Abstract
Objective
The primary purpose of this study was to evaluate the reproducibility of ambulatory blood pressure (BP) measures in African-American (AA) adolescents.
Methods
Forty-one AA adolescents (age 16.6 ± 1.3 yrs, 16F) with high-normal BP were measured on 3 occasions at 2-month intervals. Systolic BP (SBP), diastolic BP (DBP), and heart rate (HR) measures were recorded using the Spacelabs ambulatory BP monitor 90207 (Redmond, Wash) in the natural environment over 24-hour periods. Mixed model repeated measures ANOVAs were used to analyze the underlying error variance-covariance (V-C) structures as well as mean differences for the 3 visits.
Results
Daytime measures: there were no significant mean differences across visits for daytime SBP, DBP, and HR (all Ps>.57). The error V-C matrix was heterogeneous Toeplitz for daytime SBP. Correlations between visits 1 and 2, 1 and 3, and 2 and 3 for daytime SBP were rs=0.71, 0.47, and 0.71, respectively. Compound symmetry (CS) was the preferred model for daytime DBP (r=0.68) and HR (r=0.75). Nighttime measures: there were no significant mean differences across visits for nighttime SBP, DBP, and HR (all Ps>.29). The error V-C matrix was unstructured for nighttime SBP. Correlations between visits 1 and 2, 1 and 3, and 2 and 3 for SBP were rs=0.74, 0.33, and 0.33, respectively. CS was preferred for nighttime DBP (r=0.58) and HR (r=0.74).
Conclusion
Collectively, these findings demonstrate that 3 measurements of ambulatory-derived DBP and HR measures are stable across 4 months, but SBP was only stable across 2 months in African-American adolescents.
Keywords: Adolescent, Ambulatory, Blood Pressure, African American
Introduction
Essential hypertension (EH) remains a significant health problem in the United States, with approximately 50 million persons affected.1 The pathogenesis of cardiovascular disease has its origins in childhood.2 Epidemiological studies indicate that blood pressure (BP) percentile rankings for age and sex tend to track from late childhood through adolescence into adulthood.3 Teenagers with high-normal BP are at increased risk for future development of EH as adults.4–6 African Americans (AAs) in the southeastern United States have a higher prevalence of EH than those in other regions of the United States.7
Ambulatory BP monitoring (ABP) in adults has been shown to: 1) improve prediction of hypertensive complications; 2) be relatively free of placebo effects; and 3) be highly reproducible and sensitive to small changes in average BP.8 Non-invasive 24-hour ABP monitoring is widely used for evaluation of EH in adults and permits assessment of the generalization of intervention treatment effects in out-of-laboratory, real-life situations.9 Findings in youth have indicated that ABP measures are stable across periods of time varying from one to 4 years.10 Assessment of the stability of repeated measures of ABP is essential in determining precision of change scores in clinical trials comparing treatment and control groups and in comparing results of such trials. AA adolescents exhibit higher 24-hour11 and blunted nocturnal ABP levels compared with Whites.12
The feasibility of ambulatory impedance monitoring (AIM) has recently been reported and validated for measurement of hemodynamic functioning in the laboratory setting.13 Reproducibility of repeated, non-invasive vasoconstriction measures has important implications for CVD research. To our knowledge, no studies have examined the reproducibility of ABP and AIM-derived measures of hemodynamic functioning in the natural environment. The purpose of this study was to determine the reproducibility of ambulatory measures of BP and heart rate (HR) in AA adolescents with high-normal systolic BP (SBP). A sub-study was conducted to determine the feasibility and reproducibility of AIM-derived measures of hemodynamic function.
Methods
Subjects
Permission to conduct the study was granted by the superintendent of the Richmond County Public Schools and the Medical College of Georgia Human Assurance Committee. A BP screening was conducted on approximately 5,000 AA youth at inner-city high schools in Augusta, Ga. From this screening, 104 AA adolescents (age 16.6 ± 1.3 yrs, 16F) with high-normal BP (ie, SBP≥85th percentile for gender, age and height on 3 occasions3) agreed to participate in the study. These subjects were randomly assigned to either stress reduction or health education control (CTL) groups (described below).17 Only subjects from the CTL group were included in the present analysis.
