Abstract
We describe the parasitological kinetics and histopathological and immunological alterations in platelet-activating factor receptor-deficient (PAFR−/−) and wild-type mice after a single Strongyloides venezuelensis infection (subcutaneous inoculation of 500 L3 larvae). There was no difference in the numbers of worms that reached and became established in the small intestines of PAFR−/− and wild-type mice. However, at 12 days after infection, significantly more worms were recovered from PAFR−/− mice. Although PAFR−/− infected mice showed a delay in elimination of adult worms, worms established in the small intestine of these mice produced a significantly lower number of eggs due to a reduction in worm fecundity. There were also significant reductions in the number of circulating and tissue eosinophils and tumor necrosis factor levels in the small intestines of PAFR−/− mice infected for 7 days compared to the number and level in wild-type mice. Histological analysis confirmed the reduced inflammatory process and revealed that the PAFR−/− mice had a smaller number of goblet cells. The concentrations of the type 2 cytokines interleukin-4 (IL-4), IL-5, and IL-10 were lower in small intestine homogenates and in supernatants of antigen-stimulated lymphocytes from spleens or mesenteric lymph nodes of PAFR−/− mice than in the corresponding preparations from wild-type mice. Thus, in S. venezuelensis-infected PAFR−/− mice, decreased intestinal inflammation is associated with enhanced worm survival but decreased fecundity. We suggest that although a Th2-predominant inflammatory response decreases worm survival, the worm may use factors produced during this response to facilitate egg output and reproduction. PAFR-mediated responses appear to modulate these host-derived signals that are important for worm fecundity.
Gastrointestinal nematode species which have pulmonary migration in their life cycles, such as Necator americanus, Ancylostoma duodenalis, Strongyloides stercoralis, and Ascaris lumbricoides, are the most prevalent parasites in humans, infecting over one-quarter of the world's population (9, 11). Despite the high prevalence and chronic morbidity produced by these nematodes, immunopathologic and/or immunoprotective mechanisms involved in the response against these parasites are not completely understood. Experimental infections with gastrointestinal nematodes in rodents induce predominantly a Th2 type of immune response that has been associated with host protection. Nevertheless, the precise mechanisms of protection are still not clear and appear to be considerably different for different helminths (17, 18, 36).
One experimental model that has been used to study the aspects of immunoprotection and immunoregulation during gastrointestinal infection is infection of rodents with Strongyloides venezuelensis (29, 30, 40, 50), a nematode that naturally infects wild rats. In experimental infections, S. venezuelensis larvae have an obligatory migration through the lungs of the host before they become established in the duodenal mucosa (52), similar to the migration of S. stercoralis in humans, and the adult worms are spontaneously eliminated after 5 weeks in rats (55) and after 10 to 14 days of infection in mice (46). The migration of parasite larvae (37, 50) or establishment of the worm in the gut (24, 26) results in eosinophilic inflammation. Moreover, arrival of the parasite in the intestine is accompanied by intestinal eosinophilia and mastocytosis, which may be associated with the process of worm elimination (10, 24, 26).
The lipid mediator platelet-activating factor (1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorocholine) (PAF) is produced by a large number of inflammatory cells, including macrophages, neutrophils, basophils, eosinophils, platelets, and endothelial cells (7, 33). PAF is minimally expressed under normal physiological conditions; however, PAF has been implicated in a number of inflammatory conditions, including allergic inflammation (7, 21, 25, 33). Once released, PAF activates PAF receptors (PAFR), which results in diverse biological activities associated with inflammation (21), including macrophage and eosinophil activation and chemotaxis, alterations in vascular permeability, platelet activation, and induction of mucus production by epithelial cells, all of which may contribute to the course of infection with helminths. Using PAFR−/− mice (22), we analyzed the role of PAFR during the course of infection with the intestinal parasite S. venezuelensis.
MATERIALS AND METHODS
Animals.
C57BL/6 mice lacking the PAFR (PAFR−/− mice) were generated as previously described by Ishii et al. (22). These PAFR-deficient mice were provided by Takao Shimizu (Department of Biochemistry and Molecular Biology, University of Tokyo, Tokyo, Japan) and have been bred and maintained at the bioscience unit of the Institute Gonçalo Muniz (Fundação Oswaldo Cruz, Salvador, Brazil). For the experiments described here, C57BL/6 PAFR−/− and wild-type female mice that were 8 to 10 weeks old were used. All animals were kept at the Department of Biochemistry and Immunology (Federal University of Minas Gerais State, Belo Horizonte, Brazil) vivarium for infected animals during the experimental procedure, fed laboratory chow (Nuvilab; Colombo, Parana, Brazil), and given tap water ad libitum. For experimental procedures we received prior approval from the local animal ethics committee.
Parasite and parasitological techniques.
S. venezuelensis, the intestinal nematode used in all the experiments, was isolated from Rattus norvegicus (8) and has been maintained in the Department of Parasitology (Federal University of Minas Gerais State, Belo Horizonte, Brazil) by serial passage in Wistar rats. S. venezuelensis infective filiform larvae (L3) were obtained from charcoal cultures of infected-rat feces. The cultures were kept for 48 to 72 h at 28°C, and the infective larvae were collected and concentrated by using a Baermann apparatus. Subsequently, the larvae recovered were washed several times in phosphate-buffered saline (PBS) and counted, and the concentration was adjusted to 5,000 L3 per ml of PBS for infection. PAFR−/− and wild-type mice were individually inoculated via subcutaneous injection of 100 μl of PBS containing 500 infective larvae in the abdominal region.
Infectivity rates were determined by assessing fecal egg counts and numbers of worms recovered from the small intestine at 5, 7, and 12 days after infection. For recovery of worms from the small intestine, the upper half of the small intestine from each infected mouse was removed after sacrifice, washed, cut open longitudinally, and incubated in PBS at 37°C for 4 h. Worms that emerged from the intestinal tissue were quantified by stereomicroscopy. The remaining intestinal tissue was placed in PBS and incubated overnight at 4°C, the worms were quantified again, and the total number of worms was determined. After the upper half of the small intestine was separated for worm counting, the first 2 cm of the lower half of each small intestine was processed for histological analysis, and the remaining piece was frozen at −70°C for quantification of cytokines and for the eosinophil peroxidase (EPO) assay. To determine the number of nematode eggs eliminated in the feces, well-formed fecal pellets were obtained from the rectum of each infected mouse, weighed, and homogenized in a known volume of PBS. For each infected mouse, two fecal samples (100 μl) were examined by light microscopy, and the total number of parasite eggs was determined. The number of S. venezuelensis eggs was expressed per gram of mouse feces. Since S. venezuelensis-infected hosts have only female worms in the small intestine, worm fecundity was estimated by dividing the number of eggs eliminated in feces by the number of worms recovered from the intestine of each mouse at each time point.
