Abstract
Soluble fms-like tyrosine kinase receptor (sFlt-1) is a soluble form of extramembrane part of vascular endothelial growth factor receptor-1 (VEGFR-1) that has antitumor effects. Bifidobacterium Infantis is a kind of non-pathogenic and anaerobic bacteria that may have specific targeting property of hypoxic environment inside of solid tumors. The aim of this study was to construct Bifidobacterium Infantis-mediated sFlt-1 gene transferring system and investigate its antitumor effect on Lewis lung cancer (LLC) in mice. Our results demonstrated that the Bifidobacterium Infantis-mediated sFlt-1 gene transferring system was constructed successfully and the system could express sFlt-1 at the levels of gene and protein. This system could not only significantly inhibit growth of human umbilical vein endothelial cells induced by VEGF in vitro, but also inhibit the tumor growth and prolong survival time of LLC C57BL/6 mice safely. These data suggest that Bifidobacterium Infantis-mediated sFlt-1 gene transferring system presents a promising therapeutic approach for the treatment of cancer.
Keywords: antiangiogenesis, vascular endothelial growth factor, soluble fms-like tyrosine kinase receptor, Bifidobacterium Infantis
Introduction
It has been widely accepted that the growth and progression of most solid cancers are angiogenesis-dependent.1, 2 Angiogenesis, defined as the production of new capillary vessels and venules in a specific area,3 is an essential process for the primary tumor to grow and invade into the adjacent normal structures.4 It is a complex process controlled by a balance of angiogenic and angiostatic factors involved in multiple pathways that result in endothelial cell proliferation, differentiation and organization into a functional network of vascular channels.5 Antiangiogenic therapy is one of the most promising methods for the treatment of cancers.6, 7 This theory is supported by enormous experiments and clinical trials.8, 9, 10, 11, 12 For example, Avastin (bevacizumab), which is an antiangiogenesis antibody, was approved by Food and Drug Administration in 2004 for the treatment of colorectal cancer.12 Antiangiogenic agents hold much promise to serve as optimal therapeutic strategy of cancers.13, 14, 15
Among the many reported angiogenic factors, vascular endothelial growth factor (VEGF) is one of the most powerful endothelial-cell-specific mitogens that have an important role in the complicated process of angiogenesis.4 The overexpression of VEGF has been described in neoplastic cells and has also been associated with tumor growth and progression.16, 17, 18 Among the many reported angiogenic factors, VEGF is commonly overexpressed in various human tumors compared with normal tissues.5 The fms-like tyrosine kinase receptor (Flt) is a transmembrane receptor of the tyrosine kinase family, which has been identified as a receptor for VEGF. Previous studies indicate that VEGF and its receptor Flt-1 have an important role in tumor growth and metastasis, and are associated with poor prognosis in clinical human tumors.16, 17, 18 First identified in 1993, the soluble Flt (sFlt-1) was immediately found to exert powerful antiangiogenesis function.16, 17, 18 As the soluble form of extracellular part of VEGF receptor-1 (VEGFR-1), sFlt-1 can compete with VEGFR-1 for VEGF and exert its function.18 It has been confirmed that sFlt-1 can suppress both the growth and metastasis of solid tumor by many investigations.17, 18
Most solid tumors develop regions of low oxygen tension because of an imbalance in oxygen supply and consumption.4 Hypoxia in the tumor microenvironment provides a great place for anaerobic microbes to survive. Bifidobacterium Infantis is a kind of Bifidobacteria that is non-pathogenic and anaerobic, and thus they can selectively localize and proliferate in the hypoxic environment in several types of solid tumors after systemic application.19, 20, 21, 22 The aim of this study was to construct Bifidobacterium Infantis-mediated sFlt-1 gene transferring system and investigate its antitumor effect on Lewis lung cancer (LLC) in mice.
Materials and methods
Materials
A shuttle vector, PTRKH2-PsT plasmid containing erythromycinr gene, was kindly provided by Life Scientific College of Fudan University (Shanghai, China). Strains of Bifidobacterium Infantis 2001 were obtained from Key Laboratory of West China School of Stomatology, Sichuan University (Sichuan Province, China). Recombinant Escherichia coli DH5α line containing pcDNA3.1/sFlt-1 was constructed by our laboratory before.9 LLC cell line was provided by the State Key Laboratory of Biomedicine, Sichuan University (Sichuan Province, China). Female C57BL/6 mice (6–8 weeks age) weighing between 16 and 18 g were purchased from Experimental Animal Center of Sichuan University (Sichuan Province, China). Purification kit of plasmid, purification kit of polymerase chain reaction (PCR) product, plasmid mini-preparation kit, Wizard PCR Preps DNA Purification System and gel extraction kit were purchased from Omega (Bellingham, WA). PCR reaction test kit was purchased from Tiangen (Beijing, China). DNA Marker III was purchased from Tiangen or TransGen (Beijing, China). T4 DNA ligase, BamHI, SalI and One Step RNA PCR Kit were purchased from Takara (Dalian, China). Trizol reagent was purchased from TransGen. 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), lysozyme and trypsin were purchased from Sigma (Santa Clara, CA). Dulbecco's modified Eagle's medium, M199 medium and dimethylsulfoxide were purchased from Gibco (Gaithersburg, MD). Mouse monoclonal antibody for sFlt-1 was purchased from R&D (Minneapolis, MN). Mouse monoclonal antibody for CD31 and LSAB kit were purchased from Dako (Copenhagen, Denmark). Polyvinylidene difluoride membranes were purchased from Millipore (Boston, MA). The enhanced chemiluminescence detection kit and X-ray films were purchased from Roche (Basel, Switzerland).
Amplification of sFlt-1 gene
Strains of recombinant E. coli DH5α line containing pcDNA3.1/sFlt-1 were inoculated into 5 ml LB liquid medium (containing ampicillin 50 μg ml−1) with shaking, overnight at 37 °C. On the second day, genomic DNA was prepared by phenol/chloroform method and used as template DNA to perform PCR for the amplification of sFlt-1 gene. Specific primers of sFlt-1 gene were designed based on published sequences (GenBank: AF063657) and synthesized by Invitrogen (Shanghai, China). The upstream primer is 5′-TGAGGATCCATGGAGAGCAAGGT-3′ and the downstream primer is 5′-GTGGTCGACTTTTTCATGGACCCT-3′ (the underlined place was endonuclease site of BamHI and SalI). The PCR product was a 969 bp for sFlt-1 fragment. The amplification cycle was repeated 35 times with the condition of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s and extension at 72 °C for 2 min. Amplified products were purified with Wizard PCR Preps DNA Purification System and separated with electrophoresis of 0.8% agarose gel to confirm whether the amplified products had the desired size.
Digestion of sFlt-1 gene and PTRKH2-PsT plasmid
The recombinant E. coli DH5α line containing PTRKH2-PsT was resuscitated and amplified. PTRKH2-PsT plasmid was extracted from recombinant E. coli DH5α using plasmid mini-preparation kit. The sFlt-1 gene 1 μg and PTRKH2-PsT plasmid 1 μg were added into 10 μl 10 × Buffer E reactions, separately. Then, the sFlt-1 gene and plasmid were digested with dual restriction endonucleases (1.5 μl BamHI+1.5 μl SalI) at 37 °C for 4 h. Once the digestion was complete, the DNA fragments were separated by electrophoresis with 0.8% agarose gel. The desired bands of about 6.9 and 1 kb were cut out from the gel. The DNA fragments in the gel were extracted and recovered with gel extraction kit.
