Abstract
Guinea pig eosinophils were positively identified in bronchoalveolar lavage populations and in the lung granulomas of Mycobacterium tuberculosis-infected guinea pigs. It is possible that the rapid influx of these cells, and their subsequent degranulation during acute pulmonary tuberculosis, may play a key role in the susceptibility of this animal model.
The guinea pig (Cavia porcellus) is widely used in research into tuberculosis given its similarities to humans in the pathological process (7, 12). After exposure to aerosol infection (∼50 CFU), this animal develops a potent granulomatous response characterized at first by a florid influx of lymphocytes and mononuclear phagocytes, followed more slowly by increasing lesion necrosis, fibrosis, and mineralization. As a result of this process, lesions become very large, causing extensive lung involvement and damage, killing the animal in about 15 to 20 weeks after exposure to infection.
It has traditionally been assumed (2, 5) that the increasing necrosis in guinea pig lung lesions is mediated by mechanisms of acquired immunity, given the similarities to tissue-damaging delayed-type hypersensitivity. However, in a recent study (13) our investigators proposed a contrary hypothesis based upon our observation that elements of this necrosis are in fact demonstrable in very early lesions and clearly arise long before acquired T-cell-mediated immunity has had an opportunity to emerge. These small pockets of necrosis in very early lung lesions, which coalesce and form the initial lesion core structure, appear to arise from the degranulation of granulocytes that can be observed in these lesions over the first 5 to 10 days. Such granulocytes have been described in the guinea pig previously, but they have been given a variety of names, including “heterophils” and “pseudoeosinophils” (8, 12). In this brief report, we provide evidence that indicates that these particular granulocytes have, in fact, the characteristics of true eosinophils and thus should be regarded as such.
To establish a low-dose lung infection, specific-pathogen-free outbred Hartley strain guinea pigs (Charles River Breeding Laboratories, Inc., Wilmington, Mass.) were infected with approximately 50 CFU of Mycobacterium tuberculosis H37Rv (ATCC 27294; American Type Culture Collection, Rockville, Md.) via the respiratory route in a Madison infection chamber (University of Wisconsin College of Engineering Shops, Madison, Wis.). Between 1 and 5 weeks postchallenge, the animals were euthanized humanely by an intraperitoneal injection of 3 ml of sodium pentobarbital (Fort Dodge Laboratories, Inc.).
To obtain the bronchoalveolar lavage (BAL) cell population, BAL was performed by instilling ice-cold 12 mM lidocaine (10 ml) in phosphate-buffered saline (pH 7.4) with 2% heat-inactivated fetal bovine serum (FBS; Atlanta Biologicals, Norcross, Ga.) into the lungs. Lavage cells were washed twice in RPMI 1640 (Irvine Scientific, Santa Ana, Calif.) with 1% FBS by centrifugation at 320 × g for 10 min at 4°C. After the second wash, the cell pellet was resuspended in RPMI 1640 medium supplemented with 2 μM l-glutamine, 100 U of penicillin/ml, and 10% FBS. Viable BAL cells were enumerated by trypan blue exclusion (Gibco Life Technologies, Grand Island, N.Y.). An aliquot (70 μl) of the BAL cell population (106 cells) was spun for 5 min at 140 × g in a cytospin centrifuge, air dried for 1 to 2 min, and fixed and stained separately with Diff-Quik (Dade Behring Inc., Newark, Del.). After rinsing with tap water, the slides were air dried and coverslipped, and the morphology of cellular constituents was characterized with a light microscope.
Guinea pig tissue samples for histological analysis were fixed in 10% phosphate-buffered formyl saline for a minimum of 2 days and embedded in paraffin wax. Sections of 5 μm in width were cut and stained with hematoxylin and eosin and Luna's eosinophil granule stain (11) and then examined by light microscopy. In addition, major basic protein, a highly cationic protein found in the granules of eosinophils, was detected by immunohistochemistry of frozen sections of lung tissue. Routine transmission electron microscopy was conducted on paraformaldehyde-fixed tissue.
