Abstract
We report the first community-based evaluation of Shigella flexneri 2a strain SC602, a live, oral vaccine strain attenuated by deletion of the icsA (virG) plasmid virulence gene, given at 104 CFU. The primary objectives of this trial were to determine the safety and immunogenicity of the vaccine and to determine the duration of colonization. Four of 34 volunteers experienced transient fevers, and three reported diarrhea during the first 3 days of the study. Half of the volunteers mounted a positive serum immunoglobulin A (IgA) response to S. flexneri lipopolysaccharide. All but one of the volunteers excreted the vaccine in their stools for 1 to 33 days, and this excretion was often intermittent. Data from the community-based study were supplemented with an inpatient trial in which three volunteers received 103 and nine received 104 CFU. All volunteers who received 103 CFU excreted SC602 and had an IgA antibody-secreting cell response. Two of these had a serum IgA response. Six of the nine volunteers who received 104 CFU excreted SC602. One vaccinee had a transient fever and two met the definition of diarrhea. Six volunteers that received 104 CFU had an antibody-secreting cell response, and four had a serum IgA response. SC602 has now been tested at 104 CFU in a total of 58 volunteers. The cumulative results of these clinical trials, reported here and previously (Coster et al., Infect. Immun. 67:3437-3443, 1999), have demonstrated that SC602 is a substantially attenuated candidate vaccine that can evoke protection against the most severe symptoms of shigellosis in a stringent human challenge model of disease.
It is estimated that 160 million cases of shigellosis occur each year in developing countries and that an additional 1.5 million cases occur in industrialized nations (11). The intestinal manifestations of Shigella infection range from uncomplicated watery diarrhea to dysentery with frequent passage of small-volume stools containing gross blood and mucus. Dysentery is also accompanied by abdominal cramps and rectal tenesmus. Shigellosis usually includes constitutional symptoms such as fever and headache. Experimental infection of volunteers with Shigella flexneri 2a (8) or Shigella sonnei (6) elicits protection against severe shigellosis in all volunteers and against diarrhea or fever in most volunteers.
We are testing a series of genetically attenuated strains designed to asymptomatically induce the protective immune responses of naturally acquired disease (3, 10). We have described phase 1 trials of S. flexneri 2a SC602, a candidate vaccine strain with a deletion of the iscA (or virG) plasmid virulence gene (3). SC602 is the first example of a live oral Shigella vaccine tested in human trials where deletion of a specific virulence gene that is critical for pathogenesis has been used as the major attenuating feature. Shigellae with this attenuating mutation are fully invasive for tissue culture monolayers but are unable to spread to contiguous host cells (1). The icsA gene product subverts Cdc42-contolled host cell actin assembly by binding to N-WASP and activating it as an IcsA-N-WASP-Arp2/3 complex. This complex nucleates F-actin polymerization at the nongrowing poles of dividing shigellae (5). The resulting actin tail provides a motive force for intercellular spread of bacteria. An S. flexneri icsA mutant causes only asymptomatic nodular abscesses after intragastric challenge of rhesus monkeys (17). Such mutants also fail to elicit keratoconjunctivitis in the guinea pig Sereny test. In addition to icsA, the SC602 vaccine carries a mutation in the aerobactin gene (iuc) that partially attenuates S. flexneri in the Sereny test and in the rabbit ileal loop model (13).
Initial clinic-based phase 1 trials of SC602 indicated that a 104 CFU dose of vaccine was not associated with serious adverse events. Out of 15 vaccinees enrolled in two studies, one subject developed transient fever and another passed occasional diarrheal stools. None of the vaccinees ingesting 104 CFU reported constitutional or intestinal symptoms interfering with normal activities. Approximately two-thirds of vaccinees ingesting the 104-CFU dose mounted strong antibody-secreting cell responses against homologous lipopolysaccharide in peripheral blood lymphocytes. In an experimental challenge trial with 103 CFU of virulent S. flexneri 2a, all vaccinees were protected against fever and dysentery, while six of seven controls experienced severe shigellosis. Vaccination with SC602 was associated with 50% protection against watery diarrhea, and this mild manifestation of infection was seen in volunteers who had fewer immunoglobulin A (IgA) antibody-secreting cells against lipopolysaccharide following vaccination (3).
The promising safety and efficacy results seen with the 104- CFU dose of SC602 were tempered by safety issues encountered at higher vaccine doses. For example, 9 of 18 volunteers (from two studies) ingesting 106 CFU of SC602 experienced diarrhea or fever, indicating that the vaccine has a narrow range of safety (3). Furthermore, studies to date could not effectively evaluate the duration of excretion because antibiotic treatment was initiated within 8 days of vaccination.