Ligation of plasmid vector fragment and sFlt-1 gene fragment
Recovered sFlt-1 gene fragment 9 μl, recovered pTRKH2-PsT plasmid vector fragment 3 μl and T4 DNA ligase 1 μl were added into the microfuge tube. The reactions were incubated at 16 °C overnight. Then, the ligation products and recombinant pTRKH2-PsT/sFlt-1 plasmids were separated by electrophoresis to confirm whether they had the desired size.
Transformation of Bifidobacterium Infantis
Bifidobacterium Infantis 2001 was cultured in MRS solid plate medium and washed completely using ice-cold pure water and resuspended in 40 μl ice-cold sucrose (0.5 ) containing ammonium citrate (1 mm). Recombinant pTRKH2-PsT/sFlt-1 plasmid 5 μl (1 μg) was added to the bacterial suspensions, and then they were mixed and transferred to electroporation cuvette. Electroporation was carried out to transform recombinant pTRKH2-PsT/sFlt-1 plasmid into Bifidobacterium Infantis at 2.0 kV for 10 ms.
Culture of transformed Bifidobacterium Infantis
When electroporation was completed, 1 ml MRS liquid medium containing Ery 10 μg ml−1, 0.05% cysteine hydrochloride and 0.5 sucrose were added into the electroporation cuvette to resuspend bacteria. Then, 200 μl bacteria suspensions were transferred into a 1.5 ml EP tube and incubated into anaerobic environment at 37 °C. After 2.5 h, bacteria suspensions were cultured on MRS plate medium with Ery 10 μg ml−1, 0.05% cysteine hydrochloride and 0.5 sucrose, and incubated into anaerobic environment at 37 °C for 72 h.
Screening of recombinant positive Bifidobacterium Infantis
A single positively transformed bacterial colony of Bifidobacterium Infantis was picked up and inoculated into 5 ml erythromycinr MRS liquid medium into anaerobic environment at 37 °C for 24 h.
Digestion of recombinant plasmid and PCR identification
Bacteria suspensions were added into the lysozyme with a final concentration of 30 mg ml−1, and cultured at 37 °C for 40 min. The plasmid DNA was extracted by small dose plasmid extraction kit and digested by BamHI (1 μl) and SalI (1 μl) at 37 °C for 4 h. Then, the digested products were identified by 0.9% agarose gel electrophoresis. The correct recombinant plasmid DNA identified by electrophoresis was used as template and amplified through PCR using upstream and downstream primers. The amplification cycle was repeated 30 times with the condition of denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s and extension at 72 °C for 1 min 15 s. Then, the amplified products were identified by 0.9% agarose gel electrophoresis.
Sequencing of inserted gene segment of recombinant plasmid
Sequencing of inserted gene segment in recombinant plasmid extracted from positively transformed bacteria was performed using the method of Sanger dideoxynucleotide triphosphate chain termination.
Detection of expression of sFlt-1 gene of recombinant positive Bifidobacterium Infantis
The total RNA of recombinant positive colonies was extracted by Trizol reagent. Reverse transcription (RT)-PCR products were synthesized using total RNA as template (30 times amplification cycle with the condition of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s and extension at 72 °C for 1 min). The amplified products were subsequently identified through 0.9% agarose gel electrophoresis.
Detection of expression of sFlt-1 protein by western blot analysis
The positive transformed Bifidobacteria Infantis 100 μl were inoculated into 20 ml erythromycinr MRS liquid medium into anaerobic environment at 37 °C for 24 h. Then, the bacteria were harvested by centrifugation, resuspended in lysis buffer (50 m Tris-HCl, 2 m EDTA, 100 m NaCl, 0.5% Triton X-100, 1 mg ml−1 lysozyme, pH 8.5) and sonicated. Protein concentration was determined by the bicinchoninic acid method. The 30 μg protein was subjected to 4–12% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a Tris–glycine system, and then the gel was electroblotted onto polyvinylidene difluoride membrane for 45 min. The membrane was then incubated with 5% non-fat dry milk in phosphate-buffered saline for 1 h to block nonspecific binding sites, and then incubated with the appropriate primary antibody concentration (1:200 dilution for sFlt-1) for 2 h at 37 °C in 5% non-fat dry milk. The membrane was subsequently rinsed in phosphate-buffered saline, and then incubated for 2 h at 37 °C with goat anti-mouse immunoglobulin G-horse radish peroxidase at 1:2000 dilution. After incubation, the membrane was rinsed and visualized with chemiluminescence detection reagents.
Effect of recombinant positive Bifidobacterium Infantis on HUVEC proliferation
The recombinant Bifidobacteria Infantis containing pTRKH2-PsT/sFlt-1 and pTRKH2-PsT plasmids were broken by ultrasound and dialyzed to form a final solution, respectively. Human umbilical vein endothelial cells (HUVECs) were first digested with 0.25% trypsin and counted. They were then diluted and inoculated into a 96-well plate at a concentration of 1 × 104 cells per well and a volume of 0.2 ml per well in 40 wells. After culture in the M199 medium containing fetal bovine serum, the 40 wells were randomized into four groups (10 wells per group): group 1 (VEGF group) added with 0.2 ml liquid medium containing VEGF (6 ng ml−1); group 2 added with final solution of recombinant Bifidobacterium Infantis containing pTRKH2-PsT/sFlt-1 plasmid 0.2 ml and VEGF (6 ng ml−1); group 3 added with final solution of recombinant Bifidobacterium Infantis containing pTRKH2-PsT plasmid 0.2 ml and VEGF (6 ng ml−1); and group 4 (negative control group) added with 0.2 ml liquid medium. During incubation at 37 °C for 24 h, 0.02 ml MTT (5 mg ml−1 final concentration) was added to each well. The plate was then incubated at 37 °C for an additional 4 h to allow MTT to form formazan crystals by reacting with metabolically active cells. The formazan crystals were solubilized in 0.15 ml dimethylsulfoxide per well at 37 °C for 10 min. The absorbance values of the solution in each well were measured at 490 nm using a microplate reader. Cell viability (%)=absorbance of the treated group/absorbance of VEGF group × 100%.
Cell culture
LLC cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum plus ampicillin and streptomycin routinely, and incubated in 5% CO2 at 37 °C.
Design of animal experiments
All animal procedures were approved by the Animal Care and Scientific Committee of Sichuan University. The tumor tissues from LLC mice were triturated and prepared into cell suspensions (dilution 1:5 with normal saline). The cells harvested from xenografts were adjusted to the concentration of 1 × 107/ml, and 0.2 ml cell suspensions were injected subcutaneously into the armpit of right anterior superior limbs of female C57BL/6 mice.23 After 7 days, when the tumors were palpable (diameter 2–4 mm), the mice were randomized into the following three groups eight mice per group): a control group (group a) injected with 0.4 ml saline into mice through the caudal vein; a recombinant Bifidobacterium Infantis containing pTRKH2-PsT/sFlt-1 plasmid group (group b) injected with 0.4 ml suspension of Bifidobacterium Infantis containing pTRKH2-PsT/sFlt-1 plasmid (1 × 108/ml) into mice through the caudal vein; a recombinant Bifidobacterium Infantis containing pTRKH2-PsT plasmid group (group c) injected with 0.4 ml suspension of Bifidobacterium Infantis containing pTRKH2-PsT plasmid (1 × 108/ml) into mice through the caudal vein. The injection repeated six times every 3 days. All treatments lasted for 21 days.
