Abstract
The toxicity of Shiga toxins (Stx) depends on the binding of their B subunits to carbohydrate ligands on host cells. The production of antibodies against B subunits, especially immunoglobulin A (IgA) secreted on the mucosal surface, should contribute to host defense. One of the major problems in attempts to produce IgA against Stx was the poor immunogenicity of B subunits. We were able to produce serum IgA as well as IgG against Stx1B in mice of the H-2d haplotype by means of intranasal immunization with recombinant B subunits of Stx (Stx1B) together with cholera toxin as a mucosal adjuvant. Secretory IgA (S-IgA) was detected in nasal washes but not in feces. We prepared chemically cross-linked Stx1B for use as an immunogen, and the formation of stable oligomers was revealed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and mass spectrometry. When the cross-linked Stx1B was used together with cholera toxin for the intranasal immunization of BALB/c mice, strong enhancement of the immune response was observed. The S-IgA titers in nasal washes were 16- to more than 64-fold higher than those in mice immunized with native Stx1B plus cholera toxin. Furthermore, fecal IgA was detectable when the cross-linked Stx1B was used. The use of cholera toxin was necessary for the induction of high titers of S-IgA in the nasal washes. However, the effect of cross-linking was dependent on the major histocompatibility complex haplotype; that is, no enhancement of IgA production was observed in C57BL/6 mice. The present results provide a practical means of producing IgA against Stx1B in BALB/c mice.
Shiga toxins (Stx), also called verotoxins, are exotoxins and virulence factors of enterohemorrhagic Escherichia coli strains such as serotype O157:H7 (20). Two types of Stx—Stx1 and Stx2—are known to be associated with human diseases, including life-threatening complications such as hemolytic uremic syndrome and central nervous system involvement (4, 11, 23). Stx consist of a cytotoxic subunit (A subunit) and a pentamer of cell binding subunits (B subunit). The B subunit pentamers of Stx1 and Stx2 both recognize carbohydrate ligands, such as those on glycolipid globotriaosylceramide (Gb3, Galα1-4Galβ1-4Glcβ1-1Cer) (12). This carbohydrate determinant is also known as CD77, which is expressed on human germinal center B cells in secondary lymphoid follicles (7, 13, 18).
The germinal center is very important in the B-cell differentiation process. Both somatic hypermutation and immunoglobulin heavy-chain class switching take place in the germinal center (17). The toxicity against centroblasts, cells at the stage of B-cell differentiation in the germinal center, may inhibit the synthesis of high-affinity antibodies and may also inhibit class switching to immunoglobulin G (IgG) and IgA. This occurrence may prevent an efficient immune response against Stx. This concern is supported by the fact that Stx and even their B subunits have been shown to induce apoptosis in Burkitt's lymphoma cell lines (14, 15, 22), which originated from centroblasts (17, 24). Furthermore, B cells from human tonsils committed to IgG or IgA synthesis have been shown to be susceptible to Stx toxicity in vitro (5).
In contrast, we have demonstrated that mouse germinal center B cells do not express binding sites for Stx using recombinant B subunits of Stx1 (Stx1B) (9). The same reagent strongly stained human Burkitt's lymphoma cell lines (19). These results may indicate that the production of secretory IgA (S-IgA) with high affinity to Stx is feasible in mouse systems, and passive immunization by means of oral administration of such S-IgA may be of therapeutic value. However, Stx1B has been demonstrated to be a poor immunogen in mice and subject to strict major histocompatibility complex (MHC) restriction (1).
In this study, we analyzed the mucosal immune response in a mouse system to produce IgA antibodies to Stx1B. We focused on the problem of how to overcome the poor immunogenicity of Stx1B, especially with regard to mucosal immunity.
MATERIALS AND METHODS
Mice.
Specific-pathogen-free female BALB/c, C57BL/6, B10.D2, DBA/2, and C57BL/6 × DBA/2 F1 (BDF1) mice were purchased from SLC Japan (Shizuoka, Japan) and used at 6 weeks of age. Animal care and experiments were performed in accordance with the University of Shizuoka guidelines for the care and use of laboratory animals.
Reagents.
Recombinant binding subunits of Stx1 (Stx1B) were expressed in E. coli JM105 (pVT1-B5) cells, and proteins were purified by anion-exchange chromatography and chromatofocusing to homogeneity as described previously (19). Protein concentrations were determined with a bicinchoninic acid protein assay kit (Pierce, Rockford, Ill.). Rabbit anti-mouse IgG (γ-chain specific), rabbit anti-mouse IgA (α-chain specific), and horseradish peroxidase-goat anti-rabbit IgG (H+L) were purchased from Zymed (South San Francisco, Calif.); glutaraldehyde (25% solution) was from Nacalai Tesque (Kyoto, Japan); 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), phenylmethylsulfonyl fluoride, Tween 20, and glycine were from Wako Pure Chemicals (Osaka, Japan); and sodium azide was from Kanto Chemicals (Tokyo, Japan). Cholera toxin (Vibrio cholerae, type Inaba 569B, azide free) was purchased from Calbiochem (San Diego, Calif.), complete Freund's adjuvant and incomplete Freund's adjuvant were from Difco (Detroit, Mich.), and bovine serum albumin (BSA) fraction V, aprotinin, ketamine, and xylazine were from Sigma (St. Louis, Mo.). Bio-Gel P-30 (fine) was purchased from Bio-Rad (Hercules, Calif.); a gel filtration low-molecular-weight (cutoff, 3,500) calibration kit and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) protein reference standards (peptide marker kit and low-molecular-weight SDS marker kits) were from Amersham Biosciences (Piscataway, N.J.); and an SDS-PAGE standard kit II was from Daiichi Pure Chemicals (Tokyo, Japan).
Antigen modification.
