) than in ulcerative lesions (mean, 75 A.U.). IL-4, IL-13, and IL-10 mRNA levels were below the limit of detection in nodular lesions. Some IL-4 and IL-13 mRNA was detected in all patients with ulcerative lesions, albeit at very low levels (mean, 0.36 A.U. and 0.31 A.U. for IL-13 and IL-4, respectively). IL-10 mRNA, on the other hand, could be detected in ulcerative lesions at significant levels (mean, 53.5 A.U.). IFN-γ mRNA transcripts were detected in all nodular lesions (mean, 18.2 A.U.) but only in three out the nine ulcerative lesions (1.5, 1.3, and 1.0 A.U.). In conclusion, similar to the systemic responses, the local cytokine expression profile was different in nodular and ulcerative lesions, the former being characterized by high IFN-γ and low IL-10 expression and the latter by high IL-10 and low IFN-γ expression.
FIG. 4.
TNF-α, IL-4, IL-13, IL-10, and IFN-γ mRNA levels in lesions of patients with the nodular form (group1) and ulcerative form (group 2) of Buruli disease. The results are expressed as a ratio between the concentration of cytokine cDNA and that of β-actin cDNA. Each point is the mean of two or three separate determinations. Symbols used for individual patients correspond to the symbols in Fig. 3A and 3B. *, P < 0.05, Wilcoxon sum rank test, compared to production in patients with ulcers.
DISCUSSION
The results presented in this paper show for the first time a difference in intralesional cytokine expression profile in two distinct forms of Buruli disease, the nodular and necrotizing ulcerative forms. Using reverse transcription-PCR analysis, we have demonstrated high levels of IFN-γ message but undetectable IL-10 message in lesions from patients suffering from the nodular form and high levels of IL-10 but low to undetectable levels of IFN-γ message in lesions from patients with the ulcerative form. Some IL-4 and IL-13 mRNAs could be detected by reverse transcription-PCR in all ulcerative lesions, but mRNA levels were very low. This difference in intralesional cytokine expression profile was also reflected by differences in the systemic production of IL-10 and IFN-γ in PBMC cultures stimulated with M. ulcerans. Indeed, systemic IFN-γ production was also lower and IL-10 production was higher in patients with the ulcerative form than in patients with the nodular form of Buruli disease. Such differential expression of cytokines in different clinical forms of a mycobacterial disease is reminiscent of what has been described for tuberculosis (8) and leprosy (32).
A polyketide-derived macrolide called mycolactone, which can cause lesions in guinea pig skin similar to those found in Buruli ulcers in human, is considered one of the toxins responsible for the pathogenicity of M. ulcerans (9). It has been hypothesized that the anergy observed in Buruli disease could be due to the action of this mycolactone, and mycolactone purified from M. ulcerans culture filtrate is indeed known to directly inhibit stimulation of T-lymphocyte proliferation (21, 22). We have shown here that IFN-γ production in response to M. bovis BCG was comparable in the two forms of the disease but was clearly lower in response to M. ulcerans in patients presenting with the ulcerative form. This low IFN-γ production may have been the direct consequence of the accumulation of mycolactone produced by M. ulcerans during the progression of the disease. Since nodules are an early manifestation of the disease, one could speculate that their content of bacteria and mycolactone is low, resulting in only a partial unresponsiveness of T cells to mycobacterial antigens. In ulcerative lesions that develop with disease progression, mycolactone would accumulate in the infected tissue and T-cell anergy could become more pronounced. This hypothesis is also in agreement with data in a guinea pig model reproducing the clinical manifestations of Buruli disease, in which the ulceration depends on the amount of mycolactone injected (10).