Measurements
Height (via stadiometer), weight (via Detecto scale), and waist and hip circumference measurements were recorded using established protocols.14 Mean arterial pressure (MAP) and heart rate (HR) measures were recorded using the Spacelabs ambulatory BP monitor 90207 (Redmond, Wash) in the natural environment over 24-hour periods. This device uses an oscillometric method and has been previously validated.15
As a secondary analysis, a subset (N=32) of the current subject group was also evaluated on AIM-derived measures of hemodynamic function. Cardiac output (CO) measures were obtained via the AIM-8-V3 (ambulatory impedance monitor) wearable cardiac performance monitor (Bio-impedance Technology, Inc, Chapel Hill, NC) in the natural environment over 24 hours.13 The AIM unit is a micro-computer-based bio-impedance monitor and signal processing system. The computer section ensemble averages, analyzes, and stores the electrocardiogram and bio-impedance waveforms and cardiac function using the standard Minnesota system.16 The AIM unit was worn on a belt around the waist together with the Spacelabs ABP monitor and was activated to initiate a 40-second ensemble-average data acquisition together with every ABP measurement. Measurements recorded during the ambulatory period were uploaded onto a personal computer. Collectively, these units were able to track a number of hemodynamic values including total peripheral resistance (TPR), which was calculated as MAP/CO*80. AIM data was manually edited to remove spurious waveforms. Nighttime measures were recorded every 30 minutes from midnight to 6 AM and daytime measures every 20 minutes from 8 AM–10 PM on 3 occasions at 2-month intervals over 4 months.
Subjects attended a 4-month health education program based in part on the National Institutes of Health guidelines on lowering BP through weight loss, diet (reducing fat and sodium intake), and increasing physical activity. This program was provided in 15-minute sessions held daily at school.17 Subjects were paid $100 for each wearing of the ABP and AIM monitors.
Statistical Analyses
Mixed model repeated measures ANOVAs were used to analyze for differences in hemodynamic measures underlying error variance-covariance (V-C) structures, as well as mean differences for the 3 visits. Average daytime and nighttime values were calculated. Subjects with <21 daytime measures and <6 nighttime measures were excluded from the ABP analysis yielding 41 subjects. In order to retain as much data as possible for the bioimpedance data analysis, subjects with <14 daytime measures and <5 nighttime measures were excluded, yielding 32 subjects.
Variance-covariance (V-C) structures that were tested in this analysis are described as follows.18–22 Five models were considered for each variable.
Compound symmetric (CS) is the most specific structure. The variance within visits is constant and there is a common correlation between visits. Equal correlation (also known as Intraclass Correlation [ICC]) and equal variances are required. There are 2 parameters estimated: the within-subject variance and the between-subject variance. From these parameters, a correlation r value) is derived.
The heterogeneous compound symmetric (CSH) structure assumes a common correlation between visits but allows for different variances for each visit. There are 4 parameters estimated: 3 variances and the common correlation.
The Toeplitz (TOEP) structure assumes a common variance across visits but produces a banded covariance structure such that the correlations between visits separated by the same amount of time are equal. There are 3 parameters estimated: the variance, the correlation between measures 2 months apart, and the correlation between measures 4 months apart.
The heterogeneous Toeplitz (TOEPH) structure allows for different variance parameters and produces a banded covariance structure such that the correlations between visits separated by the same amount of time are equal. There are 5 parameters estimated: the 3 variances and the correlation between measures 2 months apart, and the correlation between measures 4 months apart.
The unstructured (UN) variance-covariance structure produces estimates of all 3 variances and 3 covariances (6 parameters are estimated) from which correlations are calculated. Correlations between visits and variances within visits may be different.
A likelihood ratio test (LRT) for the significance of a more general model can be constructed if one model is a sub-model of another by computing −2 times the difference between their residual log likelihoods (−2RLL). This statistic is then compared to the chi-square distribution with degrees of freedom equal to the difference in the number of parameters for the 2 models. Models are preferred where the −2RLL is smaller.
Results
Anthropometric data averaged across the three visits are shown in Table 1.
Table 1.
Descriptive characteristics*
| Anthropometrics (N = 41, 16F) | |
|---|---|
| Age (years) | 16.6 ± 1.3 |
| Weight (kg) | 86.0 ± 28.9 |
| Height (cm) | 169.9 ± 9.8 |
| Body surface area (m2) | 2.0 ± 0.3 |
| Body mass index (kg/m2) | 29.8 ± 9.8 |
| Waist-to-hip ratio | 0.8 ± 0.07 |
Values are means ± standard deviations.
