Abstract
Viral interleukin-6 (vIL-6) is a product of Kaposi's sarcoma-associated herpesvirus (KSHV) expressed in latently infected cells and to a higher degree during viral replication. A distinctive feature of vIL-6 is the ability to directly bind and activate gp130 signaling in the absence of other receptor subunits. Secretion of vIL-6 is generally poor, but vIL-6 can activate gp130 from inside the cell. Due to the wide cell distribution of gp130, vIL-6 has the potential to induce a wide range of biological effects. Expression of vIL-6 is variable in KSHV-associated Kaposi's sarcoma (KS), primary effusion lymphoma (PEL), multicentric Castleman's disease (MCD), and in a newly described MCD-like systemic inflammatory syndrome observed in human immunodeficiency virus-positive patients. PEL effusions usually contain vIL-6 at high concentrations; since vIL-6 induces vascular endothelial growth factor, vIL-6 likely contributes to vascular permeability and formation of PEL effusions. Lymph nodes affected with MCD contain vIL-6-positive cells, and vIL-6 levels rise in conjunction with flares of the disease and likely contribute to symptoms of inflammation. The development of vIL-6 inhibitors is a potentially important advance in the treatment of KSHV-associated malignancies where vIL-6 is expressed.
Introduction
Cellular interleukin-6 (IL-6), a multifunctional cytokine produced by many cell types, plays a pivotal role in a wide range of biological responses, including inflammation, acute-phase reactions, immune regulation, hematopoiesis, and tumorigenesis (Kishimoto 2010). The activities of IL-6 are mediated through its binding to the IL-6 receptor (IL-6R) and signaling initiated by the hexameric receptor complex of IL-6/IL-6R and gp130, which possesses a signaling module in the cytoplasmic tail (Yamasaki and others 1988; Taga and others 1989; Hibi and others 1990). Signaling from activated gp130 is transmitted by the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) axis and by the Ras-mitogen-activated protein kinase (MAPK) cascade (Lutticken and others 1994; Zhong and others 1994; Schiemann and others 1997).
In 1994, Chang and Moore discovered Kaposi's sarcoma-associated herpesvirus (KSHV) in association with Kaposi's sarcoma (KS) lesions and found that the virus codes for a viral homolog of cellular IL-6 (Chang and others 1994; Moore and others 1996) (Fig. 1). Besides KS, a multicentric angio-proliferative disease predominantly of the skin, KSHV infection is linked to primary effusion lymphoma (PEL) and to a subset of multicentric Castleman's disease (MCD) (Waterston and Bower 2004; Carbone and Gloghini 2008). KS, PEL, and MCD are commonly associated with human immunodeficiency virus (HIV) infection, and these diseases may co-exist (Cesarman and others 1995; Soulier and others 1995; Yarchoan and others 2005). KSHV-derived viral IL-6 (vIL-6) is detected in a proportion of KSHV-infected individuals (Aoki and others 2001b, 2001c; Uldrick and others 2010). Like cellular IL-6, vIL-6 signals through gp130 to activate the JAK-STAT pathway. Since its discovery, it was proposed that KSHV has evolved to express a homolog of cellular IL-6 for the benefit of its own survival and spread in humans (Moore and others 1996). Extensive studies have analyzed the role of vIL-6 on KSHV infection and disease pathogenesis. This review discusses aspects of this research, with an emphasis on recent molecular and clinical aspects.
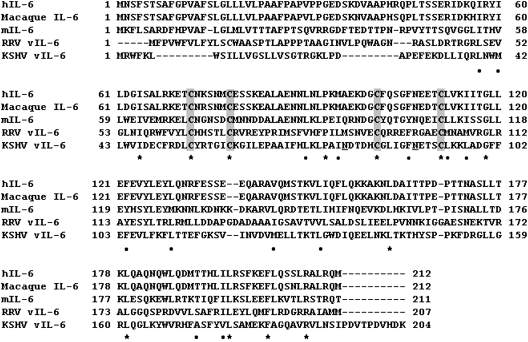
FIG. 1.
Aligment of interleukin-6 (IL-6) proteins. Comparative analysis of amino acid sequences of IL-6 from human (hIL-6: NP 000591), macaque (AAA99978), mouse (mIL-6: NP 112445), Rhesus radinovirus (RRV vIL-6: AAF59989) and Kaposi's sarcoma-associated herpesvirus (KSHV: YP 001129358) by the ClustalW program. Asterisks (identical) and dots (similar) indicate conserved residues among the proteins. Signal peptides are predicted by SignalIP 3.0 (www.cbs.dtu.dk/services/SignalP/) and shown in white letters. Conserved cysteine residues are boxed in gray. For KSHV-vIL-6, asparagine residues for N-linked glycosylation are underlined.
Human IL-6 and IL-6R-gp130
IL-6 was first purified from the culture supernatant of a T-cell lymphoma cell line as a B-cell differentiation factor (Hirano and others 1985, 1986). Soon thereafter, efforts by other laboratories linked the same IL-6 molecule to other biological activities (Hirano and others 1986; Zilberstein and others 1986; Aarden and others 1987; Coulie and others 1987; Gauldie and others 1987; Tosato and others 1988). Since these early discoveries, extensive research has defined IL-6 as a critical regulator of cell growth and differentiation, and a contributor to various diseases states, particularly inflammatory diseases (O'Shea and Murray 2008; Kishimoto 2010). Genetic studies in mice have contributed to current understanding of IL-6 biological activities. The targeted disruption of the IL-6 gene locus caused severe immunoglobulin A (IgA) deficiency and impaired immune responses to viruses and bacteria in the mouse (Kopf and others 1994; Ramsay and others 1994). Conversely, IL-6 transgenic mice (where the IL-6 gene was fused to a human Ig heavy chain enhancer) manifested a polyclonal increase in serum IgG1 and a massive plasmacytosis in thymus, lymph node and spleen, but did not induce a transplantable plasmacytoma, which is believed to require additional genetic changes such as c-Myc translocation (Suematsu and others 1989).
The biological activities of IL-6 are mediated by a protein complex of IL-6, IL-6R (IL-6R/CD126/gp80), and gp130, which activates signaling pathways mediated by the JAK-STAT and MAPK pathways (Yamasaki and others 1988; Taga and others 1989; Hibi and others 1990; Lutticken and others 1994; Stahl and others 1994; Zhong and others 1994; Schiemann and others 1997; Skiniotis and others 2005). The IL-6R does not signal, as it does not contain a signaling module in its cytoplasmic tail. Instead, gp130 not only mediates IL-6/IL-6R signaling, but also serves as a receptor component for other cytokines in the IL-6 family, including leukemia inhibitory factor, oncostatin M and IL-11, each of which engages a specific subunit to activate gp130 (Gearing and others 1992; Liu and others 1992; Yin and others 1993).