Identification of specific targeting and sFlt-1 expression in tumor tissue
Another 18 LLC bearing mice were divided into three groups and received same treatment above. After 21 days, all the mice were killed. The tumor tissue and other tissues were cut in a sterile way. Part of the tumor tissue and other tissues were homogenized using saline into a concentration of 10%, respectively. A measure of 100 μl of homogenate of all the tissues was cultured on MRS plate medium with Ery 10 μg ml−1, 0.05% cysteine hydrochloride and 0.5 sucrose, and incubated into anaerobic environment at 37 °C for 72 h. The 20 μg protein extracted from tumor tissue of each group was subjected to 4–12% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a Tris–glycine system and then the gel was electroblotted onto polyvinylidene difluoride membrane for 45 min. The membrane was then incubated with 5% non-fat dry milk in phosphate-buffered saline for 1 h to block nonspecific binding sites, and then incubated with the appropriate primary antibody concentration (1:200 dilution for sFlt-1) for 2 h at 37 °C in 5% non-fat dry milk. The membrane was subsequently rinsed in phosphate-buffered saline, and then incubated for 2 h at 37 °C with goat anti-mouse immunoglobulin G-horse radish peroxidase at 1:2000 dilution. After incubation, the membrane was rinsed and visualized with chemiluminescence detection reagents.
Tumor growth
The length, width and weight of tumor were callipered every 3 days for tumor growth, and tumor volume (TV) was estimated using the formula: TV (mm3)=(width2 × length)/2.
Observation of necrosis and signals of blood flow in tumor by color Doppler ultrasound
At 21 days after the treatment, the living mice in all groups underwent color Doppler ultrasound before being killed. The size, shape of tumor, ultrasonic echo from tumor inner to known liquefied tumor and necrotic tissues were detected under the two-dimensional ultrasound by color Doppler (ACUSON 1228ST) with 5 MHz frequency.
Signals of blood flow in tumor were detected by color Doppler flow imaging (CDFI) with color Doppler (5 MHz). Signals of blood flow in the tumor of CDFI were classified into four grades based on the criteria of Adler:24 0, no blood flow signals detected within the tumor; I, minimal blood flow (one or two dot-like or a thin- and short-like blood flow signals detected within the tumor); II, moderate blood flow (up to three dot-like blood flow signals or one longer blood flow signals detected within the tumor); and III, abundant blood flow (more than five dot-like blood flow signals or two longer blood flow signals detected within the tumor).
Inhibitive rate of the tumor
Inhibitive rate of the tumor was calculated using the formula:23, 25, 26 inhibitive rate of the tumor (%)=(1−average tumor weight in treated group/average tumor weight in control) × 100%.
Necrosis rate of the tumor
The necrosis rate of the tumor was calculated by the principle of Cavalieri27, 28 and was assessed using pathological slices way.23 The necrosis rate of the tumor was determined using the following formula: necrosis rate of the tumor=tumor necrotic area/whole tumor area × 100% (tumor necrotic area=the largest diameter × the smallest diameter of the tumor necrotic area; whole tumor area=the largest diameter × the smallest diameter of the tumor area).
Immunohistochemical detection of CD31 and detection of microvessel density
Tumor tissues were fixed immediately in 10% buffered formalin phosphate and embedded in paraffin. Immunohistochemical staining was performed using the EnVision method. Briefly, the dewaxed, rehydrated sections (5 μm) were treated with 0.3% hydrogen peroxide in methanol for 15 min to block endogenous peroxidase activity, and then washed three times with Tris-HCl-buffered saline. The sections were repaired in citric acid antigen repair solution (pH 6.0) for 40 min, followed by treatment with primary antibody at 37 °C for one night (anti-CD31 diluted for 1:100). After being washed with Tris-HCl-buffered saline, sections were then treated with EnVisionTM diluted for 1:100 at 37 °C for 45 min and washed with Tris-HCl-buffered saline. The sections were then stained with 3,3′-diaminobenzidine under the control of light microscope and counterstained with hematoxylin. Finally, the sections were dehydrated, fixed and analyzed by light microscopy.
Microvessel density (MVD) was evaluated by immunohistochemical analysis with antibodies to the endothelial marker CD31 and determined according to the method of Weidner and colleagues.29 Briefly, the immunostained sections were initially screened at low magnification ( × 40) to identify hot spots, which are the areas of highest neovascularization. Any yellow brown-stained endothelial cell or endothelial cell cluster that was clearly separate from adjacent microvessels, tumor cells and other connective tissue elements was considered a single, countable microvessel. Within the hot spot area, the stained microvessels were counted in a single high-power field ( × 200), and the average vessel count in three hot spots was considered the value of MVD. All counts were performed by three investigators in a blinded manner. Microvessel counts were compared between the observers and discrepant results were reassessed. The consensus was used as the final score for analysis.23
Side effects, quality of life and survival
Another 30 mice were randomized into groups a, b and c as mentioned above (10 mice per group) just to observe survival rate. During the experiment period, side effects such as appetite, weight loss, mental state, behavior change, reaction to stimulation, ruffling of fur and so on were observed. The survival time of every mouse was recorded until all mice died.
Statistical analysis
TV, necrosis rate of the tumor and MVD were analyzed by one-way analysis of variance, followed by the Student's t-test. Data of inhibitive rate of the tumor were analyzed by χ2 test. Survival curves were constructed according to the Kaplan–Meier method and statistical significance was determined by the log-rank test. Grade of CDFI were analyzed by Kruskal–Wallis test. All statistical analyses were performed using the SPSS 16.0 software package. All P-values were two-sided and P<0.05 was considered as the significant level of difference.
Results
PCR amplification of sFlt-1 gene
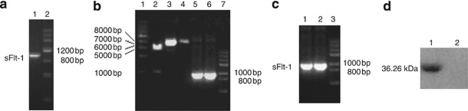
The amplified products of sFlt-1 gene were detected by 0.8% agarose gel electrophoresis, and the results showed that a specific segment with about 1 kb (Figure 1a) was obtained, which was almost the same size as that of sFlt-1 gene (969 bp).
Figure 1.
The results of electrophoresis and western blot. (a) Polymerase chain reaction (PCR) products of soluble fms-like tyrosine kinase receptor (sFlt-1). Lane 1: sFlt-1; lane 2: DNA marker III. (b) Digestion of sFlt-1 and pTRKH2-PsT by restriction endonuclease and PCR products of sFlt-1. Lane 1: 1 kb DNA ladder; lane 2: recombinant plasmid pTRKH2-PsT/sFlt-1 digested by restriction endonuclease BamHI and SalI; lane 3: recombinant plasmid pTRKH2-PsT/sFlt-1; lane 4: plasmid pTRKH2-PsT; lane 5: PCR products of sFlt-1 gene from recombinant plasmid pTRKH2-PsT/sFlt-1; lane 6: PCR products of sFlt-1 gene from recombinant plasmid pcDNA3.1/sFlt-1; lane 7: DNA marker III (TransGen). (c) Agarose gel electrophoresis of reverse transcription (RT)-PCR and PCR products from recombinant Bifidobacterium Infantis of pTRKH2-PsT/sFlt-1. Lane 1: PCR products of sFlt-1 gene from recombinant plasmid pTRKH2-PsT/sFlt-1; lane 2: RT-PCR product of RNA purified from Bifidobacterium Infantis with pTRKH2-PsT/sFlt-1; lane 3: DNA marker III (from TransGen). (d) Western blot analysis of the expression of sFlt-1 gene. A western blot band (36.26 kDa) from Bifidobacterium Infantis transformed recombinant pTRKH2-PsT/sFlt-1 plasmid was shown (lane 1), and no same band was shown from Bifidobacterium Infantis transformed recombinant pTRKH2-PsT (lane 2).
