Abstract
Female Culex nigripalpus were fed blood containing a low dose (6.3±0.01 logs plaque-forming units (PFU)/mL) or high dose (7.3±0.1 logs PFU/mL) of West Nile virus (WNV) and maintained at 28°C for incubation periods (IPs) of 6 or 12 days. Vector competence was measured using rates of infection (% with WNV-positive bodies), dissemination (% infected with WNV-positive legs), and transmission (% infected with WNV-positive saliva). Infection rates were not influenced by dose or IP. Dissemination rates were significantly higher at the high dose, and this was dependent on IP. Despite 100% infection and 90% dissemination in the most permissive treatment of high dose and 12 days, only 11% transmission was observed. Virus titers in body and leg tissues were significantly lower at the low dose and the titers were not influenced by IP. We show that not all mosquitoes with infections and/or disseminated infections transmit WNV under the conditions of this test. Therefore, characterizing the transmission ability of a vector population using infection or dissemination as indicators of transmission may provide inaccurate information. The complex relationships between infection, dissemination, and transmission must be evaluated under a variety of biological and environmental conditions to begin to assess the epidemiological risk of natural mosquito populations.
Key Words: Culex, vector competence, West Nile virus transmission
Introduction
West Nile virus (WNV; family Flaviviridae: genus Flavivirus) is maintained in an enzootic transmission cycle between birds and Culex spp. mosquitoes. Culex nigripalpus Theobald is a subtropical mosquito distributed throughout the southern United States, the Caribbean, and Central and South America (Day 2004, Darsie and Ward 2005). This species is found throughout Florida and is most abundant during summer and fall (Mitchell et al. 1980, O'Meara and Evans 1983, Day and Curtis 1989, Zyzak et al. 2002). Studies showing WNV-positive field-caught Cx. nigripalpus support its likely involvement in this arbovirus transmission cycle (Blackmore et al. 2003, Rutledge et al. 2003, Godsey et al. 2005, Vitek et al. 2008). Cx. nigripalpus was shown to transmit WNV to sentinel chickens in nature in Florida (Rutledge et al. 2003). Cx. nigripalpus is also an efficient laboratory vector of WNV (Sardelis et al. 2001, Mores et al. 2007) and a related flavivirus, St. Louis encephalitis virus (Sudia and Chamberlain 1964, Dow et al. 1964). Cx. nigripalpus shows an early- to late-season feeding shift from ornithophilic to opportunistic (Edman and Taylor 1968, Tempelis 1975). Hence, it is likely an early-season amplification vector, cycling the virus in enzootic avian hosts, and late-season bridge vector, spreading infections from avian to mammalian hosts in the epidemic cycle (Turell et al. 2005).
Vector competence of Cx. nigripalpus for a WNV strain collected in New York in 1999 (NY99) has been described (Sardelis et al. 2001). For WNV doses from 4.6 to 6.8 logs plaque-forming units (PFU)/mL, Cx. nigripalpus showed 29%–84% infection, 0%–12% dissemination, and 0%–10% transmission rates (Sardelis et al. 2001). However, studies describing vector competence for Florida WNV strains are lacking. Investigations of vector–virus interactions with regard to infection barriers are important to understanding vector competence. Mosquito midgut infection (MIB) and escape (MEB) barriers must be overcome by the virus in order for possible entry into the salivary glands. The virus must then overcome salivary gland infection and escape barriers to permit subsequent transmission to a host (Hardy et al. 1983). The current study investigates the effects of virus dose and incubation period (IP) on the vector competence of Cx. nigripalpus for a Florida WNV strain (WN-FL03) collected in 2003.
Materials and Methods
Mosquitoes and virus
Eleven- to 12-day-old Cx. nigripalpus females from a colony established from wild mosquitoes collected from Alachua County, FL, in 1995 (generation=F112) reared under standard conditions (Knight and Nayar 1999) and maintained under a 14:10 (light:dark) cycle were utilized for this study. These conditions were developed to produce adults of similar size and larval experience, to reduce potential environmental effects on vector competence. Adult mosquitoes were housed in 0.5 L cardboard cages with mesh screening on top and provided 10% sucrose and water ad libitum.
