Abstract
Background
Impairments in physical performance increase sharply with age. Low serum 25-hydroxyvitamin D (25-OHD) levels may be a modifiable risk factor for physical performance decline.
Methods
Five hundred thirty-two participants in the Women's Health Initiative Clinical Trial (WHI CT) were among a 25% randomly selected subsample of women who participated in performance-based measures of physical performance at baseline, year 1, year 3, and year 6. A physical performance summary score was derived from three tests: timed walk, chair-stand, and grip strength. Levels of 25-OHD were measured at baseline. We used the generalized estimating equations (GEE) method to examine repeated measures of physical performance as a function of follow-up time since baseline according to 25-OHD concentration.
Results
In 6 years of follow-up, participants with serum 25OHD ≥75 nmol/L had significantly higher scores for physical performance (β=2.64, 95% confidence interval [CI] 0.90-4.39) compared with the reference category (<35 nmol/L) after adjustment for age, chronic conditions, body mass index (BMI), race/ethnicity, time spent walking outside, trial arm, clinic latitude, and season of blood draw. However, the rate of decline in physical performance did not differ by level of 25OHD.
Conclusions
Higher baseline serum 25-OHD was associated with better physical performance but did not reduce decline in physical performance over the 6-year period.
Introduction
Older women are at high risk of becoming disabled, making decline in physical performance a critical health issue in this population.1 Impairments in physical performance, such as decreased muscle strength and balance, increase sharply with age.2 Declines in physical performance are associated with risks of death, disability, morbidity, and reduced quality of life,3–5 underscoring the importance of identifying modifiable factors that contribute to the risk of loss of physical performance with age.
Although the role of vitamin D in maintaining skeletal health is well known, knowledge about its role in relation to physical performance is still limited, and it is not known if vitamin D status can predict decline in physical performance.6 25-Hydroxy vitamin D (25-OHD) is crucial for calcium absorption and to maintain calcium homeostasis.7,8 Insufficiency is common among older populations7–10 and has been linked to falls,11,12 sarcopenia,13 and frailty.8 Longitudinal studies reveal mixed findings.14–17 The Women's Health Initiative Clinical Trial (WHI CT) investigators reported no effect of supplementing calcium and vitamin D on decline in physical performance, nor did they observe an interaction between serum vitamin D and supplementation in relation to physical performance.18 However, the level of vitamin D supplementation in the trial was low (400 IU vitamin D per day). New Dietary Reference Intakes (DRIs) for vitamin D recommend 600–800 IU vitamin D supplementation per day.19 Additionally, the analysis did not evaluate a comprehensive measure of performance-based function. The current analysis evaluates the association between serum 25-OHD and a global scale of physical performance among 534 women in the WHI CT.
Materials and Methods
Study population
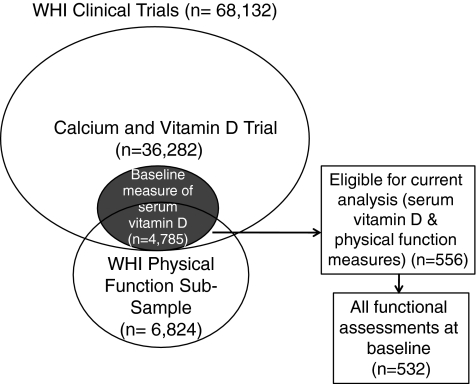
The current study includes WHI CT participants enrolled in the Calcium and Vitamin D Trial with baseline measures of serum vitamin D and physical performance. The WHI CT (n=68,132) involved three overlapping components—Dietary Modification trial (DM), Hormone Therapy trials (HT), and Calcium and Vitamin D (CaD) trial—and has been described in detail previously.20 Briefly, women 50–79 years of age were recruited through direct mailing campaigns and media awareness programs between 1993 and 1998 and randomized into one or more of the clinical trials. There were 36,282 eligible WHI participants from either the HT or DM trial enrolled in the CaD trial 12–24 months after initial enrollment.21 Baseline serum measurements of 25OHD were available for 4,785 CaD trial participants included in one of three nested case-control studies.22 As part of the WHI's overall goal to identify and prevent major causes of disability, performance-based measures of physical performance were taken from a 25% subsample of WHI CT participants aged 65–79 years (n=6,824). Five hundred fifty-six women from the CaD trial with baseline serum measurements of 25OHD also participated in the physical performance subsample and were eligible for inclusion in this study (women also participated in the DM and the HT trials, n=216 and n=284, respectively). Figure 1 describes the subsample that was included in the analysis. Levels of 25-OHD were measured using the DiaSorin Liaison chemiluminescent immunoassay system at Diasorin headquarters (Stillwater, MN) as previously described.22
FIG. 1.
