Abstract
Objective
This study examined the impact of the Transcendental Meditation (TM) program on cardiovascular (CV) reactivity in adolescents with high normal blood pressure (BP).
Method
Thirty-five adolescents [34 African Americans (AAs), 1 Caucasian American (CA); ages 15–18 years] with resting systolic blood pressure (SBP) between the 85th and 95th percentile for their age and gender on three consecutive occasions, were randomly assigned to either TM (n = 17) or health education control (CTL, n = 18) groups. The TM group engaged in 15-min meditation twice each day for 2 months including sessions during school lunch break. Primary CV outcome measures were changes in BP, heart rate (HR), and cardiac output (CO) at rest and in response to two laboratory stressors, a simulated car driving stressor and an interpersonal social stressor interview.
Results
The TM group exhibited greater decreases in resting SBP (P < .03) from pre- to post-intervention, compared to the CTL group. The TM group exhibited greater decreases from pre- to postintervention in SBP, HR, and CO reactivity (P’s < .03) to the simulated car driving stressor, and in SBP reactivity (P < .03) to the social stressor interview.
Conclusion
The TM program appears to have a beneficial impact upon CV functioning at rest and during acute laboratory stress in adolescents at-risk for hypertension.
Keywords: Transcendental Meditation, Blood pressure, Stress response, Adolescents, Cardiovascular reactivity
Introduction
Essential hypertension (EH) remains a significant health problem in the US with approximately 40 to 50 million persons affected [1]. Epidemiological studies have shown that blood pressure (BP) percentile ranking relative to age mates tends to track from late childhood through adolescence into adulthood [2,3]. Teenagers with high normal BP are at increased risk for development of EH as young adults [4–7]. From late childhood onward, African Americans (AAs) exhibit higher casual BP than Caucasian Americans (CAs) [8]. Thus, AA youth with high normal BP are at particular risk for EH [8,9].
Although the relationship between cardiovascular reactivity (CVR) and EH has been controversial, exaggerated CVR to stress has been hypothesized as contributing to the early etiology of EH [10–12]. Exaggerated CVR to laboratory stressors has been shown to predict future EH in adults [13–15], and change in BP levels and left ventricular mass 1 to 6 years later in youth [16–21]. It has been hypothesized that the high prevalence of EH among AAs is in part due to their greater exposure to chronic environmental stress related to sociostructural barriers (e.g., racism, inadequate economic resources) [11,22–24]. Similar to adult findings [22,25–27], AA youth often exhibit greater BP reactivity to stress than CA youth, and the underlying hemodynamic mechanism frequently responsible is increased total peripheral resistance (TPR) [17,28–30].
Few studies have examined whether stress reduction methods reduce casual BP in youth with high normal BP, and the results of those studies are mixed. Ewart et al. [31] examined the efficacy of progressive muscle relaxation (PMR) training in teenagers with BP above the 85th percentile. PMR instruction provided in class for 3 months reduced the systolic blood pressure (SBP) compared to a waiting-list control condition, but group BP differences 4 months later were not significant [31]. In another study, relaxation training combined with increased physical activity did not decrease BP compared to a control group in community-home boys [32].
Recent research suggests that behavioral stress reduction via Transcendental Meditation (TM) may hold promise in the reduction of casual BP and/or CVR in at-risk youth. Recent clinical trials found that TM lowered clinic BP, and reduced the risk of heart disease, carotid atherosclerosis, and mortality in hypertensive AA adults [33–37]. Further, a recent finding with long-term TM practitioners indicated that acute declines in BP during TM are due to decreases in TPR [38]. Goleman and Schwartz [39] found that TM reduced heart rate (HR) reactivity to a stressful film in normotensive CA adults, but another study of normotensive CA adults found no decreases in HR or BP reactivity to three laboratory stressors [40]. TM has also been shown to effect beneficial stress-related physiological changes, including decreased sympathetic nervous system arousal [41,42], hypothalamic–pituitary–adrenocortical axis dysregulation [43,44], cortisol levels [44], and sympathetic β-adrenergic receptor sensitivity [45]. These findings suggest that examination of the effects of TM on reducing BP and CVR in at-risk youth is warranted.
In the present study, youth with high normal BP, primarily AAs, were randomly assigned to either a 2-month TM program or a health education control (CTL) group. Based on previous findings, it was predicted that youth who practiced TM would exhibit greater decreases in resting BP and TPR, and greater decreases in CVR to laboratory stressors from pretest to 2-month posttest than controls.
Method
Participants
Permission to conduct the study was granted by the Superintendent of Richmond County Public Schools and the Medical College of Georgia Human Assurance Committee. A BP screening was conducted on a random sample of youth at an inner-city high school (200 students, 97% AAs). Thirty-five adolescents (34 AAs, 1 CA; ages 15–18 years) exhibited resting SBP in the sitting position in the ≥85th and ≤95th percentile for their age and gender on three consecutive occasions [46]. Exclusion criteria included: current involvement in a health promotion program; unwillingness to accept randomization into either study group; self-reported pregnancy or parental report of subject’s history of congenital heart defect, diabetes, asthma, or any chronic illness that requires regular pharmacological intervention. None were affected and all consented to participate in the study.
Subjects were given the preintervention as described below and randomly assigned to either a TM group (n = 17; 7 AA and 1 CA female, 9 AA males) or a CTL group (n = 18; 8 AA females, 10 AA males). The TM group engaged in 15-min sessions each day at school at 10:30 a.m. Additional 15-min individual sessions were prescribed at home, each school day, as well as 15 min twice daily individual home practice on weekends for 2 months. The CTL group was presented with seven weekly one-hour lifestyle education sessions based in part on the National Institutes of Health guidelines on lowering BP through weight loss, diet (reducing fat and sodium intake), and increasing physical activity [47]. These sessions were intended to provide comparable time and attention to the CTL subjects. Two subjects dropped out of the TM group and did not complete the post-intervention. Attendance was taken for all sessions at school and weekly “participation cards” were completed by the subjects to document meditations at home. Two months after receiving the intervention, both groups were posttested as described below. A 2-month intervention period resulted due to constraint imposed by the school semester period. Several studies using TM have reported BP reductions at 1–3 months [34,48,49]. Subjects were paid US$150 for their participation.