Cross-linking of Stx1B was carried out as follows. The purified Stx1B (1.5 mg) was dissolved in 1.1 ml of calcium- and magnesium-free Dulbecco's phosphate-buffered saline (PBS). To this solution, 1.1 ml of glutaraldehyde (0.2% in PBS) was added dropwise with stirring. The resulting mixture was incubated for 1 h at 20°C with stirring. To stop the reaction, 550 μl of 1 M glycine in PBS was added, followed by incubation for an additional 1 h at 20°C. The mixture was dialyzed against PBS by using a Spectra/Por 3 membrane (molecular weight cutoff, 3,500; Spectrum Laboratories, Rancho Dominguez, Calif.). The dialyzed solution was concentrated to 3 mg of protein per ml by using a Centricon YM-3 (Millipore, Bedford, Mass.).
Electrophoresis and gel filtration.
The increase in the apparent molecular weight upon cross-linking was revealed by Tricine-SDS-PAGE (12.5% T, 3% C) under reducing conditions (21). Proteins were stained with Coomassie brilliant blue R250 as described previously (19). SDS-PAGE (10% acrylamide) under Laemmli's reducing conditions was also performed. For gel filtration, a column (1 by 80 cm) of Bio-Gel P-30 (molecular weight range, 2,500 to 40,000) was equilibrated with PBS at 4°C. Stx1B (840 μg) dissolved in 0.6 ml of PBS was applied, and proteins were eluted with PBS. Fractions (1 ml) were collected, and the A280 of each fraction was measured. As a control, 500 μg of standard proteins or blue dextran 2000 was subjected to gel filtration under the same conditions.
Mass spectrometry.
To detect the cross-linked Stx1B, matrix-assisted laser desorption ionization-time of flight mass spectroscopy (MALDI-TOF MS) (Ultraflex; Bruker Daltonics, Bremen, Germany) analysis was performed in the linear positive-ion mode. Samples dissolved in PBS (30.8 μg/ml) were desalted by using ZipTip C4 resin (Millipore) and were then mixed with sinapinic acid (Bruker) as a matrix. The machine was calibrated with lysozyme (Bruker).
Immunization.
Mice were anesthetized by intraperitoneal injection of ketamine (80 mg/kg of body weight) and xylazine (5 mg/kg). For intranasal immunization, the purified Stx1B (40 μg) or cross-linked Stx1B (40 μg) was mixed with cholera toxin (1 μg) in 15 μl of PBS. Mice were given 7.5 μl of the antigen-cholera toxin (CT) mixture per nostril on days 0, 7, 14, and 24. For some experiments, intranasal immunization was carried out on days 0, 7, and 14. During an experiment designed to test the effects of CT, BALB/c mice received the cross-linked Stx1B with or without CT on days 0, 7, 14, and 21. For parenteral immunization, Stx1B dissolved in PBS was emulsified in complete Freund's adjuvant. Mice were given 40 μg of Stx1B by subcutaneous injection of 25-μl aliquots of the emulsion (20 μg of Stx1B) into both of the hind footpads. On day 10, 50 μl of an incomplete Freund’s adjuvant-based emulsion containing 40 μg of Stx1B was intraperitoneally injected.
Sample collection.
For intranasal immunization, serum was obtained 7 days after immunization by means of retro-orbital plexus puncture. Nasal washes were individually collected from each mouse by gently introducing 10 μl of PBS into one of the nares by using a micropipette, the liquid being immediately drawn from the nares. These procedures were repeated twice per nostril, and the samples from each of the individual mice were combined. To obtain sufficient quantities, samples collected on days 6, 7, and 8 after the fourth immunization were combined for each mouse. Fecal extracts were prepared from freshly obtained feces on day 6 after the fourth immunization. Fecal pellets were added to PBS (100 mg [wet weight]/ml) containing 1 mM phenylmethylsulfonyl fluoride, 0.3 μM aprotinin, and 0.02% NaN3. After vigorous mixing for 30 min at 4°C with a vortex mixer, the fecal suspensions were centrifuged at 17,000 × g for 10 min at 4°C. Each supernatant was collected and centrifuged again under the same conditions. The supernatants were then used as fecal extracts. For parenteral immunization, serum was obtained 4 days after the second immunization. Serum samples were diluted in PBS containing 1% BSA and 1% Tween 20 (1% BSA-Tween-PBS) or in PBS containing 0.1% BSA and 0.1% Tween 20 (0.1% BSA-Tween-PBS). Nasal washes were diluted with 0.1% BSA-Tween-PBS, and fecal extracts were diluted with 1% BSA-Tween-PBS. The buffer conditions are given in the figure legends.
Enzyme-linked immunosorbent assay (ELISA).
Antibodies against Stx1B were measured as described previously (10). In brief, the wells of a 96-well flat-bottomed enzyme immunoassay-radio immunoassay plate (Costar 9018; Corning Glass Works, Corning, N.Y.) were coated with Stx1B (100 μl, 5 μg/ml in PBS). As a control, several wells were left uncoated. After overnight incubation at 4°C, the wells were washed three times with PBS and were then blocked with 200 μl of PBS containing 1% BSA for 3 h at room temperature. Diluted samples (100 μl) were added to the wells of the Stx1B-coated plates. After 1 h of incubation at room temperature, the wells were washed three times with PBS containing 0.1% Tween 20 (PBS-Tween). To detect antibody classes specific for Stx1B, wells were incubated for 1 h at room temperature with 100 μl of rabbit anti-mouse IgG or rabbit anti-mouse IgA (1:1,000 dilution in 0.1% BSA-Tween-PBS), followed by incubation with 100 μl of horseradish peroxidase-goat anti-rabbit IgG (H+L) (1:1,000 dilution in 0.1% BSA-Tween-PBS) for 1 h at room temperature. After each incubation, the wells were washed three times with PBS-Tween. As a substrate, 100 μl of 1 mM ABTS dissolved in 0.1 M citrate buffer (pH 4.2) containing 0.034% H2O2 was added. After 10 to 30 min of incubation at room temperature, the absorbance was measured with a microplate reader (TECAN SPECTRA Readers; TECAN, Salzburg, Austria) by using a 405-nm filter. Specific absorbance was determined by subtraction of the absorbance of uncoated wells from that of Stx1B-coated wells. The end point titer of each sample was defined as the highest dilution giving a specific absorbance value (optical density) of more than 0.1.