We now report a community-based evaluation of SC602. The primary objectives of this trial were to determine the safety and immunogenicity of the vaccine for North American volunteers who were performing the normal activities of daily living and to determine the duration of colonization by this prototrophic Shigella vaccine strain. To this end, volunteers reported stooling characteristics, possible fevers (documented by Tempa-Dot), emesis, and subjective symptoms (abdominal cramps, headaches). The volunteers also performed primary plating of stool specimens for subsequent microbiological identification (18). Data from the community-based study were supplemented with an inpatient trial of 4-log and 3-log dose regimens that included daily monitoring of body temperature, stool volume and character, and stool culture by study personnel. The results from these studies will be discussed in the broader context of previous clinical trials with this live, attenuated, oral Shigella vaccine strain (3).
MATERIALS AND METHODS
Vaccine composition.
SC602 was manufactured at the Walter Reed Army Institute of Research Pilot Bioproduction Facility as a lyophilized product under current good manufacturing practices (3).
Subjects.
Volunteers were recruited from the local community. Written informed consent was obtained under protocols approved by institutional review boards under the U.S. Army Medical Research and Materiel Command. Exclusion criteria were as described previously (3), and volunteers in the outpatient study were required to provide written evidence that household contacts had been informed of their participation in the study. All vaccinations occurred at the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID) in Frederick, Md.
Statistics.
Informative tables of summary descriptive statistics and graphical displays were used to contrast variables of interest. The Mann-Whitney test was used to compare serum immune responses of inpatients and outpatients. In the outpatient trial, for each subject the total number of stools evaluated was recorded, and each stool was classified as positive or negative. Each subject was surveyed to determine whether any adverse events were associated with the collected stool. The difference in the proportions of adverse event-free periods between positive and negative stools was analyzed with the paired t test. Data were available on 31 subjects.
Outpatient study. (i) Study design.
On the day of immunization, frozen lyophilized SC602 (lot 0070) was thawed on wet ice and reconstituted with 5 ml of sterile water for irrigation USP (McGaw). Reconstituted vaccine was placed on wet ice for 10 min with gentle intermittent swirling to ensure a uniform suspension. This suspension was diluted in sterile 0.9% saline solution USP (McGaw). Thirty-four volunteers ingested 3 × 104 CFU of SC602 as determined by quantitative plate counts. The inoculation was mixed with 30 ml of sodium bicarbonate buffer (2 g of NaHCO3 per 150 ml of sterile, deionized water) and was ingested by each volunteer 2 min after ingestion of 120 ml of the sodium bicarbonate solution.
Volunteers returned to the clinic at USAMRIID or Walter Reed Army Medical Center for follow-up. The volunteers were queried by the clinical staff concerning any symptoms experienced by them or by their household contacts. Symptoms were graded by the volunteers as mild (no limitation of activity), moderate (mild to moderate limitation of activity), or severe (unable to perform daily activities). If the volunteers “felt feverish or had chills,” they used single-use thermometers (Tempa-Dot; 3M, Rochester, Minn.) and brought the device to the clinic as documentation. Fever was defined as an oral temperature of ≥100.5°F. Diarrhea was defined as ≥3 loose stools in a 24-h period, and dysentery was defined as any loose stool with gross blood. All volunteers were treated with ciprofloxacin (500 mg by mouth twice daily for 5 days) beginning on day 35. Sera were collected on days 0, 7, 14, 19, 30, 60, 90, and 120 for enzyme-linked immunosorbent assay (ELISA).
(ii) Bacteriology.
Volunteers were requested to swab two Hektoen enteric agar (HEA) plates each day that they passed a stool for 42 days and bring the plates to the clinic in an ice chest. This sampling technique has been described previously (18). After incubation of the HEA at 37°C for 24 h, non-lactose-fermenting colonies were identified as S. flexneri 2a by slide agglutination in homologous antiserum (Difco). If ten colonies tested negative in 2a antiserum, a plate was recorded as negative for S. flexneri 2a. A volunteer was defined as excreting SC602 if any stool collected that day yielded at least one S. flexneri 2a colony. Colonization was defined as the time between the first and last stools that were positive for SC602 (18). The identity of SC602 was confirmed by PCR with primers specific for icsA (18).
(iii) Immunology.
Antibody responses against S. flexneri 2a lipopolysaccharide were assessed as IgA, IgG, and IgM ELISA titers in sera. Titers were determined with endpoints derived from a linear regression analysis of eight doubling dilutions with an adjusted optical density of 0.3 (2). Seroconversion was defined as a ≥3-fold rise in titer after vaccination.
Inpatient study. (i) Study design.