Description of subsample of Women's Health Initiative (WHI) Clinical Trials included in the analytic sample.
Outcomes and follow-up
Physical performance was measured by trained, certified clinical center staff blinded to treatment group at baseline and at years 1, 3, and 6; additional details were reported previously.18,23 Timed walk and chair-stand tests provided information on gait and dynamic leg strength, respectively. Grip strength is a measure of upper extremity function and has been used as a general indicator of frailty. A physical performance summary score was derived by summing the decile ranking of the best test results for each test per visit, adapting the approach defined by Guralnik et al.4 This summary measure is reliable among women aged ≥65 and highly sensitive to change.24 A hierarchical balance test is unavailable in WHI-CT; therefore, the summary measure is modified accordingly. The internal consistency of the measure in the current analysis was assessed using the coefficient of reliability (Cronbach's alpha=0.60).
Statistical analyses
We limited our analyses to women who completed all three physical performance tests at baseline, resulting in a final study population of 534. All 534 subjects returned for their 1 year follow-up visit, 418 (97%) returned at year 3, and 498 (93%) returned at year 6. Serum 25-OHD was categorized into four categories (<25 nmol/L, 25–49 nmol/L, 50–74 nmol/L, and ≥75 nmol/L). We compared participant characteristics across categories of serum 25-OHD using F-tests and chi-square tests. Repeated measures analysis was used to fit a model between change in physical performance and baseline serum 25-OHD over time, using the generalized estimating equation (GEE) approach. This approach uses all available data from women in the study sample and accounts for the within-individual dependence in observations over time.25 Repeated measurements of physical performance from baseline through year 6 were modeled as a function of baseline age; self-reported race/ethnicity; clinic latitude: (northern>40°N), middle 35–40°N), southern<35°N); season of blood draw (December–February, March–May, June–August, and September–November); baseline body mass index (BMI); baseline comorbidity index (a modified Charlson Index previously modified for WHI data26); minutes walked outside per week (a proxy for time spent outdoors) at baseline; clinical trial arm; repeated measures of time (clinic visit). Time was modeled as a categorical variable to allow for a nonlinear relationship between time and physical performance.
The serum vitamin D term in these models represented differences in absolute physical performance averaged across the 6 years of follow-up or the cross-sectional association of vitamin D with physical performance. Interaction terms testing the degree to which baseline vitamin D level was associated with changes in physical performance over time were evaluated. The quasi -likelihood under the Independence Model Criterion (QIC) statistic was used to assess model fit. If inclusion of the interaction term resulted in a smaller QIC value, the interaction term was retained. An alpha level of 0.05 was used to determine statistical significance. All longitudinal analyses were performed using the GENMOD Procedure of SAS software version 9.1.3.27 The study protocol was approved by institutional review boards at each participating institution. De-identified data for this analysis were obtained from the WHI Coordinating Center. The study was reviewed by the Drexel Institutional Review Board and determined to be exempt.
Results
Serum vitamin D levels in this population ranged from 6.3 to 116.6 nmol/L (median 44.7). Serum 25-OHD varied significantly by race/ethnicity, season of blood draw, BMI, physical performance (Table 1). Nonwhite women were more likely to have lower levels of serum 25-OHD.
Table 1.