Identification of PCR amplified products, enzyme-digested products and sequencing
The amplified products of pTRKH2-PsT/sFlt-1 plasmid were also detected by 0.8% agarose gel electrophoresis, and the data showed that a segment with about 1 kb (Figure 1b) was obtained, which was the same size as that of sFlt-1 gene (969 bp).
The digested products of pTRKH2-PsT/sFlt-1 plasmid were also detected by agarose gel electrophoresis. The results showed that the recombinant positive pTRKH2-PsT/sFlt-1 plasmid is about 8 kb, and there were two segments of approximate 969 bp and 6.9 kb extracted from the 8 kb recombinant plasmid (Figure 1b), which were equal to the size of sFlt-1 gene and pTRKH2-PsT plasmid, respectively.
Sequencing results showed that the size and sequence of nucleotide acid of inserted gene segment were completely consistent with the size and sequence of nucleotide acid of sFlt-1 gene (GeneBank: AF063657). The full length of inserted gene fragment was 969 bp. The sequence of two ends of the inserted gene was also consistent with BamHI and SalI sites. The sequencing identified that the foreign gene, sFlt-1 gene, was correctly inserted into pTRKH2-PsT plasmid and transferred into Bifidobacterium Infantis. The data showed that targeting gene therapy system of Bifidobacterium Infantis was successfully constructed.
RT-PCR testing of the expression of sFlt-1 gene in recombinant Bifidobacterium Infantis
The results of RT-PCR electrophoresis of positive transformed Bifidobacterium Infantis showed that there was a specific segment about 1 kb (Figure 1c). It proved that the sFlt-1 gene could replicate in recombinant Bifidobacterium Infantis.
Detection of expression of sFlt-1 protein by western blot analysis
The expression of sFlt-1 protein from positive transformed Bifidobacterium Infantis was assayed by western blot analysis using a mouse monoclonal antibody for sFlt-1. The results of western blot analysis showed that there was a western blot band from Bifidobacterium infantis transformed recombinant pTRKH2-PsT/sFlt-1 plasmid (Figure 1d), which is consistent with the size predicted by Expasy proteomics tools (36.26 kDa). Furthermore, there was no same band from Bifidobacterium Infantis transformed recombinant pTRKH2-PsT plasmid (Figure 1d). It proved that sFlt-1 could be expressed at the level of protein in Bifidobacterium Infantis transformed recombinant pTRKH2-PsT/sFlt-1 plasmid.
Effect of final solution of recombinant positive Bifidobacterium Infantis on HUVECs
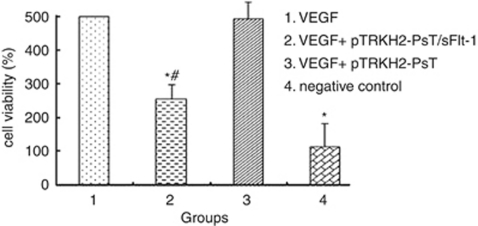
After being treated with the final solution of recombinant positive Bifidobacterium Infantis for 24 h, many HUVECs were killed and only a few grew along the wall. Many cells floated and quantities of cell debris were observed in the medium. The cells left on the wall underwent significant changes in morphology; the original shape was gone, cytoplasm became rougher, the nucleus showed pycnosis and the refraction decreased, demonstrating obvious cellular damages. In contrast, HUVECs treated with negative solution failed to demonstrate obvious morphological changes compared with the control. As shown in Figure 2, the cell viability of groups 1 and 3 had no significant difference (P>0.05). However, the cell viability of groups 2 and 4 were significantly lower than group 1 (P<0.05). The results showed the VEGF could induce the growth of HUVECs significantly and the final solution of recombinant positive Bifidobacterium Infantis could inhibit the growth of HUVECs induced by VEGF.
Figure 2.
Effect of Bifidobacterium Infantis-mediated pTRKH2-PsT/sFlt-1 delivery system on HUVECs. 1: VEGF; 2: VEGF and treated fluid of the Bifidobacterium Infantis transformed with recombinant pTRKH2-PsT/sFlt-1 plasmid; 3: VEGF and treated fluid of the Bifidobacterium Infantis transformed with pTRKH2-PsT plasmid; 4: negative control. *P<0.05 vs VEGF group; #P<0.05 vs negative control.
Specific targeting of recombinant Bifidobacterium Infantis and sFlt-1 expression in tumor tissue
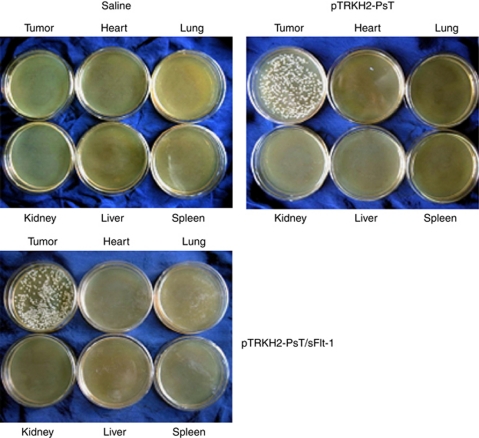
At 3 days after the incubation, we found that there were many white colonies in the medium culturing tumor tissue, and there was no colony growing in the medium culturing other tissues like the heart, liver, lung, kidney and spleen (Figure 3). These results proved that both the recombinant Bifidobacterium Infantis containing pTRKH2-PsT/sFlt-1 plasmid and pTRKH2-PsT plasmid had good targeting property to tumor tissue. Hence, we think that the Bifidobacterium Infantis had specifically targeted to the tumor tissue.
Figure 3.
Analysis of targeting property of Bifidobacterium Infantis. Saline: saline control group; pTRKH2-PsT: recombinant Bifidobacterium Infantis containing pTRKH2-PsT plasmid group; pTRKH2-PsT/sFlt-1: recombinant Bifidobacterium Infantis containing pTRKH2-PsT/sFlt-1 plasmid group.
The result of western blot (Figure 4) showed that a band about 36.26 kDa from Bifidobacterium Infantis transformed recombinant pTRKH2-PsT/sFlt-1 plasmid was shown, and no same band was shown from Bifidobacterium Infantis transformed recombinant pTRKH2-PsT or saline control group.
Figure 4.
Western blot analysis of the expression of sFlt-1 gene in tumor tissue. Saline: saline control group; pTRKH2-PsT: recombinant Bifidobacterium Infantis containing pTRKH2-PsT plasmid group; pTRKH2-PsT/sFlt-1: recombinant Bifidobacterium Infantis containing pTRKH2-PsT/sFlt-1 plasmid group. The western blot showed that a band of about 36.26 kDa from Bifidobacterium Infantis transformed recombinant pTRKH2-PsT/sFlt-1 plasmid was shown, and no same band was shown from Bifidobacterium infantis transformed recombinant pTRKH2-PsT or saline control group.