Ambulatory Hemodynamic Measures
Daytime Measures
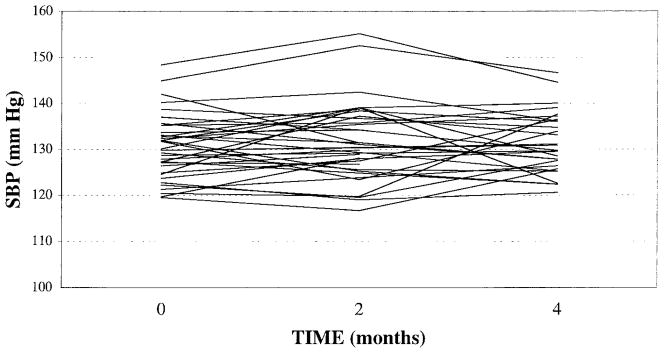
There were no significant mean differences across visits for daytime SBP (Figure 1), MAP, diastolic BP (DBP), and HR (Table 2). The error V-C matrix was TOEPH for daytime SBP, ie, the within-visit variances, were significantly different and there was a common correlation between visits separated by the same amount of time (Table 3). Correlations for daytime SBP between visits 1 and 2, 1 and 3, and 2 and 3, were rs=0.71, 0.47, and 0.71, respectively. Compound symmetry (CS) was the preferred model for daytime MAP (r=0.66), DBP (r=0.68), and HR (r=0.75), ie, the within-visit variances were not significantly different and there was an equal correlation between visits.
Fig 1.
Comparison of daytime ambulatory systolic blood pressure means across visits 1, 2 and 3, at 0, 2 and 4 months
Table 2.
Comparison of means across visits*
| (N=41) | Visit 1 | Visit 2 | Visit 3 | P Value |
|---|---|---|---|---|
| Daytime measures | ||||
| SBP | 131.2 ± 7.4 | 131.6 ± 8.7 | 131.3 ± 6.5 | .73 |
| MAP | 95.7 ± 6.7 | 95.2 ± 6.8 | 95.0 ± 6.0 | .95 |
| DBP | 76.4 ± 6.9 | 75.6 ± 6.7 | 75.3 ± 7.0 | .85 |
| HR | 82.2 ± 9.2 | 82.1 ± 10.4 | 81.6 ± 10.3 | .57 |
| Nighttime measures | ||||
| SBP | 120.9 ± 9.4 | 120.6 ± 10.3 | 121.3 ± 9.1 | .89 |
| MAP | 86.0 ± 8.2 | 84.5 ± 7.7 | 85.3 ± 7.5 | .72 |
| DBP | 65.7 ± 7.7 | 64.6 ± 7.8 | 64.8 ± 8.4 | .58 |
| HR | 72.0 ± 10.3 | 71.5 ± 10.0 | 70.0 ± 9.7 | .29 |
| 24-hour measures | ||||
| SBP | 128.4 ± 7.4 | 128.3 ± 8.7 | 127.7 ± 6.9 | .64 |
| MAP | 93.1 ± 6.5 | 92.0 ± 6.7 | 91.6 ± 6.1 | .86 |
| DBP | 73.4 ± 6.5 | 72.3 ± 6.6 | 71.9 ± 7.3 | .76 |
| HR | 79.0 ± 9.1 | 79.0 ± 9.5 | 78.2 ± 9.9 | .40 |
Values are means±standard deviations.
SBP=systolic blood pressure (mm Hg); MAP=mean arterial pressure (mm Hg); DBP=diastolic blood pressure (mm Hg); HR=heart rate (beats/min).
Table 3.
Reproducibility of hemodynamic measures*
| (N=41) | Statistical Model | Correlations (r Values)
|
||
|---|---|---|---|---|
| Visits 1 and 2 | Visits 1 and 3 | Visits 2 and 3 | ||
| Daytime measures | ||||
| SBP | TOEPH | 0.71 | 0.47 | 0.71 |
| MAP | CS | 0.66 | 0.66 | 0.66 |
| DBP | CS | 0.68 | 0.68 | 0.68 |
| HR | CS | 0.75 | 0.75 | 0.75 |
| Nighttime measures | ||||
| SBP | UN | 0.74 | 0.33 | 0.33 |
| MAP | UN | 0.78 | 0.42 | 0.37 |
| DBP | CS | 0.58 | 0.58 | 0.58 |
| HR | CS | 0.74 | 0.74 | 0.74 |
| 24-hour measures | ||||
| SBP | UN | 0.85 | 0.46 | 0.56 |
| MAP | CS | 0.74 | 0.74 | 0.74 |
| DBP | CS | 0.77 | 0.77 | 0.77 |
| HR | CS | 0.77 | 0.77 | 0.77 |
Covariance structures: TOEPH=heterogeneous Toeplitz; CS=compound symmetric; UN=unstructured.