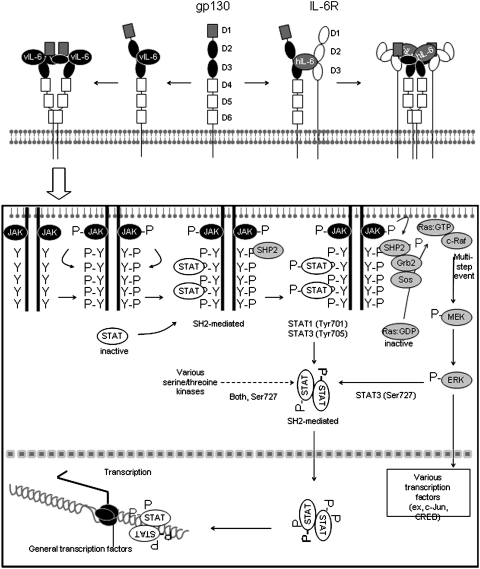
The extracellular domain of gp130 consists of distinct domains, identified as D1-D6 domains; the IL-6R also has distinct extracellular domains, identified as D1–D3. Figure 2 depicts a schematic representation (based on chrystallography results and other results) of the hexameric complex of human IL-6 (hIL-6) associated with IL-6R and gp130, in which IL-6 bridges the IL-6R D2-D3 domains to gp130 D2-D3 domains, and gp130 serves to dimerize each of the trimers (Boulanger and others 2003). IL-6/IL-6R engagement activates gp130-bound JAK1/2, which phosphorylates tyrosine residues in the cytoplasmic tail of gp130, and simultaneously causes gp130 disulfate-linked homodimerization (Murakami and others 1993). Phosphorylated gp130 at the intracytoplasmic tail provides a docking module for the Src-homology 2 (SH2) domains of STAT1 and STAT3, which are then phosphorylated by JAK kinases (STAT1 at Tyr701 and STAT3 at Tyr 705). This leads to formation of transcriptionally active heterodimers and homodimers of STAT1 and STAT3, which translocate to the nucleus to activate target genes (Fig. 2) (Leonard and O'Shea 1998). In addition, the binding of SHP2 (SH2 domain-containing phosphatase 2) to the phosphorylated tail of gp130 activates Grb2 (growth factor receptor bound protein 2) and Sos (son of sevenless homolog) resulting in the activation of Ras and initiation of the MAPK cascade, leading to extracellular signal-regulated kinase (ERK) phosphorylation and nuclear translocation. Phosphorylated ERK can also phosphorylate STAT3 (at Ser727) to modulate its function (Fig. 2) (Wen and others 1995; Chung and others 1997; Schiemann and others 1997).
FIG. 2.
Schematic representation of viral IL-6 (vIL-6) engagement of gp130 and signaling activation. The ectodomain of gp130 consists of 6 regions (D1–D6), and the ectodomain of IL-6 receptor (IL-6R) consists of 4 regions (D1–D3). The D1, D2, and D3 regions of gp130 are important for binding the complex of hIL-6 and IL-6R (involving IL-6R regions D2-D3) and formation of hexameric complexes. This model proposes that gp130 region D6 mediates binding of 2 molecules of gp130. By contrast to hIL-6, vIL-6 directly binds to the D2 and D3 regions of gp130 to form a tetramer. Although hIL-6 and vIL-6 bind to gp130 by different mechanisms, it is believed that hIL-6 and vIL-6 activate identical signaling pathways. (Box) Receptor-bound Janus kinase (JAK) is phosphorylated and activated upon ligand (hIL-6 or vIL-6) binding to gp130. Activated JAK phosphorylates tyrosine (Y) residues in the cytoplasmic tail of gp130. The phosphotyrosine motifs within the gp130 tail become recognized by Src homology 2 (SH2) from signal transducer and activator of transcription 1 (STAT1) and STAT3 (STAT 1 and 3). STAT1 and STAT3 attached to gp130 are phosporylated by JAK and form dimers capable of DNA binding. STATs are further serine-phosphorlated by other kinases, including extracellular signal-regulated kinase (ERK). Simultaneously, SHP2 (SH2 domain-containing phosphatase 2)-mediated activation of growth factor receptor binding 2 (Grb2)-son of sevenless homolog (Sos) occurs. Sos posses guanine nucleotide exchange factor (GEF) activity for Ras activation: the inactive guanosine diphosphate (GDP)-bound Ras becomes active guanosine triphosphate (GTP)-bound Ras, which binds and translocates c-Raf (v-raf-1 murine leukemia viral oncogene homolog) to the membrane. c-Raf activation is completed by a multi-step process that involves dephosphorylation by protein phosphatase 2 and phosphorylation (P) by a Src family protein, p21-activated kinase, and other unknown factors. Activated c-Raf initiates mitogen-activated protein kinase (MAPK) cascade. MEK, MAPK kinase.
KSHV and vIL-6
KSHV is a γherpesvirus discovered in association with KS tissues and found to be the causative agent of KS (Chang and others 1994). Although some aspects of the life cycle of KSHV have not yet been uncovered, the oropharynx represents the primary site of virus replication with shedding of the virus in saliva (Vieira and others 1997; Mayama and others 1998). Tonsil-derived B and T-lymphocytes can be productively infected with KSHV (Myoung and Ganem 2011), and virus in the saliva is believed to represent the principal source of infectious KSHV responsible for person-to-person transmission (Pauk and others 2000). The sexual route has also been proposed as a possible mode of transmission for KSHV (Boshoff and Weiss 2001). In the circulation, CD19-positive B-lymphocytes are believed to represent the main reservoir for KSHV in infected individuals (Boshoff and Weiss 2001).
Like other herpesviruses, KSHV is a large, enveloped DNA virus that displays 2 distinct transcriptional programs, latency and replication (Vieira and others 2001). Latency is reversible. During viral latency, most KSHV genes are not transcribed, which may serve to limit immune recognition of the virally infected cells (Moore and Chang 2001). Latent KSHV is maintained as a nuclear episome; the viral DNA is amplified by the host replication machinery once per cell cycle and is segregated during mitosis as a host chromosome, a process dependent on the viral latency-associated nuclear antigen (LANA) that teeters the viral DNA to cellular DNA (Moore and Chang 2001; Barbera and others 2006). The KSHV lytic phase is accompanied by robust expression of multiple viral genes required for production of viral progeny (Chandriani and others 2010). Lytic viral replication can lead to death of the host cell and induce an immune response to the virus-infected cells and the virus (Woodberry and others 2005).
Analysis of the KSHV genome obtained from DNA libraries of the PEL cell line BC-1, which is stably infected with KSHV and Epstein–Barr virus (EBV), identified open reading frame (ORF) K2 as a viral homolog of hIL-6; its gene product was named vIL-6 (Fig. 1) (Moore and others 1996; Russo and others 1996). The vIL-6 gene is expressed during viral latency and its expression is increased during viral replication (Moore and others 1996). In addition, activation of cellular Notch receptors in KSHV-infected cells can promote vIL-6 expression (Chang and others 2005). An IL-6 homolog gene/protein was also found in rhesus macaque radinovirus (RRV), which is linked to the development of B-cell hyperplasia and an MCD-like lymphadenopathy in rhesus macaques (Orzechowska and others 2008). However, an IL-6 homolog is not found in other mammalian γherpesviruses, such as EBV, herpesvirus saimiri, and murine gammaherpesvirus (Kaleeba and others 1999; Searles and others 1999; Nicholas 2005). An ancestor of KSHV in primates or other mammals may have captured the IL-6 gene. Consistent with this notion, RRV vIL-6 is more closely related to mammalian IL-6 than KSHV-derived vIL-6 (Fig. 1). Importantly, ORF K2 is a dispensable gene for KSHV infection and replication in a Burkitt's lymphoma cell line (Chen and Lagunoff 2007).
Structure and Function of vIL-6
The KSHV ORF K2 encodes a 204 amino acid protein with a 22 amino acid secretory signal that is highly divergent from that of cellular IL-6 (Fig. 1). Overall, vIL-6 displays ∼25% identify to hIL-6 with 4 conserved cysteines (Fig. 1). Although the calculated molecular weight of vIL-6 is 22.6 kDa, immunoblotting with specific antibodies showed a protein with higher molecular weight (∼28 kDa) attributable to N-linked glycosylation (Burger and others 1998; Dela Cruz and others 2004, 2009). Structurally, vIL-6 displays the 4-helix bundle topology typical of cellular IL-6 and other gp130-activating cytokines (Somers and others 1997; Chow and others 2001), but unique among this family of cytokines, vIL-6 can directly bind to gp130 and activate the JAK-STAT pathway downstream of gp130, much alike cellular IL-6 (Molden and others 1997; Adam and others 2009) (Fig. 2). The affinity of vIL-6 binding to gp130 was found to be ∼1,000-fold lower than the affinity of hIL-6/IL6R binding to gp130 (Aoki and others 2001a). The crystal structure of vIL-6 confirmed the direct binding of vIL-6 to the extracellular domain of gp130 and showed a tertameric structure with 2 gp130 proteins connected by 2 molecules of vIL-6 (Chow and others 2001; Boulanger and others 2004). The viral cytokine was found to use hydrophobic amino acid residues to contact gp130 at the sites II and III (Chow and others 2001; Adam and others 2009) (Fig. 2). An alignment of the primary sequence of hIL-6 and vIL-6 showed that the conserved amino acids were not the residues involved in the binding to gp130, which suggested a high degree of plasticity of ligand–receptor interactions (Chow and others 2001; Boulanger and others 2004). Recombinant vIL-6 produced in bacteria and therefore lacking glycosylation, can fold properly for structural studies, and can bind and activate gp130 in cell-based assays (Hoischen and others 2000; Chow and others 2001). However, N-glycosylation at Asn78 was reported to favor vIL-6-folding in mammalian cells (Fig. 3), and to increase vIL-6 binding to gp130 (Dela Cruz and others 2004, 2009).