TV, tumor weight and inhibitive rate of the tumor
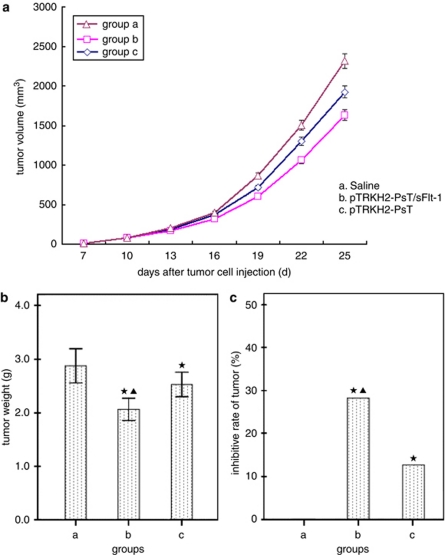
The treatment began on the seventh day after the mice were injected with tumor cells. We recorded the volume and weight of tumors and the following acceleration curve was obtained (see Figure 5a). As shown in Figure 5a, the TV of groups b and c were considerably smaller than that of group a (P<0.05), and the TV in group b was much lower than that in group c (P<0.05). The data of tumor weight showed that the tumor weight of groups b and c reduced significantly than that of group a (P<0.05), and especially in group b (P<0.05) (Figure 5b). In addition, inhibitive rate of the tumor in groups b and c showed an appreciable increase compared with group a (P<0.05), and more in group b (P<0.05) (Figure 5c). All the above findings showed that the Bifidobacterium Infantis containing pTRKH2-PsT/sFlt-1 plasmid or pTRKH2-PsT plasmid both could inhibit the growth of tumor, and the Bifidobacterium Infantis containing pTRKH2-PsT/sFlt-1 plasmid had more significant effect.
Figure 5.
Effect of Bifidobacterium Infantis-mediated pTRKH2-PsT/sFlt-1 delivery system on the growth of Lewis lung cancer C57BL/6 mice. (a) Tumor growth curve; (b) tumor weight; and (c) inhibitive rate of tumor. a: saline control group and b: recombinant Bifidobacterium Infantis containing pTRKH2-PsT/sFlt-1 plasmid group; c: recombinant Bifidobacterium Infantis containing pTRKH2-PsT plasmid group. *P<0.05 vs group a; ▴P<0.05 vs group c.
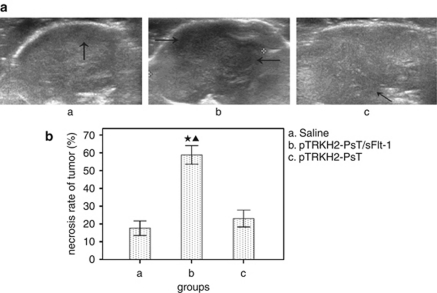
Tumor necrosis rate
The necrosis areas of tumor represented low or no echo by color Doppler ultrasound. As shown in Figure 6, there were massive necrotic areas in tumors of group b, and the necrosis rate is (58.88±6.24)%, whereas there were just small necrotic areas in groups a and c, and the necrosis rate were (17.62±4.90)% and (22.75±5.73)%, respectively. The necrosis rate of group b was significantly bigger than that of groups a and c (P<0.05).
Figure 6.
Effect of Bifidobacterium Infantis-mediated pTRKH2-PsT/sFlt-1 delivery system on tumor necrosis. (a) Ultrasound images of tumor and (b) necrosis rate of tumor. a: saline control group; b: recombinant Bifidobacterium Infantis containing pTRKH2-PsT/sFlt-1 plasmid group; and c: recombinant Bifidobacterium Infantis containing pTRKH2-PsT plasmid group. Arrows showed the necrotic area. *P<0.05 vs group a; ▴P<0.05 vs group c.
Signal of blood flow in tumor
As shown in Figure 7, CDFI showed that signals of blood flow in tumor were the worst in group b and the best in the control group. The signals of blood flow were mainly levels 0–I (5/8) in the group b, whereas 100% at level II or level III (8/8) in the control group. The signals were intermediate in group c.
Figure 7.
Observation of blood flow in tumors by color Doppler flow imaging (CDFI). (a) Blood flow image of tumor and (b) classification of blood flow signals. a: saline control group; b: recombinant Bifidobacterium Infantis containing pTRKH2-PsT/sFlt-1 plasmid group; and c: recombinant Bifidobacterium Infantis containing pTRKH2-PsT plasmid group. Grade: 0, no blood flow signals detected within the tumor; I, minimal blood flow (one or two dot-like or a thin- and short-like blood flow signals detected within the tumor); II, moderate blood flow (up to three dot-like blood flow signals or one longer blood flow signals detected within the tumor); III, abundant blood flow (more than five dot-like blood flow signals or two longer blood flow signals detected within the tumor).
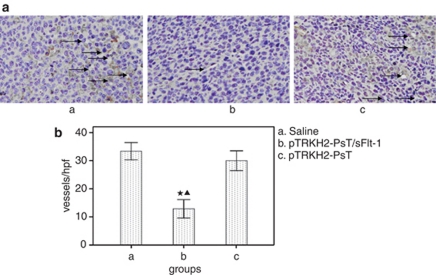
MVD
MVD was determined by counting the number of microvessels per high-power field in the section with an antibody reactive to CD31 (Figure 8a). The results showed that MVD of group b was (12.9±3.90)/vision ( × 200), whereas that of groups a and c were (33.4±3.66)/vision ( × 200) and (30.0±4.21)/vision ( × 200), respectively (Figure 8b). The MVD in group b is significantly lower than that in groups a and c (P<0.05), whereas there was no statistical significance between groups a and c (P>0.05).
Figure 8.
Detection of the tumor microvessel density (MVD) by CD31 immunohistochemistry. (a) Photomicrograph of immunohistochemical staining of CD31 ( × 200) and (b) MVD. a: Saline control group; b: recombinant Bifidobacterium Infantis containing pTRKH2-PsT/sFlt-1 plasmid group; and c: recombinant Bifidobacterium Infantis containing pTRKH2-PsT plasmid group. *P<0.05 vs group a; ▴P<0.05 vs group c. Arrows direct to the vessels.
Side effects, survival quality and analysis
All the mice in each group began to represent slight syndromes such as bad appetite, stunt response, little activity, colorless of fur on the 12th day after the treatment and the syndromes became increasingly evident in a time-dependent manner. However, there were no significant differences between each group in mental status, appetite, weight and so on.
The mice of group a began to die on the 29th day after the treatment, and all the mice of group a died on the 41st day after the treatment. The mice died from tumor deterioration, excluding improper experimental manipulation by mice anatomy. However, there were still 70% and 20% of mice in groups b and c that were alive, respectively (Figure 9). Compared with groups a and c, the survival in group b was significantly prolonged (P<0.05), whereas no statistical significance was found between groups a and c (P>0.05).
Figure 9.
Survival curves of Lewis lung cancer C57BL/6 mice. a: Saline control group; b: recombinant Bifidobacterium Infantis containing pTRKH2-PsT/sFlt-1 plasmid group; and c: recombinant Bifidobacterium Infantis containing pTRKH2-PsT plasmid group.
Discussion
Tumor angiogenesis, first reported by Aligie in 1945,30 is now believed to be one of the most crucial steps in tumor growth and development of metastasis.5 The tumor cells must gain access to the vasculature in the primary tumor, survive the circulation, arrest in the microvasculature, grow in the target organ and induce angiogenesis. Angiogenesis can also help in cancer metastasis in several ways. The production of new vessels increase tumor nutrition and oxygen supplement, allowing tumor cells to grow and providing a route of entry to the systemic circulation. It is now clear that those tumors rarely spread in the pre-vascular phase of the disease. Metastasis may be enhanced by the fragmented basement membranes of the new capillaries.3 It is reported that angiogenesis is essential for the growth of solid cancers beyond 2 mm, which is the limit of nutrient diffusion.4, 10, 31 Angiogenesis is also suggested as a prognostic factor in various solid tumors, such as breast cancer, prostate cancer, gastric and non-small-cell lung cancers.1 The antiangiogenesis therapy of cancer was developed rapidly as this theory was presented as a antitumor method by Folkman in 1971.1 Now there are many methods to treat cancer with the mechanism of antiangiogenesis, and they become an irreplaceable way to cure cancer.9, 13, 32, 33
VEGF is one of the most important stimulating factors for revascularization of tumor tissue,18 and it exerts the function through the VEGFR. The sFlt-1 is a soluble form of Flt-1, which is the extramembrane part of VEGFR-1; it has the same high affinity for VEGF, but could not conduct information for the lack of transmembrane and intramembrane parts.16, 17 In this regard, several studies have reported the application of sFlt-1 gene therapy to inhibit angiogenesis to inhibit tumor growth.34 However, there are some reports that the normal combination of VEGF and VEGFR can stimulate ischemic tissue revascularization and inhibition of angiogenic and inflammatory disorders. Hence, the usage of sFlt-1 may bring in some side effects on normal ischemic tissue and angiogenic and inflammatory disorders.35, 36, 37, 38 Therefore, there are some reports which suggest that intravenous delivery of the sFlt-1 gene via replication-deficient, infectivity-enhanced recombinant adenoviral vectors will result in the overexpression of sFlt-1 in the liver, leading to unacceptable hepatotoxicity.39 Therefore, tumor-specific targeting of the vectors and tumor-specific expression strategies should be used to ensure a clinically useful antiangiogenesis gene therapy.