Intervention
The TM technique has been described as a simple mental procedure practiced for 15 min twice a day while sitting comfortably with eyes closed [50]. The TM technique has its origin in the ancient Vedic approach to health [51], and does not require changes in personal belief, lifestyle, or philosophy [52]. No mental effort is required toward intentionally altering physiological processes (e.g., respiration rate, muscle relaxation, etc.). The ordinary thinking process becomes quiescent and a distinctive state of psychophysiological “restful alertness,” a wakeful but deeply restful state, is gained [53,54].
Procedures
Measurements
All testing was conducted at the Georgia Prevention Institute of the Medical College of Georgia. After a consent form was signed, anthropometric and hemodynamic parameters were measured, at both the pre- and posttest. At pretest, subjects completed an expectation-of-benefits questionnaire, and parents completed a demographic information form [which includes measures of socioeconomic status, and a family health history of cardiovascular (CV) disease form].
Anthropometrics
Height (via stadiometer), weight (via Detecto scale), waist and hip circumference measurements were recorded using established protocols [55,56].
Hemodynamics
Outcome measures were changes in BP, HR, cardiac output (CO), and TPR at rest and in response to a virtual reality car driving simulation task and social stressor interview. All CV measurements were conducted with the subject in the supine position. BP was monitored with a Dinamap Vital Signs Monitor 1846SX [57]. CO and HR measurements were obtained using a noninvasive thoracic electrical bio-impedance system (NCCOM-3 Model 6, Bo-Med Medical Manufacturing, Irvine, CA). The bioimpedance-measuring procedure has been validated via significant correlations between the NCCOM-3 readings and simultaneous thermo-dilution-derived estimates of CO [58,59]. CO and HR were calculated every successive 12 QRS complexes with the bioimpedance monitor while the Dinamap was inflating and calculating BP. These values were averaged to provide one measurement for each BP evaluation. BP and CO values measured simultaneously were used to calculate TPR as {TPR=(SBP + 2DBP)/3/CO} expressed as mmHg/(l/min) where DBP stands for diastolic blood pressure.
A calibration protocol was used, which adjusted impedance-derived values of stroke volume (SV), and thus CO to Doppler-derived measures [16]. During each visit, each subject’s bioimpedance system-derived SV measures at rest were calibrated based upon M-mode echocardiography-derived SV measurements, which have been shown to provide absolute measurements of SV and CO [60,61]. An M-mode echocardiographic examination using a Hewlett-Packard 5500 echocardiograph was conducted to measure aortic flow velocity. Utilizing computer analysis and the area under the velocity curve (flow velocity integral), SV and CO were calculated. The average Doppler-derived SV values during rest for each subject in conjunction with their HR values were compared to the subject’s average impedance-derived resting SVs at the same HRs. The percentage differences in SV were used as a calibration for impedance-derived SV measures during the formal evaluation. This approach resulted in standardization of all CO and TPR readings providing a more accurate means of measuring absolute responses to the stressors.
Following instrumentation with the CV monitoring equipment, all subjects were placed in a supine position and instructed to relax as completely as possible by closing the eyes, freeing the mind of distracting thoughts, and concentrating on breathing in a slow regular manner for 15 min. CV responses were simultaneously measured during minutes 10, 12, and 14. Following baseline evaluation, during both the preintervention and 2-month postintervention evaluations, the virtual reality car driving simulation and social stressor interview were presented, with the order of presentation counterbalanced among subjects but maintained within-subject for both evaluations. The car driving simulation stressor was chosen as an individual behavioral challenge and the social stressor interview was chosen as an interpersonal relationship challenge. Both stressors have been shown to increase sympathetic arousal and CVR to each has been predictive of CV risk factors in youth such as increased BP levels and left ventricular mass [20,62]. Full details of the stressor protocols have been presented in earlier reports [63,64]. The car driving stressor and social stressor interview were presented in the supine position. During each 10-min behavioral stressor, and each 15-min recovery period, CV readings were obtained every other minute.
Car driving simulation stressor
The virtual reality car driving protocol was developed in our lab [64]. The subject was fitted with a Kaiser-Optic Virtual Immersion monitor (VIM-500, Kaiser Aerospace and Electronics, Carlsbad, CA) fitted on his/her head. The VIM 500 was interfaced with a Panasonic Real 3DO Interactive Multiplayer System (Model FZ-1, Matsushita Electric of America, Secaucus, NJ) for the CD ROM “Need for Speed” (Pioneer Productions and Electronic Arts, Burnaby, Canada). The Panasonic 3DO system incorporates a handheld control pad. Standardized instructions and a demonstration were given by a trained research assistant. The subject engaged in the stressor under a condition of challenge was prompted every minute to drive as fast as possible. The research assistant recorded the average and peak driving speed. Subjects then completed nine Likert scale questions which assessed perception of task involvement, skill level, and experience with the game, and affective state changes during the stressor.
Social stressor interview
The social stressor interview was previously validated for use with adolescents [63]. During the interview, the subject discussed a recent socially stressful event. The interviewer presented the subject with a list of problems concerning school, family, friends, work, and money, which recently resulted in anger and/or frustration. Through guided imagery and reflective listening, the interviewer attempted to promote accurate reexperiencing of the event, including the subject’s affective and behavioral responses. This was followed by a summary of the outcome of the event and the subject’s perception of satisfaction with his/her responses to the event. Subjects then completed six Likert scale questions which assessed perception of task involvement and affective state changes during the stressor.
Statistical analyses
To ensure comparability between the two groups at pre-and postintervention on all sociodemographic (e.g., socioeconomic status, family history of CVD) and anthropometric variables (e.g., skinfolds, weight, height), a series of 2 (Intervention: TM vs. CTL) × 2 (Time: pre- vs. postintervention) repeated-measures analyses of variance (ANOVA) with time as the repeated measure was conducted.
Supine resting CV data (i.e., SBP, DBP, HR, CO, TPR) were analyzed as dependent variables using 2 (Intervention: TM vs. CTL) × 2 (Time: pre- vs. postintervention) repeated-measures ANOVAs with time as the repeated measure. Multivariate repeated-measures analyses of covariance (MANCOVAs) were conducted separately for each stressor using the mean CV responses (i.e., SBP, DBP, HR, CO, TPR) as the dependent variables with phase (prestressor vs. stress response) and time (pre- vs. postintervention) as repeated-measures factors, intervention (TM vs. CTL) as the grouping factor, and supine resting CV values as covariates. Follow-up comparisons were not needed because there were only two levels for each of the independent variables.