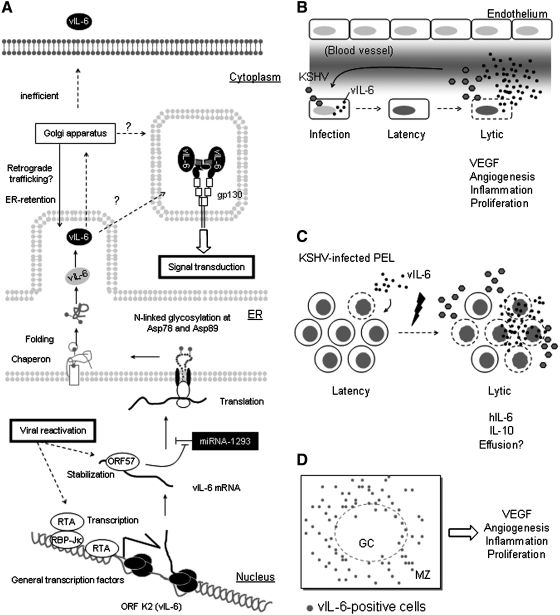
FIG. 3.
Transcription, translation, and secretion of vIL-6. (A) Viral reactivation occurring during KSHV lytic replication initiates vIL-6 [open reading frame (ORF) K2] transcription via viral replication and transcription activator (RTA) and the cellular transcription factor recombination signal binding protein for immunoglobulin kappa J region (RBP-Jκ). mRNA of vIL-6 is a target of miR-1293 at a site also bound by the viral ORF57 protein. miR-1293 and viral ORF 57 protein compete with each other for binding to miR-1293. vIL-6 polypeptide undergoes N-linked glycosylation at the endoplasmic reticulum (ER); N-glycan at Asp89 may facilitate intrinsic or chaperon-mediated protein folding. Immature vIL-6 may be glycosylated further at the Golgi apparatus and may relocated to ER by retrograde traffic. In the proximity of the ER, vIL-6 engages gp130 for autocrine signal transduction. In addition, vIL-6 is secreted although inefficiently via an unidentified sorting pathway still undefined (?). (B) vIL-6 in KS tissue or normal endothelium. Primary infection of KSHV is associated with viral latency in vitro and in vivo, where KSHV DNA is maintained at a low copy number and vIL-6 expression is not detected or detected at very low levels. Hypoxia or other signals can induce KSHV lytic production associated with massive release of vIL-6 found in serum or culture supernatant. vIL-6 induces vascular endothelial growth factor (VEGF) production, which promotes angiogenesis and vascular permeability. (C) vIL-6 in primary effusion lymphoma (PEL). PEL cells contain a small population of cells undergoing lytic replication, which express vIL-6. vIL-6 and other growth factors, particularly IL-10, are prominent survival factors for PEL. Lytic replication, induced by the hypoxic environment of effusions or other signals, leads to increased vIL-6 production: body cavity effusions and culture supernatants contain higher levels of vIL-6. Since vIL-6 promotes VEGF secretion, vIL-6 indirectly contributes to the accumulation of body cavity effusions. (D) vIL-6-producing cells in lymph node affected with multicentric Castleman's disease. vIL-6-positive B-cells are located in the vicinity of germinal centers (GC) and marginal zones (MZ).
The crystal structure of vIL-6 bound to gp130 showed that the site on vIL-6 corresponding to the IL-6R binding site on hIL-6 in unoccupied (Chow and others 2001). Additional studies showed that vIL-6 can bind IL-6R (Li and others 2001), and under some experimental conditions, the IL-6R was found to enhance vIL-6 activity (Wan and others 1999; Hu and Nicholas 2006), a result supported by studies with chimeric proteins of vIL-6 and hIL-6 (Adam and others 2009).
The conditioned medium of cells transduced with the vIL-6 gene contains vIL-6 protein that is biologically active (Aoki and others 1999; Jones and others 1999), but vIL-6 secretion is inefficient as most of the protein is retained inside the cell (Meads and Medveczky 2004; Chen and others 2009a). In this intracellular location, vIL-6 has been shown to be active and to engage and activate gp130 in the cytoplasm resulting in autocrine cell stimulation (Fig. 3) (Meads and Medveczky 2004; Chen and others 2009b). Defective secretion is also observed with fibroblast growth factor-2 (FGF-2), which lacks the signal peptide for secretion (Quarto and others 1989). However, unlike the situation with FGF2, the signal peptide of vIL-6 is functional, as it has been shown to support hIL-6 secretion (Meads and Medveczky 2004). Most of the vIL-6 is localized in the endoplasmic reticulum (ER) in conjunction with calnexin; gp130 promotes the exit of vIL-6 from the ER, but not its secretion (Meads and Medveczky 2004; Chen and others 2009a), which may require yet unidentified factors. However, cell death of KSHV-infected cells subsequent to viral replication or cytotoxic T-cell killing may trigger a massive vIL-6 release (Nicholas and others 1998). vIL-6 can induce cellular IL-6 expression in a variety of cell lines (Mori and others 2000). In bioassays, vIL-6 is ∼1,000-fold less potent than hIL-6 (Aoki and others 2001a).
Role of vIL-6 in KSHV-Associated Diseases
Three diseases are associated with KSHV infection: KS, PEL, and MCD (Chang and others 1994; Cesarman and others 1995; Soulier and others 1995). Since vIL-6 is expressed in latently infected cells and its expression is increased upon viral replication and by activation of cellular Notch receptors (Moore and others 1996; Chang and others 2005), vIL-6 has the potential to contribute to the pathogenesis of all these diseases. Early preclinical studies analyzed the effects of vIL-6 overexpression in T-cell immunodeficient mice (Aoki and others 1999). When injected with NIH3T3 cell transduced with a vIL-6 expression plasmid, the mice developed tumors that were highly vascularized and expressed high levels of vascular endothelial growth factor (VEGF). The size of the tumor appeared to be directly related to the levels of vIL-6 detected in the blood. The lymph nodes displayed a prominent plasma cell infiltration, and liver and spleen were enlarged. In addition, there was evidence of increased hematopoiesis. Thus, these experiments indicated that vIL-6 promotes angiogenesis, tumor growth, and plasmocytosis in the mouse (Aoki and others 1999). The role of vIL-6 in KS, PEL, and MCD appears to differ, at least in part due to the different degrees to which the virus undergoes lytic replication.