To obtain L3 soluble antigen, a large number of infective larvae were extensively washed with PBS, resuspended in PBS containing protease inhibitor cocktail (one tablet in 25 ml of PBS; Boehringer Mannheim, Indianapolis, Ind.), and disrupted by vortexing with glass beads (five cycles, 1 min each) and by sonication with a cell sonic disrupter (PGC Scientific, Gaithersburg, Md.) by using 10 1-min cycles at the highest power allowed for the standard microtip (48). After the insoluble particles were removed by centrifugation of the larval homogenate at 400 × g for 30 min, the supernatant was removed, and the protein concentration was determined before division into aliquots and storage at −20°C. The antigen preparation was used to stimulate cells obtained from mesenteric lymph nodes (MLN) and spleens.
Leukocytes in blood and BAL fluid of infected mice.
At 7 and 12 days after S. venezuelensis infection, PAFR−/− and wild-type mice were anesthetized and bled via the tail vein, and the blood samples were used to estimate the circulating cell compositions. Bronchoalveolar lavage (BAL) was performed 5 and 7 days after infection by intratracheal instillation of three aliquots of 1 ml of PBS containing 0.3% bovine serum albumin (Sigma) and protease inhibitor cocktail (one tablet in 50 ml of PBS; Boehringer Mannheim). The lavage fluid was centrifuged at 200 × g for 7 min, and the cell pellet from the BAL fluid was resuspended in 1 ml of PBS containing 0.3% bovine serum albumin.
The total numbers of leukocytes in blood and BAL fluid were estimated by using a Neubauer chamber. Cytospin slides prepared from BAL fluid and blood smears were stained with May-Grünwald-Giemsa stain. Cells were differentiated into mononuclear cells, mature eosinophils, and mature neutrophils by using standard morphological criteria, and at least 200 cells were counted per slide by light microscopy.
Histopathology.
As mentioned above, a section of the intestine collected from the jejunal portion was fixed in 10% buffered formalin and embedded in paraffin, and 5-μm sections were prepared for histology. Sections were stained with hematoxylin and eosin for assessment of the overall inflammatory response. Goblet cell and mucus production was analyzed by using periodic acid-Schiff-stained slides.
EPO assay.
The EPO assay, performed as described by Silveira et al. (50), was used to estimate the numbers of eosinophils in the lungs and intestine.
Cell culture.
Cytokine production was measured in supernatants from spleens and MLN cells restimulated with L3 total antigen or concanavalin A (ConA). For these experiments, uninfected PAFR−/− and wild-type mice and PAFR−/− and wild-type mice that were infected with S. venezuelensis for 7 and 12 days were used. Briefly, the spleen and MLN were aseptically removed, a single-cell suspension was prepared, and red blood cells were lysed. Individual spleens and MLN from four or five mice per group were used at each time. The cells obtained from these organs were centrifuged at 200 × g for 5 min, counted, plated in 96-well, flat-bottom microplates (NUNC, Naperville, Ill.) at a concentration of 1 × 106 cells/well in RPMI 1640 containing 25 mM HEPES and sodium bicarbonate (Sigma, St. Louis, Mo.) and supplemented with 10% fetal calf serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 10 mM sodium pyruvate, and 2 mM l-glutamine (Sigma), and cultured at 37°C in the presence of 5% CO2 for 72 h. Cells were cultured in duplicate in 200 μl either alone or in the presence of 2 μg of ConA (Sigma) per ml or 100 μg of parasite antigen per ml. Supernatants were collected after 72 h, divided into aliquots, and kept at −20°C for quantification of interleukin-4 (IL-4), IL-5, IL-10, and gamma interferon (IFN-γ).
Measurement of cytokines in intestinal homogenates and spleen and MLN culture supernatants.
Cytokines were quantified in cell culture supernatants and in homogenates prepared from the small intestine. The latter samples were obtained by homogenizing 100 mg of the lower half of the small intestine from PAFR−/− or wild-type mice obtained 7 and 12 days after infection with S. venezuelensis. The intestinal samples were homogenized in 1 ml of PBS containing protease inhibitors (0.1 mM phenylmethylsulfonyl fluoride, 0.1 mM benzethonium chloride, 10 mM EDTA, 20 KI aprotinin A, and 0.05% Tween 20). Each sample was then centrifuged for 10 min at 3,000 × g, and the supernatant was used for an enzyme-linked immunosorbent assay (ELISA).
Concentrations of IL-4, IL-5, IL-10, IFN-γ, and tumor necrosis factor alpha (TNF-α) were determined by an ELISA technique with commercially available antibody pairs used according to the instructions supplied by the manufacturer (R&D Systems). Known concentrations of the recombinant proteins were used to generate a standard curve to convert optical density values of samples to picograms per milliliter. The sensitivity of the assays was 16 pg/ml.
Statistical analysis.
Data are reported below as means ± standard errors of the means and were analyzed by using Student's t test (two groups) or one-way analysis of variance. In the latter analysis, P values were assigned by using Tukey post hoc analysis. Differences were considered significant at a P value of >0.05.
RESULTS
PAFR-deficient mice have delayed elimination of S. venezuelensis from the small intestine.
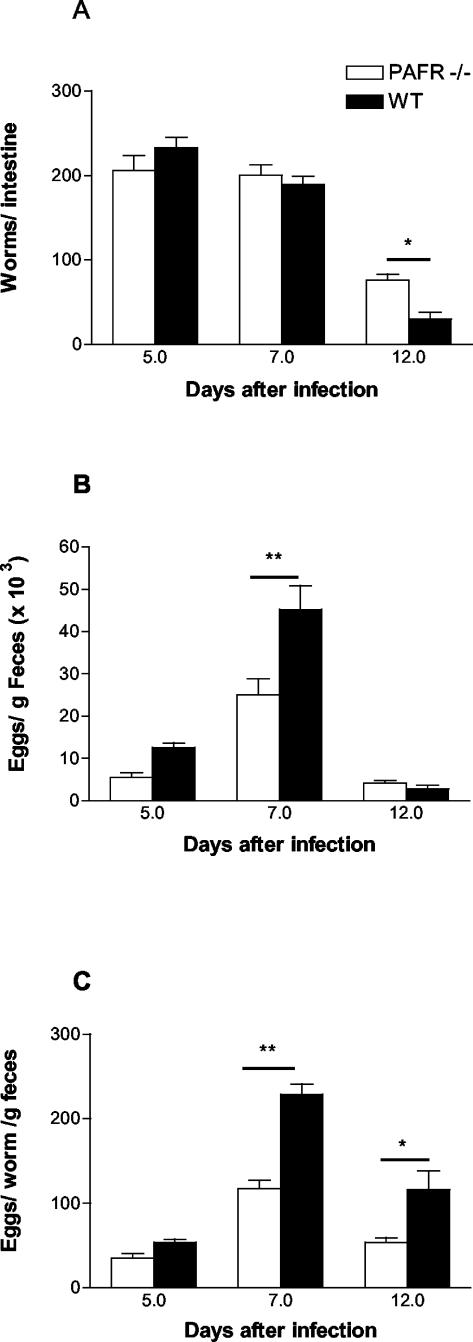
The kinetics of S. venezuelensis infection in mice (Fig. 1A) revealed that there was no statistical difference in the numbers of worms that arrived and became established in the small intestines of PAFR−/− and wild-type mice at 7 days after infection (201 ± 12 worms in PAFR−/− mice and 189 ± 10 worms in wild-type mice). However, at 12 days after infection, there were 76 ± 7 worms/mouse in PAFR−/− infected mice, while only 30 ± 8 worms/mouse were recovered from the wild-type controls (P < 0.05).