Results
Descriptive characteristics
The average attendance of the TM and CTL groups at the school sessions was 67.8% and 68.2%, respectively. The average self-reported compliance with TM practice at home was 76.6%. The percentage of students attending at least 60% of the total possible sessions was 80% for the TM groups and 58% for the CTL group. Descriptive characteristics of the TM and CTL groups are presented in Tables 1a and 2a. There were no significant main (i.e., intervention, time) or interaction effects for any parameter (all Ps > .36) indicating that the groups did not differ significantly at preintervention in expectation of benefits nor at pre- or postintervention in any anthropometric or demographic parameters.
Table 1.
Preintervention descriptive characteristics and cardiovascular responsivity to stressors by intervention
| (a) Preintervention descriptive characteristics by intervention
| ||
|---|---|---|
| TM (n = 15) | CTL (n = 18) | |
| Anthropometric/demographic | ||
| Age (years) | 16.5 ± 1.1 | 16.6 ± 1.1 |
| Weight (kg) | 87.1 ± 21.8 | 91.2 ± 25.7 |
| Height (cm) | 170.6 ± 6.8 | 171.0 ± 8.2 |
| Body surface area (m2) | 1.98 ± 0.25 | 2.02 ± 0.27 |
| Body mass index (kg/m2) | 29.7 ± 6.2 | 31.2 ± 8.6 |
| Ponderal index (kg/m3) | 17.4 ± 3.4 | 18.3 ± 5.2 |
| Waist-to-hip ratio | 0.81 ± 0.07 | 0.81 ± 0.09 |
| Baseline resting CV function | ||
| SBP (mmHg) | 124.7 ± 9.1 | 118.8 ± 8.2 |
| DBP (mmHg) | 61.6 ± 7.1 | 59.7 ± 5.8 |
| HR (bpm) | 65.3 ± 9.5 | 66.0 ± 12.8 |
| CO (l/min) | 4.9 ± 1.2 | 5.1 ± 1.3 |
| TPR (mmHg/(l/min)) | 17.7 ± 4.5 | 16.4 ± 5.3 |
| (b) Preintervention cardiovascular responsivity to stressors by intervention
| ||||
|---|---|---|---|---|
| TM
|
CTL
|
|||
| Prestressor level | Mean stressor level | Prestressor level | Mean stressor level | |
| Car driving | ||||
| SBP (mmHg) | 125.9 ± 8.2 | 139.5 ± 8.3 | 119.3 ± 7.8 | 127.2 ± 13.2 |
| DBP (mmHg) | 61.7 ± 7.8 | 75.2 ± 10.2 | 63.5 ± 8.1 | 70.0 ± 8.3 |
| HR (bpm) | 64.6 ± 9.6 | 79.1 ± 9.7 | 65.7 ± 7.3 | 72.8 ± 11.2 |
| CO (l/min) | 4.9 ± 1.3 | 5.4 ± 1.3 | 5.2 ± 1.4 | 5.2 ± 1.5 |
| TPR (mmHg/(l/min)) | 17.9 ± 4.7 | 19.1 ± 5.1 | 17.4 ± 7.2 | 18.7 ± 6.1 |
| Social stressor interview | ||||
| SBP (mmHg) | 123.7 ± 8.5 | 140.0 ± 10.2 | 118.1 ± 6.1 | 133.8 ± 7.2 |
| DBP (mmHg) | 62.0 ± 8.1 | 73.0 ± 9.9 | 62.6 ± 8.3 | 72.9 ± 9.4 |
| HR (bpm) | 66.0 ± 9.3 | 73.0 ± 10.4 | 66.4 ± 13.6 | 70.9 ± 9.5 |
| CO (l/min) | 5.0 ± 1.3 | 4.9 ± 1.3 | 5.1 ± 1.4 | 5.1 ± 1.3 |
| TPR (mmHg/(l/min)) | 17.3 ± 4.4 | 20.8 ± 5.7 | 17.2 ± 6.6 | 20.0 ± 6.8 |
Values are means ± standard deviation. SBP = systolic blood pressure; DBP = diastolic blood pressure; HR = heart rate; CO = cardiac output; TPR = total peripheral resistance. All TM vs. CTL effects = not significant.
Table 2.
Postintervention descriptive characteristics and cardiovascular responsivity to stressors by intervention and time
| (a) Postintervention descriptive characteristics by intervention
| |||
|---|---|---|---|
| TM (n = 15) | CTL (n = 18) | Significant effect | |
| Anthropometric/demographic | |||
| Age (years) | 16.7 ± 1.1 | 16.8 ± 1.1 | |
| Weight (kg) | 88.1 ± 20.8 | 92.0 ± 26.1 | |
| Height (cm) | 170.5 ± 6.7 | 171.0 ± 8.2 | |
| Body surface area (m2) | 1.99 ± 0.24 | 2.03 ± 0.27 | |
| Body mass index (kg/m2) | 30.1 ± 6.1 | 31.4 ± 8.8 | |
| Ponderal index (kg/m3) | 17.6 ± 3.4 | 18.4 ± 5.3 | |
| Waist-to-hip ratio | 0.83 ± 0.08 | 0.83 ± 0.08 | |
| Baseline rest CV function | |||
| SBP (mmHg) | 119.9 ± 9.1 | 121.4 ± 11.2 | ab * |
| DBP (mmHg) | 58.1 ± 8.5 | 60.9 ± 7.9 | ab * |
| HR (bpm) | 66.2 ± 11.2 | 64.5 ± 8.5 | ns |
| CO (l/min) | 5.0 ± 1.3 | 5.3 ± 1.3 | ns |
| TPR (mmHg/(l/min)) | 16.4 ± 5.3 | 16.3 ± 4.6 | ns |
| (b) Postintervention cardiovascular responsivity to stressors by intervention and time
| |||||
|---|---|---|---|---|---|
| TM
|
CTL
|
||||
| Prestressor level | Mean stressor level | Prestressor level | Mean stressor level | Significant effects | |
| Car driving | |||||
| SBP (mmHg) | 121.1 ± 9.5 | 130.2 ± 10.9 | 119.8 ± 12.3 | 131.2 ± 13.9 | abc * |
| DBP (mmHg) | 59.1 ± 10.0 | 69.5 ± 11.2 | 60.7 ± 7.6 | 71.8 ± 10.1 | |
| HR (bpm) | 66.2 ± 11.1 | 76.9 ± 12.7 | 64.8 ± 8.9 | 75.3 ± 14.9 | abc * |
| CO (l/min) | 4.8 ± 1.4 | 4.9 ± 1.5 | 5.4 ± 1.3 | 5.7 ± 1.3 | abc * |
| TPR (mmHg/(l/min)) | 17.8 ± 5.2 | 20.1 ± 6.0 | 15.4 ± 3.5 | 19.1 ± 9.4 | |
| Social stressor interview | |||||
| SBP (mmHg) | 126.0 ± 10.8 | 133.1 ± 10.9 | 121.0 ± 10.9 | 133.1 ± 12.2 | abc * |
| DBP (mmHg) | 64.5 ± 7.1 | 70.2 ± 9.5 | 61.5 ± 6.3 | 69.7 ± 6.8 | bc * |
| HR (bpm) | 70.0 ± 10.5 | 74.7 ± 9.7 | 66.0 ± 9.9 | 71.7 ± 12.4 | |
| CO (l/min) | 4.7 ± 1.6 | 4.6 ± 1.4 | 5.3 ± 1.4 | 5.7 ± 1.6 | ab * |
| TPR (mmHg/(l/min)) | 21.3 ± 11.4 | 22.9 ± 11.8 | 16.3 ± 4.8 | 17.2 ± 5.0 | ab **, bc |
Values are means ± standard deviation.