KS is a multicentric lymphoproliferative disease often localized to the skin and less frequently in the oral cavity, the gastrointestinal tract, and the lung (Boshoff and Weiss 2002; Yarchoan and others 2005). Typical KS lesions are brown, purple, or dark red nodules and plaques found on the skin. Histologically, KS lesions are characterized by spindle cells infected with KSHV, as reflected by immunostaining for the nuclear antigen LANA. Some of the spindle cells express CD31/PECAM-1 (platelet endothelial cell adhesion molecule-1) and markers for the lymphatic endothelium such as Prox1 (prospero homeobox 1) and LYVE1 (lymphatic vessel endothelial hyaluronan receptor 1), suggestive of the endothelial origin of KS (Carroll and others 2004; Hong and others 2004; Sivakumar and others 2008; Sakakibara and Tosato 2009). The KSHV-infected spindle cells form poorly organized vascular structures, referred to as vascular slits replete with red cells and breakdown products of red cells that account for the characteristic brown-purple color of the lesions (Boshoff and Weiss 2002; Yarchoan and others 2005). KS lesions also contain many infiltrating leukocytes, particularly CD68-positive monocytes and/or macrophages, which often produce VEGF, a principal stimulator of endothelial cell survival, growth, and migration (Parravicini and others 2000; Sakakibara and others 2009). KSHV is mostly latent in KS tissues, but viral replication is often detected and is believed to play an important role in sustaining the disease (Boshoff and Weiss 2001; Moore and Chang 2001). Despite the presence of many KSHV-infected cells in KS lesion, vIL-6 expression has been detected infrequently in KS tissues (Parravicini and others 1997, 2000; Cannon and others 1999), and only a proportion of KS patients had detectable vIL-6 in the circulation (Aoki and others 2001c). By contrast, KS tissues often express cellular IL-6, which is induced at least in part by the KSHV genes vFLIP and vGPCR that are expressed in KS (Montaner and others 2004; Sakakibara and others 2009).
PEL is an aggressive immunoblastic lymphoma, which usually presents as an effusion malignancy in the body cavities of patients with acquired immune deficiency syndrome, and is almost always infected with KSHV and co-infected with EBV in 30%–50% of cases (Cesarman and others 1995; Nador and others 1996). The pleural and peritoneal effusions from patients with PEL generally contain vIL-6 at high concentrations (Parravicini and others 1997; Aoki and others 2000). They also contain IL-6 and IL-10 (Aoki and others 2000). Two of these cytokines, vIL-6 and IL-10, promote autocrine growth of PEL cells (Tosato and others 1993; Jones and others 1999). In culture, PEL cell lines generally express vIL-6 mRNA at low levels (Katano and others 2000). This difference between vIL-6 production by PEL in vivo and in vitro has been attributed to the occurrence of spontaneous KSHV reactivation in vivo induced by hypoxia, which is prominent in effusions (Davis and others 2001). Consistently, in vitro treatment of PEL with 12-O-tetradodecanoyl-phorbol-13-acetate (TPA) markedly enhances vIL-6 expression at the RNA and protein levels (Moore and others 1996; Parravicini and others 2000). This is attributable to TPA-induced activation of KSHV ORF50/replication and transcription activator (RTA), which acts as a strong transactivator for many viral genes, including ORF K2/vIL-6, ORF K5, viral poly-adenylated nuclear RNA, ORF K9/vIRF-1, ORF59, and ORF50 itself (Sun and others 1999; Chen and others 2000; Haque and others 2000; Sakakibara and others 2001; West and Wood 2003; Deng and others 2007). This transactivating effect is attributable both to direct binding of ORF50/RTA to the promoter regions of target genes and to indirect binding mediated by recombination signal binding protein for Ig kappa J region (RBP-Jκ), which is a Notch-induced transcription factor (Deng and others 2002; Chang and others 2005) or other cellular factors (West and Wood 2003). Consistently, activation of Notch signaling, which is mediated by RBP-Jκ, initiates vIL-6 expression in KSHV infected cells (Chang and others 2005). A recent report revealed some of the complexities of vIL-6 regulation: KSHV ORF 57 protein, a viral RNA processor that is expressed during the lytic phase (Fig. 3) (Kang and others 2011), stimulates vIL-6 expression through its binding to an ORF57-responsive element MTA-responsive element (MREB) present in vIL-6 RNA and promoting vIL-6 translation; MREB can also be bound by miR-1293 (micro RNA-1293), and ORF57 competes with miR-1293 for binding and function as a vIL-6 silencer (Kang and others 2011).
MCD is a rare disease that affects primarily the lymph nodes, and is associated with KSHV infection in a substantial proportion of cases, especially when it arises in immunosuppressed individuals infected with HIV (Chang and others 1994; Soulier and others 1995). When untreated, the disease is rapidly fatal (Uldrick and others 2010). The diagnosis of MCD is based on histological characteristics of the affected lymph nodes, generally showing altered architecture with hypocellular germinal centers that are vascularized and perifollicular infiltration with plasmablastic cells that usually express IgM λ, and varying degrees of hyaline degeneration (Teruya-Feldstein and others 1998; Du and others 2001; Chadburn and others 2008; Cronin and Warnke 2009). A proportion of the plasmablastic cells surrounding the germinal center are infected with KSHV and express vIL-6 (Parravicini and others 2000; Aoki and others 2001b). Patients with MCD often have prominent inflammatory manifestations, which wax and wane (Oksenhendler and others 1996; Waterston and Bower 2004), and have been attributed at least in part to elevations in circulating levels of vIL-6, IL-6, and IL-10 (Oksenhendler and others 1996; Aoki and others 2001b). In a well-documented case, elevations in vIL-6 coincided with the development of severe symptoms of inflammation (Aoki and others 2001b). Recently, a cohort of patients co-infected with HIV and KSHV with recurrent inflammatory symptoms typical of MCD but without other evidence of MCD was identified, and found to have high levels of circulating vIL-6, IL-6, and IL-10 comparable to those found in patients with MCD (Uldrick and others 2010).
Approaches to vIL-6 Neutralization
There is evidence supporting the potential utility of vIL-6 neutralization at least in the context of MCD and perhaps PEL. Since vIL-6 is uniquely a viral product, and plays no role in physiological conditions, specific vIL-6 neutralization is expected to have a favorable toxicity profile. Early efforts at generating specific antibodies to vIL-6 produced a panel of 4 mouse monoclonal antibodies that effectively neutralize vIL-6 biological activity by interfering with vIL-6 binding to gp130 (Aoki and others 2001a). Mapping studies showed that these neutralizing antibodies bind to a region of vIL-6 that does not contribute to gp130 binding, suggesting that the antibodies produce allosteric changes in vIL-6 that prevent its binding to gp130 or render the molecule resistant to conformational changes necessary for gp130 binding (Aoki and others 2001a).
Using phage-display screening of human synthetic antibody libraries, a human monoclonal antibody specific to vIL-6 (named MAV) was identified, which inhibited vIL-6-dependent growth of the PEL cell line BCBL1, and vIL-6-dependent STAT3 activation in the HepG2 cell line (Kovaleva and others 2006). MAV binds to a site of vIL-6, which interacts with gp130 and prevents vIL-6 binding to gp130 (Kovaleva and others 2006).
A soluble form of gp130, which inhibits hIL-6-mediated signaling, is detected in human sera (Narazaki and others 1993). Similarly, gp130 fused to the constant fragment (Fc)-region of human IgG can also be used to neutralize vIL-6 (Jostock and others 2001). In vitro, soluble gp130-Fc prevented vIL-6-induced growth of the PEL cell line BCBL1 and inhibited vIL-6 induced STAT3 activation in HepG2 cells (Kovaleva and others 2006). Unlike the specific antibodies to vIL-6, soluble gp130-Fc can also inhibit gp130 binding of the complex composed of cellular IL-6 and IL-6R. Thus, transgenic mice overexpressing gp130-Fc displayed a reduced recruitment of myeloid cells/macrophages in a model of local inflammation that is dependent upon IL-6/IL6R signaling (Rabe and others 2008). Thus, soluble gp130-Fc has the potential to simultaneously neutralize vIL-6 and at least in part cellular IL-6.
Based on the crystal structure of the vIL-6/gp130 complex, synthetic peptides mimicking the gp130 binding sites of vIL-6 have been generated and shown to have specific IL-6 neutralizing activity in vitro (Sudarman and others 2008).