FIG. 1.
Parasite burden of wild-type (WT) and PAFR−/− mice infected with S. venezuelensis (one subcutaneous inoculation of 500 L3/mouse). The total number of worms recovered from the small intestine (A), the total number of eggs eliminated in the feces (B), and the total number of eggs eliminated per adult worm per gram of feces (fecundity) (C) were evaluated at 5, 7, and 12 days after infection with S. venezuelensis. The bars and error bars indicate the means and standard errors of the means, respectively, for 8 to 10 mice in each group in two separate experiments. One asterisk and two asterisks indicate that the P values are <0.05 and <0.01, respectively, for a comparison of wild-type and PAFR−/− mice.
Interestingly, the number of S. venezuelensis eggs eliminated in the feces of PAFR−/− infected mice did not reflect the kinetics of adult worm presence in the intestine (Fig. 1B). At 7 days after infection, a time when the numbers of worms recovered from the small intestine were similar for the two groups, PAFR−/− infected mice eliminated significantly fewer S. venezuelensis eggs in the feces than infected controls eliminated (25,000 ± 3,800 eggs for PAFR−/− mice compared to 44,760 ± 5,000 eggs for wild-type controls). At 12 days after infection, PAFR−/− mice and infected wild-type controls eliminated similar numbers of eggs, even though the former group of animals had more worms in the small intestine. The smaller number of parasite eggs eliminated in the feces of PAFR−/− infected mice was a consequence of the lower fecundity of the worms established in the small intestine of these animals (Fig. 1C). Indeed, the fecundity of worms from PAFR−/− mice was lower than that of worms from wild-type controls at 7 and 12 days after infection (Fig. 1C).
Cellular response in blood and BAL fluid of S. venezuelensis-infected PAFR−/− mice.
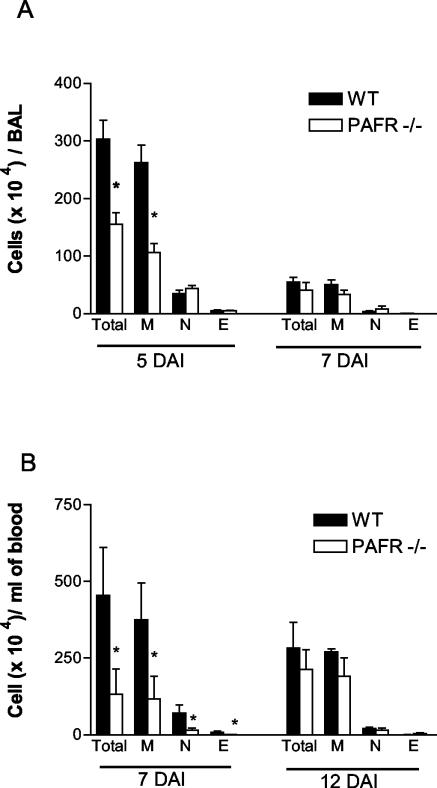
The total number of leukocytes obtained from the BAL fluid collected 5 days after infection of PAFR−/− mice was lower than the total number of leukocytes found in the wild-type counterparts (Fig. 2A). The reduction was due to a reduced number of mononuclear cells present in the BAL fluid of PAFR−/− infected mice. The numbers of eosinophils and neutrophils in the BAL fluid of both groups were similar (Fig. 2A). Seven days after infection, the amounts of cellular infiltrate in the lungs of PAFR−/− and control mice were very small and were similar in the two groups (Fig. 2A).
FIG. 2.
Leukocyte infiltration in the lungs and blood of wild-type (WT) and PAFR−/− mice infected with S. venezuelensis. The total numbers of leukocytes (Total) and the numbers of macrophages (M), neutrophils (N), and eosinophils (E) in BAL fluid (A) and blood (B) were evaluated at different times after subcutaneous infection with 500 L3/mouse. The bars and error bars indicate the means and standard errors of the means, respectively, for four to six mice in each group. An asterisk indicates that the P value is <0.05 for a comparison of wild-type and PAFR−/− mice. DAI, days after infection.
A lower number of leukocytes was also observed in the blood of PAFR−/− mice than in the blood of the infected wild-type controls (133 × 104 ± 80 × 104 and 454 × 104 ± 150 × 104 cells/ml at 7 days after infection, respectively; P < 0.05). The reduction in the total number of blood leukocytes was due to a significant reduction in the number of circulating eosinophils, neutrophils, and mononuclear cells (Fig. 2B). After 12 days of infection there were no significant differences between PAFR−/− and wild-type mice.
Histopathological changes in the intestine of S. venezuelensis-infected PAFR−/− mice.
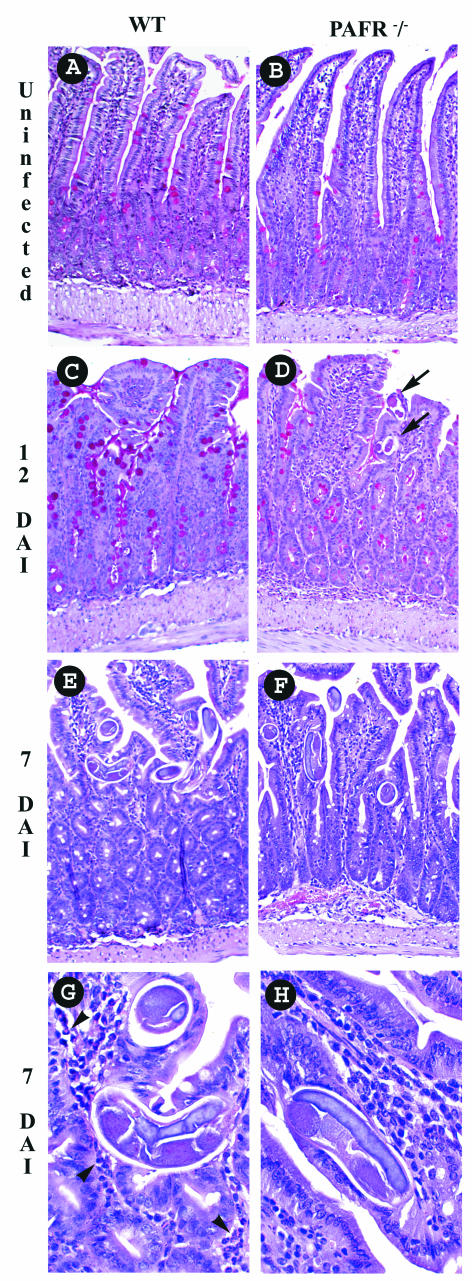
There were no apparent differences in the small intestines of wild-type and PAFR−/− uninfected mice (Fig. 3A and B). Histopathological analysis of the small intestine of infected mice revealed many worms localized mainly beneath the epithelial layer (Fig. 3E and F). In wild-type mice, this was accompanied by intense cellular infiltration into the lamina propria of the villi, along with the presence of eosinophils (Fig. 3G). Eosinophil-rich infiltrates were not evident in the small intestine of PAFR−/− infected mice (Fig. 3H). At 12 days after infection, adult worms were still present in the small intestine of PAFR−/− infected mice, confirming the delay in elimination of worms (Fig. 3D). Moreover, at 12 days after infection, there were more goblet cells filled with mucus in the epithelial layer of wild-type infected mice (Fig. 3C) than in the epithelial layer of PAFR−/− infected mice (Fig. 3D).