In recent years, the lack of specific transfer system is one of the main blockages in gene therapy of cancer.40 Many vectors such as virus or liposome always could not reach the interior of tumor; moreover, they can cause many serious side effects, such as the pathogenic effects of virus, the induction of tumor and so on.41 However, Bifidobacterium Infantis is recently confirmed to be one of the most perfect vectors that can reach the inside of tumor specifically and cause nearly no side effects on the body.20 In fact, Bifidobacterium Infantis is a kind of non-pathogenic Gram-positive anaerobes that live in the large intestine and lower part of small intestine of human and other mammals. They keep a coordinate commensalisms relationship between our body and act as a protective function of our body. It has been demonstrated by researches that Bifidobacterium Infantis has a lot of physiological functions, such as inhibiting pathogenic bacteria, stimulating immune function, activating macrophage, enhancing the anti-infectious and antitumor function of the body.42 It has already been widely used in food industry areas. As it is well established, the center of the solid tumor is hypoxic because of rapid proliferation,10 which lead to the specific targeting function of Bifidobacterium Infantis. Yazawa et al.43 injected non-pathological strains of Bifidobacterium 105-A and 108-A into mice bearing lung cancer. After 168 h, both strains of bacteria were observed to have targeted and colonized in the tumors, whereas no bacteria were found in the liver, spleen, kidney, normal lung tissues or other normal tissues.43 When the anaerobic bacteria were used as the gene transfer vector, it could specifically proliferate and directly express foreign gene products in tumor tissues. There is no need to transform cancer cells, which highly improves the gene expression.20, 42 For aspects of the specific targeting function and safety, we consider Bifidobacterium Infantis to be the tumor-specific vector of sFlt-1 gene to exert its antitumor effects.
In this study, we constructed the pTRKH2-PsT/sFlt-1 plasmid by a series of endogenous nucleases and ligase; ultimately, the Bifidobacterium Infantis-mediated sFlt-1 gene transferring system was constructed by electroporation. All the products were identified by electrophoresis or western blot. We chose the positive colony after culture, and the plasmid was cleaved by endogenous enzymes and then identified through electrophoresis. The two fragments on the electrophoresis gel were about 969 bp and 6.9 kb, which corresponded to the size of sFlt-1 gene and pTRKH2-PsT plasmid. In addition, the sequencing results showed that it is consistent with sFlt-1 sequence in GenBank. The results demonstrated that the pTRKH2-PsT/sFlt-1 plasmid was successfully transferred to the Bifidobacterium Infantis. The results of RT-PCR electrophoresis and western blot demonstrated that the sFlt-1 gene was integrated into the genome of Bifidobacterium Infantis and it could be expressed at the levels of gene and protein.
In our in vitro experiment, the growth of HUVECs added with VEGF and final solution extracted from recombinant positive Bifidobacterium Infantis were inhibited significantly. Many HUVECs were killed and only a few grew along the wall. Many cells floated and quantities of cell debris were observed in the medium. The cells left on the wall underwent significant changes in morphology; the original shape was gone, cytoplasm became rougher, the nucleus showed pycnosis and the refraction decreased. The effectiveness of our in vitro experiment advocates a basis for our further study in vivo.
After our study on tumor bearing mice, our results showed that both the recombinant Bifidobacterium Infantis containing pTRKH2-PsT/sFlt-1and pTRKH2-PsT plasmids had good targeting property to tumor tissue. The sFlt-1 protein can be expressed in tumor tissue in recombinant positive group. The tumor growth of the recombinant Bifidobacterium Infantis containing pTRKH2-PsT/sFlt-1 plasmid group was inhibited significantly (P<0.05) and the survival was prolonged significantly (P<0.05). All these results demonstrated a very evident inhibitive effect of tumor of the recombinant Bifidobacterium Infantis containing pTRKH2-PsT/sFlt-1 plasmid. Besides, as shown in the Result section, there were some inhibition effects of tumor by Bifidobacterium Infantis itself comparing group c with group a. The mechanism is still not certain and it may be possible that the Bifidobacterium Infantis can stimulate the inflammatory response, induce the accumulation of immune cell and promote the secreting of antitumor factors by macrophage. It is also reported that the structure of cell wall of Bifidobacterium Infantis had antitumor effects.20 The local proliferation of Bifidobacterium Infantis competing the nutrition with tumor cells may contribute to its antitumor effects, too. However, the expression of sFlt-1 may have a more significant role comparing group b with group c.
The results above showed a significant inhibition effect of tumor growth. Then, we know it is correlated with antiangiogenesis. Hence, we detected the tumor necrosis rate and blood flow signals by color Doppler ultrasound and measured the MVD through CD31 immunohistochemistry to evaluate whether it is correlated with antiangiogenesis. Our results showed that there were massive necrotic areas in tumors of the recombinant Bifidobacterium Infantis containing pTRKH2-PsT/sFlt-1 plasmid group and only a few dot-like blood flow signals near the edge of tumors. However, there were just small necrotic areas and abundant blood flow signals in tumors of the saline control group (group a) and the recombinant Bifidobacterium Infantis containing pTRKH2-PsT plasmid group (group c). Compared with groups a and c, the tumor necrosis rate was significantly increased and the blood flow signals and MVD were significantly decreased in the recombinant Bifidobacterium Infantis containing pTRKH2-PsT/sFlt-1 plasmid group. These results demonstrated that the significant antitumor effects of the recombinant Bifidobacterium Infantis containing pTRKH2-PsT/sFlt-1 plasmid group were correlated with the antiangiogenesis mechanism. The antiangiogenesis mechanism of sFlt-1 is still not for sure. It may inhibit angiogenesis through competing with VEGFR-1; also, it can combine to VEGFR to form heterodimer to interfere with the combination between VEGF and VEGFR.7, 44
As we mentioned before, the Bifidobacteria Infantis are the normal bacteria living in the body of human and other mammals and they keep a coordinate commensalism relationship between our bodies. In fact, the Bifidobacterium Infantis is widely used in the food industry areas. Besides, it has been proved in animal experiments that Bifidobacterium has no obvious influence on body weight, peripheral leukocytes, temperature or survival time of mice, hamsters, guinea pigs or rabbits. Moreover, anaerobic bacteria are highly vulnerable to antibiotics and very low doses of antibiotics are enough to kill them. Even in the case of overgrowth, the bacteria can be easily controlled by antibiotics, which further ensures its safety.20 In addition, Yazawa et al.42, 43 had proved the high specific targeting property of Bifidobacterium to tumor tissue. Our previous experiment also showed that intravenous delivery of Bifidobacterium Infantis was safe.20 In this experiment, there were also no obvious side effects on tumor bearing mice after intravenous delivery of Bifidobacterium Infantis-mediated sFlt-1 gene transferring system. Hence, we consider this system is safe enough.