Statistical analyses.
Student's t test, the Welch test, and the Mann-Whitney U test were used to carry out statistical analyses.
RESULTS
Poor immunogenicity of Stx1B.
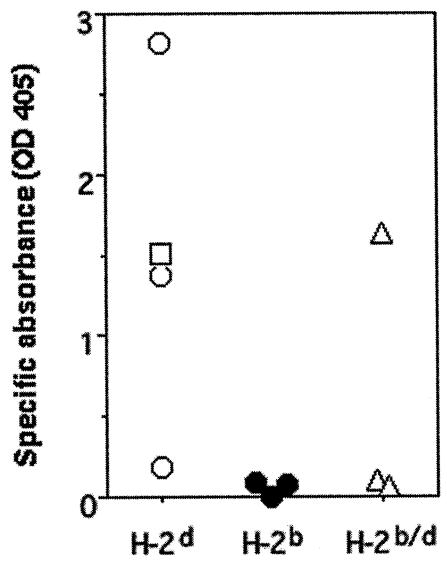
It has been demonstrated that the immune response against Stx1B is restricted to H-2 haplotypes. Mice of the H-2b haplotype have been shown to be nonresponders to Stx1B by parenteral immunization (1). We compared the levels of serum IgG specific for Stx1B by using mice of the H-2d and H-2b haplotypes and their F1 hybrids after secondary parenteral immunization with purified native Stx1B (Fig. 1). As already reported, H-2d mice (B10.D2 and DBA/2) substantially responded to the native Stx1B, while H-2b mice (C57BL/6) did not. Because MHC is codominant, we expected a substantial response in BDF1 (H-2b/d) mice. However, BDF1 mice responded to Stx1B rather poorly. These results suggested that Stx1B is a poor immunogen, and the immune response against Stx1B appears to be largely dependent on the density of appropriate MHC class II molecules on antigen-presenting cells.
FIG. 1.
H-2 restriction of the immune response against Stx1B. Mice were parenterally immunized with the purified native Stx1B with Freund's adjuvant. Sera from individual B10.D2 (open circles), DBA/2 (open square), C57BL/6 (filled circles), and BDF1 (open triangles) mice were analyzed for Stx1B-specific IgG antibodies (ordinate) by means of ELISA at a 1:1,000 dilution. Samples were diluted with 1% BSA-Tween-PBS.
Stx1B-specific serum IgG and IgA after intranasal immunization.
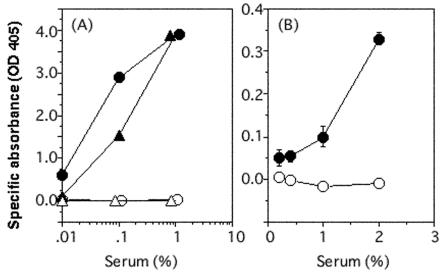
The immune response against Stx1B was studied after mucosal immunization via the intranasal route by using CT as a mucosal adjuvant (6, 25). Three immunizations at weekly intervals with Stx1B in the presence of CT resulted in the production of serum IgG in DBA/2 mice (Fig. 2A). In contrast, mice receiving CT alone did not produce Stx1B-specific serum IgG. IgG antibodies were also detected after secondary immunization with a lower titer (Fig. 2A). Serum IgA specific for Stx1B was detected after three weekly immunizations with Stx1B in the presence of CT; however, the antibody level was low (Fig. 2B). CT alone did not induce Stx1B-specific serum IgA either. Secretory IgA specific for Stx1B was not detected in feces after the third immunization (data not shown).
FIG. 2.
Production of serum IgG and IgA antibodies in DBA/2 mice on intranasal immunization with Stx1B. (A) Serum IgG specific for Stx1B. Sera were collected 7 days after secondary (triangles) or tertiary (circles) immunization with Stx1B plus CT (filled symbols) or CT alone (open symbols). (B) Serum IgA specific for Stx1B. Sera were collected 7 days after tertiary immunization with Stx1B plus CT (filled circles) or CT alone (open circles). Pooled serum samples from three mice (Stx1B plus CT) or two mice (CT alone) were used. Samples were diluted with 1% BSA-Tween-PBS. The binding of antibodies was colorimetrically determined (ordinate) in response to the serum dilution (abscissa). Data represent means ± standard deviations of triplicate determinations by ELISA.
Cross-linking of Stx1B and its characterization.
The experiments described above suggested that the immunogenicity of Stx1B is not sufficient for the production of IgA, especially S-IgA. The low response observed for the F1 hybrid mice suggested that immunogenicity appeared to be greatly dependent on the efficiency of antigen presentation. Therefore, we next examined whether the poor immunogenicity of Stx1B could be improved by increasing the number of putative T-cell epitopes displayed on antigen-presenting cells. To this end, we cross-linked purified Stx1B, using glutaraldehyde as a bifunctional reagent to increase the number of putative T-cell epitopes per molecule.