FIG. 3.
Small intestines of wild-type (WT) and PAFR−/− mice infected with S. venezuelensis. (A, C, E, and G) Sections from wild-type mice; (B, D, F, and H) sections from PAFR−/− mice. Uninfected mice were evaluated (A and B), and mice infected with S. venezuelensis were evaluated 12 days (C and D) or 7 days (E to H) after infection. Note the greater number of goblet cells (C) and the eosinophilic tissue infiltration (G) in wild-type mice. In panel D, the arrows indicate sections of worms in the small intestine of a PAFR−/− mouse after 12 days of S. venezuelensis infection. In panel G, the arrowheads indicate the presence of eosinophils. The tissue was fixed with buffered formalin and embedded in paraffin, and 5-μm sections were stained with periodic acid-Schiff stain (A to D) or hematoxylin and eosin (E to G). (A to F) Magnification, ×200; (G and H) magnification, ×400. DAI, days after infection.
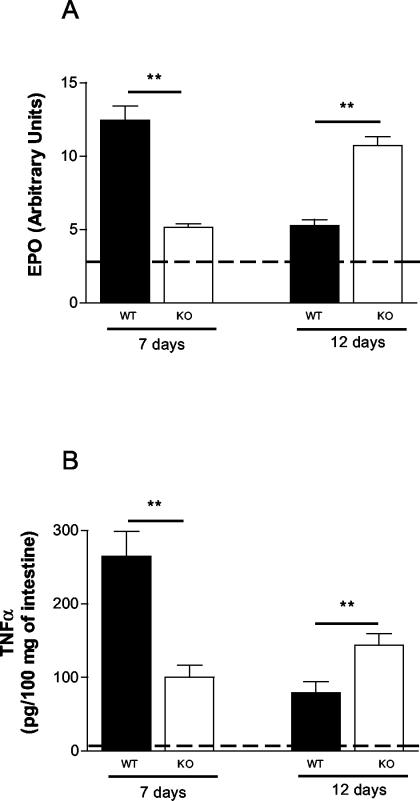
With the intent of quantifying the inflammatory response in the small intestine, we assessed eosinophil infiltration indirectly by measuring the activity of EPO (Fig. 4A) and the TNF-α concentrations by ELISA in tissue homogenates (Fig. 4B). As shown in Fig. 4, the levels of EPO and TNF-α were significantly lower in PAFR−/− infected mice than in infected wild-type controls at 7 days after infection. Interestingly, both values were greater for PAFR−/− infected mice than for wild-type controls at 12 days after infection (Fig. 4).
FIG. 4.
Levels of EPO (A) and TNF-α (B) in small intestine homogenates 7 and 12 days after S. venezuelensis infection of wild-type (WT) and PAFR−/− mice. EPO activity was measured by a colorimetric assay, and the TNF-α level was estimated by an ELISA, as described in Materials and Methods. The dashed lines indicate the background levels of EPO and TNF-α in wild-type mice. The background levels in PAFR−/− mice were not significantly different from the background levels in wild-type mice and are not shown for clarity. The bars and error bars indicate the means and standard errors of the means, respectively, for four mice. Two asterisks indicate that the P value is <0.01 for a comparison of wild-type and PAFR−/− mice. KO, PAFR−/− mice.
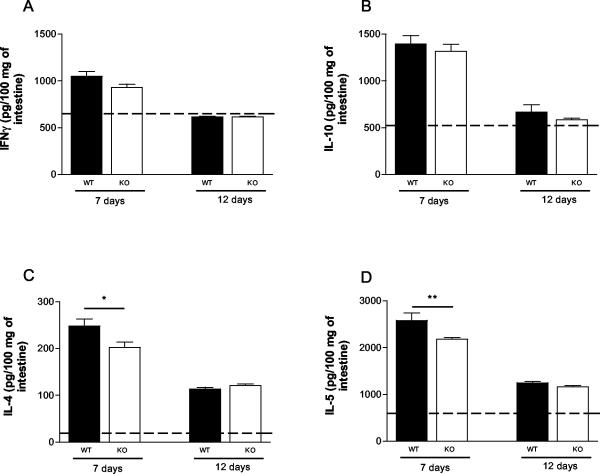
S. venezuelensis-infected PAFR−/− mice expressed lower levels of Th2 cytokines.
Cytokine analysis of homogenates of the small intestine showed that there was a discrete increase in the concentration of IFN-γ 7 days after infection, but the concentration returned to the normal level 12 days after S. venezuelensis infection in both PAFR−/− and wild-type mice (Fig. 5A). The concentrations of IL-10 exhibited similar kinetics; i.e., there was a threefold increase above the levels found in noninfected animals at day 7, and background concentrations were observed at day 12 after infection (Fig. 5B). The concentrations of the Th2 cytokines IL-4 and IL-5 exhibited the greatest increases above the values found in noninfected animals. Moreover, elevated concentrations of both IL-4 and IL-5 were found even after 12 days of infection (Fig. 5C and D). At day 7 after infection, the concentrations of IL-4 and IL-5 were significantly lower in PAFR−/− mice than in the small intestines of the wild-type controls (Fig. 5C and D).
FIG. 5.
Levels of IFN-γ (A), IL-10 (B), IL-4 (C), and IL-5 (D) in small intestine homogenates 7 and 12 days after S. venezuelensis infection of wild-type (WT) and PAFR−/− mice. Cytokine levels were measured by ELISA, as described in Materials and Methods. The dashed lines indicate the background levels of cytokines in wild-type mice. The background levels in PAFR−/− mice were not significantly different from the background levels in wild-type mice and are not shown for clarity. The bars and error bars indicate the means and standard errors of the means, respectively, for four mice. One asterisk and two asterisks indicate that the P values are <0.05 and <0.01, respectively, for a comparison of wild-type and PAFR−/− mice. KO, PAFR−/− mice.