Three inpatient volunteers received 3 × 103 CFU of SC602 and nine received 2 × 104 CFU. Volunteers ingested 150 ml of sodium bicarbonate followed by SC602 in 30 ml of water. Volunteers were randomized, and the study was handled in a double-blind manner regarding dose. Signs and symptoms were monitored daily and graded as described for the outpatient study. All stools were collected, weighed, and graded as follows: 1, hard (normal); 2, soft (normal); 3, thick liquid (loose); 4, opaque watery liquid (loose); or 5, clear watery (loose). Diarrhea was defined by two or more grade 3 to 5 stools within 48 h totaling at least 200 ml or a single grade 3 or greater stool of >300 ml in 24 h. Dysentery was defined by a stool of grade 3 or higher with gross blood. All volunteers were treated with ciprofloxacin beginning on day 8 and were released from the study ward on day 10 to 12 after passing stools negative for S. flexneri 2a on two consecutive days. Sera were collected on days 0, 7, 14, and 28 for ELISA and whole blood was collected on days 0, 7, and 9 for the antibody-secreting cell assay.
(ii) Bacteriology.
All stools were cultured on HEA until the volunteer was discharged from the unit. Detection of SC602 was as described for the outpatient study.
(iii) Immunology.
Antibody responses against S. flexneri 2a lipopolysaccharide were assessed as IgA, IgG, and IgM ELISA titers in sera (19). Titers were determined as described for the outpatient study. The enzyme-linked immunospot assay was used to enumerate IgA, IgG, and IgM antibody-secreting cells per 106 peripheral blood lymphocytes. No lipopolysaccharide-specific antibody-secreting cells were detected before vaccination. In the absence of a calculated standard deviation, a positive response was arbitrarily defined as ≥10 antibody-secreting cells per 106 peripheral blood lymphocytes (3).
RESULTS
Clinical response in outpatients.
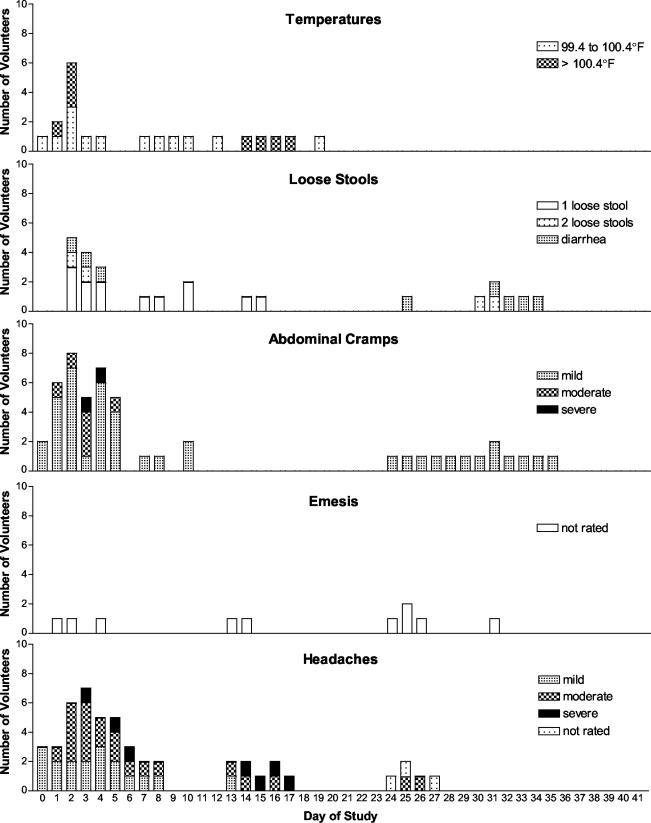
Thirty-four subjects (aged 18 to 56 years) received 3 × 104 CFU of SC602. The time course of reported adverse events is shown in Fig. 1. Five of 34 volunteers (15%) had fevers ranging from 100.6 to 102.8°F. Four of these fevers occurred on study day 1 or 2. The highest fever, 102.8°F, occurred on days 14 to 17 and was associated with severe headache, severe aches, severe loss of appetite, moderate nausea, malaise, and three episodes of emesis. However, this volunteer reported no abdominal cramps, loose stools, dysentery, or diarrhea. This volunteer's stool was positive for S. flexneri 2a the day before symptoms began, but no samples were provided during this period (see volunteer number 1 in Fig. 3A).
FIG. 1.
Time course of adverse events in 34 subjects of the outpatient trial. The bars represent the total number of volunteers reporting symptoms per day.
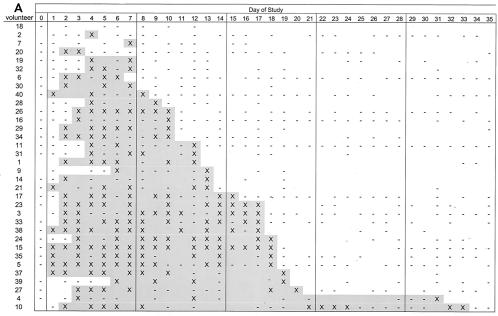
FIG. 3.