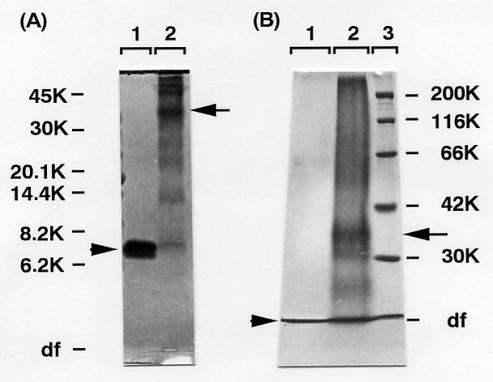
The native Stx1B gave a single band corresponding to an apparent molecular weight of 7,500 by Tricine-SDS-PAGE carried out under reducing conditions (Fig. 3A). After treatment with glutaraldehyde, there was no formation of a precipitate or a decrease in solubility. Nevertheless, a ladder of protein bands was observed by Tricine-SDS-PAGE. Although a band representing the monomer was still observed, the majority of the proteins formed oligomers that were resistant to denaturation by SDS and reducing agents (Fig. 3A). The oligomers consist of the dimer, trimer, tetramer, and pentamer and oligomers of higher molecular weight. The predominant species among the oligomers was the pentamer (apparent molecular weight, 37,000) as judged according to staining intensity. By Tris-glycine-SDS-PAGE (Laemmli's standard reducing conditions), native Stx1B proteins ran at the dye frontal position because of their low molecular weight. After cross-linking, a protein band corresponding to the pentamer (apparent molecular weight, 35,000) and bands corresponding to higher-molecular-weight materials were observed (Fig. 3B).
FIG. 3.
SDS-PAGE analysis of cross-linked Stx1B. Proteins were stained with Coomassie blue. The positions of standards are indicated by their molecular weights. (A) Tricine-SDS-PAGE for peptides. Native (not cross-linked) Stx1B (lane 1, 20 μg) and cross-linked Stx1B (lane 2, 20 μg) were electrophoresed under reducing conditions. The molecular weight standards were ovalbumin (45K), carbonic anhydrase (30K), trypsin inhibitor (20.1K), myoglobin peptide I + II (14.4K), myoglobin peptide I (8.2K), and myoglobin peptide II (6.2K). The Stx1B monomer (an arrowhead) and the band corresponding to the pentamer (an arrow) are indicated. (B) Tris-glycine-SDS-PAGE (10% acrylamide) for proteins. Native Stx1B (lane 1, 20 μg) and cross-linked Stx1B (lane 2, 40 μg) were electrophoresed under reducing conditions. The molecular weight standards (lane 3) were myosin (200K), β-galactosidase (116K), BSA (66K), aldolase (42K), and carbonic anhydrase (30K). Native Stx1B ran completely at the dye front (arrowhead). The band corresponding to the pentamer is indicated by an arrow. df, dye front.
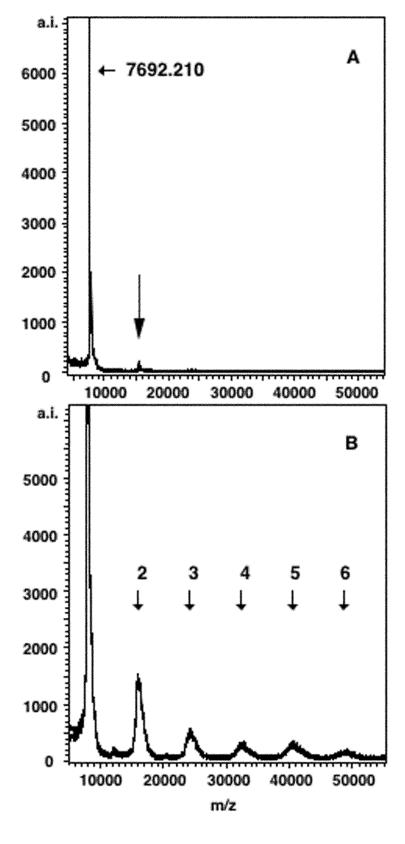
By mass spectrometry, native Stx1B gave a major signal, corresponding to a molecular weight of 7,692, which represents a molecular cation (Fig. 4A). A small signal corresponding to the dimer was also observed. After cross-linking, a series of peaks corresponding to oligomers was observed (Fig. 4B). The results also indicated the formation of stable oligomers after cross-linking.
FIG. 4.
Mass spectrum of the native and cross-linked Stx1B. MALDI-TOF MS was performed in the linear positive-ion mode. The abscissa indicates the m/z values. (A) Native Stx1B. A peak representing the monomer is indicated by a short arrow together with the observed m/z value. A small peak corresponding to the dimer is indicated by a long arrow. (B) Cross-linked Stx1B. Short arrows with the numbers of B subunits indicate the signals of oligomers.
Oligomer formation of native Stx1B in solution.
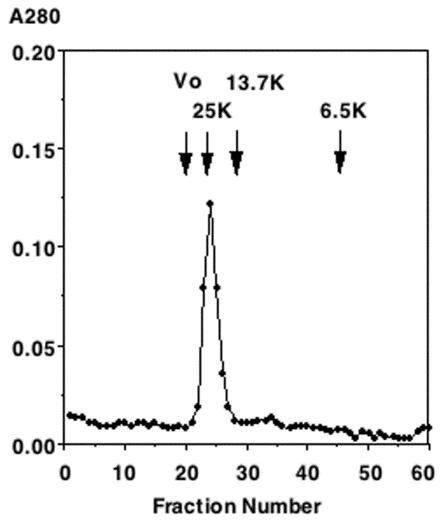
Although treatment with detergents and reducing agents completely dissociates native Stx1B into its subunits, it may form oligomers in solution. A mass spectrum revealed the presence of oligomers even after laser desorption ionization (Fig. 4A). The elution pattern as observed by gel filtration suggested oligomerization of the native Stx1B in PBS (Fig. 5). The average apparent molecular weight of the native Stx1B was calculated to be between 23,500 and 25,000 based on the elution positions of standard proteins.
FIG. 5.