We used WNV strain WN-FL03 (Genbank accession number DQ983578), originally isolated from a pool of Cx. nigripalpus collected in Indian River County, FL, in 2003, passaged five times in African green monkey kidney (Vero) cells and once in baby hamster kidney cells.
Mosquito infection
Seventy-two hours before use, 100 Cx. nigripalpus females were transferred to each of 40 0.5 L cardboard cages, provided 10% sugar solution and water ad libitum, and housed in incubators at 28°C and 90%–100% humidity. These environmental conditions and acclimatization period have been shown to increase the membrane feeding rate of the Cx. nigripalpus from this colony (unpublished data). Mosquitoes were deprived of sugar for 48 h and water for 24 h before being allowed to obtain blood through a hog gut membrane (The Sausage Maker, Inc., Buffalo, NY) affixed to the base of water-jacketed glass membrane feeders (Lillie Glassblowers, Smyrna, GA). Mosquitoes were allowed to feed for 45 min on 3 mL of warm (37°C) defibrinated bovine blood (Hemostat, Dixon, CA) containing either a low or high WNV dose. The low dose was created using a 1:100 dilution of freshly propagated virus + media:blood, whereas the high dose was a 1:10 dilution. Subsequent to feeding, mosquitoes were immobilized with cold and fully engorged mosquitoes were transferred to 0.5 L cardboard cages (∼20 mosquitoes/cage) with mesh screening, placed in incubators, provided 10% sugar solution and water ad libitum, and held for IPs of 6 or 12 days at 28°C and 90%–100% humidity. Unfed or partially engorged mosquitoes were discarded.
Mosquito processing
At each IP and for each dose, ≥15 surviving mosquitoes were removed from cages. At the 6-day IP, 15 mosquitoes were removed randomly across cages, whereas at the 12-day IP all remaining surviving mosquitoes were removed from cages, thereby ensuring ≥15 mosquitoes per treatment. For each mosquito, legs were detached and transferred to individual sample tubes containing 1.0 mL BA-1 diluent (Lanciotti et al. 2000) with two 4.5 mm zinc-plated beads. Wings were removed and discarded to limit mosquito mobility during salivation. Saliva was collected using standard conditions as previously described (Hurlbut 1966, Anderson et al. 2010) so that live mosquitoes were allowed to salivate for 45 min into capillary tubes containing immersion oil. Bodies and saliva were then transferred to separate tubes labeled for each individual mosquito. All sample tubes contained 1.0 mL BA-1 diluent with two 4.5 mm zinc-plated beads. Thus, each mosquito had three tubes corresponding to bodies, legs, and saliva.
Virus assay
All samples (i.e., bodies, legs, and saliva) were homogenized at 25 Hz for 3 min (TissueLyser; Qiagen, Inc., Valencia, CA), centrifuged at 4°C and 3148 g for 4 min, and nucleic acids extracted as previously described (Richards et al. 2009). The amount of viral RNA in each sample was determined using quantitative real-time TaqMan reverse transcriptase–polymerase chain reaction using a previously established program (Richards et al. 2007).
The infection rate was defined as the percentage of all mosquitoes tested having infected bodies. The dissemination rate was the percentage of mosquitoes with infected bodies that also had infected legs. The transmission rate was the percentage of mosquitoes with infected bodies that also had infected saliva. The calculation of both dissemination and transmission rates as a function of infection rate allowed for comparisons between rates.