Besides direct suppression by the mycolactone, other suppressive mechanisms and particularly production of cytokines with IFN-γ downregulating activities may also be involved in the T-cell anergy associated with Buruli disease. Therefore, we analyzed the production of the Th2-type cytokines IL-4 and IL-13, which are known to exert profound IFN-γ downregulating activities in response to antigenic stimulation, particularly in the context of Leishmania infections (1). Intralesional IL-4 and IL-13 mRNA was detected only in very minute amounts, and only in lesions from patients with the ulcerative form. As IL-4 or IL-13 levels in antigen-stimulated PBMC culture supernatants from Buruli patients were below the detection limit, it is difficult to conclude from our results that the development of a truly Th2-biased immune response is the cause of the T-cell anergy observed in Buruli disease.
This is somewhat in contrast to findings by Gooding et al. (14), but a formal comparison of both reports is difficult because in that study IL-4 mRNA expression was not quantified (but analyzed for the presence or absence of a detectable DNA band on agarose gel stained with ethidium bromide) and actual cytokine production in the culture supernatant was not measured. Also, reverse transcription-PCR analysis was performed on blood samples and not on skin biopsies (as in our study) and furthermore the patient population was different from ours, as in Gooding's report only one patient had active disease and the others had healed the M. ulcerans infection at the time of the study (14). Anyway, it must be underlined that our failure to detect IL-4 and IL-13 is unlikely to have been caused by technical problems, as we recently used the same reverse transcription-PCR method to demonstrate that IL-13 is the predominant Th2 cytokine in localized cutaneous leishmaniasis, the prototype of an infectious disease linked to Th2-type T-cell function (4).
In contrast to IL-4 and IL-13, elevated levels of IL-10 could be detected in all patients with Buruli disease, but not in healthy controls. Since it is well known that IL-10 is also a powerful downregulator of IFN-γ production (20), our data suggest, although they do not formally prove, that IL-10 may be responsible for the T-cell anergy observed in patients with Buruli disease. Recently, Cooper and colleagues convincingly demonstrated the pivotal role of IL-10 as an immunosuppressive cytokine in chronic or progressive tuberculosis infection (29). It is not clear for the moment which cell type(s) is responsible for the elevated production of IL-10 in Buruli disease, but cells of myeloid origin such as macrophages and regulatory T cells (6) are among the possible candidates. Finally, it is possible that neither IL-10, IL-4, nor IL-13 but yet another factor is responsible for the observed decreased production in IFN-γ in these patients.
TNF-α could be one of the additional factors involved in the pathology of Buruli disease, but its precise role in the development of the skin lesions and the underlying thrombosis and adipocyte destruction remains to be elucidated. mRNA for TNF-α was detected in ulcerative but even more so in nodular lesions, which was somewhat surprising because mycolactone was reported to suppress production of this cytokine in vitro (10). TNF-α is essential in the protection against mycobacteria, and TNF-deficient mice infected with M. tuberculosis are highly susceptible and fail to mount the typical protective, granulomatous response in the infected organs (3). Histopathological findings in Buruli disease do not show this typical granuloma structure, and M. ulcerans apparently can survive in an extracellular state (15).
Mycolactone is known to cause an arrest in the G0/G1 phase of the cell cycle within 48 h, but that is not sufficient to explain the extensive necrosis observed in Buruli patients even if this toxin is also able to induce apoptosis of macrophages (9, 10). At least two other enzymes, a phospholipase C and a hemolysin, may also exert toxic effects in M. ulcerans infection (11, 12). In this context it has been demonstrated that strongly hemolytic isolates of mycobacteria induced stronger TNF-α production than weakly hemolytic bacilli (25), and the TNF-α detected in M. ulcerans lesions might therefore be due to hemolysin secretion.
Ag85 is a major protein component from BCG culture filtrate, which possesses an enzymatic mycolyltransferase activity. Ag85 is actually a family of three proteins Ag85 A, B, and C, which are encoded by three distinct but paralogous genes (31), and homologues of Ag85 have been described in all mycobacterial species tested so far. Ag85-specific, IFN-γ-producing T cells are found in the majority of healthy subjects infected with M. tuberculosis and in BCG-vaccinated mice and humans (16, 18). On the other hand, most tuberculosis patients with active or even cured disease show low lymphoproliferative and IFN-γ responses to this antigen (18). Also, highly cross-reactive cellular immune responses against the BCG protein can be detected in healthy Mycobacterium leprae-infected individuals, but not in leprosy patients (19).