Baseline Characteristics of Women's Health Initiative Participants, According to Serum 25-OHD Classification (n=534)
| |
Serum 25-OHD concentration (nmol/L) |
|||||
|---|---|---|---|---|---|---|
| |
Total |
<25 |
25–49 |
50–74 |
≥75 |
|
| n | 534 | 67 | 255 | 148 | 64 | p value |
| Serum 25-OHD, nmol/L* | 48.2±21.4 | 18.7±4.7 | 38.1±6.7 | 61.2±6.8 | 88.5±12.4 | |
| Age, years, mean±SD | 70.3±3.7 | 70.5±3.9 | 70.5±3.6 | 69.8±3.8 | 70.7±3.6 | 0.16 |
| Clinic latitude, n(%) | ||||||
| Southern:<35°N | 128 | 13 (10.2) | 58 (45.3) | 40 (31.2) | 17 (13.3) | 0.70 |
| Middle: 35–40°N | 202 | 28 (13.9) | 96 (47.5) | 58 (28.7) | 20 (9.9) | |
| Northern:>40°N | 204 | 26 (12.7) | 101 (49.5) | 50 (24.5) | 27 (13.2) | |
| Season of blood draw,*n(%) | ||||||
| Winter | 127 | 14 (11.0) | 78 (61.4) | 26 (20.5) | 9 (7.1) | 0.0013 |
| Spring | 119 | 25 (21.0) | 50 (42.0) | 32 (26.9) | 12 (10.1) | |
| Summer | 162 | 19 (11.7) | 70 (43.2) | 46 (28.4) | 27 (16.7) | |
| Fall | 126 | 9 (7.2) | 57 (45.2) | 44 (34.9) | 16 (12.7) | |
| Ethnicity,*n(%) | ||||||
| White, non-Hispanic | 489 | 56 (11.4) | 237 (48.5) | 139 (28.4) | 57 (11.7) | 0.05 |
| Nonwhite/other | 44 | 11 (25.0) | 17 (38.6) | 9 (20.4) | 7 (16.0) | |
| BMI (kg/m2),* mean±SD | 28.7±5.2 | 30.2±5.0 | 29.1±5.3 | 28.0±5.0 | 26.9±4.3 | 0.0007 |
| Time walked outside, min/week, mean±SD | 50.2±68.1 | 42.5±61.1 | 46.2±60.4 | 56.7±74.2 | 59.0±86.2 | 0.25 |
| Chronic conditions,a mean±SD | 0.4±0.6 | 0.4±0.6 | 0.4±0.6 | 0.3±0.6 | 0.3±0.5 | 0.26 |
| Dietary Modification (DM) Trial, n(%) | ||||||
| Intervention | 128 | 20 (15.6) | 67 (52.4) | 32 (25.0) | 9 (7.0) | 0.37 |
| Control | 190 | 20 (10.5) | 87 (45.8) | 56 (29.5) | 27 (14.2) | |
| Not enrolled | 216 | 27 (12.5) | 101 (46.8) | 60 (27.8) | 28 (12.9) | |
| Calcium/Vitamin D (CaD) Trial, n(%) | ||||||
| Intervention | 261 | 35 (13.4) | 126 (48.3) | 67 (25.7) | 33 (12.6) | 0.73 |
| Control | 273 | 32 (11.7) | 129 (47.2) | 81 (29.7) | 31 (11.4) | |
| Hormone Therapy (HT) Trial, n(%) | ||||||
| E-alone intervention | 56 | 6 (10.7) | 22 (39.3) | 22 (39.3) | 6 (10.7) | 0.59 |
| E-alone control | 57 | 9 (15.8) | 27 (47.4) | 14 (24.5) | 7 (12.3) | |
| E+P intervention | 89 | 10 (11.2) | 43 (48.3) | 20 (22.5) | 16 (18.0) | |
| E+P control | 82 | 12 (14.6) | 43 (48.3) | 20 (22.5) | 7 (12.3) | |
| Not enrolled | 250 | 30 (12.0) | 120 (48.0) | 72 (28.8) | 28 (11.2) | |
| Physical performance summary,* mean±SD | 15.0±6.2 | 12.6±6.1 | 14.9±6.2 | 15.6±5.9 | 16.5±6.2 | 0.0009 |
p<0.05.
Values are means±standard deviation (SD) or number (%).
Modified Charlson Index includes weighted sum of myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, connective tissues disease, ulcerative disease, liver disease, diabetes, leukemia, lymphoma, solid tumor.
BMI, body mass index; E, estrogen; P, progesterone.
The longitudinal associations of time (clinic visit) and serum 25-OHD with physical performance are presented in Table 2. We observed a significant, positive linear relationship between serum 25-OHD at baseline and absolute physical performance averaged across the 6 years of follow-up (p for trend<0.001) (Table 2). In fully adjusted models, participants with baseline serum 25-OHD ≥75 nmol/L scored almost 3 points higher on the physical performance summary scale (relative risk 2.64, 95% confidence interval [CI] 0.90-4.39) compared with the reference category (<75 nmol/L) (Table 2, Model 2). Physical performance declined steadily over time. Overall, participants' summary score declined by 3.50 points over the 6-year follow-up period (95 % CI−4.01-−2.99, p for trend<0.001). However, the decline in physical performance over time did not differ by level of serum 25-OHD (time*serum 25-OHD p=0.848). In other words, baseline serum vitamin D had no effect on rate of change in physical performance among women in this study sample. Results for change in each of the component tests of physical performance were qualitatively similar to our results for the overall score in terms of direction of effect, although the p for trend for timed chair-stand and timed walk was of borderline significance (p for trend=0.050) (data not shown).