Post hoc power analyses were used to determine the power we had to detect vector competence differences at small (w=0.1), medium (w=0.3), and large (w=0.5) effect sizes (Faul et al. 2007). The effect size is a measure of the distance between the null hypothesis and the hypothesis under investigation, and the power to detect different effect sizes is strongly related to sample size (Faul et al. 2007). The power analyses were based on analyses that determined differences in infection and dissemination rates between doses at each IP and indicate our ability to differentiate between hypotheses given the sample sizes achieved with limited feeding success.
Statistical analysis
All statistical analyses were completed using SAS (SAS Institute 2002) with the exception of power analyses that were conducted using G*Power (Faul et al. 2007). Individual mosquitoes were treated as the experimental units. χ2 tests were used to detect significant differences (p<0.05) in infection, dissemination, and transmission rates between virus doses at each IP. χ2 tests were also used to detect significant differences (p<0.05) in these rates between IPs for each virus dose. Virus titers of blood meals, bodies, legs, and saliva were log-transformed [log (x +1)] to improve normality before analysis. Analyses of variance (ANOVA, PROC GLM) using SAS determined significant (p<0.05) differences in blood meal titers between doses, as well as differences in the titers in bodies, legs, and saliva between IPs, doses, and the IP–dose interaction. If significant differences were observed, then a Duncan multiple comparison test was used to determine which means were significantly different.
Results
Feeding rate
Although 4000 female mosquitoes were exposed to infectious blood meals, only ∼5% of these mosquitoes fed to full engorgement and we observed roughly a 38% total mortality rate over the 12-day IP. These factors resulted in a total sample size of 77 engorged mosquitoes at the end of the IP, out of the total 4000.
Virus titer of blood meal
Mosquitoes were fed blood meals containing significantly different virus titers (mean±standard error) of either 6.3±0.01 (low dose) or 7.3±0.1 (high dose) logs PFU WNV/mL.
Effects of virus dose and IP on vector competence
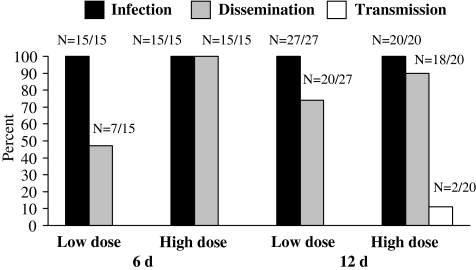
Infection, dissemination, and transmission rates are shown in Figure 1. Transmission was only observed in one treatment group under the most permissive conditions of high dose and 12 days, so statistical comparisons to other treatments could not be made for this variable. Infection rates were 100% in all treatments, and therefore were not affected by the dose or IP used here. Dissemination rates were greater in the high dose, but this difference was only significant at 6 days (6 days: χ2=10.94, df=1, p=0.001; 12 days: χ2=1.88, df=1, p=0.170). Dissemination rates were not significantly different between IPs, regardless of dose (low dose: χ2=3.15, df=1, p=0.076; high dose: χ2=1.59, df=1, p=0.207). Despite 100% infection and 90% dissemination in the most permissive treatment, only 11% transmission was observed.
FIG. 1.
Rates of WNV infection, dissemination, and transmission in Culex nigripalpus at 6 and 12 days postinfection at 28°C.
Virus titers of bodies, legs, and saliva are shown in Table 1. Virus titers in body and leg tissues were significantly affected by dose, but not by IP (Table 2). The significant IP–dose interaction shows that differences between IPs were affected by dose. The treatment group of low dose and 6 days had significantly lower titers than other treatments (Table 1).
Table 1.
The Mean Titers (Logs Plaque-Forming Units West Nile Virus /mL)±Standard Error for Culex nigripalpus Fed a Low or High Virus Dose and Held at 28°C for 6 or 12 Days Postinfection
| |
|
Body titer |
Leg titer |
Saliva titer |
|---|---|---|---|---|
| Incubation period (days) | No. tested | Low dose | ||
| 6 | 15 | 5.6±0.1b | 3.2±0.4b | — |
| 12 | 27 | 6.0±0.1a | 3.9±0.1a | — |
Treatment groups with the same superscript letter in each column are not significantly different by means comparisons.