Thus, it is possible to neutralize vIL-6 activity, at least in vitro, by use of monoclonal antibodies, soluble gp130, or peptide mimics of vIL-6 binding interface with gp130. However, studies by 2 groups have provided compelling evidence suggesting that vIL-6 can activate gp130 signaling within the cell (Meads and Medveczky 2004; Kovaleva and others 2006; Chen and others 2009b). Although it is clear from preclinical studies that soluble vIL-6 is biologically active in mice (Aoki and others 2001a), the potential contribution of intracellular vIL-6 signaling in the context of in vivo KSHV infection is currently unclear. Nonetheless, preliminary results have shown that it possible to neutralize intracellular vIL-6 by targeting a vIL-6 neutralizing antibody to the ER (where most intracellular vIL-6 is detected) (Kovaleva and others 2006).
Perspective and Concluding Remarks
Since its discovery as a gene product of KSHV, much has been learned about the regulation of vIL-6 expression, structure, receptor engagement, signaling, and function, including its contribution to KSHV-associated diseases. There is now compelling evidence suggesting that vIL-6 is responsible for some of the inflammatory symptoms in MCD, and thus vIL-6 may represent an important therapeutic target at least in the context of MCD. In this light, several areas of investigation may be useful. First, it is important to establish the relative contribution of secreted vIL-6 as opposed to intracellular vIL-6 in KSHV-associated malignancies. Depending on this assessment the approaches to vIL-6 neutralization would be different, and perhaps complementary. Second, since vIL-6 and cellular IL-6 share gp130 signaling, display similar biological activities, and often co-exist in KSHV-associated diseases, it is important to further dissect their relationship in terms of regulation of expression and specific contributions to disease. We know that vIL-6 can promote IL-6 expression, but to which extent vIL-6 is responsible for cellular IL-6 expression in KSHV-associated malignancies is unclear. With the advent of an anti-IL-6R antibody that specifically neutralizes hIL-6 (tocilizumab) and its approval for the treatment of rheumatoid arthritis, it may be important to assess its effects in the treatment of KSHV-associated MCD. Third, it is important to develop clinical-grade neutralizing agents for vIL-6 to test in preclinical and clinical setting. To this end, it could be very useful to develop a good preclinical model of MCD.
Acknowledgment
This work was supported by the intramural research program at Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD. The authors thank Dr. R. Yarchoan for his comments.
Author Disclosure Statement
G.T. is a co-inventor on a patent describing the measurement of KSHV vIL-6. This invention was made when Dr. Tosato was an employee of the U.S. Government under 45 Code of Federal Regulations Part 7. All rights, title, and interest to this patent have been assigned to the U.S. Department of Health and Human Services. The government conveys a portion of the royalties it receives to its employee inventors under the Federal Technology Transfer Act of 1986 (P.L. 99-5-02).
References
- Aarden LA. De Groot ER. Schaap OL. Lansdorp PM. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Adam N. Rabe B. Suthaus J. Grotzinger J. Rose-John S. Scheller J. Unraveling viral interleukin-6 binding to gp130 and activation of STAT-signaling pathways independently of the interleukin-6 receptor. J Virol. 2009;83(10):5117–5126. doi: 10.1128/JVI.01601-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y. Jaffe ES. Chang Y. Jones K. Teruya-Feldstein J. Moore PS. Tosato G. Angiogenesis and hematopoiesis induced by Kaposi's sarcoma-associated herpesvirus-encoded interleukin-6. Blood. 1999;93(12):4034–4043. [PubMed] [Google Scholar]
- Aoki Y. Narazaki M. Kishimoto T. Tosato G. Receptor engagement by viral interleukin-6 encoded by Kaposi sarcoma-associated herpesvirus. Blood. 2001a;98(10):3042–3049. doi: 10.1182/blood.v98.10.3042. [DOI] [PubMed] [Google Scholar]
- Aoki Y. Tosato G. Fonville TW. Pittaluga S. Serum viral interleukin-6 in AIDS-related multicentric Castleman disease. Blood. 2001b;97(8):2526–2527. doi: 10.1182/blood.v97.8.2526. [DOI] [PubMed] [Google Scholar]
- Aoki Y. Yarchoan R. Braun J. Iwamoto A. Tosato G. Viral and cellular cytokines in AIDS-related malignant lymphomatous effusions. Blood. 2000;96(4):1599–1601. [PubMed] [Google Scholar]
- Aoki Y. Yarchoan R. Wyvill K. Okamoto S. Little RF. Tosato G. Detection of viral interleukin-6 in Kaposi sarcoma-associated herpesvirus-linked disorders. Blood. 2001c;97(7):2173–2176. doi: 10.1182/blood.v97.7.2173. [DOI] [PubMed] [Google Scholar]
- Barbera AJ. Chodaparambil JV. Kelley-Clarke B. Joukov V. Walter JC. Luger K. Kaye KM. The nucleosomal surface as a docking station for Kaposi's sarcoma herpesvirus LANA. Science. 2006;311(5762):856–861. doi: 10.1126/science.1120541. [DOI] [PubMed] [Google Scholar]
- Boshoff C. Weiss R. AIDS-related malignancies. Nat Rev Cancer. 2002;2(5):373–382. doi: 10.1038/nrc797. [DOI] [PubMed] [Google Scholar]
- Boshoff C. Weiss RA. Epidemiology and pathogenesis of Kaposi's sarcoma-associated herpesvirus. Philos Trans R Soc Lond B Biol Sci. 2001;356(1408):517–534. doi: 10.1098/rstb.2000.0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger MJ. Chow DC. Brevnova E. Martick M. Sandford G. Nicholas J. Garcia KC. Molecular mechanisms for viral mimicry of a human cytokine: activation of gp130 by HHV-8 interleukin-6. J Mol Biol. 2004;335(2):641–654. doi: 10.1016/j.jmb.2003.10.070. [DOI] [PubMed] [Google Scholar]
- Boulanger MJ. Chow DC. Brevnova EE. Garcia KC. Hexameric structure and assembly of the interleukin-6/IL-6 alpha-receptor/gp130 complex. Science. 2003;300(5628):2101–2104. doi: 10.1126/science.1083901. [DOI] [PubMed] [Google Scholar]
- Burger R. Neipel F. Fleckenstein B. Savino R. Ciliberto G. Kalden JR. Gramatzki M. Human herpesvirus type 8 interleukin-6 homologue is functionally active on human myeloma cells. Blood. 1998;91(6):1858–1863. [PubMed] [Google Scholar]
- Cannon JS. Nicholas J. Orenstein JM. Mann RB. Murray PG. Browning PJ. DiGiuseppe JA. Cesarman E. Hayward GS. Ambinder RF. Heterogeneity of viral IL-6 expression in HHV-8-associated diseases. J Infect Dis. 1999;180(3):824–828. doi: 10.1086/314956. [DOI] [PubMed] [Google Scholar]
- Carbone A. Gloghini A. KSHV/HHV8-associated lymphomas. Br J Haematol. 2008;140(1):13–24. doi: 10.1111/j.1365-2141.2007.06879.x. [DOI] [PubMed] [Google Scholar]
- Carroll PA. Brazeau E. Lagunoff M. Kaposi's sarcoma-associated herpesvirus infection of blood endothelial cells induces lymphatic differentiation. Virology. 2004;328(1):7–18. doi: 10.1016/j.virol.2004.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesarman E. Chang Y. Moore PS. Said JW. Knowles DM. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332(18):1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- Chadburn A. Hyjek EM. Tam W. Liu Y. Rengifo T. Cesarman E. Knowles DM. Immunophenotypic analysis of the Kaposi sarcoma herpesvirus (KSHV; HHV-8)-infected B cells in HIV+ multicentric Castleman disease (MCD) Histopathology. 2008;53(5):513–524. doi: 10.1111/j.1365-2559.2008.03144.x. [DOI] [PubMed] [Google Scholar]