Elution profile of the native Stx1B on a column of Bio-Gel P30. Protein elution was assessed as the A280 (ordinate) of each fraction (abscissa). As molecular weight standards, marker proteins or blue dextran 2000 were subjected to gel filtration under the same conditions. Blue dextran 2000 and ovalbumin (43K) were eluted in the void volume. The void volume (V0) and elution positions for chymotrypsinogen A (25K), RNase A (13.7K), and aprotinin (6.5K) are indicated by arrows.
Enhanced immunogenicity of cross-linked Stx1B for production of S-IgA in BALB/c mice.
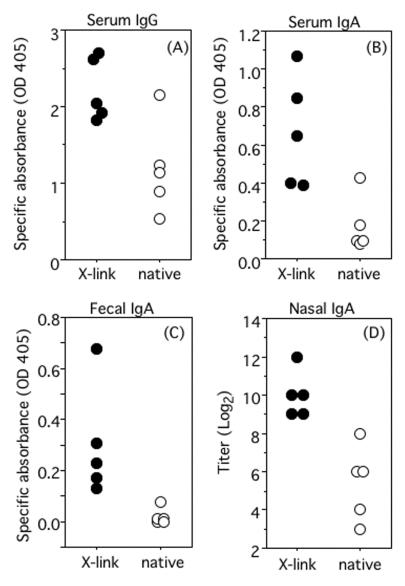
We examined whether the cross-linked Stx1B induces a stronger immune response upon mucosal immunization. BALB/c mice were intranasally immunized with either native Stx1B plus CT or cross-linked Stx1B plus CT four times at weekly intervals. The Stx1B-specific serum IgG (P < 0.02, t test) and IgA (P < 0.01, t test) levels were significantly higher in mice immunized with cross-linked Stx1B (Fig. 6A and B). Although it was difficult to induce fecal IgA by means of intranasal immunization with native Stx1B plus CT, significant levels of fecal IgA specific for Stx1B were observed (P < 0.05, Welch test) in mice immunized with cross-linked Stx1B plus CT (Fig. 6C). More dramatic enhancement of the titers of Stx1B-specific S-IgA was observed in the nasal washes on immunization with cross-linked Stx1B plus CT (P < 0.01, U test). The median titer after immunization with cross-linked Stx1B was 1:1,024, whereas that after immunization with native Stx1B was 1:64. The enhancement was thus 16-fold (Fig. 6D).
FIG. 6.
Enhanced immunogenicity of cross-linked Stx1B upon intranasal immunization of BALB/c mice. Stx1B-specific serum IgG (A), serum IgA (B), fecal IgA (C), and secreted IgA in nasal washes (D) were examined. Sera were collected on day 7 (panels A and B), fresh fecal extracts were prepared on day 6 (panel C), and nasal washes were collected on days 6, 7, and 8 (panel D) after the fourth immunization with cross-linked Stx1B plus CT (filled circles) or untreated Stx1B plus CT (open circles). The nasal washes from each mouse were combined as a sample that represents each mouse. Serum samples and nasal washes were diluted with 0.1% BSA-Tween-PBS, and fecal extracts were diluted with 1% BSA-Tween-PBS. Data for individual mice are shown as absorbance readings with 1:10,000 dilution (panel A), 1:200 dilution (panel B), and 1:2 dilution (panel C) of samples, or the data are shown as titers (maximal dilution giving a specific absorbance reading of 0.1 or more) in panel D.
H-2-restricted enhancement of immunogenicity of Stx1B on cross-linking.
The enhanced immunogenicity of cross-linked Stx1B in BALB/c mice may be due to an increase in the number of appropriate peptides, which are presented on MHC class II molecules of the H-2d haplotype and generated from each cross-linked molecule. If a lack of appropriate peptide sequences presented on I-Ab molecules in Stx1B is the reason for the unresponsiveness, then cross-linking should not improve the immunogenicity of Stx1B in C57BL/6 mice. In this case, nonresponder C57BL/6 mice (H-2b) may not exhibit a stronger immune response against cross-linked Stx1B. Thus, we next compared the effects of cross-linking of Stx1B on IgA production in BALB/c and C57BL/6 mice in parallel.
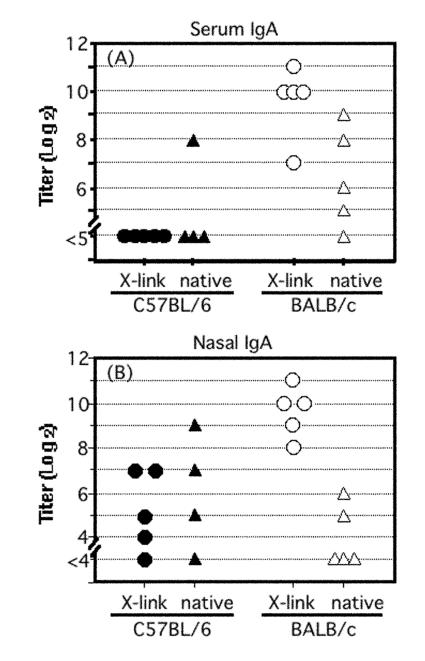
In BALB/c mice, the serum IgA titers significantly increased (P < 0.05, U test) with cross-linking of Stx1B (Fig. 7A). In contrast, cross-linking of Stx1B did not enhance the production of Stx1B-specific serum IgA in C57BL/6 mice, while one mouse immunized with native Stx1B plus CT responded exceptionally. The S-IgA titers in the nasal washes from BALB/c mice after immunization with cross-linked Stx1B plus CT were again significantly higher (P < 0.01, U test) than in the case of those immunized with native Stx1B plus CT (Fig. 7B). The median titer after immunization with cross-linked Stx1B was 1:1,024, whereas that after immunization with native Stx1B was less than 1:16. The enhancement was thus at least 64-fold. Unlike in the case of parenteral immunization, there were significant titers of S-IgA in the nasal washes in some C57BL/6 mice; however, the titers were generally low. The titers of S-IgA in C57BL/6 mice did not increase upon cross-linking of Stx1B (Fig. 7B). That is, the median titer after immunization with cross-linked Stx1B was 1:32, while that after immunization with native Stx1B was 1:64. The same mouse produced the highest titer of serum IgA and of S-IgA in nasal washes among the C57BL/6 mice.