Table 2.
Analysis of Variance Showing Differences in the Mean Titers of Bodies and Legs Between Doses and Incubation Periods
| |
df (num., denom.) |
F |
p |
|---|---|---|---|
| Variable | Body titer | ||
| IP | 1, 73 | 2.07 | 0.155 |
| Dose | 1, 73 | 18.85 | <0.0001 |
| IP×dose | 3, 73 | 6.94 | 0.0004 |
| Leg titer | |||
|---|---|---|---|
| IP | 1, 56 | 0.01 | 0.924 |
| Dose | 1, 56 | 4.38 | 0.041 |
| IP×dose | 3, 56 | 2.94 | 0.041 |
Significant P-values are in bold.
num, numerator; denom, denominator; IP, incubation period.
Our sample sizes for infection and dissemination rates were higher at the 12-day compared to 6-day IP. Post hoc power analyses for the 6-day IP showed we were able to detect large differences (w=0.5) in infection rates and dissemination rates with 78% and 65% power, respectively. Power analyses for the 12-day IP showed that we could detect large differences in infection and dissemination rates with 93% and 87% power, respectively. For both treatments, the power to detect medium (w=0.3) or small (w=0.1) differences in rates was low (≤54%). Power analyses were not conducted for the transmission rate as transmission was only observed in one treatment group.
Discussion
Under the conditions of this test, Cx. nigripalpus did not exhibit an MIB (body infection). However, body titer was influenced by dose and the IP–dose interaction, showing that differences between IPs were affected by the dose. The MEB (leg infection) and leg titer were also influenced by dose and the IP–dose interaction. At the low dose, virions took longer to replicate in the bodies and legs, whereas titers in mosquitoes fed the high dose appear to plateau more quickly. This demonstrates the importance of studying the interactive effects between factors when evaluating vector competence. Previously, we have shown the complex nature of biological and environmental interactions that contribute to vector competence and that the degree of these interactions changes from population to population (Richards et al. 2009, 2010). The low transmission rate observed in the current study suggests that Cx. nigripalpus exhibits a salivary gland infection and/or escape barrier. This has been observed in field investigations showing that WNV infection rates of pooled Florida Cx. nigripalpus do not always reflect transmission rates to sentinel chickens (Rutledge et al. 2003, Vitek et al. 2008). However, in the previous studies, the failure to observe transmission in infected mosquitoes was likely influenced by the variation in IPs of the field-collected mosquitoes. In the current study, all mosquitoes had the same IP and disseminated infection did not always lead to transmission. While we cannot extrapolate the results of these laboratory studies to specific field populations, these results demonstrate that relationships between infection, dissemination, and transmission are complex. This must be considered when estimating the importance of mosquitoes to WNV transmission cycles in nature.
The infection and dissemination rates we observed in Cx. nigripalpus after a 12-day IP at 28°C (WN-FL03 strain) for both doses are higher than others have observed for the NY99 WNV strain. With 6.3–6.7 logs PFU WNV/mL (NY99 strain), 14-day IP at 26°C, Mores et al. (2007) showed 81% infection and 33% dissemination. At higher doses (6.9–7.4 logs PFU WNV/mL), infection was 95% and dissemination was 38% (Mores et al. 2007) and was still lower than we observed. Another study with Cx. nigripalpus utilizing a dose of 6.8 logs PFU/mL (NY99 strain) showed 84% infection and 12% dissemination with a 12–14-day IP at 26°C (Sardelis et al. 2001). Further, the capillary tube transmission rates observed here (11%) are higher than observed by others (0%) that used Cx. nigripalpus and the NY99 strain of WNV (Mores et al. 2007). Even though the WN-FL03 strain is similar to the NY99 strain (Chisenhall and Mores 2009), it is likely that genetic variation in virus strains and mosquito populations alter virus–mosquito interactions, and consequently variation in vector competence. Our observations may also be due to the higher extrinsic incubation temperature we used, which is known to significantly increase infection, dissemination, or transmission (Kilpatrick et al. 2008, Richards et al. 2010). Others have reported increased vector competence in Cx. pipiens pipiens and Cx. tarsalis with the WN02 strain compared to NY99 strain (Moudy et al. 2007). Further studies should be conducted evaluating the vector competence of Cx. nigripalpus from different wild populations for different WNV strains.