Table 2.
Results from Longitudinal Analysis of Physical Performance Score Over 6-Year Period as Function of Vitamin D Categories, Time, and Other Factors, Women's Health Initiative, 1993–2005
| |
Model 1a |
Model 2b |
||
|---|---|---|---|---|
| Relative risk | (95 % confidence interval) | Relative risk | (95 % confidence interval) | |
| Serum 25-OHD (nmol/L) | ||||
| < 25 | Reference (0) | Reference (0) | ||
| 25–49 | 1.64 | (0.28–3.01) | 1.02 | (−0.28–2.32) |
| 50–74 | 2.32 | (0.89–3.75) | 1.05 | (−0.32–2.43) |
| ≥75 | 3.66 | (1.88–5.45) | 2.64 | (0.90–4.39) |
| p for trend | <0.001 | 0.010 | ||
| Time (visit) | ||||
| Baseline | Reference (0) | Reference (0) | ||
| Time 1 (year 1) | −0.62 | (−1.03–−0.20) | −0.93 | (−1.39–−0.48) |
| Time 2 (year 3) | −1.84 | (−2.27–−1.40) | −1.78 | (−2.22–−1.34) |
| Time 3 (year 6) | −3.56 | (−4.06–−3.05) | −3.50 | (−4.01–−2.99) |
| p for trend | <0.001 | <0.001 | ||
| Serum 25-OHD * time | ||||
| Serum 25-OHD * time 1 | NA | 0.0032 | (−0.62–0.62) | |
| Serum 25-OHD * time 2 | NA | 0.2459 | (−0.40–0.89) | |
| Serum 25-OHD * time 3 | NA | −0.0223 | (−0.78–0.74) | |
Physical performance score ranged from 3 (scoring in the lowest 10% for all three measures) to 30 (scoring in the highest 10% for all three measures).
Adjusted for clinic latitude and season.
Adjusted for model 1 plus age, chronic conditions, BMI, race/ethnicity, time walked outside, HT trial arm, DM trial arm.
NA, not applicable.
Discussion
Higher levels of serum 25-OHD were associated with better physical performance but did not predict rate of change in physical performance during follow-up. Our results are consistent with two longitudinal observational studies in postmenopausal women reporting no association between baseline levels of 25-OHD and change in physical performance over ≥3 years of follow-up.15,16 In contrast, two other longitudinal cohort studies reported significant association between baseline levels of serum vitamin D and change in physical performance.14,17 Wicherts et al.14 reported significantly greater 3-year declines in summary performance scores comparing participants with low serum 25-OHD (≤50 nmol/L) to those with high levels (75 nmol/L) in a study including 979 men and women in the Longitudinal Aging Study Amsterdam (LASA). This study did not report results separately for men and women. Another longitudinal study including 656 women in the Rancho Bernardo Study (RBS) reported that women in the lowest 25-OHD quartile (<80 nmol/L) compared to the highest quartile had accelerated rates of decline in the timed up and go test and timed chair-stands over 2.5 years of follow-up.17 These two longitudinal studies reporting a significant association between vitamin D and declines in physical performance differ from our study in terms of the distribution of vitamin D in the population. While the mean level of serum 25-OHD in the LASA population was similar to that in our study (53.9 nmol/L vs. 48.2 nmol/L), the percent of the study population with the highest levels of vitamin D in LASA (≥75 nmol/L) was almost twice the percent in the WHI (18% vs. 11%).14 The mean level of serum 25-OHD in the RBS cohort was much higher than the level in the current study (100.8 vs. 48.2 nmol/L).17 Perhaps the observed significant association between serum vitamin D and change in functional performance in these two latter trials with greater variability of serum vitamin D suggests that the benefits of vitamin D are observed only among those with greater levels of vitamin D. A small trial (n=139) of vitamin D-deficient older adults reported a significant improvement in an aggregate measure of functional performance in the intervention group compared to a decline in the control group over a 6-month period.28 However, very large doses of vitamin D such as were administered in that trial may have unintended consequences, such as an increase in falling.29 Further, this follow-up period was very short, and it is not clear if the improvement would be sustained over a longer follow-up period.