Excretion of SC602 by volunteers. Symbols represent culture-positive stools (X) and culture-negative stools (−). No entry for a collection day indicates that no sample was collected. The shaded area represents the duration of colonization. (A) Outpatient study: volunteers received antibiotics from days 35 to 39. (B) Inpatient study: volunteers received antibiotics from days 8 to 12.
Fifteen percent (5 of 34) of the outpatient volunteers met the criteria for diarrhea. Half of the episodes of diarrhea occurred during the first week of the study (Fig. 1), and these stools were positive for S. flexneri 2a, while the other half occurred 4 to 5 weeks after vaccination and were not associated with excretion of shigellae. Diarrhea lasted only one day, except for volunteer number 15, who reported diarrhea for two days (days 32 to 33) in the absence of SC602 excretion. That volunteer had abdominal cramps every day from days 24 through 35 along with loss of appetite, nausea, and fatigue, and the volunteer reported emesis on days 24 to 27. This volunteer did not report fever, headache, or bloody stools. He did not shed SC602 during days 28 to 35, and there were no microbiology samples on days 20 to 27. Therefore, the relationship of this illness to SC602 vaccination is uncertain.
Most subjective complaints were mild, but the most common moderate-severe complaints in the outpatient study were headaches (35% of volunteers), abdominal cramps (24%), and generalized aches (24%). Most of the moderate and severe symptoms occurred on days 2 and 3. A total of 16 volunteers (47%) experienced at least one moderate or severe symptom or fever or diarrhea within the first week. Emesis did not appear to be related to vaccine administration.
Immune response in outpatients.
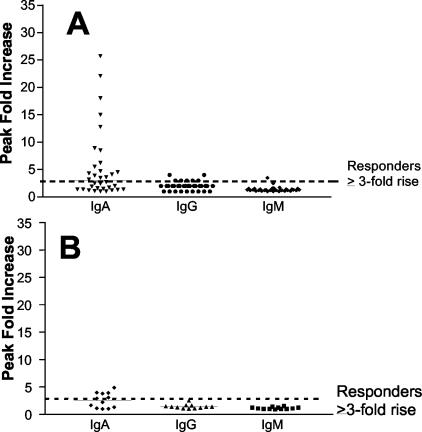
A positive ELISA IgA response to S. flexneri 2a lipopolysaccharide was seen in 17 (50%) volunteers in the outpatient study (Fig. 2A). We detected minimal IgG and IgM responses to lipopolysaccharide. The geometric mean titer of peak IgA responses to lipopolysaccharide was 299, an increase from the baseline geometric mean titer of 91. The geometric mean titer of peak IgG responses was 2,220, increased from a baseline geometric mean titer of 1,190. The baseline geometric mean titer of IgM was 855, and the peak titer was 1,130. Median increases (n = fold) for serum IgA peaked on day 14 and declined thereafter, while the modest levels in IgG reached similar levels on days 14, 19, and 30, followed by a gradual decline. There was no apparent relationship between the magnitude of serum IgA response against lipopolysaccharide and the duration of colonization with SC602.
FIG. 2.
Fold increase in serum IgA, IgG, and IgM antibody titers to S. flexneri 2a lipopolysaccharide. (A) Outpatient study. (B) Inpatient study. The dashed line identifies the threshold of response. The solid lines define the median increase (n = fold).
Excretion in outpatients.
Volunteers were followed for 42 days (18) (Fig. 3A). S. flexneri 2a was detected from all but one of the volunteers, and that volunteer failed to submit stools on 3 days during the first week. The first positive stool was detected on day 1 and the last positive stool was detected on day 33. The average length of excretion was 11.6 days, with a standard deviation of 7.4 days. As many as 6 days with negative cultures were bracketed by days with positive cultures, and two subjects (volunteers 10 and 4, Fig. 3A) were intermittently culture positive for over 4 weeks. SC602 was not detected in volunteers' stools after antibiotic treatment began on day 35. There was no relationship between positive stool culture and reported symptoms; adverse events were not associated with excretion of SC602 in the stool. The two variables acted independently (t = 0.41, P = 0.683).
Clinical response in inpatients.
Nine volunteers (aged 18 to 56) received 2.1 × 104 CFU of SC602, and three volunteers (aged 19 to 28) received 2.8 × 103 CFU of SC602 after admission to the study ward. One of the volunteers who received 104 CFU had a transient fever (100.6°F) on day 1. Two volunteers out of nine receiving 104 CFU met the criteria for diarrhea. One volunteer experienced diarrhea on days 1 and 3, and the other volunteer experienced diarrhea on days 4 and 5. All complaints for either dose were mild, except one volunteer at the 104-CFU dose complained of a severe headache on day 3. The most common complaints for the entire inpatient study were abdominal cramps (66%) and headaches (42%). Most symptoms were experienced on days 1 to 3. However, unlike the outpatient study, all symptoms but one in the inpatient study were mild.
Immune response in inpatients.