The infection and dissemination rates we observed for Cx. nigripalpus at the low dose are also higher than rates observed in two colonies of Culex pipiens quinquefasciatus fed a similar dose (ranges 31%–98% and 27%–55%, respectively, fed 6.0 logs PFU WNV/mL WN-FL03 strain; IP 13 days at 28°C) (Richards et al. 2010). This shows a greater MIB and MEB in the two Cx. p. quinquefasciatus colonies compared to our Cx. nigripalpus colony under these conditions. Transmission rates for Cx. nigripalpus at the high dose are lower than observed rates (29%) for Cx. p. quinquefasciatus (fed 6.8 logs PFU WNV/mL of WN-FL03; IP 14 days at 28°C) (Richards et al. unpublished). Previous studies showed that vector competence can change due to interactions occurring under different biological (mosquito age and population) and environmental (extrinsic incubation temperature and virus dose) conditions in complex nonlinear ways (Richards et al. 2009, 2010). In-depth studies are needed to evaluate these complex interactive effects. Studies are also needed to evaluate how vector–virus interactions between different populations of Cx. nigripalpus and other WNV strains influence vector competence. We show only one example using a single colony of how environmental variation can affect vector–virus interactions at different points during the extrinsic IP. This needs to be studied further using different field populations to determine the impact of various environmental factors on the role of different natural populations of Cx. nigripalpus in WNV epidemiological cycles.
Large sample sizes are important to distinguish differences between treatment groups in vector competence studies (Lord et al. 2006, Richards et al. 2009, 2010). However, some mosquito species or populations (including the Cx. nigripalpus colony used here) exhibit low feeding and/or survival rates in laboratory experiments. Hence, it is often difficult to obtain the sample sizes required to provide sufficient power of analyses. These factors must be considered when designing experiments because some species may be prone to producing mosquito populations, which lead to low sample sizes, thereby affecting the ability to interpret the results. We showed that our sample sizes were appropriate for distinguishing large differences with 65%–93% power, depending on the IP. However, we did not have sufficient power to differentiate smaller effect sizes. The high infection rates, low transmission rates, and limited feeding success restricted our analysis of some factors. Future studies interested in evaluating additional factors and/or levels of factors must consider the tradeoff in sample sizes with regard to power.
We have shown that vector competence for virus transmission is influenced by a host of factors that interact with one another in complex nonlinear ways such that any assessment of vector competence ultimately requires characterization at multiple levels to develop even a basic understanding of the potential influence on vector-borne disease epidemiology (Richards et al. 2009, 2010). Even with low sample sizes and not being able to analyze some factors, interactions occur in Cx. nigripalpus as well as Cx. p. quinquefasciatus. We are only beginning to address the complexity of the factors that influence vector competence. These factors must be considered further when assessing vector importance in transmission cycles in natural field environments.
Acknowledgments
We are thankful to Sara Ortiz, Heather Robinson, Chelsea Smartt, Tanise Stenn, and Samantha Yost for laboratory assistance, as well as Roxanne Connelly, Carol Thomas, and two anonymous reviewers for critically reviewing earlier drafts of the article. This research was supported by the National Institutes of Health grant AI-42164. A University of Florida Graduate Alumni Award was used to support Sheri Anderson.
Disclosure Statement
No competing financial interests exist.