Cytokine concentrations were also measured in supernatants of spleen and MLN cells collected from infected mice and restimulated in vitro with L3 antigen. Similar to the concentrations of cytokines in the intestine, there were lower concentrations of cytokines in the supernatants of spleen and MLN cell cultures from PAFR−/− mice infected for 7 days than in the supernatants of spleen and MLN cultures from the wild-type controls (Table 1). Specifically, the concentrations of IL-5 and IFN-γ in supernatants of spleen cell cultures and the concentrations of IL-4, IL-5, and IFN-γ in supernatants of MLN cell cultures were significantly lower for PAFR−/− mice than for wild-type mice (Table 1). Cytokine levels were reduced after 12 days of infection in both PAFR−/− and wild-type mice (data not shown). Stimulation with ConA resulted in greater and more persistent production of IL-4 and IL-5 in both spleen and MLN cells collected from infected mice. ConA-induced IFN-γ production by spleen and MLN cells from normal animals was not different than ConA-induced IFN-γ production by spleen and MLN cells from infected animals (data not shown). Moreover, there were no significant differences between the ConA-stimulated cytokine levels in PAFR−/− and wild-type mice (data not shown).
TABLE 1.
Concentrations of cytokines in supernatants of spleen and MLN cells stimulated with S. venezuelensis L3 antigen
| Cytokine | Concn (pg/ml of supernatant) ina:
|
|||
|---|---|---|---|---|
| Spleen cells
|
MLN cells
|
|||
| Wild-type mice | PAFR−/− mice | Wild-type mice | PAFR−/− mice | |
| IL-4 | 22.9 ± 3.2 | 6.7 ± 6.6b | 26.0 ± 9.5 | 23.8 ± 19.4 |
| IL-5 | 372 ± 50 | 173 ± 89b | 285 ± 97 | 99 ± 57b |
| IL-10 | 5,074 ± 740 | 4,876 ± 232 | 966 ± 268 | 71 ± 66b |
| IFN-γ | 1,880 ± 356 | 509 ± 226b | 1,291 ± 393 | 1,087 ± 476 |
Spleens and MLN were obtained 7 days after infection with S. venezuelensis. The values are means ± standard errors of the means for five mice in each group. The experiment was repeated twice, and similar results were obtained.
Value is significantly different (P < 0.05) from the value for wild-type mice.
DISCUSSION
PAF is a potent phospholipid mediator that has been shown to mediate a broad spectrum of biological activities, including leukocyte recruitment and activation, airway constriction, and vascular hyperpermeability, through its G-protein-coupled receptor (21). In addition, several experiments have linked PAF to allergic processes (25; reviewed in reference 21). It has been demonstrated previously that S. venezuelensis infection induces eosinophilic lung inflammation that is accompanied by airway hyperreactivity in rats (37, 50). A similar inflammatory reaction is observed in the small intestine of Strongyloides-infected mice (15, 24, 32). The participation of PAF or the PAFR during gastrointestinal nematode infection has been poorly documented; Nippostrongylus brasiliensis-infected rats showed elevated levels of PAF, systemically and in the intestinal lumen, only during the process of elimination of worms (34). Moreover, the adult worms secreted an acetylhydrolase that functionally inhibited PAF activity (6, 19). PAF has also been associated with the development of dilated cardiomyopathy observed in chronic Trichinella spiralis-infected rats (42).
In the present work, it was demonstrated that S. venezuelensis larval migration and intestinal establishment were not affected by the absence of the PAFR, at least during the primary parasite infection. However, PAFR−/− infected mice exhibited delayed elimination of adult worms, and there was decreased worm fecundity compared to that in the infected wild-type controls. The delay in elimination of adult worms coincided with lower leukocyte recruitment and activation, as detected by the decreased number of eosinophils and goblet cells in tissue and diminished cytokine production, respectively. Specifically, there was a reduction in the production of Th2 cytokines (IL-4, IL-5, and IL-10) in the small intestine tissue and by specific antigen-stimulated spleen or MLN cells. Interestingly, the IL-4 level was not reduced significantly in MLN cells, suggesting that IL-4 could be regulated independent of IL-5 and IL-10 in this compartment.
The induction of a predominant Th2 immune response, characterized by production of the IL-4, IL-5, IL-9, IL-10, and IL-13 cytokines (1), is associated with protective immunity against gastrointestinal nematodes in experimental models of infection by Trichuris muris, Heligmosomoides polygyrus, N. brasiliensis, and T. spiralis (5, 16, 17, 18, 28). In Strongyloides-infected mice the relevance of IL-4-mediated responses for elimination of worms has not been directly confirmed (54). However, there is evidence in humans infected by S. stercoralis (38), as well as in experimental models of this infection (24, 27, 29, 30), that a predominant Th2 type of immune response is related to host protection. Increased levels of IL-5 and the consequent eosinophilia have been associated with protection in Strongyloides-infected hosts (10, 15, 29, 41). However, it appeared that activated eosinophils were relevant for the destruction of migrating larvae rather than for elimination of worms (10, 15), suggesting that the observed effects on IL-5 and eosinophils are unlikely to account for the delay in elimination of worms in our system. In contrast, Strongyloides ratti-infected IL-5-deficient mice showed higher fecundity (41). Therefore, although IL-5 and eosinophils may not contribute to elimination of worms, further studies are needed to evaluate their contribution to changes in worm fecundity.
Another aspect of the Th2 immune response induced by Strongyloides infection is the intestinal mastocytosis and goblet cell hyperplasia. It has been demonstrated that IL-4 acting in synergy with IL-13 regulates intestinal mastocytosis (27, 54), and IL-13 has been associated with the control of goblet cell hyperplasia (31) observed during elimination of worms. Moreover, experiments with mast cell-deficient W/WV and nude mice (2, 24, 27, 35) have suggested that there is an association between intestinal mastocytosis and the ability of mice to expel S. venezuelensis and S. ratti adult worms.
Recently, Maruyama et al. (30) suggested that sulfated carbohydrates, including mast cell glycosaminoglycans, inhibit attachment of adult worms and subsequent invasion of S. venezuelensis worms in the intestinal epithelium. Similarly, heavily sulfated mucins from goblet cells of S. venezuelensis-infected hamsters or rats were associated with elimination of worms (23, 48). These studies suggested that production of mucus, especially sulfated mucins, favors elimination of intestinal parasites. PAF, through the activation of its receptor, may function as a mucus secretagogue on airway epithelium and appears to be an important activator of goblet cell secretion during airway inflammation (13, 44). In at least one study the workers described an effect of PAF on intestinal epithelium mucin secretion (51). In our study, goblet cell hyperplasia was prevented in S. venezuelensis-infected PAFR−/− mice, suggesting that PAFR plays a role in mediating mucus production following intestinal helminth infection. Based on the discussion above, it is possible that the diminished goblet cell hyperplasia observed in S. venezuelensis-infected PAFR−/− mice also contributes to the delay in elimination of adult worms.