Intervention effect (TM vs. CTL).
Time effect (pre- vs. postintervention).
Phase effect (prestressor vs. task level).
P < .05
P < .10
Resting CV evaluations
The unadjusted means and standard deviations for the resting CV parameters as a function of intervention status and time are presented in Tables 1b and 2b. Analyses revealed no main effects for intervention on any CV parameter (Ps >.42). A Group × Time interaction (Fig. 1, P < .03) for SBP indicated that at postintervention, the TM group exhibited lower while the CTL group exhibited slightly higher resting SBP than at preintervention. An Intervention × Time interaction indicated a trend such that the TM group exhibited lower while the CTL group exhibited higher resting DBP at postintervention (P < .06).
Fig. 1.

Change in supine resting SBP (pre- minus postintervention) after a 2-month intervention.
CV responsivity evaluations
Car driving simulation stressor
For the MANCOVA analyses, the most important term to be tested was the three-way interaction of Intervention × Phase × Time. The MANCOVAs revealed significant phase effects for all CV parameters (Ps < .04), indicating that subjects exhibited significant CV arousal to the stressors. For the car driving stressor, Intervention × Phase × Time interactions were observed for SBP (Fig. 2, P < .03), CO (P < .01), and HR (P < .03) and for a trend was noted for DBP (P < .07). The nature of these interactions was such that differences between the prestressor and stress responses (reactivity) were smaller at postintervention for the TM compared to the CTL group which showed a slight increase.
Fig. 2.
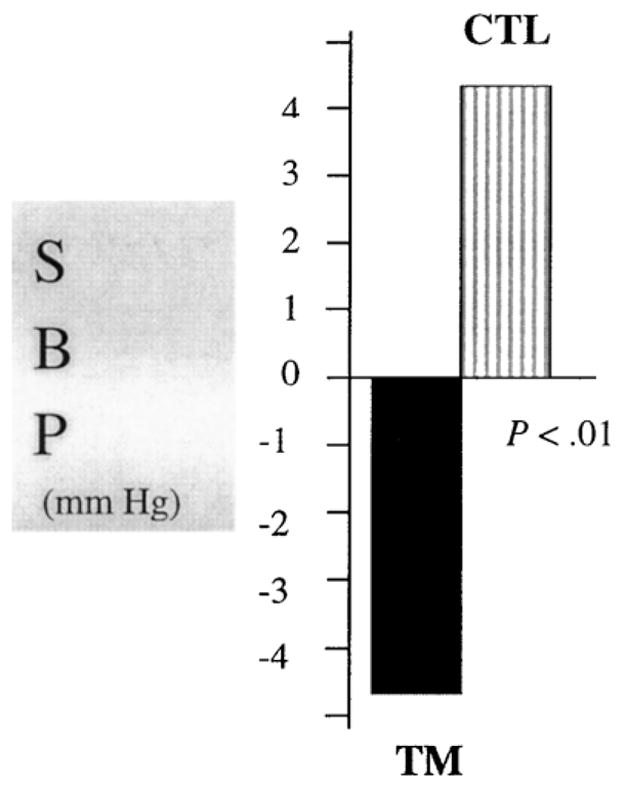
Change in SBP reactivity (mean stressor minus prestressor level) to the car driving simulation stressor (pre- minus postintervention) comparing TM vs. CTL groups after a 2-month intervention.
There were no significant differences between the groups in self-reported task involvement, and affective responses to the stressors at pre- or postintervention (Ps >.14). At pre-intervention, there were no significant differences between the groups on maximum (TM: 113.8 ± 14.1; CTL: 114.6 ± 7.9 mph) and average (TM: 70.8 ± 6.0; CTL: 66.5 ± 11.3 mph) driving speeds (Ps >.24) during the car driving stressor. At postintervention during the car driving simulation task, the TM group exhibited higher maximum (TM: 119.3 ± 10.1; CTL: 114.8 ± 8.7 mph, P < .04), but similar average (TM: 74.4 ± 7.8; CTL: 72.9 ± 13.3 mph) driving speeds.
Social stressor interview
A less consistent pattern of findings was noted for the social stressor interview. The MANCOVAs revealed significant phase effects for all CV parameters (Ps < 0.04), except for CO (P >.10), indicating that subjects exhibited significant CV arousal to the stressors. For SBP, there was a significant Group × Phase × Time interaction (Fig. 3, P < .03). The interaction was such that the differences between the prestressor and stress response were markedly smaller at post- compared with preintervention for the TM compared to the CTL group for which the differences were slightly smaller at post- compared with preintervention.
Fig. 3.

Change in SBP reactivity (mean stressor minus prestressor level) to the social stressor interview (pre- minus postintervention) comparing TM vs. CTL groups after a 2-month intervention.