IFN-γ production in response to Ag85 from M. bovis BCG was lower in PBMC cultures from Buruli disease patients than in cultures from healthy purified protein derivative-positive controls and lower in the ulcerative form than in the nodular form of the disease (although the difference was not statistically significant). It is interesting that the decreased response to Ag85 paralleled the IFN-γ production to M. ulcerans, whereas responses against whole M. bovis BCG bacilli were in the same range for the two forms of the disease. We have previously reported on a similar phenomenon in lepromatous leprosy patients, in whom the reactivity to Ag85 from M. bovis BCG is also more correlated with the response to M. leprae than to M. bovis BCG (19).
The Ag85A component from M. ulcerans was recently sequenced by us, and we demonstrated that it shows 91% identical or conserved amino acid residues with the Ag85A component from M. bovis BCG (27). Furthermore, vaccination with a DNA vaccine encoding Ag85A from BCG, which is effective against an experimental tuberculosis infection in mice (17), can also protect mice against an experimental footpad challenge with M. ulcerans (27). All these results taken together strongly favor the hypothesis that Ag85 is also a protective antigen in infection with M. ulcerans and that Ag85 could indeed be used in a vaccine against Buruli disease. The fact that several studies have demonstrated a protective role of BCG vaccination against Buruli disease is also in accordance with these findings (5, 23, 26).
In conclusion, we have demonstrated that a different cytokine expression profile exists (both in PBMC cultures and in skin lesions) in patients suffering from the nodular and from the ulcerative forms of Buruli disease. Nodules were associated with higher IFN-γ and lower IL-10 productions, and ulcers were associated with lower IFN-γ and higher IL-10 production. The exact role of IL-10 in the progression of M. ulcerans infection and in the T-cell anergy described in these patients has to be analyzed further, and characterization of the cells and the antigen(s) responsible for this IL-10 production may be important for prevention of the disease.
Acknowledgments
We are grateful to F. Portaels (ITG Antwerp) for the gift of M. ulcerans strain 5150.
This work was partially supported by grants from the Damiaanaktie Belgium and from FWO-Vlaanderen (G.0266.00).
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. [DOI] [PubMed] [Google Scholar]
- 2.Asiedu, K., W. Meyers, and P. Agbernoku. 2000. Clinical features and treatment. In K. Asiedu, R. Scherpbier, and M. Raviglione (ed.), Buruli ulcer. Mycobacterium ulcerans infection. Global Buruli Ulcer Initiative, World Health Organization, Geneva, Switzerland.
- 3.Bean, A. G., D. R. Roach, H. Briscoe, M. P. France, H. Korner, J. D. Sedgwick, and W. J. Britton. 1999. Structural deficiencies in granuloma formation in TNF gene targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 162:3504-3511. [PubMed] [Google Scholar]
- 4.Bourreau, E., G. Prévot, R. Pradinaud, and P. Launois. 2001. Interleukin (IL)-13 is the predominant Th2 cytokine in localized cutaneous leishmaniasis lesions and renders specific CD4+ T cells unresponsive to IL-12. J. Infect. Dis. 158:953-959. [DOI] [PubMed] [Google Scholar]
- 5.Bradley, D. J., M. S. R. Hutt, J. W. M. Kiryabwire, A. Kisuule, A. Lloyd, J. Mafigiri, R. H. Morrow, W. Passon, I. Phillips, M. C. Pike, P. A. Pike, and D. L. Revill. 1969. BCG vaccination against Mycobacterium ulcerans infection (Buruli Ulcer). First results of a trial in Uganda. Lancet i:111-115. [PubMed]