S. venezuelensis-infected PAFR−/− mice had reduced tissue levels of IL-10 and TNF-α. Interestingly, T. muris-infected IL-10-deficient mice failed to expel the parasite after 20 days of infection and displayed increased morbidity and mortality. This increased susceptibility to the parasite was correlated with increased production of type 1 cytokines (IFN-γ and TNF-α), increased inflammation at the intestinal site, decreased serum eosinophilia, and the absence of goblet cell hyperplasia and mucus production (47). Similarly, T. spiralis-infected TNF-α receptor-deficient mice showed increased enteropathology, but no difference was observed in the kinetics of elimination of worms, suggesting that TNF-α was not associated with the mechanism of nematode expulsion (28).
Altogether, the studies described above suggest that Th2 cytokines and effector mechanisms (mastocytosis and eosinophilia) play a role in mediating resistance against Strongyloides infection in rodent models. PAFR−/− mice infected with S. venezuelensis exhibited decreased production of Th2 cytokines in tissues and after stimulation of immune cells with specific antigen. Although the reduction in cytokine levels in tissue was not marked (around 20 to 30% inhibition), this reduction was sufficient to affect the recruitment of cells and the production of proinflammatory cytokines, such as TNF-α. Thus, it is possible that the observed reduction in the production of Th2 cytokines and the ensuing inflammation accounted for the observed delay in parasite expulsion but did not prevent parasite expulsion. It is not clear at present why a Th2 immune response would be defective or partially suppressed in PAFR−/− mice, as there is no direct evidence which suggests that PAFR activation plays a role in determining Th2 cytokine production. One possible explanation stems from the important role of PAFR in the phagocytosis of particles (4, 53). In the absence of PAFR or when there is a blockade of PAFR, phagocytosis and, consequently, antigen presentation could be impaired. One alternative possibility stems from the work of Shinkai and colleagues (49), who showed that parasites are capable of inducing innate cells, such as eosinophils, to produce Th2 cytokines, such as IL-4. As eosinophil migration is defective early in the course of S. venezuelensis infection, it is also possible that a defective Th2 response is secondary to reduced eosinophil influx in PAFR−/− mice. These possibilities clearly deserve further study.
In PAFR−/− mice infected for 7 days, in which the number of adult worms in the small intestine was similar to the number in wild-type mice infected for 7 days, the number of eggs eliminated and worm fecundity were significantly lower. Lower worm fecundity was accompanied by less intestinal inflammation, as assessed histologically and by TNF-α and EPO levels. Thus, whereas parasite survival was enhanced in PAFR−/− mice, worm fecundity was greatly suppressed. These apparently contradictory results suggest that the effector mechanisms that lead to elimination of adult worms may be different from the mechanisms that are involved in an antifecundity immune response. Indeed, parasites are able to use host-derived signals for growth and developmental control (45). There is evidence which suggests that T cells might be a source of some of these host signals, as severe combined immunodeficiency (SCID), nude, RAG−/−, or T-cell-depleted mice showed reduced Schistosoma mansoni and Schistosoma japonicum growth and fecundity (3, 14, 20, 43). Although the findings are controversial (12), Amiri et al. (3) have shown that TNF-α can enhance the fertility of female schistosome worms. In this way, treatment of S. mansoni-infected SCID mice with TNF-α restored the reproductive capacity of the schistosome. Moreover, it has been shown that inhibition of intestinal mucosal mast cell hyperplasia with anti-stem cell factor was accompanied by a reduction in fecal egg output in N. brasiliensis-infected rats (39). A situation may arise in which decreased intestinal inflammation (as observed in S. venezuelensis-infected PAFR−/− mice or in S. mansoni-infected SCID mice) is associated with enhanced survival of worms but decreased fecundity. On the other hand, the greater host inflammatory response against the worms (as observed in wild-type mice) may result in reduced survival but significantly greater worm fecundity. This is an interesting example of an evolutionary trade-off used by the parasite to enhance its survival ability; i.e., although a predominant Th2 inflammatory response decreases survival of the worms, the worms may use factors produced during this response to facilitate egg output and reproduction (for example, S. mansoni may use TNF-α and N. brasiliensis may use stem cell factor). The factors that might modulate the fecundity of S. venezuelensis remain to be determined.
Overall, our results indicate that the PAFR participates in the cascade of events leading to elimination of S. venezuelensis from the small intestine. Moreover, PAFR-mediated responses appear to modulate host-derived signals that are important for worm fecundity. Host-derived signals necessary for worm fecundity may be good targets for strategies aimed at control of helminth transmission.
Acknowledgments
We are grateful to Satoshi Ishii and Takao Shimizu of the Department of Biochemistry and Molecular Biology, University of Tokyo, Tokyo, Japan, for providing the PAFR−/− mice used in the experiments. We also thank Jeferson do Carmo Bernardes for technical support in all the experiments reported here.