Discussion
This study examined the impact of a 2-month participation in the TM technique on CV function at rest and during acute stress in adolescents with high normal BP. With respect to resting CV function, the TM group exhibited greater decreases in resting SBP and a trend for greater decreases in DBP compared to the control group. These findings were not attributable to differences in anthropometrics or demographics since the TM and control groups were similar on these parameters at pre- and postintervention, and both groups were similar in their expectations of health benefits at preintervention. Eisenberg et al. [65] were unable to document the efficacy of various types of stress reduction approaches upon EH (e.g., biofeedback, relaxation, stress management, and/or non-TM meditation) under controlled conditions. However, the present findings are consistent with adult studies which have shown that TM reduced BP significantly in older hypertensive AAs [34] at 3 months, CAs [49] at 3 months, normotensive Asian medical students at 1.5 and 3 months [48], and in normotensive CA college students over 4 months [40].
Few BP-related stress reduction studies have been conducted in youth and findings have been mixed. Relaxation training combined with increased physical activity for 4 months failed to yield any BP differences in community-home boys compared to a control group [32]. A daily PMR program conducted for 4 months at school in teenagers with high normal BP showed a 5.3 mmHg greater decrease in their SBP compared to a waiting-list control condition [31]. The present findings compare favorably with the results of that study [31] in that the TM group exhibited a 4.8-mmHg greater decrease in SBP compared to a 2.6-mmHg increase in the CTL group.
Only a few studies have examined the impact of TM upon CVR and all have involved adults. TM was shown to decrease HR reactivity to viewing a stressful film in normotensive adults [39]. No significant changes were reported in either BP or HR reactivity to mental arithmetic, mirror star tracing, public speaking, or an isometric handgrip task in a 4-month study comparing TM to a stress education control group in normotensive CA college students [40]. The present findings in youth extend these reports. Greater decreases in SBP, HR, and CO reactivity and a trend for a greater decrease in DBP reactivity were observed in the TM group compared to increases in the control group during the car driving simulation task. With respect to the social stressor interview, both groups exhibited decreases in SBP reactivity but a greater decrease was observed in the TM group compared to the control group.
Why TM had a more consistent impact on CVR to car driving simulation is unknown. Perhaps TM subjects exhibited decreased sympathetic arousal to the car driving simulation on the postevaluation due in part to improved perceptual–motor coordination and/or faster reaction time previously observed to be a result of TM [66,67]. Partial support for this hypothesis is provided by the higher peak driving speed for the TM group on the second visit. No other measures of driving-related performance (e.g., number of wrecks, time off of road, etc.) were recorded. Future stress reduction studies evaluating possible CVR reduction to perceptual–motor or cognitive skills stressors may benefit from inclusion of relevant performance measures. Reasons for the increased CVR to car driving simulation in the CTL group are also unknown. Although many studies have reported a decrease in CVR to the same stressor over time, findings have been mixed and a number have reported increases in CVR. For example Mills et al. [68] reported increases in SBP (6 mmHg), DBP (4 mmHg), and HR (3 bpm) reactivity to standing and math stressors given 10 days apart.
Although intriguing and supported by the adult literature, the present findings should be interpreted cautiously since impact of health education on change in lifestyle-related moderators, such as diet (e.g. sodium intake), physical activity, and environmental stress were not evaluated. However, all subjects resided in the same geographical locale (i.e., lower socioeconomic status neighborhoods), and none participated in any formal sports or lifestyle programs during the intervention period, besides physical education. Further, there were no significant differences between the groups in changes in weight or adiposity, which would provide some indication of significant diet and/or physical activity lifestyle changes. The study did not provide the groups with knowledge regarding the impact of stress on BP and other CV variables. Although efforts were made to provide comparable instruction time and attention at school to both groups, the TM group did receive greater direct contact. While TM is not conventionally practiced during lunch breaks, this facilitated fitting a daily session into the school schedule without taking time from the academic schedule. In other studies, neither lifestyle modification education matched for direct attention, nor waiting-list control groups showed significant decreases in resting BP [31,32,34]. Further, one of these studies found the CTL group to exhibit slight increases in clinic BP at follow-up compared to the decrease exhibited by the TM group [34].
To our knowledge, no studies have shown whether relaxation-reduced CVR in youth prevents the development of EH. The health-damaging effects of CVR are thought to be due to the accumulation of repeated occurrences that eventually produce detrimental alterations in CV structure and function [69]. Since it is frequently many years before children display overt clinical symptoms of CV disease, longitudinal studies are needed to examine the impact of reduced CVR or the development of preclinical manifestation of increased EH risk (e.g., left ventricular hypertrophy, endothelial dysfunction, increased resting BP). Future studies would benefit from inclusion of follow-up evaluation to determine whether impact of TM is lasting. Also, evaluation of possible moderators such as diet, physical activity, and social support would be beneficial.
To our knowledge, this is the first controlled randomized study of reduction of resting BP and CVR to acute stress in at-risk youth using behavioral stress reduction via TM. The successful implementation of the intervention points to the potential of school-based stress reduction programs to prevent early onset of EH in high-risk youth, particularly AAs. The results of this study contribute to the currently limited knowledge of the efficacy of stress reduction programs in reducing resting BP and CVR to acute stress.
Acknowledgments
We would like to thank Dr. Charles Larke, Superintendent; Dr. Rush Utley and Mr. Quentin Motley, Principals, Richmond County Public Schools in Augusta, GA for their cooperation in providing the facilities for this study. This study was supported in part by National Institutes of Health Grant #HL62976 to Dr. Treiber and an American Heart Association Scientist Development Grant #9930073N to Dr. Barnes.