FIG. 7.
Comparison of the enhanced immunogenicity of cross-linked Stx1B between BALB/c and C57BL/6 mice. Stx1B-specific serum IgA (A) and secreted IgA in nasal washes (B) from C57BL/6 mice (filled symbols) or from BALB/c mice (open symbols) were examined. Serum samples and nasal washes were obtained as described in the legend of Fig. 6 after the fourth immunization with cross-linked Stx1B plus CT (circles) or untreated Stx1B plus CT (triangles). The nasal washes from each mouse were combined as a sample that represents each mouse. Samples were diluted with 0.1% BSA-Tween-PBS. Data for individual mice are shown as titers. Enhanced immunogenicity of the cross-linked Stx1B in the production of specific IgA was observed in BALB/c mice but not in C57BL/6 mice.
For BALB/c mice, cross-linked Stx1B induced similar levels of S-IgA, with small variation in the titers among individuals (titer range, 1:256 to 1:2,048). In contrast, the titers of S-IgA in C57BL/6 mice immunized with cross-linked Stx1B ranged from less than 1:16 to 1:128. The titers in three out of five C57BL/6 mice were not higher than 1:32.
Adjuvant requirement.
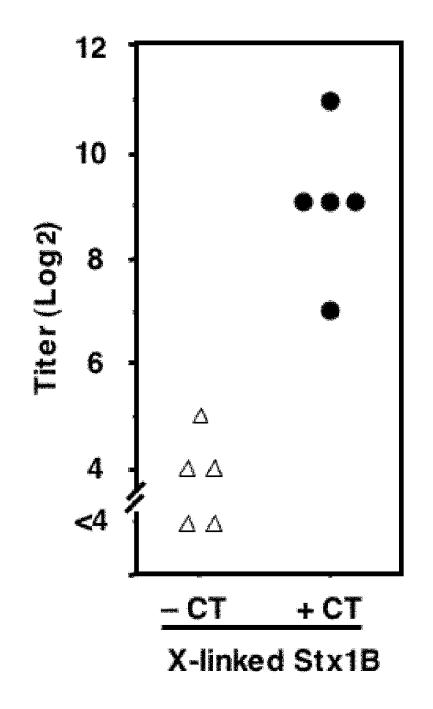
The dramatic effect of cross-linking of Stx1B may imply that intranasal inoculation of cross-linked Stx1B is sufficient for the induction of high-titer S-IgA. BALB/c mice were intranasally immunized with cross-linked Stx1B with or without using CT, and then the S-IgA titers against Stx1B in the nasal washes were compared after the fourth immunization (Fig. 8). The use of CT was essential for the induction of the high-titer S-IgA against Stx1B (P < 0.01, U test). The median titer was 1:512 when CT was used as an adjuvant, whereas it was 1:16 without CT.
FIG. 8.
Effect of CT during the intranasal immunization with cross-linked Stx1B. BALB/c mice were intranasally immunized with the cross-linked Stx1B in the presence (filled circles) or absence (open triangles) of CT. Nasal washes were obtained as described in the legend of Fig. 6 after the fourth immunization. The nasal washes from each mouse were combined as a sample that represents each mouse. Samples were diluted with 0.1% BSA-Tween-PBS. Data for individual mice are shown as titers. CT was required for the production of high-titer S-IgA against Stx1B.
DISCUSSION
We investigated mucosal immunity by using an antigen relevant to the host-pathogen interaction on the mucosal surface rather than model antigens such as ovalbumin. We chose Stx1B because it is not only responsible for the binding to the carbohydrate ligands expressed on host cells but is also nontoxic. However, the difficulty is that Stx1B is a poor immunogen in mice. It is important to increase the immunogenicity of Stx1B to produce high-affinity antibodies of the IgA isotype that have potential immunotherapeutic value.
The low immunogenicity of Stx1B in mice is not due to the adverse effect of Stx1B on binding to germinal center B cells (9). Limitation of the polypeptide sequence that can be presented on MHC class II molecules appears to be the reason. That is, any peptide derived from Stx1B may not be presentable on MHC class II molecules of the H-2b haplotype, which is a nonresponder (1). Such limitation of the presentable sequence seems to have a quantitative effect, by which the low responder phenotype of the F1 hybrid, between the responder (H-2d) and nonresponder (H-2b) haplotypes, can be explained (Fig. 1).
We successfully immunized mice of the H-2d haplotype by mucosal immunization via the intranasal route. Class-switched IgG antibodies were observed in serum. This finding indicated that germinal centers are functional in mice even when Stx1B is used as the immunogen. In contrast to the successful production of IgG antibodies, the production of IgA antibodies is still difficult. Serum IgA antibodies produced in response to specific immunization were detectable, but the antibody level was low (Fig. 2). Furthermore, S-IgA was not detectable in feces after intranasal immunization with Stx1B plus CT (Fig. 6). There have been several previously published reports indicating the successful production of S-IgA in the gastrointestinal tract upon intranasal immunization. Fecal IgA against ovalbumin was detected by using zonula occludens toxin as a mucosal adjuvant (16), fecal IgA against tetanus toxoid using interleukin 12 (2), and fecal IgA against influenza virus vaccine using CT (8). These results further revealed the difficulty of Stx1B-specific IgA production, especially of its secreted form. It should be noted that intragastric immunization with Stx1B plus CT did not produce better results with regard to S-IgA production (data not shown). It should also be noted that only marginal signals were obtained, if any, for Stx1B-specific IgA in intestinal mucosal washings collected 1 week after the third intranasal immunization with Stx1B plus CT (data not shown).