- Chandriani S. Xu Y. Ganem D. The lytic transcriptome of Kaposi's sarcoma-associated herpesvirus reveals extensive transcription of noncoding regions, including regions antisense to important genes. J Virol. 2010;84(16):7934–7942. doi: 10.1128/JVI.00645-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. Dittmer DP. Shin YC. Hong Y. Jung JU. Role of Notch signal transduction in Kaposi's sarcoma-associated herpesvirus gene expression. J Virol. 2005;79(22):14371–14382. doi: 10.1128/JVI.79.22.14371-14382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. Cesarman E. Pessin MS. Lee F. Culpepper J. Knowles DM. Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266(5192):1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- Chen D. Choi YB. Sandford G. Nicholas J. Determinants of secretion and intracellular localization of human herpesvirus 8 interleukin-6. J Virol. 2009a;83(13):6874–6882. doi: 10.1128/JVI.02625-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D. Sandford G. Nicholas J. Intracellular signaling mechanisms and activities of human herpesvirus 8 interleukin-6. J Virol. 2009b;83(2):722–733. doi: 10.1128/JVI.01517-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. Ueda K. Sakakibara S. Okuno T. Yamanishi K. Transcriptional regulation of the Kaposi's sarcoma-associated herpesvirus viral interferon regulatory factor gene. J Virol. 2000;74(18):8623–8634. doi: 10.1128/jvi.74.18.8623-8634.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. Lagunoff M. The KSHV viral interleukin-6 is not essential for latency or lytic replication in BJAB cells. Virology. 2007;359(2):425–435. doi: 10.1016/j.virol.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow D. He X. Snow AL. Rose-John S. Garcia KC. Structure of an extracellular gp130 cytokine receptor signaling complex. Science. 2001;291(5511):2150–2155. doi: 10.1126/science.1058308. [DOI] [PubMed] [Google Scholar]
- Chung J. Uchida E. Grammer TC. Blenis J. STAT3 serine phosphorylation by ERK-dependent and -independent pathways negatively modulates its tyrosine phosphorylation. Mol Cell Biol. 1997;17(11):6508–6516. doi: 10.1128/mcb.17.11.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulie PG. Vanhecke A. Van Damme J. Cayphas S. Poupart P. De Wit L. Content J. High-affinity binding sites for human 26-kDa protein (interleukin 6, B cell stimulatory factor-2, human hybridoma plasmacytoma growth factor, interferon-beta 2), different from those of type I interferon (alpha, beta), on lymphoblastoid cells. Eur J Immunol. 1987;17(10):1435–1440. doi: 10.1002/eji.1830171008. [DOI] [PubMed] [Google Scholar]
- Cronin DM. Warnke RA. Castleman disease: an update on classification and the spectrum of associated lesions. Adv Anat Pathol. 2009;16(4):236–246. doi: 10.1097/PAP.0b013e3181a9d4d3. [DOI] [PubMed] [Google Scholar]
- Davis DA. Rinderknecht AS. Zoeteweij JP. Aoki Y. Read-Connole EL. Tosato G. Blauvelt A. Yarchoan R. Hypoxia induces lytic replication of Kaposi sarcoma-associated herpesvirus. Blood. 2001;97(10):3244–3250. doi: 10.1182/blood.v97.10.3244. [DOI] [PubMed] [Google Scholar]
- Dela Cruz CS. Lee Y. Viswanathan SR. El-Guindy AS. Gerlach J. Nikiforow S. Shedd D. Gradoville L. Miller G. N-linked glycosylation is required for optimal function of Kaposi's sarcoma herpesvirus-encoded, but not cellular, interleukin 6. J Exp Med. 2004;199(4):503–514. doi: 10.1084/jem.20031205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dela Cruz CS. Viswanathan SR. El-Guindy AS. Shedd D. Miller G. Complex N-linked glycans on Asn-89 of Kaposi sarcoma herpes virus-encoded interleukin-6 mediate optimal function by affecting cytokine protein conformation. J Biol Chem. 2009;284(43):29269–29282. doi: 10.1074/jbc.M109.039115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H. Chu JT. Rettig MB. Martinez-Maza O. Sun R. Rta of the human herpesvirus 8/Kaposi sarcoma-associated herpesvirus up-regulates human interleukin-6 gene expression. Blood. 2002;100(5):1919–1921. doi: 10.1182/blood-2002-01-0015. [DOI] [PubMed] [Google Scholar]
- Deng H. Liang Y. Sun R. Regulation of KSHV lytic gene expression. Curr Top Microbiol Immunol. 2007;312:157–183. doi: 10.1007/978-3-540-34344-8_6. [DOI] [PubMed] [Google Scholar]
- Du MQ. Liu H. Diss TC. Ye H. Hamoudi RA. Dupin N. Meignin V. Oksenhendler E. Boshoff C. Isaacson PG. Kaposi sarcoma-associated herpesvirus infects monotypic (IgM lambda) but polyclonal naive B cells in Castleman disease and associated lymphoproliferative disorders. Blood. 2001;97(7):2130–2136. doi: 10.1182/blood.v97.7.2130. [DOI] [PubMed] [Google Scholar]
- Gauldie J. Richards C. Harnish D. Lansdorp P. Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing DP. Comeau MR. Friend DJ. Gimpel SD. Thut CJ. McGourty J. Brasher KK. King JA. Gillis S. Mosley B. The IL-6 signal transducer, gp130: an oncostatin M receptor and affinity converter for the LIF receptor. Science. 1992;255(5050):1434–1437. doi: 10.1126/science.1542794. others. [DOI] [PubMed] [Google Scholar]
- Haque M. Chen J. Ueda K. Mori Y. Nakano K. Hirata Y. Kanamori S. Uchiyama Y. Inagi R. Okuno T. Yamanishi K. Identification and analysis of the K5 gene of Kaposi's sarcoma-associated herpesvirus. J Virol. 2000;74(6):2867–2875. doi: 10.1128/jvi.74.6.2867-2875.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi M. Murakami M. Saito M. Hirano T. Taga T. Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990;63(6):1149–1157. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- Hirano T. Taga T. Nakano N. Yasukawa K. Kashiwamura S. Shimizu K. Nakajima K. Pyun KH. Kishimoto T. Purification to homogeneity and characterization of human B-cell differentiation factor (BCDF or BSFp-2) Proc Natl Acad Sci U S A. 1985;82(16):5490–5494. doi: 10.1073/pnas.82.16.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T. Yasukawa K. Harada H. Taga T. Watanabe Y. Matsuda T. Kashiwamura S. Nakajima K. Koyama K. Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324(6092):73–76. doi: 10.1038/324073a0. others. [DOI] [PubMed] [Google Scholar]
- Hoischen SH. Vollmer P. Marz P. Ozbek S. Gotze KS. Peschel C. Jostock T. Geib T. Mullberg J. Mechtersheimer S. Fischer M. Grotzinger J. Galle PR. Rose-John S. Human herpes virus 8 interleukin-6 homologue triggers gp130 on neuronal and hematopoietic cells. Eur J Biochem. 2000;267(12):3604–3612. doi: 10.1046/j.1432-1327.2000.01389.x. [DOI] [PubMed] [Google Scholar]
- Hong YK. Foreman K. Shin JW. Hirakawa S. Curry CL. Sage DR. Libermann T. Dezube BJ. Fingeroth JD. Detmar M. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat Genet. 2004;36(7):683–685. doi: 10.1038/ng1383. [DOI] [PubMed] [Google Scholar]