References
- 1.Burt VL, Whelton P, Rocella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population: results from the Third National Health and Nutrition Examination survey, 1988–1991. Hypertension. 1995;25:305–13. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 2.Baron A, Freyer B, Fixler D. Longitudinal blood pressure in Blacks, Whites, and Mexican Americans during adolescence and early adulthood. Am J Epidemiol. 1986;123:809–17. doi: 10.1093/oxfordjournals.aje.a114310. [DOI] [PubMed] [Google Scholar]
- 3.Gillman MW, Cook NR, Rosner B, Evans DA, Keough ME, Taylor JO, Hennekens CH. Identifying children at high risk for the development of essential hypertension. J Pediatr. 1993;1226:837–46. doi: 10.1016/s0022-3476(09)90005-1. [DOI] [PubMed] [Google Scholar]
- 4.Berenson GS, Wattigney WA, Bao W, Nicklas TA, Jiang X, Rush JA. Epidemiology of early primary hypertension and implications for prevention: the Bogalusa Heart Study. J Hum Hypertens. 1994;85:303–11. [PubMed] [Google Scholar]
- 5.Beckett LA, Rosner B, Roche AF, Guo S. Serial changes in blood pressure from adolescence into adulthood. Am J Epidemiol. 1992;13510:1166–77. doi: 10.1093/oxfordjournals.aje.a116217. [DOI] [PubMed] [Google Scholar]
- 6.Hofman A, Valkenburg HA, Maas J, Groustra FN. The natural history of blood pressure in childhood. Int J Epidemiol. 1985;141:91–6. doi: 10.1093/ije/14.1.91. [DOI] [PubMed] [Google Scholar]
- 7.Shear CL, Burke GL, Freedman DS, Berenson GS. Value of childhood blood pressure measurements and family history in predicting future blood pressure status: results from 8 years of follow-up in the Bogalusa Heart Study. Pediatrics. 1986;776:862–9. [PubMed] [Google Scholar]
- 8.Alpert BS, Fox ME. Racial aspects of blood pressure in children and adolescents. Pediatr Clin North Am. 1993;40:13–20. doi: 10.1016/s0031-3955(16)38477-2. [DOI] [PubMed] [Google Scholar]
- 9.Alpert BS, Murphy JK, Treiber FA. Essential hypertension: approaches to prevention in children. Med Exercise Nutr Health. 1994;3:296–307. [Google Scholar]
- 10.Manuck SB, Kasprowicz AL, Muldoon MF. Behaviorally-evoked cardiovascular reactivity and hypertension: conceptual issues and potential associations. Ann Behav Med. 1990;12:17–29. [Google Scholar]
- 11.Manuck SB. Cardiovascular reactivity in cardiovascular disease: “Once more unto the breach”. Int J Behav Med. 1994;11:4–31. doi: 10.1207/s15327558ijbm0101_2. [DOI] [PubMed] [Google Scholar]
- 12.Fredrikson M, Matthews KA. Cardiovascular responses to behavioral stress and hypertension: a meta-analytic review. Ann Behav Med. 1990;12:30–9. [Google Scholar]
- 13.Jackson AS, Squires WG, Grimes G, Beard EF. Prediction of future resting hypertension from exercise blood pressure. J Cardiac Rehab. 1983;3:263–8. [Google Scholar]
- 14.Menkes MS, Matthews KA, Krantz DS, Lundberg U, Mead LA, Qaqish B, Liang KY, Thomas CB, Pearson TA. Cardiovascular reactivity to the cold pressor test as a predictor of hypertension. Hypertension. 1989;145:524–30. doi: 10.1161/01.hyp.14.5.524. [DOI] [PubMed] [Google Scholar]
- 15.Matthews KA, Woodall KL, Allen MT. Cardiovascular reactivity to stress predicts future blood pressure status. Hypertension. 1993;22:479–85. doi: 10.1161/01.hyp.22.4.479. [DOI] [PubMed] [Google Scholar]
- 16.Treiber FA, Turner JR, Davis H, Strong WB. Prediction of resting cardiovascular functioning in youth with family histories of essential hypertension: a 5-year follow-up. Int J Behav Med. 1997;4:278–91. doi: 10.1207/s15327558ijbm0404_2. [DOI] [PubMed] [Google Scholar]
- 17.Treiber FA, Rauniker A, Davis H, Fernandez T, Levy M, Strong WB. One-year stability and prediction of cardiovascular functioning at rest and during laboratory stressors in youth with family histories of essential hypertension. Int J Behav Med. 1994;14:335–53. doi: 10.1207/s15327558ijbm0104_4. [DOI] [PubMed] [Google Scholar]
- 18.Treiber F, DeRosario J, Davis H, Gutin B, Strong WB. Cardiovascular stress responses predict cardiovascular functioning: a four-year follow-up. In: Armstrong N, editor. Children and exercise. London: Chapman & Hall; 1997. pp. 366–72. [Google Scholar]
- 19.Treiber FA, Turner JR, Davis H, Thompson W, Levy M, Strong WB. Young children’s cardiovascular stress responses predict resting cardiovascular functioning 2 1/2 years later. J Cardiovasc Risk. 1996;3:95–100. [PubMed] [Google Scholar]
- 20.Murdison KA, Treiber FA, Mensah G, Davis H, Thompson W, Strong WB. Prediction of left ventricular mass in youth with family histories of essential hypertension. Am J Med Sci. 1998;3152:118–23. doi: 10.1097/00000441-199802000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Malpass D, Treiber FA, Turner JR, Davis H, Thompson W, Strong WB, Levy M. Relationships between children’s cardiovascular stress responses and resting cardiovascular functioning 1 year later. Int J Psychophysiol. 1997;25:139–44. doi: 10.1016/s0167-8760(96)00736-2. [DOI] [PubMed] [Google Scholar]
- 22.Anderson NB, McNeilly M, Myers H. A biopsychosocial model of race differences in vascular reactivity. In: Blascovich J, Katkin ESE, editors. Cardiovascular reactivity to psychological stress and disease. Washington (DC): American Psychological Association; 1993. pp. 83–108. [Google Scholar]
- 23.Anderson NB, McNeilly M, Myers H. Autonomic reactivity and hypertension in Blacks: a review and proposed model. Ethn Dis. 1991;12:154–70. [PubMed] [Google Scholar]
- 24.Barnes VA, Schneider RH, Alexander CN, Staggers F. Stress, stress reduction and hypertension in African Americans: an updated review. J Natl Med Assoc. 1997;897:464–76. [PMC free article] [PubMed] [Google Scholar]