References
- Anderson SL. Richards SL. Smartt CT. A simple method for examining arbovirus transmission in mosquitoes. J Am Mosq Control Assoc. 2010;26:108–111. doi: 10.2987/09-5935.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore CGM. Stark LM. Jeter WC. Oliveri RL, et al. Surveillance results from the first West Nile virus transmission season in Florida, 2001. Am J Trop Med Hyg. 2003;69:141–150. [PubMed] [Google Scholar]
- Chisenhall DM. Mores CN. Diversification of West Nile virus in a subtropical region. Virol J. 2009;6:106. doi: 10.1186/1743-422X-6-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsie RF. Ward RA. Identification and geographic distribution of the mosquitoes of North America, north of Mexico. Florida: University Press of Florida; 2005. [Google Scholar]
- Day JF. The Florida SLE mosquito, Culex nigripalpus Theobald (Insecta: Diptera: Culicidae). Electronic Data Information System: University of Florida/IFAS Extension 2004, ENY-010. http://edis.ifas.ufl.edu/IN136 http://edis.ifas.ufl.edu/IN136
- Day JF. Curtis GA. Influence of rainfall on Culex nigripalpus (Diptera: Culicidae) blood feeding in Indian River County, Florida. Ann Entomol Soc Am. 1989;82:32–37. [Google Scholar]
- Dow RP. Coleman PH. Meadows KE. Work TH. Isolation of St. Louis encephalitis virus from mosquitoes in the Tampa Bay area of Florida during the epidemic of 1962. Am J Trop Med Hyg. 1964;13:462–468. doi: 10.4269/ajtmh.1964.13.462. [DOI] [PubMed] [Google Scholar]
- Edman JD. Taylor DJ. Culex nigripalpus: Seasonal shift in the bird-mammal feeding ratio in a mosquito vector of human encephalitis. Science. 1968;161:67–68. doi: 10.1126/science.161.3836.67. [DOI] [PubMed] [Google Scholar]
- Faul F. Erdfelder E. Lang AG. Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Godsey MS., Jr. Blackmore MS. Panella NA. Burkhalter K, et al. West Nile virus epizootiology in the southeastern United States, 2001. Vector Borne Zoonotic Dis. 2005;5:82–89. doi: 10.1089/vbz.2005.5.82. [DOI] [PubMed] [Google Scholar]
- Hardy JL. Houk EJ. Kramer LD. Reeves WC. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Ann Rev Entomol. 1983;28:229–262. doi: 10.1146/annurev.en.28.010183.001305. [DOI] [PubMed] [Google Scholar]
- Hurlbut HS. Mosquito salivation and virus transmission. Am J Trop Med Hyg. 1966;15:989–993. doi: 10.4269/ajtmh.1966.15.989. [DOI] [PubMed] [Google Scholar]
- Kilpatrick AM. Meola MA. Moudy RM. Kramer LD. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Path. 2008;4:e1000092. doi: 10.1371/journal.ppat.1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight JW. Nayar JK. Colonization of Culex nigripalpus Theobald (Diptera: Culicidae) by stimulation of mating using males of other mosquito species. J Am Mosq Control Assoc. 1999;15:72–73. [PubMed] [Google Scholar]
- Lanciotti RS. Kerst AJ. Nasci RS. Godsey MS. Mitchell CJ. Savage HM. Komar N. Panella NA. Allen BC. Volpe KE. Davis BS. Roehrig JT. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord CC. Rutledge CR. Tabachnick WJ. Relationships between host viremia and vector susceptibility for arboviruses. J Med Entomol. 2006;43:623–630. doi: 10.1603/0022-2585(2006)43[623:rbhvav]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CJ. Monath TP. Sabattini MS. Transmission of St. Louis encephalitis virus from Argentina by mosquitoes of the Culex pipiens (Diptera: Culicidae) complex. J Med Entomol. 1980;17:282–285. doi: 10.1093/jmedent/17.3.282. [DOI] [PubMed] [Google Scholar]