Nighttime Measures
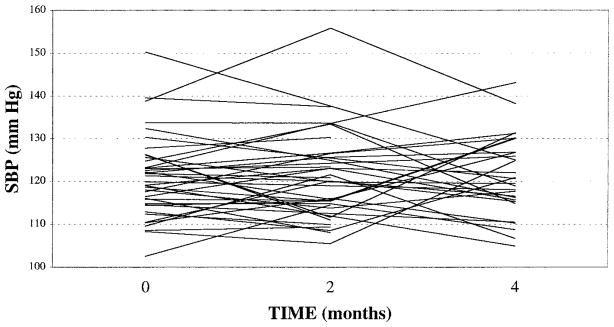
There were no significant mean differences across visits for nighttime SBP (Figure 2), MAP, DBP, and HR (Table 2). The error V-C matrix was unstructured for nighttime MAP and SBP, ie, the within-visit variances were significantly different and there were no structures to the correlations between visits (Table 3). Correlations for nighttime SBP between visits 1 and 2, 1 and 3, and 2 and 3, were rs=0.74, 0.33, and 0.33, respectively. Correlations for nighttime MAP between visits 1 and 2, 1 and 3, and 2 and 3, were rs=0.78, 0.42, and 0.37, respectively. Compound symmetry was preferred for nighttime DBP (r=0.58), and HR (r=0.77).
Fig 2.
Comparison of nighttime ambulatory systolic blood pressure means across visits 1, 2 and 3, at 0, 2 and 4 months
24-Hour Measures
There were no significant mean differences across visits for 24-hour SBP, MAP, DBP, and HR (Table 2). The error V-C matrix was unstructured for 24-hr SBP. Correlations for 24-hr SBP between visits 1 and 2, 1 and 3, and 2 and 3, were rs=0.85, 0.46, and 0.56, respectively (Table 3). Compound symmetry was preferred for 24-hr MAP, (r=0.74), DBP (r=0.77), and HR (r=0.77).
Ambulatory Bioimpedance Measures
A trend was observed for mean differences across visits for daytime CO (P<.09, Table 4). The means for daytime TPR were significantly different for visits 1, 2, and 3, respectively (P<.03). Compound symmetry (CS) was the preferred model for daytime CO (r=0.52, Table 5). Heterogeneous CS was preferred for daytime TPR (r=0.51), ie, the within-visit variances were significantly different while there was a common correlation between visits. Nighttime CO and TPR means were not significantly different across visits. Compound symmetry (CS) was preferred for nighttime CO (r=0.47) and TPR (r=0.39).
Table 4.
Comparison of bioimpedance measures across visits*
| (N=32) | Visit 1 | Visit 2 | Visit 3 | P Value |
|---|---|---|---|---|
| Daytime measures | ||||
| CO | 8.3 ± 1.7 | 8.4 ± 1.8 | 7.5 ± 2.3 | .09 |
| TPR | 994 ± 223 | 977 ± 208 | 1146 ± 327 | .03 |
| Nighttime measures | ||||
| CO | 7.1 ± 1.9 | 7.7 ± 2.1 | 6.5 ± 2.6 | .21 |
| TPR | 1023 ± 306 | 959 ± 294 | 1157 ± 358 | .15 |
Values are means±standard deviations.
CO=cardiac output (L/min); TPR=total peripheral resistance (dyn**s*cm−5).
Table 5.
Reproducibility of bioimpedance measures*
| (N=32) | Statistical Model | Correlation (r Values)
|
||
|---|---|---|---|---|
| Visits 1 and 2 | Visits 1 and 3 | Visits 2 and 3 | ||
| Daytime measures | ||||
| CO | CS | 0.52 | 0.52 | 0.52 |
| TPR | CSH | 0.51 | 0.51 | 0.51 |
| Nighttime measures | ||||
| CO | CS | 0.47 | 0.47 | 0.47 |
| TPR | CS | 0.39 | 0.39 | 0.39 |
Covariance structures: CS=compound symmetric; CSH=heterogeneous compound symmetric.