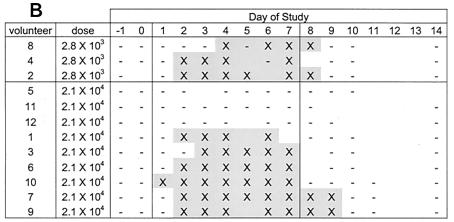
A positive serum IgA response to S. flexneri 2a lipopolysaccharide was seen in five volunteers (an additional volunteer had a titer of 2.9 that was just below the cutoff) (Table 1 and Fig. 2B). We observed minimal IgG and IgM responses to lipopolysaccharide. The geometric mean titer of peak IgA responses to lipopolysaccharide was 441, an increase from the baseline geometric mean titer of 201. The geometric mean titer of peak IgG responses was 1,856, increased from a baseline geometric mean titer of 1,369. The baseline geometric mean titer of IgM was 357, with no appreciable increase (peak of 395). Median increases for serum IgA peaked on day 14 and declined thereafter, while the modest increases in IgG peaked on day 14 and remained elevated on day 28. A positive IgA antibody-secreting cell response was seen in 9 of 12 volunteers (three volunteers were from the group receiving 103 CFU). The three volunteers who did not shed the vaccine were the same three volunteers who did not mount a positive IgA antibody-secreting cell response (one volunteer did mount a positive IgG antibody-secreting cell response).
TABLE 1.
Immune response to S. flexneri 2a lipopolysaccharide in the inpatient trial
| Dose (CFU) | Volunteer no. | No. of ASC/ 106 cellsa
|
Peak increase (fold)
|
Excretion | ||||
|---|---|---|---|---|---|---|---|---|
| IgA | IgG | IgM | IgA | IgG | IgM | |||
| 104 | 5 | 0 | 0 | 1 | 1.1 | 1.1 | 1.0 | No |
| 12 | 4 | 43 | 5 | 1.0 | 1.2 | 0.9 | Nob | |
| 11 | 5 | 5 | 0 | 1.0 | 1.1 | 1.0 | Nob | |
| 6 | 40 | 13 | 55 | 3.9 | 1.4 | 1.5 | Yes | |
| 9 | 85 | 36 | 33 | 2.9 | 1.6 | 1.2 | Yes | |
| 10 | 110 | 2 | 19 | 1.7 | 1.2 | 1.2 | Yes | |
| 1 | 113 | 33 | 42 | 2.2 | 1.4 | 1.1 | Yes | |
| 3 | 179 | 173 | 133 | 3.8 | 1.3 | 1.1 | Yes | |
| 7 | 186 | 60 | 90 | 4.8 | 2.5 | 1.2 | Yes | |
| 103 | 2 | 10 | 1 | 10 | 1.3 | 1.1 | 1.0 | Yes |
| 4 | 75 | 2 | 35 | 3.1 | 1.4 | 1.4 | Yes | |
| 8 | 277 | 175 | 340 | 4.0 | 1.6 | 1.0 | Yes | |
| GMTc | 30 | 11 | 18 | 2.2 | 1.4 | 1.1 | ||
Peak number of antibody-secreting cells (ASC) per 106 peripheral blood lymphocytes.
PCR positive.
GMT, geometric mean titer (determined for both doses).
Excretion in inpatients.
In the inpatient study, volunteers were followed for up to 14 days. Three of the volunteers were culture negative (Fig. 3B). The first positive stool was detected on day 1, and most volunteers began excretion on day 2. No volunteers excreted SC602 after 2 days of antibiotic treatment. The volunteer who reported a severe headache never excreted SC602.
DISCUSSION
Of the 34 vaccinees in the community-based trial, four reported fever of a few hours’ duration during the first two study days. Similarly, two of 21 vaccinees experienced fever during this time frame in clinic-based trials reported here and previously (3) (Table 2). The cumulative 12% rate of transient, febrile reactions is comparable to the rate of febrile reactions attributed to licensed parenteral vaccines such as those for diphtheria, Haemophilus influenzae type b, Lyme disease, measles-mumps-rubella, and varicella zoster (www.cdc.gov/nip). An additional outpatient vaccinee suffered a more protracted febrile illness, with no change in bowel habits 2 weeks after vaccination. The timing and nature of this illness raise some doubt as to whether it was vaccine related. Nonetheless, we have included this occurrence in our cumulative reactogenicity data in Table 2.
TABLE 2.