Several reasons for the lack of an observed association between serum 25-OHD and change in physical performance in the current study should be considered. There is a lack of agreement about definitive categorizations of 25-OHD deficiency, although the 25-OHD cutoffs used in this study were previously found to be appropriate30 and the trends observed in the current study provide credibility for their use. Current evidence suggests that serum vitamin D levels <50 nmol/L indicate deficiency.30 It is also possible that the specificity of our physical performance tests may have been inadequate to detect the mechanisms by which 25-OHD is thought to affect neuromuscular response, such as by affecting a specific muscle type (e.g., fast twitch or slow twitch fibers), muscle contraction speed, or nerve conduction velocity.11 Error in our measurement of physical performance may have also biased our results toward the null. The physical performance summary score was a construct in which its internal consistency was measured with a Cronbach's alpha value of 0.60, indicating only fair reliability. Removal analysis indicated that of the three measures available for use in this study, grip strength was the least correlated with the total (r=0.32). Despite its shortcomings, the summary score is important, as it gives a broader measure of physical performance that is not confined to the parameters of a single test. Finally, although all models controlled for baseline characteristics, health status, and physical activity, residual confounding from other factors related to decline in physical performance may still be present.
The WHI is a diverse longitudinal study; however, the results of this substudy, drawing from a sample that was disproportionately white (92%, 510 women), must be interpreted cautiously with respect to the general population. Furthermore, given racial disparities in disability,31 this study may underestimate the influence of serum 25-OHD in relation to change in physical performance.
In this longitudinal, observational study, higher serum 25-OHD was associated with better physical performance but did not predict change in physical performance over the 6-year period. Additional studies in diverse populations, including randomized clinical trials of moderate to high dose vitamin D supplementation, are warranted.
Supplementary Material
Acknowledgments
A short list of WHI investigators is provided as supplemental material (available online at www.liebertonline.com).
Disclosure Statement
No competing financial interests exist. J.E.M. and colleagues at Brigham and Women's Hospital, Harvard Medical School, are recipients of funding from the National Institutes of Health to conduct the VITamin D and OmegA-3 TriaL (VITAL), a large-scale randomized trial of vitamin D and omega-3s in the prevention of cancer and cardiovascular disease.
References
- 1.Leveille SG. Penninx BW. Melzer D. Izmirlian G. Guralnik JM. Sex differences in the prevalence of mobility disability in old age: The dynamics of incidence, recovery, and mortality. J Gerontol B Psychol Sci Soc Sci. 2000;55:S41–S50. doi: 10.1093/geronb/55.1.s41. [DOI] [PubMed] [Google Scholar]
- 2.Guralnik JM. Ferrucci L. Simonsick EM. Salive ME. Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Penninx BW. Ferrucci L. Leveille SG. Rantanen T. Pahor M. Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55:M691–M697. doi: 10.1093/gerona/55.11.m691. [DOI] [PubMed] [Google Scholar]
- 4.Guralnik JM. Ferrucci L. Pieper CF, et al. Lower extremity function and subsequent disability. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cesari M. Kritchevsky SB. Penninx BWHJ, et al. Prognostic value of usual gait speed in well-functioning older people—Results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 6.Annweiler C. Schott A. Berrut G. Fantino B. Beauchet O. Vitamin D-related changes in physical performance: A systematic review. J Nut Health Aging. 2009;13:893–898. doi: 10.1007/s12603-009-0248-x. [DOI] [PubMed] [Google Scholar]
- 7.Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92:4–8. doi: 10.1016/j.pbiomolbio.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Shardell M. Hicks GE. Miller RR, et al. Association of low vitamin D levels with the frailty syndrome in men and women. J Gerontol A Biol Sci Med Sci. 2009;64A:69–75. doi: 10.1093/gerona/gln007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: Consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 10.Looker AC. Dawson-Hughes B. Calvo MS. Gunter EW. Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30:771–777. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 11.Mowe M. Haug E. Bohmer T. Low serum calcidiol concentration in older adults with reduced muscular function. J Am Geriatr Soc. 1999;47:220–226. doi: 10.1111/j.1532-5415.1999.tb04581.x. [DOI] [PubMed] [Google Scholar]