Conclusions
This study suggested that Bifidobacterium Infantis-mediated sFlt-1 gene transferring system was successfully constructed and could express sFlt-1 at the levels of gene and protein. This system could significantly inhibit growth of HUVECs induced by VEGF in vitro. Moreover, it could inhibit the tumor growth and prolong survival time of LLC C57BL/6 mice safely. This may be a promising therapeutic strategy for cancer patients.
Acknowledgments
This work was supported by grants from the National Natural Scientific Foundation of China (Nos. 30570693 and 81070313).
Author contributions: HZ, ZJL, DDC, SHM, LCD, JPH, CY and YH designed and performed the experiments, and contributed to manuscript writing; SHM and TGL performed pathology experiments; BYM performed ultrasound experiments; and STZ and YQZ analyzed the data. All authors read and approved the final manuscript.
Glossary
- Flt
fms-like tyrosine kinase receptor
- VEGF
vascular endothelial growth factor
- MVD
microvessel density
- CDFI
color Doppler flow imaging
The authors declare no conflict of interest.
References
- Sonmezer M, Gungor M, Ensari A, Ortac F. Prognostic significance of tumor angiogenesis in epithelial ovarian cancer: in association with transforming growth factor [beta] and vascular endothelial growth factor. Int J Gynecol Cancer. 2004;14:82–88. doi: 10.1111/j.1048-891x.2004.14202.x. [DOI] [PubMed] [Google Scholar]
- Li W, Xu RJ, Zhang HH, Jiang LH. Overexpression of cyclooxygenase-2 correlates with tumor angiogenesis in endometrial carcinoma. Int J Gynecol Cancer. 2006;16:1673–1678. doi: 10.1111/j.1525-1438.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- Cantu De Leon D, Lopez-Graniel C, Mendivil MF, Vilchis GC, Gomez C, Salazar JDLG. Significance of microvascular density (MVD) in cervical cancer recurrence. Int J Gynecol Cancer. 2003;13:856–862. doi: 10.1111/j.1525-1438.2003.13399.x. [DOI] [PubMed] [Google Scholar]
- Chen WT, Huang CJ, Wu MT, Yang SF, Su YC, Chai CY. Hypoxia-inducible factor-1[alpha] is associated with risk of aggressive behavior and tumor angiogenesis in gastrointestinal stromal tumor. Jpn J Clin Oncol. 2005;35:207–213. doi: 10.1093/jjco/hyi067. [DOI] [PubMed] [Google Scholar]
- Joo YEMD, Rew JSMD, Seo YHMD, Choi SKMD, Kim YJMD, Park CSMD, et al. Cyclooxygenase-2 overexpression correlates with vascular endothelial growth factor expression and tumor angiogenesis in gastric cancer. J Clin Gastroenterol. 2003;37:28–33. doi: 10.1097/00004836-200307000-00009. [DOI] [PubMed] [Google Scholar]
- Jordan K, Wolf HH, Voigt W, Kegel T, Mueller LP, Behlendorf T, et al. Bevacizumab in combination with sequential high-dose chemotherapy in solid cancer, a feasibility study. Bone Marrow Transplant. 2010;45:1704–1709. doi: 10.1038/bmt.2010.50. [DOI] [PubMed] [Google Scholar]
- Soltau J, Drevs J. Mode of action and clinical impact of VEGF signaling inhibitors. Expert Rev Anticancer Ther. 2009;9:649–662. doi: 10.1586/era.09.19. [DOI] [PubMed] [Google Scholar]
- Marom EMMD, Martinez CHMD, Truong MTMD, Lei XP, Sabloff BSMD, Munden RFMD, et al. Tumor cavitation during therapy with antiangiogenesis agents in patients with lung cancer. J Thorac Oncol. 2008;3:351–357. doi: 10.1097/JTO.0b013e318168c7e9. [DOI] [PubMed] [Google Scholar]
- Ji L, Mao S, Liu H, Xu S, Yang Y, Yi C, et al. Construction and expression of soluble vascular endothelial growth factor receptor-1 eukaryotic expression vector and its effect on proliferation of vascular endothelial cells. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2010;27:369–372. [PubMed] [Google Scholar]
- Harris AL. Antiangiogenesis for cancer therapy. Lancet. 1997;349:13sII–15sII. doi: 10.1016/s0140-6736(97)90014-3. [DOI] [PubMed] [Google Scholar]
- Kommareddy S, Amiji M. Antiangiogenic gene therapy with systemically administered sFlt-1 plasmid DNA in engineered gelatin-based nanovectors. Cancer Gene Ther. 2007;14:488–498. doi: 10.1038/sj.cgt.7701041. [DOI] [PubMed] [Google Scholar]
- FDA approves first antiangiogenesis therapy for treating cancer. Expert Rev Anticancer Ther. 2004;4:167. [Google Scholar]
- Camp-Sorrell DMSNFNPA Antiangiogenesis: the fifth cancer treatment modality. Oncol Nurs Forum. 2003;30:934–944. doi: 10.1188/03.ONF.934-944. [DOI] [PubMed] [Google Scholar]
- Li C, Bowles DE, van Dyke T, Samulski RJ. Adeno-associated virus vectors: potential applications for cancer gene therapy. Cancer Gene Ther. 2005;12:913–925. doi: 10.1038/sj.cgt.7700876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne M, Stellmach V, Cornwell M, Chung C, Doll JA, Lee EJ, et al. Gene transfer of pigment epithelium-derived factor suppresses tumor growth and angiogenesis in a hepatoblastoma xenograft model. Pediatr Res. 2006;60:282–287. doi: 10.1203/01.pdr.0000232789.86632.91. [DOI] [PubMed] [Google Scholar]
- Ye C, Feng C, Wang S, Wang KZQ, Huang N, Liu X, et al. sFlt-1 gene therapy of follicular thyroid carcinoma. Endocrinology. 2004;145:817–822. doi: 10.1210/en.2003-1106. [DOI] [PubMed] [Google Scholar]
- Liu J, Li J, Su C, Huang B, Luo S. Soluble Fms-like tyrosine kinase-1 expression inhibits the growth of multiple myeloma in nude mice. Acta Biochim Biophys Sin. 2007;39:499–506. doi: 10.1111/j.1745-7270.2007.00310.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura I, Mizuguchi Y, Miyajima A, Asano T, Tadakuma T, Hayakawa M. Suppression of lung metastasis of renal cell carcinoma by the intramuscular gene transfer of a soluble form of vascular endothelial growth factor receptor I. J Urol. 2004;171:2467–2470. doi: 10.1097/01.ju.0000117801.04926.a8. [DOI] [PubMed] [Google Scholar]
- McCarthy J, O'Mahony L, O'Callaghan L, Sheil B, Vaughan EE, Fitzsimons N, et al. Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut. 2003;52:975–980. doi: 10.1136/gut.52.7.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi C, Huang Y, Guo ZY, Wang SR. Antitumor effect of cytosine deaminase/5-fluorocytosine suicide gene therapy system mediated by Bifidobacterium infantis on melanoma 1. Acta Pharmacol Sin. 2005;26:629–634. doi: 10.1111/j.1745-7254.2005.00094.x. [DOI] [PubMed] [Google Scholar]