Cumulative reactogenicity for all SC602 trials at a 104-CFU dosea
| Symptom | Severity | 104 CFU of SC602 (n = 58)
|
Placebo (n = 16)
|
||
|---|---|---|---|---|---|
| No. (%) with complaint | 95% CI (%) | No. (%) with complaint | 95% CI (%) | ||
| Shigellosis | 0 | 0-5 | 0 | 0-17 | |
| Dysentery | 0 | 0-5 | 0 | 0-17 | |
| Headache | Mild or moderate | 21 (36) | 24-50 | 7 (44) | 20-70 |
| Severe | 4 (7) | 2-17 | 0 | 0-17 | |
| Abdominal cramps | Mild or moderate | 24 (41) | 29-55 | 6 (38) | 15-68 |
| Severe | 2 (3) | 0.4-12 | 0 | 0-17 | |
| Fever | 7 (12) | 5-23 | 1 (6) | 0.3-30 | |
| Diarrhea | 6 (10) | 4-21 | 1 (6) | 0.3-30 | |
Data are from this report and reference 3. Data are limited to the 2 weeks after vaccination. CI, confidence interval.
Because of the circumstances of data collection, diarrhea was defined by a specific number of liquid stools (three to five) for outpatients and by a specific weight of liquid stools for inpatients. Although different definitions were applied, the overall rates of diarrhea were similar in all of the inpatient (9%) trials and in the outpatient (12%) trial. The cumulative rate of diarrhea for all SC602 trials was 10% (Table 2). With the caveat of different parameters of diarrhea and different methods for detecting fever in inpatient and outpatient trials, a total of 11 of 58 vaccinees receiving the 104-CFU dose of SC602 experienced either fever or diarrhea, and one vaccinee experienced both symptoms, for a cumulative rate of 19% for any clinical reaction. In placebo controls from an earlier dose-ranging trial of SC602, 2 of 16 volunteers had diarrhea or fever, for a cumulative rate of 12%.
We also collected data on subjective symptoms that might have an impact on consumer acceptance of SC602. Headache and abdominal cramps were cited by 5 of 58 vaccinees (9%) as “severe” complaints inhibiting normal activities (Table 2). None of the placebo controls in the initial dose-ranging trial reported any severe symptoms, but the rate of mild to moderate symptoms was similar to that of vaccinees (Table 2) (3). We tentatively conclude that the rate of short-term fever, diarrhea, severe headache, or severe intestinal cramps elicited by a 104- CFU dose of SC602 in North American adults is approximately 30%, compared to 13% in placebo controls (Table 2). However, an expanded double-blind, placebo-controlled, community-based phase 2 trial of SC602 would be required to formally assess the rate of reactogenicity of this vaccine.
The ELISPOT antibody-secreting cell assay for peripheral blood lymphocytes expressing anti-2a lipopolysaccharide is a sensitive assay for quantifying immune responses evoked in the intestinal mucosa. When IgA antibody-secreting cell data from the 12 inpatients receiving a 3-log or 4-log dose of SC602 were compared to those for a previous group of 12 patients receiving a single 4-log dose (3), the geometric mean titers were almost identical (46 versus 47 antibody-secreting cells/106 peripheral blood lymphocytes). Eight vaccinees had a positive antibody-secreting cell response (≥10 antibody-secreting cells) for at least one antibody isotype in the first inpatient trial, and 10 vaccinees were positive in the present trial (overall positive response rates of 75% for any isotype and 66% for IgA).
As a point of reference, volunteers experiencing severe shigellosis after challenge with virulent S. flexneri 2a had an IgA geometric mean titer of 174 antibody-secreting cells/106 peripheral blood lymphocytes, with an 86% positive response (3). Threefold serum IgA ELISA responses were detected in half the outpatient and inpatient vaccinees, and this level of antibody response correlated with protection against all symptoms of shigellosis (including diarrhea) in the previous efficacy trial of SC602 vaccinees. All vaccinees were protected against fever, dysentery, and severe shigellosis, but not against diarrhea.
The self-plating technique devised for the outpatient trial (18) successfully detected excretion of vaccine organisms by 33 of 34 vaccinees, with a mean duration of colonization of approximately 12 days. An unexpected finding in the outpatient trial was the intermittent isolation of shigellae. As many as six negative cultures over the course of 12 days were bracketed by periods of positive cultures, and two subjects were intermittently culture positive for over 4 weeks. Infrequent long-term excretion of shigellae (15, 14) and Shigella flexneri (4, 12, 16) is consistent with past reports, showing that SC602 is not attenuated regarding excretion. Because the original criterion of two consecutive culture-negative stools did not prove to be a reliable standard for determining the persistence of SC602, all volunteers were asked to return as many inoculated HEA plates as possible for 1 month.
In order to clearly terminate the outpatient trial, all volunteers were treated with ciprofloxacin on days 35 to 40. Since we were unable to always detect SC602 in stool samples from vaccinees who were colonized with SC602, the duration of colonization in a small proportion of vaccinees could be longer than 35 days. In the inpatient trial, SC602 was not detected by microbiological culture in the stools of three of nine volunteers ingesting a 4-log dose of SC602, while all three inpatients ingesting the 3-log dose excreted vaccine up to the end of the 7-day study. In previous inpatient trials, all vaccinees receiving a 4-log dose of SC602 were culture positive (3). Cumulative analysis of inpatient and outpatient trials indicates that 90% of vaccinees receiving this dose of SC602 were culture positive on day 7. SC602 was not transmitted to control volunteers during the initial inpatient dose-ranging trials (3).