- Hu F. Nicholas J. Signal transduction by human herpesvirus 8 viral interleukin-6 (vIL-6) is modulated by the nonsignaling gp80 subunit of the IL-6 receptor complex and is distinct from signaling induced by human IL-6. J Virol. 2006;80(21):10874–10878. doi: 10.1128/JVI.00767-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KD. Aoki Y. Chang Y. Moore PS. Yarchoan R. Tosato G. Involvement of interleukin-10 (IL-10) and viral IL-6 in the spontaneous growth of Kaposi's sarcoma herpesvirus-associated infected primary effusion lymphoma cells. Blood. 1999;94(8):2871–2879. [PubMed] [Google Scholar]
- Jostock T. Mullberg J. Ozbek S. Atreya R. Blinn G. Voltz N. Fischer M. Neurath MF. Rose-John S. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem. 2001;268(1):160–167. doi: 10.1046/j.1432-1327.2001.01867.x. [DOI] [PubMed] [Google Scholar]
- Kaleeba JA. Bergquam EP. Wong SW. A rhesus macaque rhadinovirus related to Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 encodes a functional homologue of interleukin-6. J Virol. 1999;73(7):6177–6181. doi: 10.1128/jvi.73.7.6177-6181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JG. Pripuzova N. Majerciak V. Kruhlak M. Le SY. Zheng ZM. Kaposi's sarcoma-associated herpesvirus orf57 promotes escape of viral and human interleukin-6 from microRNA-mediated suppression. J Virol. 2011;85(6):2620–2630. doi: 10.1128/JVI.02144-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katano H. Sato Y. Kurata T. Mori S. Sata T. Expression and localization of human herpesvirus 8-encoded proteins in primary effusion lymphoma, Kaposi's sarcoma, and multicentric Castleman's disease. Virology. 2000;269(2):335–344. doi: 10.1006/viro.2000.0196. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. IL-6: from its discovery to clinical applications. Int Immunol. 2010;22(5):347–352. doi: 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- Kopf M. Baumann H. Freer G. Freudenberg M. Lamers M. Kishimoto T. Zinkernagel R. Bluethmann H. Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368(6469):339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- Kovaleva M. Bussmeyer I. Rabe B. Grotzinger J. Sudarman E. Eichler J. Conrad U. Rose-John S. Scheller J. Abrogation of viral interleukin-6 (vIL-6)-induced signaling by intracellular retention and neutralization of vIL-6 with an anti-vIL-6 single-chain antibody selected by phage display. J Virol. 2006;80(17):8510–8520. doi: 10.1128/JVI.00420-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard WJ. O'Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- Li H. Wang H. Nicholas J. Detection of direct binding of human herpesvirus 8-encoded interleukin-6 (vIL-6) to both gp130 and IL-6 receptor (IL-6R) and identification of amino acid residues of vIL-6 important for IL-6R-dependent and -independent signaling. J Virol. 2001;75(7):3325–3334. doi: 10.1128/JVI.75.7.3325-3334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. Modrell B. Aruffo A. Marken JS. Taga T. Yasukawa K. Murakami M. Kishimoto T. Shoyab M. Interleukin-6 signal transducer gp130 mediates oncostatin M signaling. J Biol Chem. 1992;267(24):16763–16766. [PubMed] [Google Scholar]
- Lutticken C. Wegenka UM. Yuan J. Buschmann J. Schindler C. Ziemiecki A. Harpur AG. Wilks AF. Yasukawa K. Taga T. Association of transcription factor APRF and protein kinase Jak1 with the interleukin-6 signal transducer gp130. Science. 1994;263(5143):89–92. doi: 10.1126/science.8272872. others. [DOI] [PubMed] [Google Scholar]
- Mayama S. Cuevas LE. Sheldon J. Omar OH. Smith DH. Okong P. Silvel B. Hart CA. Schulz TF. Prevalence and transmission of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in Ugandan children and adolescents. Int J Cancer. 1998;77(6):817–820. doi: 10.1002/(sici)1097-0215(19980911)77:6<817::aid-ijc2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Meads MB. Medveczky PG. Kaposi's sarcoma-associated herpesvirus-encoded viral interleukin-6 is secreted and modified differently than human interleukin-6: evidence for a unique autocrine signaling mechanism. J Biol Chem. 2004;279(50):51793–51803. doi: 10.1074/jbc.M407382200. [DOI] [PubMed] [Google Scholar]
- Molden J. Chang Y. You Y. Moore PS. Goldsmith MA. A Kaposi's sarcoma-associated herpesvirus-encoded cytokine homolog (vIL-6) activates signaling through the shared gp130 receptor subunit. J Biol Chem. 1997;272(31):19625–19631. doi: 10.1074/jbc.272.31.19625. [DOI] [PubMed] [Google Scholar]
- Montaner S. Sodhi A. Servitja JM. Ramsdell AK. Barac A. Sawai ET. Gutkind JS. The small GTPase Rac1 links the Kaposi sarcoma-associated herpesvirus vGPCR to cytokine secretion and paracrine neoplasia. Blood. 2004;104(9):2903–2911. doi: 10.1182/blood-2003-12-4436. [DOI] [PubMed] [Google Scholar]
- Moore PS. Boshoff C. Weiss RA. Chang Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science. 1996;274(5293):1739–1744. doi: 10.1126/science.274.5293.1739. [DOI] [PubMed] [Google Scholar]
- Moore PS. Chang Y. Molecular virology of Kaposi's sarcoma-associated herpesvirus. Philos Trans R Soc Lond B Biol Sci. 2001;356(1408):499–516. doi: 10.1098/rstb.2000.0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori Y. Nishimoto N. Ohno M. Inagi R. Dhepakson P. Amou K. Yoshizaki K. Yamanishi K. Human herpesvirus 8-encoded interleukin-6 homologue (viral IL-6) induces endogenous human IL-6 secretion. J Med Virol. 2000;61(3):332–335. doi: 10.1002/1096-9071(200007)61:3<332::aid-jmv8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Murakami M. Hibi M. Nakagawa N. Nakagawa T. Yasukawa K. Yamanishi K. Taga T. Kishimoto T. IL-6-induced homodimerization of gp130 and associated activation of a tyrosine kinase. Science. 1993;260(5115):1808–1810. doi: 10.1126/science.8511589. [DOI] [PubMed] [Google Scholar]
- Myoung J. Ganem D. Infection of primary human tonsillar lymphoid cells by KSHV reveals frequent but abortive infection of T cells. Virology. 2011;413(1):1–11. doi: 10.1016/j.virol.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nador RG. Cesarman E. Chadburn A. Dawson DB. Ansari MQ. Sald J. Knowles DM. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpes virus. Blood. 1996;88(2):645–656. [PubMed] [Google Scholar]
- Narazaki M. Yasukawa K. Saito T. Ohsugi Y. Fukui H. Koishihara Y. Yancopoulos GD. Taga T. Kishimoto T. Soluble forms of the interleukin-6 signal-transducing receptor component gp130 in human serum possessing a potential to inhibit signals through membrane-anchored gp130. Blood. 1993;82(4):1120–1126. [PubMed] [Google Scholar]
- Nicholas J. Human gammaherpesvirus cytokines and chemokine receptors. J Interferon Cytokine Res. 2005;25(7):373–383. doi: 10.1089/jir.2005.25.373. [DOI] [PubMed] [Google Scholar]
- Nicholas J. Zong JC. Alcendor DJ. Ciufo DM. Poole LJ. Sarisky RT. Chiou CJ. Zhang X. Wan X. Guo HG. Reitz MS. Hayward GS. Novel organizational features, captured cellular genes, and strain variability within the genome of KSHV/HHV8. J Natl Cancer Inst Monogr. 1998;(23):79–88. doi: 10.1093/oxfordjournals.jncimonographs.a024179. [DOI] [PubMed] [Google Scholar]
- Oksenhendler E. Duarte M. Soulier J. Cacoub P. Welker Y. Cadranel J. Cazals-Hatem D. Autran B. Clauvel JP. Raphael M. Multicentric Castleman's disease in HIV infection: a clinical and pathological study of 20 patients. AIDS. 1996;10(1):61–67. [PubMed] [Google Scholar]