Discussion
Collectively, these findings demonstrate that 3 repeated measurements of ambulatory-derived DBP, HR, and daytime and 24-hr MAP measures are stable across 4 months in African-American adolescents. Systolic blood pressure (SBP) is stable across 2 months, but not across 4 months in this sample. Previous studies have reported correlations that range from 0.72–0.93 (SBP) and 0.53–0.87 (DBP).23 Reasons for the SBP instability are unclear but may relate to the small sample size relative to other studies.24 Correlations of 0.73 for daytime SBP and 0.67 for nighttime SBP have been reported for African-American youth for a sample of 94 on ABP recordings performed 2 years apart.24
Increased vascular tone has been implicated as playing an important role in the early development of EH in AAs.25 Collectively, the present findings demonstrate that ambulatory derived measurement of vasoconstrictive tone (ie, TPR) is feasible but is not stable across 4 months in youth. Limited TPR stability observed in the present study may be partially attributable to uncontrolled posture and physical activity levels. Both of these parameters have been associated with alterations in ambulatory hemodynamic function.13 However, a pattern of increasing stability was observed with repeated wearings across visits that may be attributable to an adaptation response. Further study using momentary event sampling with user-friendly personal digital assistant methodology would enable postural changes and physical activity to be controlled in the data analyses, which would be expected to increase reproducibility. Comparison with other ambulatory bioimpedance monitors would be fruitful in future studies.
Collectively, the present findings indicate that ambulatory derived measures of vasoconstrictive tone, ie, TPR, are not stable across 4 months in youth. Internal validity factors that may have affected the stability of the measures include: several upgrades by the manufacturer to improve the reliability of the AIM; lack of inclusion of diary data; and problems with wearing the AIM including displaced electrodes, obstructed air hoses, and other factors. Lower stability of nighttime measures may be attributable, in part, to inclusion of fewer measures in the analyses compared to daytime, and difficulties inherent in wearing the monitors while sleeping. The present ABP findings should be considered in view of the added burden of wearing the AIM unit with its associated electrode configuration. The ABP stability may have been impacted by the occasional partial obstruction of the air hose by the AIM unit pressure switch, which caused the Spacelabs ABP monitor to shut down. Reproducibility of repeated measures of ABP and TPR has important implications for studies examining the pathogenesis of CVD, for example, intervention trials attempting to improve hemodynamic function.
This is the first report of the feasibility and reproducibility of ambulatory measures of CO and TPR in youth in the natural setting. Further study with a larger sample and/or more post-testing may improve the reproducibility findings. This methodology may be useful in cardiovascular research studies requiring the determination of the efficacy of 24-hour circadian patterns of hemodynamic performance in the natural environment.
Acknowledgments
We would like to thank Dr. C. Larke, superintendent, and the principals of Richmond County Public Schools in Augusta, Georgia for their cooperation in providing the facilities for this study. This study was supported in part by National Institutes of Health Grant #HL62976 to Dr. Treiber and an American Heart Association Scientist Development Grant #9930073N to Dr. Barnes.
Footnotes
Author Contributions
Design and concept of study: Barnes, Treiber
Acquisition of data: Barnes, Dekkers
Data analysis and interpretation: Barnes, Johnson, Treiber
Manuscript draft: Barnes, Johnson, Dekkers, Treiber
Statistical expertise: Barnes, Johnson
Acquisition of funding: Barnes, Treiber
Administrative, technical, or material assistance: Barnes, Dekkers
Supervision: Barnes, Treiber
References
- 1.Gillum RF. Cardiovascular disease in the United States: an epidemiological overview. In: Saunders E, editor. Cardiovascular Disease in Blocks. Philadelphia, Penn: FA Davis; 1991. [Google Scholar]
- 2.Sorof JM, Portman RJ. Ambulatory blood pressure monitoring in the pediatric patient. J Pediatr. 2000;136(5):578–586. doi: 10.1067/mpd.2000.106230. [DOI] [PubMed] [Google Scholar]