- Mores CN. Turell MJ. Dohm DJ. Blow JA, et al. Experimental transmission of West Nile virus by Cx. nigripalpus from Honduras. Vector Borne Zoonotic Dis. 2007;7:279–284. doi: 10.1089/vbz.2006.0557. [DOI] [PubMed] [Google Scholar]
- Moudy RM. Meola M. A. Morin L. L. Ebel G. D., et al. A newly emergent genotype of West Nile virus is transmitted earlier and more efficiently by Culex mosquitoes. Am J Trop Med Hyg. 2007;77:365–370. [PubMed] [Google Scholar]
- O'Meara G. Evans F. Seasonal patterns of abundance among three species of Culex mosquitoes in a South Florida Wastewater Lagoon. Entomol Soc Amer. 1983;76:130–134. [Google Scholar]
- Richards SL. Lord CC. Pesko KA. Tabachnick WJ. Environmental and biological factors influencing Culex pipiens quinquefasciatus Say (Diptera: Culicidae) vector competence for Saint Louis encephalitis virus. Am J Trop Med Hyg. 2009;81:264–272. [PMC free article] [PubMed] [Google Scholar]
- Richards SL. Lord CC. Pesko KA. Tabachnick WJ. Environmental and biological factors influencing Culex pipiens quinquefasciatus Say (Diptera: Culicidae) vector competence for West Nile virus. Am J Trop Med Hyg. 2010;83:126–134. doi: 10.4269/ajtmh.2010.09-0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards SL. Mores CN. Lord CC. Tabachnick WJ. Impact of extrinsic incubation temperature and virus exposure on vector competence of Culex pipiens quinquefasciatus Say (Diptera: Culicidae) for West Nile virus. Vector Borne Zoonotic Dis. 2007;7:629–636. doi: 10.1089/vbz.2007.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge CR. Day JF. Lord CC. Stark LM, et al. West Nile virus infection rates in Culex nigripalpus (Diptera: Culicidae) do not reflect transmission rates in Florida. J Med Entomol. 2003;40:253–258. doi: 10.1603/0022-2585-40.3.253. [DOI] [PubMed] [Google Scholar]
- Sardelis MR. Turell MJ. Dohm DJ. O'Guinn ML. Vector competence of selected North American Culex and Coquillettidia mosquitoes for West Nile virus. Emerg Infect Dis. 2001;7:1018–1022. doi: 10.3201/eid0706.010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute. computer program, version By. SAS; North Carolina: 2002. SAS/STAT User's guide for personal computers. [Google Scholar]
- Sudia WD. Chamberlain RW. Experimental infection of Culex nigripalpus Theobold with the virus of St. Louis Encephalitis. Am J Trop Med Hyg. 1964;13:469–471. doi: 10.4269/ajtmh.1964.13.469. [DOI] [PubMed] [Google Scholar]
- Tempelis CH. Host-feeding patterns of mosquitoes, with a review of advances in analysis of blood meals by serology. J Med Entomol. 1975;11:635–653. doi: 10.1093/jmedent/11.6.635. [DOI] [PubMed] [Google Scholar]
- Turell MJ. Dohm DJ. Sardelis MR. O'Guinn ML, et al. An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit West Nile Virus. J Med Entomol. 2005;42:57–62. doi: 10.1093/jmedent/42.1.57. [DOI] [PubMed] [Google Scholar]
- Vitek CJ. Richards SL. Mores CN. Day JF, et al. Arbovirus transmission by Culex nigripalpus in Florida, 2005. J Med Entomol. 2008;45:483–493. doi: 10.1603/0022-2585(2008)45[483:atbcni]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyzak MT. Loyless S. Cope M. Wooster M, et al. Seasonal abundance of Culex nigripalpus Theobald and Culex salinarius Coquillet in north Florida, USA. J Vector Ecol. 2002;27:155–162. [PubMed] [Google Scholar]