Dose selection trials of a second icsA (virG) candidate (S. sonnei WRSS1) have yielded promising safety and immunogenicity results in phase 1 trials (10). More recent community-based trials of WRSS1 have included active microbiological monitoring of stools of all household contacts for adventitious spread of the excreted vaccine. Like SC602, WRSS1 colonizes a majority of volunteers who ingest as few as 103 CFU after bicarbonate buffer, and the vaccine strain was excreted for an average of 5 days. However, when 32 household contacts were exposed to vaccinees excreting WRSS1 for a cumulative total of 164 days, there was no evidence of accidental transmission of the vaccine (unpublished data). These data suggest that adventitious spread of attenuated Shigella vaccines to individuals with normal standards of hygiene will be an uncommon event. A similar community-based study of SC602 household contacts is planned as a key step in further development of this vaccine. Regardless of the outcome of such phase 1 trials, however, the possibility remains that excreted Shigella vaccines will occasionally colonize bystanders.
Fortunately, the icsA gene deletion is a powerful attenuation that prevents development of hemorrhagic lesions in the intestinal mucosa even when 108 CFU of SC602 is ingested with bicarbonate (3). In primates inoculated with an icsA mutant, small foci of inflammation occur in lymphoid follicles, but the infection does not progress to colonic lesions, presumably because the shigellae cannot invade contiguous epithelial cells (17). The failure of shigellae to propagate by intercellular spread is an attenuating feature that should be effective even in immunocompromised individuals. The Food and Drug Administration has recently addressed the issue of live, attenuated vaccines with a potential for adventitious transmission by approving the live, attenuated FluMist intranasal vaccine for general use only in healthy people aged 18 to 50 years. Prescriptions are required for youths aged 5 to 17 years, and the killed influenza vaccine is preferred to FluMist for anyone who comes into close contact with people with weakened immune systems. Likewise, live vaccines such as SC602 and WRSS1 would presumably be contraindicated for consumers with immunocompromised household or workplace contacts.
The cumulative results of SC602 clinical trials have demonstrated that this icsA (virG) mutant is a substantially attenuated candidate vaccine strain that can evoke protection against the most severe symptoms of shigellosis in a stringent human challenge model of disease. The short-term symptoms associated with SC602 (headache, cramps, diarrhea, and fever) have thus far accompanied all invasive Shigella vaccines that induce substantial immune responses (9). These symptoms could presumably be lessened by palliative treatments without sacrificing vaccine efficacy, and for certain high-risk customers (e.g., soldiers and adventure travelers), the reactions would probably represent an acceptable inconvenience. As noted previously, further studies of SC602 would be required to confirm the safety profile of the vaccine for vaccinees and for bystanders.
Acknowledgments
We are indebted to the staff of the Clinical Studies Department and the Biometrics and Information Management Division at USAMRIID for conduct of the study and to the Department of Biometrics at WRAIR for assistance in data analysis. We acknowledge the seminal contributions of P. J. Sansonetti of the Institut Pasteur that made these studies possible.
This work was supported by the Military Infectious Diseases Research Program and the U.S. Army Medical Materiel Development Activity of the U.S. Army Medical Research and Materiel Command, Fort Detrick, Md.