- 25.Light KC, Turner JR, Hinderliter AL, Sherwood A. Race and gender comparisons: I. Hemodynamic responses to a series of stressors. Health Psychol. 1993;12:354–65. doi: 10.1037//0278-6133.12.5.354. [DOI] [PubMed] [Google Scholar]
- 26.Light KC, Sherwood A. Race, borderline hypertension, and hemodynamic responses to behavioral stress before and after beta-adrenergic blockade. Health Psychol. 1989;85:577–95. doi: 10.1037//0278-6133.8.5.577. [DOI] [PubMed] [Google Scholar]
- 27.Saab PG, Llabre MM, Hurwitz BE, Frame CA, Reineke LJ, Fins AI, McCalla J, Cieply LK, Schneiderman N. Myocardial and peripheral vascular responses to behavioral challenges and their stability in Black and White Americans. Psychophysiology. 1992;294:384–97. doi: 10.1111/j.1469-8986.1992.tb01712.x. [DOI] [PubMed] [Google Scholar]
- 28.Treiber FA, Musante L, Braden D, Arensman F, Strong WB, Levy M, Leverett S. Racial differences in hemodynamic responses to the cold face stimulus in children and adults. Psychosom Med. 1990;52:286–96. doi: 10.1097/00006842-199005000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Treiber FA, McCaffrey F, Musante L, Rhodes T, Davis H, Strong WB, Levy M. Ethnicity, family history of hypertension and patterns of hemodynamic reactivity in boys. Psychosom Med. 1993;55:70–7. doi: 10.1097/00006842-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Dysart JM, Treiber FA, Pfleiger K, Davis H, Strong WB. Ethnic differences in the myocardial and vascular reactivity to stress in normotensive girls. Am J Hypertens. 1994;7:15–22. doi: 10.1093/ajh/7.1.15. [DOI] [PubMed] [Google Scholar]
- 31.Ewart CK, Harris WL, Iwata MM, Coates TJ, Bullock R, Simon B. Feasibility and effectiveness of school-based relaxation in lowering blood pressure. Health Psychol. 1987;65:399–416. doi: 10.1037//0278-6133.6.5.399. [DOI] [PubMed] [Google Scholar]
- 32.Rauhala E, Alho H, Hanninen O, Helin P. Relaxation training combined with increased physical activity lowers the psychophysiological activation in community-home boys. Int J Psychophysiol. 1990;101:63–8. doi: 10.1016/0167-8760(90)90046-g. [DOI] [PubMed] [Google Scholar]
- 33.Schneider RH, Nidich SI, Salerno JW. The transcendental meditation program: reducing the risk of heart disease and mortality and improving quality of life in African Americans. Ethn Dis. 2001;111:159–60. [PubMed] [Google Scholar]
- 34.Schneider RH, Staggers F, Alexander CN, Sheppard W, Rainforth M, Kondwani K, Smith S, Gaylord C. A randomized controlled trial of stress reduction for hypertension in older African Americans. Hypertension. 1995;26:820–7. doi: 10.1161/01.hyp.26.5.820. [DOI] [PubMed] [Google Scholar]
- 35.Schneider RH, Alexander CN, Rainforth M, Salerno JW, Aguilar M, Hartz A. Randomized controlled trials of effects of the transcendental meditation program on cancer, cardiovascular, and all-cause mortality: a meta-analysis. Ann Behav Med. 1999;21(Supplement):S012. [Google Scholar]
- 36.Alexander CN, Schneider RH, Staggers F, Sheppard W, Clayborne BM, Rainforth M, Salerno J, Kondwani K, Smith S, Egan B. Trial of stress reduction for hypertension in older African Americans: Part II. Sex and risk subgroup analysis. Hypertension. 1996;28:228–37. doi: 10.1161/01.hyp.28.2.228. [DOI] [PubMed] [Google Scholar]
- 37.Castillo-Richmond A, Schneider R, Alexander C, Cook R, Myers H, Nidich S, Haney C, Rainforth M, Salerno J. Effects of stress reduction on carotid atherosclerosis in hypertensive African Americans. Stroke. 2000;313:568–73. doi: 10.1161/01.str.31.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnes VA, Treiber FA, Turner JR, Davis H, Strong WB. Acute effects of transcendental meditation on hemodynamic functioning in middle aged adults. Psychosom Med. 1999;614:525–31. doi: 10.1097/00006842-199907000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goleman DJ, Schwartz GE. Meditation as an intervention in stress reactivity. J Consult Clin Psychol. 1976;443:456–66. doi: 10.1037//0022-006x.44.3.456. [DOI] [PubMed] [Google Scholar]
- 40.Wenneberg SR, Schneider RH, Walton KG, MacLean CRK, Levitsky DK, Salerno JW, Wallace RK, Mandarino JV, Rainforth MV, Waziri R. A controlled study on the effects of transcendental meditation on cardiovascular reactivity and ambulatory blood pressure. Int J Neurosci. 1997;89:15–28. doi: 10.3109/00207459708988461. [DOI] [PubMed] [Google Scholar]
- 41.Jevning R, Wilson AF, Smith WR. Adrenocortical activity during meditation. Horm Behav. 1978;101:54–60. doi: 10.1016/0018-506x(78)90024-7. [DOI] [PubMed] [Google Scholar]
- 42.Walton KG, Pugh NDC, Gelderloos P, Macrea P. Stress reduction and preventing hypertension: preliminary support for a psychoneuroendocrine mechanism. J Altern Compl Med. 1995;13:263–83. doi: 10.1089/acm.1995.1.263. [DOI] [PubMed] [Google Scholar]
- 43.MacLean CR, Walton KG, Wenneberg SR, Levitsky DK, Mandarino JP, Waziri R, Hillis SL, Schneider RH. Effects of the transcendental meditation program on adaptive mechanisms: changes in hormone levels and responses to stress after 4 months. Psychoneuroendocrinology. 1997;224:277–95. doi: 10.1016/s0306-4530(97)00003-6. [DOI] [PubMed] [Google Scholar]
- 44.MacLean CR, Walton KG, Wenneberg SR, Levitsky DK, Mandarino JV, Waziri R, Schneider RH. Altered responses of cortisol, GH, TSH and testosterone to acute stress after four months’ practice of transcendental meditation (TM) Ann NY Acad Sci. 1994;746:381–4. doi: 10.1111/j.1749-6632.1994.tb39261.x. [DOI] [PubMed] [Google Scholar]