Cross-linking of Stx1B resulted in dramatic improvement of the immunogenicity in BALB/c mice (H-2d). Levels of both IgG and IgA specific for Stx1B in serum were increased. Even fecal IgA became detectable, although the levels were still low. In the case of nasal washes, a 16-fold to more than 64-fold increase in the median antibody titer of S-IgA was observed after immunization with the cross-linked Stx1B compared with the titer after immunization with intact Stx1B (Fig. 6 and 7). In contrast, no enhancement of the immunogenicity of Stx1B was observed for C57BL/6 mice (H-2b) (Fig. 7).
Characterization of cross-linked Stx1B by SDS-PAGE indicated the presence of a series of oligomers of Stx1B (Fig. 3). The subunits in the native Stx1B completely dissociated under the denaturing conditions, while gel filtration analysis of the native Stx1B revealed the formation of oligomers in PBS (Fig. 5). In contrast, the oligomers in the cross-linked Stx1B preparation survived such denaturing conditions. A band with a relatively strong signal corresponded to the pentamer, which may be a reflection of the oligomerization state in solution (3). A ladder of Stx1B oligomers has been demonstrated by cross-linking using dimethylpimelimidate (3). In this case, the majority of the oligomers were reported to be the dimer and trimer (3).
We also characterized the cross-linked Stx1B by MALDI-TOF MS (Fig. 4). Most of the native Stx1B dissociated into subunits during laser ionization, while some oligomers (mainly dimers) were still observed. After cross-linking, a series of oligomers was also observed. These results clearly demonstrate that stable oligomers are formed upon cross-linking with glutaraldehyde. Without covalent cross-linking, Stx1B oligomers are not stable enough to survive denaturing conditions or the absorption of energy.
In the case of intranasal immunization, some mice of the C57BL/6 strain showed a positive immune response to serum IgA and S-IgA production (Fig. 7). These results are different from those obtained for parenteral immunization (Fig. 1) (1). It is not clear at present whether this difference is due to some differences in the characteristics of the B cells involved in the mucosal immune system and the systemic immune system. Alternatively, this difference may be due to some differences in the antigen presentation depending on the route of immunization. However, from the practical point of view, C57BL/6 mice did not respond to Stx1B consistently. In contrast, BALB/c mice consistently exhibited high titers of S-IgA, with little variance observed among individuals in response to intranasal immunization with cross-linked Stx1B.
The reason for the effectiveness of cross-linked Stx1B can be speculated to be as follows. Stx1B may offer limited numbers of peptides presentable in association with I-Ad or I-Ed MHC class II molecules. Native Stx1B can generate limited numbers of presentable peptides upon endocytosis at one time. On the other hand, when antigen-presenting cells (especially B cells in the secondary response) endocytose one cross-linked molecule, many presentable peptides can be generated at one time in endosomes. Therefore, the efficiency of antigen presentation for Stx1B can be greatly improved through cross-linking. In contrast, no peptide, or a very limited number of peptides, if any, may be generated from Stx1B in antigen-presenting cells in association with I-Ab molecules in C57BL/6 mice. One can imagine that cross-linking does not increase the number of presentable peptides generated in endosomes in these mice. For this reason, cross-linked Stx1B may not be more immunogenic than are native Stx1B in C57BL/6 mice.
One may expect that it would be useful if the immunogenicity of the cross-linked Stx1B were strong enough to make the use of CT dispensable. However, the results indicated that the use of CT was still required for the production of high-titer S-IgA in the nasal washes (Fig. 8).
In conclusion, we succeeded in producing S-IgA against Stx1B in BALB/c mice by intranasal immunization with cross-linked Stx1B and CT. Because high titers of S-IgA with little variation among individual mice were obtained, this procedure will constitute a practical method of immunization for producing S-IgA against Stx1B.
Acknowledgments
The COE Program in the 21st Century was supported by a grant from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
We thank Kazuo Yamamoto (Laboratory of Molecular Medicine, Department of Integrated Biosciences, Graduate School of Frontier Sciences, The University of Tokyo) for helpful suggestions. We thank Shigenori Kumazawa (Laboratory of Functional Food Science, University of Shizuoka) for help with mass spectrometry. We thank Chie Kobayashi (Department of Microbiology, University of Shizuoka School of Pharmaceutical Sciences) for technical assistance.