- 6.Caramalho, I., T. Lopes-Carvalho, D. Ostler, S. Zelenay, M. Haury, and J. Demengeot. 2003. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J. Exp. Med. 197:403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Bruyn, J., K. Huygen, R. Bosmans, M. Fauville, R. Lippens, J.-P. Van Vooren, P. Falmagne, M. Weckx, H. G. Wiker, M. Harboe, and M. Turneer. 1987. Purification, characterization and identification of a 32 kDa protein antigen of Mycobacterium bovis BCG. Microb. Pathog. 2:351-366. [DOI] [PubMed] [Google Scholar]
- 8.Dlugovitzky, D., M. L. Bay, and L. Rateni. 1999. In vitro synthesis of interferon-gamma, interleukin-4, transforming growth factor and interleukin 1 beta by peripheral blood mononuclear cells from tuberculosis patients: relationship with the severity of pulmonary involvement. Scand. J. Immunol. 48:210-217. [DOI] [PubMed] [Google Scholar]
- 9.George, K. M., D. Chatterjee, G. Gunawardana, D. Welty, J. Hayman, R. Lee, and P. Small. 1999. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science 283:854-857. [DOI] [PubMed] [Google Scholar]
- 10.George, K. M., L. Pascopella, D. M. Welty, and P. L. Small. 2000. A Mycobacterium ulcerans toxin, mycolactone, causes apoptosis in guinea pig ulcers and tissue culture cells. Infect. Immun. 68:877-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez, A., A. Mve-Obiang, B. Vray, J. Remacle, K. Chemlal, W. M. Meyers, F. Portaels, and P.-A. Fonteyne. 2000. Biochemical and genetic evidence for phospholipase C activity in Mycobacterium ulcerans. Infect. Immun. 68:2995-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez, A., A. Mve-Obiang, B. Vray, W. Rudnicka, I. C. Shamputa, F. Portaels, W. M. Meyers, P.-A. Fonteyne, and L. Realini. 2001. Detection of phospholipase C in non-tuberculous mycobacteria and its possible role in hemolytic activity. J. Clin. Microbiol. 39:1396-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gooding, T. M., P. D. R. Johnson, D. E. Campbell, J. A. Hayman, E. Hartland, A. S. Kemp, and R. M. Robins-Browne. 2001. Immune response to infection with Mycobacterium ulcerans. Infect. Immun. 69:1704-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gooding, T. M., P. D. R. Johnson, M. Smith, A. S. Kemp, and R. M. Robins-Browne. 2002. Cytokine profiles of patients infected with Mycobacterium ulcerans and unaffected household contacts. Infect. Immun. 70:5562-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayman, J., and A. McQueen. 1985. The pathology of Mycobacterium ulcerans infection. Pathology 17:594-600. [DOI] [PubMed] [Google Scholar]
- 16.Huygen, K., D. Abramowicz, P. Vandenbussche, F. Jacobs, J. De Bruyn, A. Kentos, A. Drowart, J.-P. Van Vooren, and M. Goldman. 1992. Spleen cell cytokine secretion in Mycobacterium bovis BCG-infected mice. Infect. Immun. 60:2880-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huygen, K., J. Content, O. Denis, D. L. Montgomery, A. M. Yawman, R. R. Deck, C. M. DeWitt, I. M. Orme, S. Baldwin, C. D'Souza, A. Drowart, E. Lozes, P. Vandenbussche, J.-P. Van Vooren, M. A. Liu, and J. B. Ulmer. 1996. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat. Med. 2:893-898. [DOI] [PubMed] [Google Scholar]
- 18.Huygen, K., J.-P. Van Vooren, M. Turneer, R. Bosmans, P. Dierckx, and J. De Bruyn. 1988. Specific lymphoproliferation, gamma interferon production, and serum immunoglobulin G directed against a purified 32 kDa mycobacterial protein antigen (P32) in patients with active tuberculosis. Scand. J. Immunol. 27:187-194. [DOI] [PubMed] [Google Scholar]