The views expressed herein are those of the authors and do not necessarily represent those of the Department of the Army or Department of Defense.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Bernardini, M. L., J. Mounier, H. d'Hauteville, M. Coquis-Rondon, and P. J. Sansonetti. 1989. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc. Natl. Acad. Sci. USA 86:3867-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen, D., S. Ashkenazi, M. S. Green, M. Gdalevich, G. Robin, R. Slepon, M. Yavzori, N. Orr, C. Block, I. Ashkenazi, J. Shemer, D. N. Taylor, T. L. Hale, J. C. Sadoff, D. Pavliakova, R. Schneerson, and J. B. Robbins. 1997. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet 349:155-159. [DOI] [PubMed] [Google Scholar]
- 3.Coster, T. S., C. W. Hoge, L. L. van de Verg, A. B. Hartman, E. V. Oaks, M. M. Venkatesan, D. Cohen, G. Robin, A. Fontaine-Thompson, P. J. Sansonetti, and T. L. Hale. 1999. Vaccination against shigellosis with attenuated Shigella flexneri 2a strain SC602. Infect. Immun. 67:3437-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DuPont, H. L., R. B. Hornick, A. T. Dawkins, M. J. Snyder, and S. B. Formal. 1969. The response of man to virulent Shigella flexneri 2a. J. Infect. Dis. 119:296-299. [DOI] [PubMed] [Google Scholar]
- 5.Egile, C., T. P. Loisel, V. Laurent, R. Li, D. Pantaloni, P. J. Sansonetti, and M. F. Carlier. 1999. Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J. Cell Biol. 146:1319-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herrington, D. A., L. L. Van de Verg, S. B. Formal, T. L. Hale, B. D. Tall, S. J. Cryz, E. C. Tramont, and M. M. Levine. 1990. Studies in volunteers to evaluate candidate Shigella vaccines: further experience with a bivalent Salmonella typhi-Shigella sonnei vaccine and protection conferred by previous Shigella sonnei disease. Vaccine 8:353-357. [DOI] [PubMed] [Google Scholar]
- 7.Houng, H. S., O. Sethabutr, and P. Echeverria. 1997. A simple polymerase chain reaction technique to detect and differentiate Shigella and enteroinvasive Escherichia coli in human feces. Diagn. Microbiol. Infect. Dis. 28:19-25. [DOI] [PubMed] [Google Scholar]
- 8.Kotloff, K. L., J. P. Nataro, G. A. Losonsky, S. S. Wasserman, T. L. Hale, D. N. Taylor, J. C. Sadoff, and M. M. Levine. 1995. A modified Shigella volunteer challenge model in which the inoculum is administered with bicarbonate buffer: clinical experience and implications for Shigella infectivity. Vaccine 13:1488-1494. [DOI] [PubMed] [Google Scholar]
- 9.Kotloff, K. L., F. R. Noriega, T. Samandari, et al. 2000. Shigella flexneri 2a strain CVD 1207, with specific deletions in virG, sen, set, and guaBA, is highly attenuated in humans. Infect. Immun. 68:1034-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotloff, K. L., D. N. Taylor, M. B. Sztein, S. S. Wasserman, G. A. Losonsky, J. P. Nataro, M. Venkatesan, A. Hartman, W. D. Picking, D. E. Katz, J. D. Campbell, M. M. Levine, and T. L. Hale. 2002. Phase I evaluation of ΔvirG Shigella sonnei live, attenuated, oral vaccine strain WRSS1 in healthy adults. Infect. Immun. 70:2016-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotloff, K. L., J. P. Winickoff, B. Ivanoff, J. D. Clemens, D. L. Swerdlow, P. J. Sansonetti, G. K. Adak, and M. M. Levine. 1999. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull. W. H. O. 77:651-666. [PMC free article] [PubMed] [Google Scholar]
- 12.Levine, M. M., H. L. DuPont, M. Khodabandelou, and R. B. Hornick. 1973. Long-term Shigella-carrier state. N. Engl. J. Med. 288:1169-1171. [DOI] [PubMed] [Google Scholar]
- 13.Nassif, X., M. C. Mazert, J. Mounier, and P. J. Sansonetti. 1987. Evaluation with an iuc::Tn10 mutant of the role of aerobactin production in the virulence of Shigella flexneri. Infect. Immun. 55:1963-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neter, E. 1943. Bacteriological, epidemiological, immunological, and chemotherapeutic aspects of bacillary dysentery. Part I. Gastroenterology 1:366-382. [Google Scholar]
- 15.Pickering, L. K., H. L. DuPont, D. G. Evans, D. J. Evans, Jr., and J. Olarte. 1977. Isolation of enteric pathogens from asymptomatic students from the United States and Latin America. J. Infect. Dis. 135:1003-1005. [DOI] [PubMed] [Google Scholar]
- 16.Ribner, B. S., and E. H. Freimer. 1978. Oxolinic acid for the treatment of chronic gastrointestinal Shigella carriers. Am. J. Trop. Med. Hyg. 27:840-842. [DOI] [PubMed] [Google Scholar]
- 17.Sansonetti, P. J., and J. Arondel. 1989. Construction and evaluation of a double mutant of Shigella flexneri as a candidate for oral vaccination against shigellosis. Vaccine 7:443-450. [DOI] [PubMed] [Google Scholar]
- 18.Teska, J. D., T. Coster, W. R. Byrne, J. R. Colbert, D. Taylor, M. Venkatesan, and T. L. Hale. 1999. Novel self-sampling culture method to monitor excretion of live, oral Shigella flexneri 2a vaccine SC602 during a community-based phase 1 trial. J. Lab. Clin. Med. 134:141-146. [DOI] [PubMed] [Google Scholar]
- 19.Van de Verg, L. L., D. A. Herrington, J. R. Murphy, S. S. Wasserman, S. B. Formal, and M. M. Levine. 1990. Specific immunoglobulin A-secreting cells in peripheral blood of humans following oral immunization with a bivalent Salmonella typhi-Shigella sonnei vaccine or infection by pathogenic S. sonnei. Infect. Immun. 58:2002-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]