- 12.Pfeifer M. Begerow B. Minne H. Suppan K. Fahrleitner-Pammer A. Dobnig H. Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos Int. 2009;20:315–322. doi: 10.1007/s00198-008-0662-7. [DOI] [PubMed] [Google Scholar]
- 13.Visser M. Deeg DJH. Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): The Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88:5766–5772. doi: 10.1210/jc.2003-030604. [DOI] [PubMed] [Google Scholar]
- 14.Wicherts IS. van Schoor NM. Boeke AJP, et al. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92:2058–2065. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 15.Verreault R. Semba RD. Volpato S. Ferrucci L. Fried LP. Guralnik JM. Low serum vitamin D does not predict new disability or loss of muscle strength in older women. J Am Geriatr Soc. 2002;50:912–917. doi: 10.1046/j.1532-5415.2002.50219.x. [DOI] [PubMed] [Google Scholar]
- 16.Faulkner K. Cauley J. Zmuda J, et al. Higher 1,25-dihydroxyvitamin D concentrations associated with lower fall rates in older community-dwelling women. Osteoporos Int. 2006;17:1318–1328. doi: 10.1007/s00198-006-0071-8. [DOI] [PubMed] [Google Scholar]
- 17.Dam TT. von Mühlen D. Barrett-Connor E. Sex-specific association of serum vitamin D levels with physical function in older adults. Osteoporos Int. 2009;20:751–760. doi: 10.1007/s00198-008-0749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunner RL. Cochrane B. Jackson RD, et al. Calcium, vitamin D supplementation, and physical function in the Women's Health Initiative. J Am Diet Assoc. 2008;108:1472–1479. doi: 10.1016/j.jada.2008.06.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross AC. Manson JE. Abrams SA, et al. The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: What clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Design of the Women's Health Initiative Clinical Trial and Observational Study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 21.Jackson RD. Lacroix AZ. Cauley JA. McGowan J. The Women's Health Initiative Calcium-Vitamin D Trial: Overview and baseline characteristics of participants. Ann Epidemiol. 2003;13:S98–S106. doi: 10.1016/s1047-2797(03)00046-2. [DOI] [PubMed] [Google Scholar]
- 22.Millen AE. Wactawski-Wende J. Pettinger M, et al. Predictors of serum 25-hydroxyvitamin D concentrations among postmenopausal women: The Women's Health Initiative Calcium plus Vitamin D Clinical Trial. Am J Clin Nutr. 2010;91:1324–1325. doi: 10.3945/ajcn.2009.28908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michael YL. Gold R. Manson JE, et al. Hormone therapy and physical function change among older women in the Women's Health Initiative: A randomized controlled trial. Menopause. 2010;17:295–302. doi: 10.1097/gme.0b013e3181ba56c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostir GV. Volpato S. Fried LP. Chaves P. Guralnik JM. Reliability and sensitivity to change assessed for a summary measure of lower body function: Results from the Women's Health and Aging Study. J Clin Epidemiol. 2002;55:916–921. doi: 10.1016/s0895-4356(02)00436-5. [DOI] [PubMed] [Google Scholar]
- 25.Zeger SL. Liang K-Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 26.Gold R. Michael YL. Whitlock EP, et al. Race/ethnicity, socioeconomic status, and lifetime morbidity burden in the Women's Health Initiative: A cross-sectional analysis. J Womens Health. 2006;15:1161–1173. doi: 10.1089/jwh.2006.15.1161. [DOI] [PubMed] [Google Scholar]
- 27.SAS. 9.1.3 Help and documentation. Cary, NC: SAS Institute; 2000–2004. [Google Scholar]
- 28.Dhesi JK. Jackson SHD. Bearne LM, et al. Vitamin D supplementation improves neuromuscular function in older people who fall. Age Ageing. 2004;33:589–595. doi: 10.1093/ageing/afh209. [DOI] [PubMed] [Google Scholar]
- 29.Sanders KM. Stuart AL. Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: A randomized controlled trial. JAMA. 2010;303:1815–1822. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 30.Lips P. Which circulating level of 25-hydroxyvitamin D is appropriate? J Steroid Biochem Mol Biol. 2004;89–90:611–614. doi: 10.1016/j.jsbmb.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 31.Mendes de Leon CF. Barnes LL. Bienias JL. Skarupski KA. Evans DA. Racial disparities in disability: Recent evidence from self-reported and performance-based disability measures in a population-based study of older adults. J Gerontol B Psychol Sci Soc Sci. 2005;60:S263–S271. doi: 10.1093/geronb/60.5.s263. [DOI] [PubMed] [Google Scholar]
- 32.Annweiler C. Bridenbaugh S. Schott A-M. Berrut G. Kressig RW. Beauchet O. Vitamin D and muscle function: New prospects? BioFactors. 2009;35:3–4. doi: 10.1002/biof.4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.