This work was supported by the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (Minas Gerais State Foundation for Scientific Research), by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (National Council for Scientific and Technological Development), and by the Programa de Apoio ao Desenvolvimento Científico e Tecnológico (Program for the Support of Scientific and Technological Development).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature (London) 383:787-793. [DOI] [PubMed] [Google Scholar]
- 2.Abe, T., and Y. Nawa. 1988. Worm expulsion and mucosal mast cell response induced by repetitive IL-3 administration in Strongyloides ratti-infected nude mice. Immunology 63:181-185. [PMC free article] [PubMed] [Google Scholar]
- 3.Amiri, P., R. M. Locksley, T. G. Parslow, M. Sadick, E. Rector, D. Ritter, and J. H. McKerrow. 1992. Tumour necrosis factor alpha restores granulomas and induces parasite egg-laying in schistosome-infected SCID mice. Nature 356:604-607. [DOI] [PubMed] [Google Scholar]
- 4.Au, B. T., M. M. Teixeira, P. D. Collins, and T. J. Williams. 2001. Blockade of PAF receptors controls interleukin-8 production by regulating the activation of neutrophil CD11/CD18. Eur. J. Pharmacol. 425:65-71. [DOI] [PubMed] [Google Scholar]
- 5.Bancroft, A. J., A. N. McKenzie, and R. K. Grencis. 1998. A critical role for IL-13 in resistance to intestinal nematode infection. J. Immunol. 160:3453-3461. [PubMed] [Google Scholar]
- 6.Blackburn., C. C., and M. E. Selkirk. 1992. Inactivation of platelet-activating factor by a putative acetylhydrolase from the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunology 75:41-46. [PMC free article] [PubMed] [Google Scholar]
- 7.Braquet, P., L. Touqui, T. Y. Shen, and B. B. Vargaftig. 1987. Perspectives in platelet-activating factor research. Pharmacol. Rev. 39:97-145. [PubMed] [Google Scholar]
- 8.Brener, Z., and G. Chaia. 1960. Isolamento e manutenção do Strongyloides ratti (Sandground, 1925) em condições de laboratório. Rev. Bras. Biol. 20:447-451. [Google Scholar]
- 9.Bundi, D. A. 1994. Immunoepidemiology of intestinal helminthic infections. 1. The global burden of intestinal nematode disease. Trans. R. Soc. Trop. Med. Hyg. 88:259-261. [DOI] [PubMed] [Google Scholar]
- 10.Cara, D. C., D. Negrão-Corrêa, and M. M Teixeira. 2000. Mechanisms underlying eosinophil trafficking and their relevance in vivo. Histol. Histopathol. 15:899-920. [DOI] [PubMed] [Google Scholar]
- 11.Chan, M.-S. 1997. The global burden of intestinal nematode infections. Fifty years on. Parasitol. Today 13:438-443. [DOI] [PubMed] [Google Scholar]
- 12.Cheever, A. W., R. W. Poindexter, and T. A. Wynn. 1999. Egg laying is delayed but worm fecundity is normal in SCID mice infected with Schistosoma japonicum and S. mansoni with or without recombinant tumor necrosis factor alpha treatment. Infect. Immun. 67:2201-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christie, P. E., and W. R. Henderson, Jr. 2002. Lipid inflammatory mediators: leukotrienes, prostaglandins, platelet-activating factors. Clin. Allergy Immunol. 16:233-254. [PubMed] [Google Scholar]
- 14.Davies, S. J., J. L. Grogan, R. B. Blank, K. C. Lim, R. M. Locksley, and J. H. McKerrow. 2001. Modulation of blood fluke development in the liver by hepatic CD4+ lymphocytes. Science 294:1358-1361. [DOI] [PubMed] [Google Scholar]
- 15.El-Malky, M., H. Maruyama, Y. Hirabayashi, S. Shimada, A. Yoshida, T. Amano, A. Tominaga, K. Takatsu, and N. Ohta. 2003. Intraepithelial infiltration of eosinophils and their contribution to the elimination of adult intestinal nematode Strongyloides venezuelensis in mice. Parasitol. Int. 52:71-79. [DOI] [PubMed] [Google Scholar]
- 16.Else, K. J., F. D. Finkelman, C. R. Maliszewski, and R. K. Grencis. 1994. Cytokine-mediated regulation of chronic intestinal helminth infection. J. Exp. Med. 179:347-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finkelman, F. D., T. Shea-Donohue, J. Goldhill, C. A. Sullivan, S. C. Morris, K. B. Madden, W. C. Gause, and J. F. Urban, Jr. 1997. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu. Rev. Immunol. 15:505-533. [DOI] [PubMed] [Google Scholar]
- 18.Gause, W. C., J. F. Urban, and M. J. Stadecker. 2003. The immune response to parasitic helminths: insights from murine models. Trends Immunol. 24:269-277. [DOI] [PubMed] [Google Scholar]
- 19.Grigg, M. E., K. Gounaris, and M. E. Selkirk. 1996. Characterization of a platelet-activating factor acetylhydrolase secreted by the nematode parasite Nippostrongylus brasiliensis. Biochem. J. 317:541-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison, R. A., and M. J. Doenhoff. 1983. Retarded development of Schistosoma mansoni in immunosuppressed mice. Parasitology 86:429-438. [DOI] [PubMed] [Google Scholar]
- 21.Ishii, S., and T. Shimizu. 2000. Platelet-activating factor (PAF) receptor and genetically engineered PAF receptor mutant mice. Prog. Lipid Res. 39:41-82. [DOI] [PubMed] [Google Scholar]
- 22.Ishii, S., T. Kuwaki, T. Nagase, K. Maki, F. Tashiro, S. Sunaga, W. H. Cao, K. Kume, Y. Fukuchi, K. Ikuta, J. Miyazaki, M. Kumada, and T. Shimizu. 1998. Impaired anaphylactic responses with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J. Exp. Med. 187:1779-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishikawa, N., B. B. Shi, A. I. Khan, and Y. Nawa. 1995. Reserpine-induced sulphomucin production by goblet cells in the jejunum of rats and its significance in the establishment of intestinal helminths. Parasite Immunol. 17:581-586. [DOI] [PubMed] [Google Scholar]
- 24.Khan, A. I., Y. Horii, Y. Tiuria, Y. Sato, and Y. Nawa. 1993. Mucosal mast cells and the expulsive mechanisms of mice against Strongyloides venezuelensis. Int. J. Parasitol. 23:551-555. [DOI] [PubMed] [Google Scholar]
- 25.Klein, A., V. Pinho, A. L. Alessandrini, T. Shimizu, S. Ishii, and M. M. Teixeira. 2002. Platelet-activating factor drives eotaxin production in an allergic pleurisy in mice. Br. J. Pharmacol. 135:1213-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korenaga, M., Y. Hitoshi, N. Yamaguchi, Y. Sato, K. Takatsu, and I. Tada. 1991. The role of interleukin-5 in protective immunity to Strongyloides venezuelensis infection in mice. Immunology 72:502-507. [PMC free article] [PubMed] [Google Scholar]
- 27.Lantz, C. S., J. Boesiger, C. H. Song, N. Mach, T. Kobayashi, R. C. Mulligan, Y. Nawa, G. Dranoff, and S. J. Galli. 1998. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature 392:90-93. [DOI] [PubMed] [Google Scholar]
- 28.Lawrence, C. E., J. C. M. Paterson, L. M. Higgins, T. T. MacDonald, M. W. Kennedy, and P. Garside. 1998. IL-4-regulated enteropathy in an intestinal nematode infection. Eur. J. Immunol. 28:2672-2684. [DOI] [PubMed] [Google Scholar]