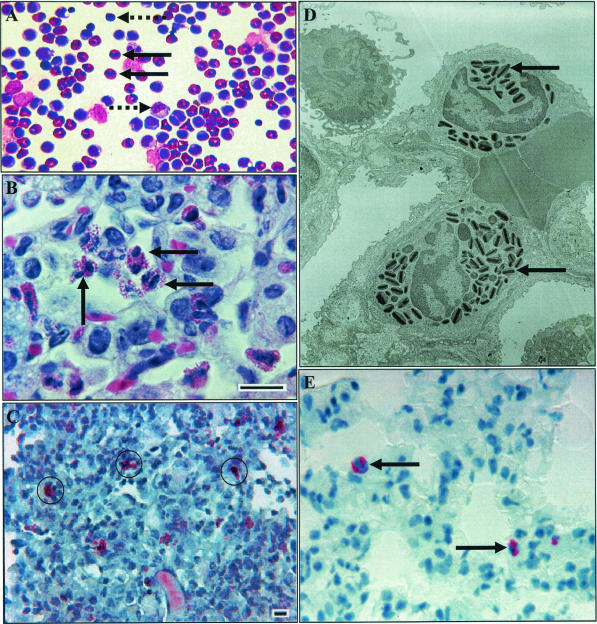
Following low-dose aerosol infection with M. tuberculosis, we report for the first time high numbers of eosinophils associated with both resident BAL populations and granulomata seen in guinea pig lung sections. Figure 1A depicts a photomicrograph of guinea pig leukocytes from a BAL procedure conducted at 5 weeks postinfection. The eosinophils could be identified by their distinctive, round to bilobed nucleus and large, globular, densely packed granules that fill the cytoplasm. In addition, guinea pig neutrophils, referred to by some as pseudoeosinophils due to their cytoplasmic granules (8), could be distinguished from eosinophils by the characteristic pyknotic, hypersegmented neutrophil nucleus and punctate eosinophilic granules. Figure 1A clearly demonstrates the predominance of eosinophils in the BAL fluid, which represented approximately 20% of the total cell population. As expected, appreciable numbers of large mononuclear cells (most likely cells from the monocyte/macrophage lineage, but which cannot be distinguished from type II pneumocytes) as well as the smaller lymphocytes were seen in these populations. In comparison to uninfected guinea pig BAL differentials, both the neutrophil and lymphocyte cell subpopulations increased approximately 600%. In contrast, eosinophils in the BAL fluid decreased by 100% (unpublished data).
FIG. 1.
Accumulation of eosinophils in experimental pulmonary tuberculosis in the guinea pig. (A) Representative photomicrograph of bronchoalveolar cells collected at 5 weeks postinfection with M. tuberculosis, with eosinophils (solid black arrows), large mononuclear cells (right-pointing dashed arrow), and lymphocytes (left-pointing dashed arrow). Magnification, ×40. (B) Representative photomicrograph of a guinea pig tuberculous granuloma 11 days after an aerosol infection. Note the multiple eosinophils (black arrows). Hematoxylin and eosin stain was used. Bar = 10 μm. (C) Representative photomicrograph of a guinea pig tuberculous granuloma 11 days after aerosol infection, stained with Luna's eosinophil granule stain. Positive cells are surrounded by black rings. Bar = 10 μm. (D) Representative transmission electron micrograph of a guinea pig tuberculous granuloma 11 days after aerosol infection. Note the two eosinophils with distinctive intracytoplasmic granules (arrows). (E) Representative photomicrograph of a guinea pig tuberculous granuloma stained for major basic protein at 30 days postinfection with low-dose M. tuberculosis. Magnification, ×100.
Figure 1B is a representative photomicrograph of a granuloma 11 days after an aerosol infection, stained with hematoxylin and eosin. Note the multiple eosinophils. Figure 1C is a representative photomicrograph of a granuloma 11 days after an aerosol infection, stained with Luna's eosinophil granule stain. Positive cells were surrounded by a black ring. Panel D is a representative transmission electron micrograph of a granuloma 11 days after the aerosol challenge. Note the two eosinophils with distinctive intracytoplasmic granules. Panel E is a representative photomicrograph of a granuloma stained for major basic protein at 30 days postinfection with low-dose M. tuberculosis infection. In comparison to normal guinea pig lung tissue, the alveolar walls of the M. tuberculosis-infected animals were moderately to markedly widened by mixed mononuclear cells, comprised primarily of histiocytic macrophages, and moderate to large numbers of eosinophils. The inflammation, particularly the eosinophils, often extended into the alveolar spaces. In contrast, the control animals possessed only rare alveolar macrophages and no eosinophils.
The influx of eosinophils into the lungs is usually associated with the pathogenesis of allergic diseases such as asthma and allergic rhinitis (15). Eosinophils can mediate antibody-dependent cellular cytotoxicity and interact closely with TH2-type CD4 T cells in mediating cytokine production, chemokine receptor expression, and cellular influx into the respiratory tract (6). It is thus paradoxical that they appear to rapidly accumulate in the very early lesions seen in guinea pigs infected with M. tuberculosis. They have been noted in the lesions of human patients with tuberculosis (3, 14), but their role has never been investigated.