Editor: J. T. Barbieri
REFERENCES
- 1.Bast, D. J., J. Sandhu, N. Hozumi, B. Barber, and J. Brunton. 1997. Murine antibody responses to the verotoxin 1 B subunit: demonstration of major histocompatibility complex dependence and an immunodominant epitope involving phenylalanine 30. Infect. Immun. 65:2978-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyaka, P. N., M. Marinaro, R. J. Jackson, S. Menon, H. Kiyono, E. Jirillo, and J. R. McGhee. 1999. IL-12 is an effective adjuvant for induction of mucosal immunity. J. Immunol. 162:122-128. [PubMed] [Google Scholar]
- 3.Calderwood, S. B., D. W. K. Acheson, M. B. Goldberg, S. A. Boyko, and A. Donohue-Rolfe. 1990. A system for production and rapid purification of large amounts of the Shiga toxin/Shiga-like toxin I B subunit. Infect. Immun. 58:2977-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calderwood, S. B., F. Auclair, A. Donahue-Rolfe, G. T. Keusch, and J. J. Mekalanos. 1987. Nucleotide sequence of the Shiga-like toxin genes of Escherichia coli. Proc. Natl. Acad. Sci. USA 84:4364-4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen, A., V. Madrid-Marina, Z. Estrov, M. H. Freedman, C. A. Lingwood, and H. M. Dosch. 1990. Expression of glycolipid receptors to Shiga-like toxin on human B lymphocytes: a mechanism for the failure of long-lived antibody response to dysenteric disease. Int. Immunol. 2:1-8. [DOI] [PubMed] [Google Scholar]
- 6.Freytag, L. C., and J. D. Clements. 1999. Bacterial toxins as mucosal adjuvants. Curr. Top. Microbiol. Immunol. 236:215-236. [DOI] [PubMed] [Google Scholar]
- 7.Fyfe, G., J. A. Cebra-Thomas, E. Mustain, J. M. Davie, C. D. Alley, and M. H. Nahm. 1987. Subpopulations of B lymphocytes in germinal centers. J. Immunol. 139:2187-2194. [PubMed] [Google Scholar]
- 8.Hodge, L. M., M. Marinaro, H. P. Jones, J. R. Mcghee, H. Kiyono, and J. W. Simecka. 2001. Immunoglobulin A (IgA) responses and IgE-associated inflammation along the respiratory tract after mucosal but not systemic immunization. Infect. Immun. 69:2328-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imai, Y., T. Fukui, A. Ikegaya, T. Ishikawa, Y. Ono, and K. Kurohane. 2002. Lack of Shiga-like toxin binding sites in germinal centres of mouse lymphoid tissues. Immunology 105:509-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imai, Y., Y. Matsuura, Y. Ono, T. Ishikawa, and Y. Ito. 2001. Demonstration of the pH sensitive binding of multivalent carbohydrate ligands to immobilized Shiga-like toxin 1 B subunits. J. Biochem. (Tokyo) 130:665-670. [DOI] [PubMed] [Google Scholar]
- 11.Jackson, M. P., R. J. Neill, A. D. O'Brien, R. K. Holmes, and J. W. Newland. 1987. Nucleotide sequence analysis and comparison of the structural genes for Shiga-like toxin I and Shiga-like toxin II encoded by bacteriophages from Escherichia coli 933. FEMS Microbiol. Lett. 44:109-114. [DOI] [PubMed] [Google Scholar]
- 12.Lingwood, C. A. 1996. Role of verotoxin receptors in pathogenesis. Trends Microbiol. 4:147-153. [DOI] [PubMed] [Google Scholar]
- 13.Madassery, J. V., B. Gillard, D. M. Marcus, and M. H. Nahm. 1991. Subpopulations of B cells in germinal centers. III. HJ6, a monoclonal antibody, binds globoside and a subpopulation of germinal center B cells. J. Immunol. 147:823-829. [PubMed] [Google Scholar]
- 14.Mangeney, M., C. A. Lingwood, S. Taga, B. Caillou, T. Tursz, and J. Wiels. 1993. Apoptosis induced in Burkitt's lymphoma cells via Gb3/CD77, a glycolipid antigen. Cancer Res. 53:5314-5319. [PubMed] [Google Scholar]
- 15.Marcato, P., G. Mulvey, and G. D. Armstrong. 2002. Cloned Shiga toxin 2 B subunit induces apoptosis in Ramos Burkitt's lymphoma B cells. Infect. Immun. 70:1279-1286. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Marinaro, M., A. Di Tommaso, S. Uzzau, A. Fasano, and M. T. De Magistris. 1999. Zonula occludens toxin is a powerful mucosal adjuvant for intranasally delivered antigens. Infect. Immun. 67:1287-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin, A., P. D. Bardwell, C. J. Woo, M. Fan, M. J. Shulman, and M. D. Scharff. 2002. Activation-induced cytidine deaminase turns on somatic hypermutation in hybridomas. Nature 415:802-806. [DOI] [PubMed] [Google Scholar]
- 18.McCloskey, N., J. D. Pound, M. J. Holder, J. M. Williams, L. M. Roberts, J. M. Lord, and J. Gordon. 1999. The extrafollicular-to-follicular transition of human B lymphocytes: induction of functional globotriaosylceramide (CD77) on high threshold occupancy of CD40. Eur. J. Immunol. 29:3236-3244. [DOI] [PubMed] [Google Scholar]
- 19.Miyashita, S., Y. Matsuura, D. Miyamoto, Y. Suzuki, and Y. Imai. 1999. Development of recombinant B subunit of Shiga-like toxin 1 as a probe to detect carbohydrate ligands in immunochemical and flow cytometric application. Glycoconjugate J. 16:697-705. [DOI] [PubMed] [Google Scholar]
- 20.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schägger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 22.Taga, S., K. Carlier, Z. Mishal, C. Capoulade, M. Mangeney, and Y. Lécluse. 1997. Intracellular signaling events in CD77-mediated apoptosis of Burkitt's lymphoma cells. Blood 90:2757-2767. [PubMed] [Google Scholar]
- 23.Takao, T., T. Tanabe, Y.-M. Hong, Y. Shimonishi, H. Kurazono, T. Yutsudo, C. Sasakawa, M. Yoshikawa, and Y. Takeda. 1988. Identity of molecular structure of Shiga-like toxin I (VT1) from Escherichia coli O157:H7 with that of Shiga toxin. Microb. Pathog. 5:357-369. [DOI] [PubMed] [Google Scholar]
- 24.Tuscano, J. M., K. M. Druey, A. Riva, J. Pena, C. B. Thompson, and J. H. Kehrl. 1996. Bcl-x rather than Bcl-2 mediates CD40-depedent centrocyte survival in the germinal center. Blood 88:1359-1364. [PubMed] [Google Scholar]
- 25.Williams, N. A., T. R. Hirst, and T. O. Nashar. 1999. Immune modulation by the cholera-like enterotoxins: from adjuvant to therapeutic. Immunol. Today 20:95-101. [DOI] [PubMed] [Google Scholar]