- 19.Launois, P., K. Huygen, J. De Bruyn, M. N′Diaye, B. Diouf, J. L. Sarthou, J. Grimaud, and J. Millan. 1991. T-cell response to purified filtrate antigen 85 from Mycobacterium bovis bacillus Calmette-Guérin (BCG) in leprosy patients. Clin. Exp. Immunol. 86:286-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore, K. W., A. O'Garra, R. de Waal Malefyt, P. Vieira, and T. R. Mosmann. 1993. Interleukin-10. Annu. Rev. Immunol. 11:165-190. [DOI] [PubMed] [Google Scholar]
- 21.Pahlevan, A. A., D. J. Wright, C. Andrews, M. George, P. L. Small, and B. M. Foxwell. 1999. The inhibitory action of Mycobacterium ulcerans soluble factor on monocyte/T-cell cytokine production and NF-kappa B function. J. Immunol. 163:3928-3935. [PubMed] [Google Scholar]
- 22.Pimsler, M., T. A. Sponsler, and W. M. Meyers. 1988. Immunosuppressive properties of the soluble toxin from Mycobacterium ulcerans. J. Infect. Dis. 157:577-580. [DOI] [PubMed] [Google Scholar]
- 23.Portaels, F., J. Aguiar, M. Debacker, C. Steunou, C. Zinsou, A. Guedenon, and W. M. Meyers. 2002. Prophylactic effect of Mycobacterium bovis BCG vaccination against osteomyelitis in children with Mycobacterium ulcerans disease (Buruli ulcer). Clin. Diagn. Lab. Immunol. 9:1389-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross, B. C., L. Marino, F. Oppediasano, R. Edwards, R. M. Robins-Browne, and P. D. R. Johnson. 1997. Development of a PCR for rapid diagnosis of Mycobacterium ulcerans infection. J. Clin. Microbiol. 35:1696-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rudnicka, W., M. Brzychcy, M. Klink, A. G. Lopez, P.-A. Fonteyne, S. Rusch-Gerdes, and R. B. 1999. The production of nitric oxide and Tumor Necrosis Factor by murine macrophages infected with mycobacterial strains differing by hemolytic activity. Microbiol. Immunol. 43:637-644. [DOI] [PubMed] [Google Scholar]
- 26.Smith, P. G., W. D. L. Revill, E. Lukwago, and Y. P. Rykushin. 1976. The protective effect of BCG against Mycobacterium ulcerans disease: a controlled trial in an endemic area of Uganda. Trans. R. Soc. Trop. Med. Hyg. 70:449-457. [DOI] [PubMed] [Google Scholar]
- 27.Tanghe, A., J. Content, J.-P. Van Vooren, F. Portaels, and K. Huygen. 2001. Protective efficacy of a DNA vaccine encoding Ag85A from Mycobacterium bovis BCG against Buruli ulcer. Infect. Immun. 69:5403-5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thangaraj, H. S., M. R. Evans, and M. H. Wansbrough-Jones. 1999. Mycobacterium ulcerans disease; Buruli ulcer. Trans. R. Soc. Trop. Med. Hyg. 93:337-340. [DOI] [PubMed] [Google Scholar]
- 29.Turner, J., M. Gonzalez-Juarrero, D. L. Ellis, R. J. Basaraba, A. Kipnis, I. M. Orme, and A. M. Cooper. 2002. In vivo IL-10 production reactivates chronic pulmonary tuberculosis in C57BL/6 mice. J. Immunol. 169:6343-6351. [DOI] [PubMed] [Google Scholar]
- 30.van der Werf, T. S., W. T. A. van der Graaf, J. W. Tappero, and K. Asiedu. 1999. Mycobacterium ulcerans infection. Lancet 354:1013-1018. [DOI] [PubMed] [Google Scholar]
- 31.Wiker, H. G., and M. Harboe. 1992. The antigen 85 Complex: A major secretion product of Mycobacterium tuberculosis. Microbiol. Rev. 56:648-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamura, M., K. Uyemura, R. J. Deans, K. Weinberg, T. H. Rea, B. R. Bloom, and R. L. Modlin. 1991. Defining protective responses to pathogens: Cytokine profiles in leprosy lesions. Science 254:277-279. [DOI] [PubMed] [Google Scholar]