Results from animal models have suggested that granulocytes, especially neutrophils, may play a minor protective role during the early stages of mycobacterial infection (4, 10). In the guinea pig model it is possible that the local inflammatory conditions may attract eosinophils into developing lesions and, once there, their presence could be detrimental due to their degranulation, which may create the initial necrosis, setting in motion a process that eventually kills the animal. It is tempting to speculate, given the susceptibility of the guinea pig, that an inherent immunological deficit is at play. For example, a reduced ability to produce gamma interferon may be tied to the highly granulocytic cellular influx which gives rise to tissue destruction and death, as in the gamma interferon-deficient mouse model (9). However, a recent study has suggested that eosinophil granules contain a peroxidase capable of killing mycobacteria (1), but as yet we have been unable to show any direct interaction between these cells and mycobacteria in the lesions, and in any case such activity would be minimal given the progressive nature of the course of the infection at this time.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Borelli, V., F. Vita, S. Shankar, M. R. Soranzo, E. Banfi, G. Scialino, C. Brochetta, and G. Zabucchi. 2003. Human eosinophil peroxidase induces surface alteration, killing, and lysis of Mycobacterium tuberculosis. Infect. Immun. 71:605-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dannenberg, A. M., Jr. 1991. Delayed-type hypersensitivity and cell-mediated immunity in the pathogenesis of tuberculosis. Immunol. Today 12:228-233. [DOI] [PubMed] [Google Scholar]
- 3.Flores, M., J. Merino-Angulo, J. G. Tanago, and C. Aguirre. 1983. Late generalized tuberculosis and eosinophilia. Arch. Intern. Med. 143:182-187. [PubMed] [Google Scholar]
- 4.Fulton, S. A., S. M. Reba, T. D. Martin, and W. H. Boom. 2002. Neutrophil-mediated mycobacteriocidal immunity in the lung during Mycobacterium bovis BCG infection in C57BL/6 mice. Infect. Immun. 70:5322-5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lurie, M. B. 1942. Studies on the mechanism of immunity to tuberculosis. The fate of tubercle bacilli ingested by mononuclear cells derived from normal and immunized animals. J. Exp. Med. 75:247-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacKenzie, J. R., J. Mattes, L. A. Dent, and P. S. Foster. 2001. Eosinophils promote allergic disease of the lung by regulating CD4+ Th2 lymphocyte function. J. Immunol. 167:3146-3155. [DOI] [PubMed] [Google Scholar]
- 7.McMurray, D. N. 1994. Guinea pig model of pulmonary tuberculosis, p. 135-147. In B. R. Bloom (ed.), Tuberculosis: pathogenesis, protection, and control. ASM Press, Washington, D.C.
- 8.Moore, D. M. 2000. Hematology of the guinea pig, p. 1107-1115. In C. Feldman, M. Zinkl, and J. Jain (ed.), Schalm's veterinary hematology. Lippincott, Williams, and Wilkins, Baltimore, Md.
- 9.Pearl, J. E., B. M. Saunders, S. Ehlers, I. M. Orme, and A. M. Cooper. 2001. Inflammation and lymphocyte activation during mycobacterial infection in the interferon-γ-deficient mouse. Cell. Immunol. 211:43-50. [DOI] [PubMed] [Google Scholar]
- 10.Pedrosa, J., B. M. Saunders, R. Appelberg, I. M. Orme, M. T. Silva, and A. M. Cooper. 2000. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect. Immun. 68:577-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheehan, D. C., and B. B. Hrapchak. 1980. Special cells and tissues, p. 282-283. In C. V. Mosby (ed.), Theory and practice of histotechnology, 2nd ed. The C.V. Mosby Company, St. Louis, Mo.
- 12.Turner, O. C., R. J. Basaraba, A. A. Frank, and I. M. Orme. 2003. Granuloma formation in mouse and guinea pig models of experimental tuberculosis, p. 65-84. In D. L. Boros (ed.), Granulomatous infections and inflammations: cellular and molecular mechanisms. ASM Press, Washington, D.C.
- 13.Turner, O. C., R. J. Basaraba, and I. M. Orme. 2003. Immunopathogenesis of pulmonary granulomas in the guinea pig after infection with Mycobacterium tuberculosis. Infect. Immun. 71:864-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vijayan, V. K., A. M. Reetha, M. S. Jawahar, K. Sankaran, and R. Prabhakar. 1992. Pulmonary eosinophilia in pulmonary tuberculosis. Chest 101:1708-1709. [DOI] [PubMed] [Google Scholar]
- 15.Wardlaw, A. J., R. Moqbel, and A. B. Kay. 1995. Eosinophils: biology and role in disease. Adv. Immunol. 60:151-163. [DOI] [PubMed] [Google Scholar]