- 3.National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents. Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: a working group report from the National High Blood Pressure Education Program. Pediatrics. 1996;98(4 pt 1):649–658. [PubMed] [Google Scholar]
- 4.Nelson M, Ragland D, Syme SL. Longitudinal prediction of adult blood pressure from juvenile blood pressure levels. Am J Epidemiol. 1992;136:633–645. doi: 10.1093/oxfordjournals.aje.a116543. [DOI] [PubMed] [Google Scholar]
- 5.Lauer RM, Clarke WR, Beaglehole R. Level, trend, and variability of blood pressure during childhood: The Muscatine Study. Circulation. 1984;69:242–249. doi: 10.1161/01.cir.69.2.242. [DOI] [PubMed] [Google Scholar]
- 6.Beckett LA, Rosner B, Roche AF, Guo S. Serial changes in blood pressure from adolescence into adulthood. Am J Epidemiol. 1992;135(10):1166–1177. doi: 10.1093/oxfordjournals.aje.a116217. [DOI] [PubMed] [Google Scholar]
- 7.Burt VL, Whelton P, Rocella EJ, et al. Prevalence of hypertension in the US adult population: results from the Third National Health and Nutrition Examination survey, 1988–1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien E, Staessen J. Normotension and hypertension as defined by 24-ambulatory blood pressure monitoring. Blood Pressure. 1995;4:266–282. doi: 10.3109/08037059509077607. [DOI] [PubMed] [Google Scholar]
- 9.Bald M, Kubel S, Rascher W. Validity and reliability of 24h blood pressure monitoring in children and adolescents using a portable, oscillometric device. J Hum Hypertens. 1994;8(5):363–366. [PubMed] [Google Scholar]
- 10.Treiber FA, Murphy JK, Davis H, Rauniker A, Pflieger K, Strong WB. Pressor reactivity, ethnicity, and 24-hour ambulatory monitoring in children from hypertensive families. Behav Med. 1994;20:133–142. doi: 10.1080/08964289.1994.9934628. [DOI] [PubMed] [Google Scholar]
- 11.Chase HP, Garg SK, Icaza G, Carmain JA, Walravens CF, Marshall G. 24-h ambulatory blood pressure monitoring in healthy young adult Anglo, Hispanic, and African-American subjects. Am J Hypertens. 1997;10(1):18–23. doi: 10.1016/s0895-7061(96)00260-9. [DOI] [PubMed] [Google Scholar]
- 12.Harshfield GA, Treiber FA. Racial differences in ambulatory blood pressure monitoring-derived 24 h patterns of blood pressure in adolescents. Blood Press Monit. 1999;4(3–4):107–110. [PubMed] [Google Scholar]
- 13.Sherwood A, McFetridge J, Hutcheson JS. Ambulatory impedance cardiography: a feasibility study. J Appl Physiol. 1998;85(6):2365–2369. doi: 10.1152/jappl.1998.85.6.2365. [DOI] [PubMed] [Google Scholar]
- 14.National Center for Health Statistics. NHA-NES III Anthropometric Procedures. Washington, DC: US Dept of Health and Human Services, US Government Printing Office; 1988. [videotape] [Google Scholar]
- 15.O’Brien E, Mee F, Atkins N, O’Malley K. Accuracy of the SpaceLabs 90207 determined by the British Hypertension Society protocol. J Hypertens. 1991;9(6):573–574. doi: 10.1097/00004872-199106000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, van Doornen LJ. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27(1):1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- 17.Barnes VA, Treiber FA, Davis H. Impact of Transcendental Meditation on cardiovascular function at rest and during acute stress in adolescents with high normal blood pressure. J Psychosom Res. 2001;51(4):597–605. doi: 10.1016/s0022-3999(01)00261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolfinger RD. A Tutorial on Mixed Models. Cary, NC: SAS Institute, Inc; 1992. [Google Scholar]
- 19.Littel RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. Cary, NC: SAS Institute, Inc; 1996. [Google Scholar]
- 20.SAS Institute Inc. SAS/STAT User’s Guide: Version 6. 4. Vol. 2. Cary, NC: SAS Institute, Inc; 1989. [Google Scholar]
- 21.SAS Institute Inc. SAS Technical Report P-229, SAS/STAT Software: Changes and Enhancements, Release 6.07. Cary, NC: SAS Institute, Inc; 1992. [Google Scholar]
- 22.SAS Institute Inc. SAS/STAT Software: Changes and Enhancements, Release 6.10. Cary, NC: SAS Institute, Inc; 1994. [Google Scholar]
- 23.Pickering TG. Ambulatory Monitoring and Blood Pressure Variability. London: Science Press; 1991. [Google Scholar]
- 24.Harshfield GA, Treiber FA, Wilson ME, Kapuku GK, Davis HC. A longitudinal study of ethnic differences in ambulatory blood pressure patterns in youth. Am J Hypertens. 2002;15:525–530. doi: 10.1016/s0895-7061(02)02267-7. [DOI] [PubMed] [Google Scholar]
- 25.Anderson NB. Ethnic differences in resting and stress-induced cardiovascular and humoral activity. In: Schneiderman N, Weiss SM, Kaufman PG, editors. Handbook of Research Methods in Cardiovascular Behavioral Medicine. New York, NY: Plenum Press; 1989. [Google Scholar]