- 45.Mills PJ, Schneider RH, Hill D, Walton KG, Wallace RK. Beta-adrenergic receptor sensitivity in subjects practicing transcendental meditation. J Psychosom Res. 1990;341:29–33. doi: 10.1016/0022-3999(90)90005-o. [DOI] [PubMed] [Google Scholar]
- 46.National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents. Update on the 1987 task force report on high blood pressure in children and adolescents: a working group report from the National High Blood Pressure Education Program. Pediatrics. 1996;984(Pt 1):649–58. [PubMed] [Google Scholar]
- 47.Kaplan NM. The 6th Joint National Committee report (JNC-6): new guidelines for hypertension therapy from the USA. Keio J Med. 1998;472:99–105. doi: 10.2302/kjm.47.99. [DOI] [PubMed] [Google Scholar]
- 48.Bagga OP, Gandhi A. A comparative study of the effect of transcendental meditation (TM) and Shavasana practice on cardiovascular system. Indian Heart J. 1983;351:39–45. [PubMed] [Google Scholar]
- 49.Alexander CN, Langer EJ, Newman RI, Chandler HM, Davies JL. Transcendental meditation, mindfulness, and longevity: an experimental study with the elderly. J Pers Soc Psychol. 1989;576:950–64. doi: 10.1037//0022-3514.57.6.950. [DOI] [PubMed] [Google Scholar]
- 50.Roth R. Maharishi Mahesh Yogi’s transcendental meditation. Washington (DC): Primus; 1994. [Google Scholar]
- 51.Yogi MM. Maharishi’s Vedic approach to health. Vlodrop (Holland): Maharishi Vedic Univ. Press; 1995. [Google Scholar]
- 52.Nader T. Human physiology—expression of Veda and the Vedic literature. Vlodrop (Holland): Maharishi Vedic Univ. Press; 1994. [Google Scholar]
- 53.Wallace RK, Benson H, Wilson AF. A wakeful hypometabolic physiologic state. Am J Physiol. 1971;221:795–9. doi: 10.1152/ajplegacy.1971.221.3.795. [DOI] [PubMed] [Google Scholar]
- 54.Wallace KR. The physiology of consciousness. Fairfield: Maharishi International Univ. Press; 1993. [Google Scholar]
- 55.Barnes VA, Treiber FA, Davis H, Kelley T, Strong WB. Central adiposity and hemodynamic functioning at rest and during stress in adolescents. Int J Obesity. 1998;2211:1079–83. doi: 10.1038/sj.ijo.0800730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.National Center for Health Statistics. NHANES III Anthropometric procedures (video) Washington (DC): US Dept of Health and Human Services, US Government Printing Office; 1988. [Google Scholar]
- 57.Rosner BA, Appel LJ, Raczynski JM, Hebert PR, Whelton PK, Murphy JK, Miller ST, Oberman A. A comparison of two automated monitors in the measurement of blood pressure reactivity. Ann Epidemiol. 1990;1:57–69. doi: 10.1016/1047-2797(90)90019-o. [DOI] [PubMed] [Google Scholar]
- 58.Braden DS, Leatherbury L, Treiber FA, Strong WB. Noninvasive assessment of cardiac output in children using impedance cardiography. Am Heart J. 1990;120:1166–72. doi: 10.1016/0002-8703(90)90132-h. [DOI] [PubMed] [Google Scholar]
- 59.Mattar JA, Baruzzi A, Diament D, Szynkier RT, Felippe J, Luz PL, Auler JO, Lage D, Pileggi F, Jatene A. A clinical comparison between cardiac output measured by thermodilution versus noninvasive thoracic electrical bioimpedance. Acute Care. 1986;12:58–60. [PubMed] [Google Scholar]
- 60.Coats AJ. Doppler ultrasonic measurement of cardiac output: reproducibility and validation. Eur Heart J. 1990;11(Suppl I):49–61. doi: 10.1093/eurheartj/11.suppl_i.49. [DOI] [PubMed] [Google Scholar]
- 61.Lacolley PJ, Pannier BM, Levy BI, Safar ME. Non-invasive study of cardiac performance using Doppler ultrasound in patients with hypertension. Eur Heart J. 1990;11(Suppl I):62–6. doi: 10.1093/eurheartj/11.suppl_i.62. [DOI] [PubMed] [Google Scholar]
- 62.Kapuku GK, Treiber FA, Davis HC, Harshfield GA, Cook BB, Mensah GA. Hemodynamic function at rest, during acute stress, and in the field: predictors of cardiac structure and function 2 years later in youth. Hypertension. 1999;345:1026–31. doi: 10.1161/01.hyp.34.5.1026. [DOI] [PubMed] [Google Scholar]
- 63.Ewart CK, Kolodner KB. Social competence interview for assessing physiological reactivity in adolescents. Psychosom Med. 1991;53:289–304. doi: 10.1097/00006842-199105000-00003. [DOI] [PubMed] [Google Scholar]
- 64.Turner JR, Treiber FA, Davis H, Rectenwald J, Pipkin W, Strong WB. Virtual reality technology: application to laboratory CV reactivity studies. Behav Res Methods Instrum Comput. 1997;293:386–9. [Google Scholar]
- 65.Eisenberg DM, Delbanco TL, Berkey CS, Kaptchuk TJ, Kupelnick B, Kuhl J, Chalmers TC. Cognitive behavioral techniques for hypertension: are they effective? Ann Intern Med. 1993;11812:964–72. doi: 10.7326/0003-4819-118-12-199306150-00009. [DOI] [PubMed] [Google Scholar]
- 66.Holt WR, Caruso JL, Riley JB. Transcendental meditation vs. pseudo-meditation on visual choice reaction time. Percept Mot Skills. 1978;463(Pt 1):726. doi: 10.2466/pms.1978.46.3.726. [DOI] [PubMed] [Google Scholar]
- 67.Appelle S, Oswald LE. Simple reaction time as a function of alertness and prior mental activity. Percept Mot Skills. 1974;383:1263–8. doi: 10.2466/pms.1974.38.3c.1263. [DOI] [PubMed] [Google Scholar]
- 68.Mills PJ, Berry CC, Dimsdale JE, Nelesen RA, Ziegler MG. Temporal stability of task-induced cardiovascular, adrenergic, and psychological responses: the effects of race and hypertension. Psychophysiology. 1993;302:197–204. doi: 10.1111/j.1469-8986.1993.tb01732.x. [DOI] [PubMed] [Google Scholar]
- 69.Folkow B. The structural factor in hypertension. In: Laragh JH, Brenner SM, editors. Hypertension: pathophysiology, diagnosis and management. New York: Raven Press; 1990. pp. 5–58. [Google Scholar]