- 29.Maruyama, H., Y. Osada, A. Yoshida, M. Futakuchi, H. Kawaguchi, R. Zhang, J. Fu, T. Shirai, S. Kojima, and N. Ohta. 2000. Protective mechanisms against the intestinal nematode Strongyloides venezuelensis in Schistosoma japonicum-infected mice. Parasite Immunol. 22:279-286. [DOI] [PubMed] [Google Scholar]
- 30.Maruyama, H., Y. Yabu, A. Yoshida, Y. Nawa, and N. Ohta. 2000. A role of mast cell glycosaminoglycans for the immunological expulsion of intestinal nematode, Strongyloides venezuelensis. J. Immunol. 164:3749-3754. [DOI] [PubMed] [Google Scholar]
- 31.McKenzie, G. J., A. Bancroft, R. K. Grencis, and A. N. McKenzie. 1998. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr. Biol. 8:339-342. [DOI] [PubMed] [Google Scholar]
- 32.Mimori, T., Y. Nawa, M. Korenaga, and I. Tada. 1982. Strongyloides ratti: mast cell and goblet cell responses in the small intestine of infected rats. Exp. Parasitol. 54:366-370. [DOI] [PubMed] [Google Scholar]
- 33.Montrucchio, G., G. Alloatti, and G. Camussi. 2000. Role of platelet-activating factor in vascular cardiovascular pathophysiology. Physiol. Rev. 80:1669-1699. [DOI] [PubMed] [Google Scholar]
- 34.Moqbel, R., A. J. MacDonald, A. B. Kay, and H. R. P. Miller. 1989. Platelet activating factor (PAF) release during intestinal anaphylaxis in rats. FASEB J. 3:A1337. [Google Scholar]
- 35.Nawa, Y., M. Kiyota, M. Korenaga, and M. Kotani. 1985. Defective protective capacity of W/Wv mice against Strongyloides ratti infection and its reconstitution with bone marrow cells. Parasite Immunol. 7:429-438. [DOI] [PubMed] [Google Scholar]
- 36.Negrão-Corrêa, D. 2001. Importance of immunoglobulin E (IgE) in the protective mechanism against gastrointestinal nematode infection: looking at the intestinal mucosae. Rev. Inst. Med. Trop. Sao Paulo 43:291-299. [DOI] [PubMed] [Google Scholar]
- 37.Negrão-Corrêa, D., M. R. Silveira, C. M. Borges, D. G. Souza, and M. M. Teixeira. 2003. Changes in pulmonary function and parasite burden in rats infected with Strongyloides venezuelensis concomitant with induction of allergic airway inflammation. Infect. Immun. 71:2607-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neva, F. A., J. Oliveira Filho, A. A. Gam, R. Thompson, V. Freitas, A. Melo, and E. M. Carvalho. 1998. Interferon-γ and interleukin-4 responses in relation to serum IgE levels in persons infected with human T lymphotropic virus type I and Strongyloides stercoralis. J. Infect. Dis. 178:1856-1859. [DOI] [PubMed] [Google Scholar]
- 39.Newlands, G. F. J., H. R. P. Miller, A. Mackeller, and S. J. Galli. 1995. Stem cell factor contributes to intestinal mucosal mast cell hyperplasia in rats infected with Nippostrongylus brasiliensis or Trichinella spiralis, but anti-stem cell factor treatment decreases parasite egg production during N. brasiliensis infection. Blood 86:1968-1976. [PubMed] [Google Scholar]
- 40.Onah, D. N., F. Uchiyama, Y. Nagakui, M. Ono, T. Takai, and Y. Nawa. 2000. Mucosal defense against gastrointestinal nematodes: responses of mucosal mast cells and mouse mast cell protease 1 during primary Strongyloides venezuelensis infection in FcRγ-knockout mice. Infect. Immun. 68:4968-4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ovington, K. S., K. McKie, K. I. Matthaei, I. G. Young, and C. A. Behm. 1998. Regulation of primary Strongyloides ratti infections in mice: a role for interleukin-5. Immunology 95:488-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paolocci, N., M. Sironi, M. Bettini, G. Batoli, S. Michalak, C. Bandi, F. Magni, and F. Bruschi. 1998. Immunopathological mechanisms underlying the time-course of Trichinella spiralis cardiomyopathy in rats. Virchows Arch. 432:261-266. [DOI] [PubMed] [Google Scholar]
- 43.Ridi, R. E., T. Ozaki, T. Inabi, M. Ito, and H. Kamiya. 1997. Schistosoma mansoni oviposition in vitro reflects worm fecundity in vivo: individual-, parasite age- and host-dependent variations. Int. J. Parasitol. 27:381-387. [DOI] [PubMed] [Google Scholar]
- 44.Rieves, R. D., J. Goff, T. Wu, P. Larivee, C. Logun, and J. H. Shelhamer. 1992. Airway epithelial cell mucin release: immunologic quantitation and response to platelet-activating factor. Am. J. Respir. Cell. Mol. Biol. 6:158-167. [DOI] [PubMed] [Google Scholar]
- 45.Salzet, M., A. Capron, and G. B. Stefano. 2000. Molecular crosstalk in host-parasite relationships: schistosome- and leech-host interactions. Parasitol. Today 16:536-540. [DOI] [PubMed] [Google Scholar]
- 46.Sato, Y., and H. Toma. 1990. Strongyloides venezuelensis infections in mice. Int. J. Parasitol. 20:57-62. [DOI] [PubMed] [Google Scholar]
- 47.Schopf, L. R., K. F. Hoffmann, A. W. Cheever, J. F. Urban, Jr., and T. A. Wynn. 2002. IL-10 is critical for host resistance and survival during gastrointestinal helminth infection. J. Immunol. 168:2383-2892. [DOI] [PubMed] [Google Scholar]
- 48.Shi, B. B., N. Ishikawa, H. Itoh, H. Ide, K. Tsuchiya, Y. Horii, F. Uchiyama, and Y. Nawa. 1994. Goblet cell mucins of four genera of the subfamily Cricetinae with reference to the protective activity against Strongyloides venezuelensis. Parasite Immunol. 16:553-559. [DOI] [PubMed] [Google Scholar]
- 49.Shinkai, K., M. Mohrs, and R. M. Locksley. 2002. Helper T cells regulate type-2 innate immunity in vivo. Nature 420:825-829. [DOI] [PubMed] [Google Scholar]
- 50.Silveira, M. R., K. P. Nunes, D. C. Cara, D. G. Souza, A. Corrêa, Jr., M. M. Teixeira, and D. Negrão-Corrêa. 2002. Infection with Strongyloides venezuelensis induces transient airway eosinophilic inflammation, an increase in immunoglobulin E, and hyperresponsiveness in rats. Infect. Immun. 70:6263-6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sperber, K., J. Shim, M. Mehra, A. Lin, I. George, S. Ogata, L. Mayer, and S. Itzkowitz. 1998. Mucin secretion in inflammatory bowel disease: comparison of a macrophage-derived secretagogue (MMS-68) to conventional secretagogues. Inflamm. Bowel Dis. 4:12-17. [DOI] [PubMed] [Google Scholar]
- 52.Takamure, A. 1995. Migration route of Strongyloides venezuelensis in rodents. Int. J. Parasitol. 25:907-911. [DOI] [PubMed] [Google Scholar]
- 53.Talvani, A., F. S. Machado, G. C. Santana, A. Klein, L. Barcelos, J. S. Silva, and M. M. Teixeira. 2002. Leukotriene B(4) induces nitric oxide synthesis in Trypanosoma cruzi-infected murine macrophages and mediates resistance to infection. Infect. Immun. 70:4247-4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watanabe, K., S. Hamano, S. Yada, K. Noda, K. Kishihara, K. Nomoto, and I. Tada. 2001. The effect of interleukin-4 on the induction of intestinal mast cells and chronological cytokine profiles during intestinal nematode Strongyloides ratti infection. Parasitol. Res. 87:149-154. [DOI] [PubMed] [Google Scholar]
- 55.Wertheim, G. 1970. Growth and development of Strongyloides venezuelensis Brumpt, 1934 in the albino rat. Parasitology 61:381-388. [DOI] [PubMed] [Google Scholar]