- Hu B, Kou L, Li C, Zhu LP, Fan YR, Wu ZW, et al. Bifidobacterium longum as a delivery system of TRAIL and endostatin cooperates with chemotherapeutic drugs to inhibit hypoxic tumor growth. Cancer Gene Ther. 2009;16:655–663. doi: 10.1038/cgt.2009.7. [DOI] [PubMed] [Google Scholar]
- Li X, Fu GF, Fan YR, Liu WH, Liu XJ, Wang JJ, et al. Bifidobacterium adolescentis as a delivery system of endostatin for cancer gene therapy: selective inhibitor of angiogenesis and hypoxic tumor growth. Cancer Gene Ther. 2003;10:105–111. doi: 10.1038/sj.cgt.7700530. [DOI] [PubMed] [Google Scholar]
- Liu TG, Huang Y, Cui DD, Huang XB, Mao SH, Ji LL, et al. Inhibitory effect of ginsenoside Rg3 combined with gemcitabine on angiogenesis and growth of lung cancer in mice. BMC Cancer. 2009;9:250. doi: 10.1186/1471-2407-9-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicer A, Cabanas F, Perez-Higueras A, Garcia-Alix A, Quero J. Neural migration disorders studied by cerebral ultrasound and colour Doppler flow imaging. Arch Dis Childhood Fetal Neonat Edn. 1995;73:55F–61F. doi: 10.1136/fn.73.2.f55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Fu GF, Fan YR, Liu WH, Liu XJ, Wang JJ, et al. Bifidobacterium adolescentis as a delivery system of endostatin for cancer gene therapy: selective inhibitor of angiogenesis and hypoxic tumor growth. Cancer Gene Ther. 2003;10:105–111. doi: 10.1038/sj.cgt.7700530. [DOI] [PubMed] [Google Scholar]
- Chen G, Zhou J, Gao Q, Huang X, Li K, Zhuang L, et al. Oncolytic adenovirus-mediated transfer of the antisense chk2 selectively inhibits tumor growth in vitro and in vivo. Cancer Gene Ther. 2006;13:930–939. doi: 10.1038/sj.cgt.7700967. [DOI] [PubMed] [Google Scholar]
- Yoruk OMD, Dane SMD, Ucuncu HMD, Aktan BMD, Can IP. Stereological evaluation of laryngeal cancers using computed tomography via the Cavalieri method: correlation between tumor volume and number of neck lymph node metastases. J Craniofac Surg. 2009;20:1504–1507. doi: 10.1097/SCS.0b013e3181b09bc3. [DOI] [PubMed] [Google Scholar]
- Akbas HMD, Sahin BPD, Eroglu LMD, Odaci EMDPD, Bilgic SPD, Kaplan SPD, et al. Estimation of breast prosthesis volume by the Cavalieri principle using magnetic resonance images. Aesthet Plast Surg. 2004;28:275–280. doi: 10.1007/s00266-004-0022-8. [DOI] [PubMed] [Google Scholar]
- Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- Oreilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- Mahendra G, Kumar S, Isayeva T, Mahasreshti PJ, Curiel DT, Stockardt CR, et al. Antiangiogenic cancer gene therapy by adeno-associated virus 2-mediated stable expression of the soluble FMS-like tyrosine kinase-1 receptor. Cancer Gene Ther. 2005;12:26–34. doi: 10.1038/sj.cgt.7700754. [DOI] [PubMed] [Google Scholar]
- Machein MR, Plate KH. VEGF in brain tumors. J Neuro-Oncol. 2000;50:109–120. doi: 10.1023/a:1006416003964. [DOI] [PubMed] [Google Scholar]
- Sokoloff MH, Bradley M, Zhau HE, Simons JW, Chung LWK. VEGF inhibits human prostate cancer (HPC) growth and potentiates anti-angiogenesis therapy. J Urol. 1999;161:53. [Google Scholar]
- Ramachandra S, D'Souza SS, Gururaj AE, Shaila MS, Salimath BP. Paracrine action of sFLT-1 secreted by stably-transfected Ehrlich ascites tumor cells and therapy using sFLT-1 inhibits ascites tumor growth in vivo. J Gene Med. 2009;11:422–434. doi: 10.1002/jgm.1309. [DOI] [PubMed] [Google Scholar]
- Zhao QMD, Egashira KMD, Inoue SMD, Usui MMD, Kitamoto SMD, Ni WMD, et al. Vascular endothelial growth factor is necessary in the development of arteriosclerosis by recruiting/activating monocytes in a rat model of long-term inhibition of nitric oxide synthesis. Circulation. 2002;105:1110–1115. doi: 10.1161/hc0902.104718. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Egashira K, Hiasa K-I, Ishibashi M, Inoue S, Ohtani K, et al. Essential role of vascular endothelial growth factor and Flt-1 signals in neointimal formation after periadventitial injury. Arterioscler Thromb Vasc Biol. 2004;24:2284–2289. doi: 10.1161/01.ATV.0000147161.42956.80. [DOI] [PubMed] [Google Scholar]
- Medina MA, Muñoz-Chápuli R, Quesada AR. Challenges of antiangiogenic cancer therapy: trials and errors, and renewed hope. J Cell Mol Med. 2007;11:374–382. doi: 10.1111/j.1582-4934.2007.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu FMD, Longo MMDP, Tamayo E, Maner WBS, Al-Hendy AMDP, Anderson GDMD, et al. The effect of over-expression of sFlt-1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice. Am J Obstet Gynecol. 2007;196:396e1–396e7. doi: 10.1016/j.ajog.2006.12.024. [DOI] [PubMed] [Google Scholar]
- Mahasreshti PJ, Kataram M, Wang MH, Stockard CR, Grizzle WE, Carey D, et al. Intravenous delivery of adenovirus-mediated soluble FLT-1 results in liver toxicity. Clin Cancer Res. 2003;9:2701–2710. [PubMed] [Google Scholar]
- Liu SC, Minton NP, Giaccia AJ, Brown JM. Anticancer efficacy of systemically delivered anaerobic bacteria as gene therapy vectors targeting tumor hypoxia/necrosis. Gene Therapy. 2002;9:291–296. doi: 10.1038/sj.gt.3301659. [DOI] [PubMed] [Google Scholar]
- Fukuhara T, Taketomi A, Motomura T, Okano S, Ninomiya A, Abe T, et al. Variants in IL28B in liver recipients and donors correlate with response to peg-interferon and ribavirin therapy for recurrent hepatitis C. Gastroenterology. 2010;139:1577–1585. doi: 10.1053/j.gastro.2010.07.058. [DOI] [PubMed] [Google Scholar]
- Yazawa K, Fujimori M, Nakamura T, Sasaki T, Amano J, Kano Y, et al. Bifidobacterium longum as a delivery system for gene therapy of chemically induced rat mammary tumors. Breast Cancer Res Treat. 2001;66:165–170. doi: 10.1023/a:1010644217648. [DOI] [PubMed] [Google Scholar]
- Yazawa K, Fujimori M, Amano J, Kano Y, Taniguchi S. Bifidobacterium longum as a delivery system for cancer gene therapy: Selective localization and growth in hypoxic tumors. Cancer Gene Ther. 2000;7:269–274. doi: 10.1038/sj.cgt.7700122. [DOI] [PubMed] [Google Scholar]
- Wang R, Zhang XW, Wang GQ, Chen XC, Tian L, Yang HS, et al. Systemic inhibition of tumor growth by soluble Flk-1 gene therapy combined with cisplatin. Cancer Gene Ther. 2006;13:940–947. doi: 10.1038/sj.cgt.7700958. [DOI] [PubMed] [Google Scholar]