- Orzechowska BU. Powers MF. Sprague J. Li H. Yen B. Searles RP. Axthelm MK. Wong SW. Rhesus macaque rhadinovirus-associated non-Hodgkin lymphoma: animal model for KSHV-associated malignancies. Blood. 2008;112(10):4227–4234. doi: 10.1182/blood-2008-04-151498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea JJ. Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28(4):477–487. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parravicini C. Chandran B. Corbellino M. Berti E. Paulli M. Moore PS. Chang Y. Differential viral protein expression in Kaposi's sarcoma-associated herpesvirus-infected diseases: Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. Am J Pathol. 2000;156(3):743–749. doi: 10.1016/S0002-9440(10)64940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parravicini C. Corbellino M. Paulli M. Magrini U. Lazzarino M. Moore PS. Chang Y. Expression of a virus-derived cytokine, KSHV vIL-6, in HIV-seronegative Castleman's disease. Am J Pathol. 1997;151(6):1517–1522. [PMC free article] [PubMed] [Google Scholar]
- Pauk J. Huang ML. Brodie SJ. Wald A. Koelle DM. Schacker T. Celum C. Selke S. Corey L. Mucosal shedding of human herpesvirus 8 in men. N Engl J Med. 2000;343(19):1369–1377. doi: 10.1056/NEJM200011093431904. [DOI] [PubMed] [Google Scholar]
- Quarto N. Talarico D. Sommer A. Florkiewicz R. Basilico C. Rifkin DB. Transformation by basic fibroblast growth factor requires high levels of expression: comparison with transformation by hst/K-fgf. Oncogene Res. 1989;5(2):101–110. [PubMed] [Google Scholar]
- Rabe B. Chalaris A. May U. Waetzig GH. Seegert D. Williams AS. Jones SA. Rose-John S. Scheller J. Transgenic blockade of interleukin 6 transsignaling abrogates inflammation. Blood. 2008;111(3):1021–1028. doi: 10.1182/blood-2007-07-102137. [DOI] [PubMed] [Google Scholar]
- Ramsay AJ. Husband AJ. Ramshaw IA. Bao S. Matthaei KI. Koehler G. Kopf M. The role of interleukin-6 in mucosal IgA antibody responses in vivo. Science. 1994;264(5158):561–563. doi: 10.1126/science.8160012. [DOI] [PubMed] [Google Scholar]
- Russo JJ. Bohenzky RA. Chien MC. Chen J. Yan M. Maddalena D. Parry JP. Peruzzi D. Edelman IS. Chang Y. Moore PS. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci U S A. 1996;93(25):14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara S. Pise-Masison CA. Brady JN. Tosato G. Gene regulation and functional alterations induced by Kaposi's sarcoma-associated herpesvirus-encoded ORFK13/vFLIP in endothelial cells. J Virol. 2009;83(5):2140–2153. doi: 10.1128/JVI.01871-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara S. Tosato G. Regulation of angiogenesis in malignancies associated with Epstein-Barr virus and Kaposi's sarcoma-associated herpes virus. Future Microbiol. 2009;4(7):903–917. doi: 10.2217/fmb.09.49. [DOI] [PubMed] [Google Scholar]
- Sakakibara S. Ueda K. Chen J. Okuno T. Yamanishi K. Octamer-binding sequence is a key element for the autoregulation of Kaposi's sarcoma-associated herpesvirus ORF50/Lyta gene expression. J Virol. 2001;75(15):6894–6900. doi: 10.1128/JVI.75.15.6894-6900.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann WP. Bartoe JL. Nathanson NM. Box 3-independent signaling mechanisms are involved in leukemia inhibitory factor receptor alpha- and gp130-mediated stimulation of mitogen-activated protein kinase. Evidence for participation of multiple signaling pathways which converge at Ras. J Biol Chem. 1997;272(26):16631–16636. doi: 10.1074/jbc.272.26.16631. [DOI] [PubMed] [Google Scholar]
- Searles RP. Bergquam EP. Axthelm MK. Wong SW. Sequence and genomic analysis of a Rhesus macaque rhadinovirus with similarity to Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8. J Virol. 1999;73(4):3040–3053. doi: 10.1128/jvi.73.4.3040-3053.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakumar R. Sharma-Walia N. Raghu H. Veettil MV. Sadagopan S. Bottero V. Varga L. Levine R. Chandran B. Kaposi's sarcoma-associated herpesvirus induces sustained levels of vascular endothelial growth factors A and C early during in vitro infection of human microvascular dermal endothelial cells: biological implications. J Virol. 2008;82(4):1759–1776. doi: 10.1128/JVI.00873-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiniotis G. Boulanger MJ. Garcia KC. Walz T. Signaling conformations of the tall cytokine receptor gp130 when in complex with IL-6 and IL-6 receptor. Nat Struct Mol Biol. 2005;12(6):545–551. doi: 10.1038/nsmb941. [DOI] [PubMed] [Google Scholar]
- Somers W. Stahl M. Seehra JS. 1.9 A crystal structure of interleukin 6: implications for a novel mode of receptor dimerization and signaling. EMBO J. 1997;16(5):989–997. doi: 10.1093/emboj/16.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulier J. Grollet L. Oksenhendler E. Cacoub P. Cazals-Hatem D. Babinet P. d'Agay MF. Clauvel JP. Raphael M. Degos L. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995;86(4):1276–1280. others. [PubMed] [Google Scholar]
- Stahl N. Boulton TG. Farruggella T. Ip NY. Davis S. Witthuhn BA. Quelle FW. Silvennoinen O. Barbieri G. Pellegrini S. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science. 1994;263(5143):92–95. doi: 10.1126/science.8272873. others. [DOI] [PubMed] [Google Scholar]
- Sudarman E. Bollati-Fogolin M. Hafner M. Muller W. Scheller J. Rose-John S. Eichler J. Synthetic mimetics of the gp130 binding site for viral interleukin-6 as inhibitors of the vIL-6-gp130 interaction. Chem Biol Drug Des. 2008;71(5):494–500. doi: 10.1111/j.1747-0285.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- Suematsu S. Matsuda T. Aozasa K. Akira S. Nakano N. Ohno S. Miyazaki J. Yamamura K. Hirano T. Kishimoto T. IgG1 plasmacytosis in interleukin 6 transgenic mice. Proc Natl Acad Sci U S A. 1989;86(19):7547–7551. doi: 10.1073/pnas.86.19.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R. Lin SF. Staskus K. Gradoville L. Grogan E. Haase A. Miller G. Kinetics of Kaposi's sarcoma-associated herpesvirus gene expression. J Virol. 1999;73(3):2232–2242. doi: 10.1128/jvi.73.3.2232-2242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga T. Hibi M. Hirata Y. Yamasaki K. Yasukawa K. Matsuda T. Hirano T. Kishimoto T. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989;58(3):573–581. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- Teruya-Feldstein J. Zauber P. Setsuda JE. Berman EL. Sorbara L. Raffeld M. Tosato G. Jaffe ES. Expression of human herpesvirus-8 oncogene and cytokine homologues in an HIV-seronegative patient with multicentric Castleman's disease and primary effusion lymphoma. Lab Invest. 1998;78(12):1637–1642. [PubMed] [Google Scholar]
- Tosato G. Jones K. Breinig MK. McWilliams HP. McKnight JL. Interleukin-6 production in posttransplant lymphoproliferative disease. J Clin Invest. 1993;91(6):2806–2814. doi: 10.1172/JCI116523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosato G. Seamon KB. Goldman ND. Sehgal PB. May LT. Washington GC. Jones KD. Pike SE. Monocyte-derived human B-cell growth factor identified as interferon-beta 2 (BSF-2, IL-6) Science. 1988;239(4839):502–504. doi: 10.1126/science.2829354. [DOI] [PubMed] [Google Scholar]
- Uldrick TS. Wang V. O'Mahony D. Aleman K. Wyvill KM. Marshall V. Steinberg SM. Pittaluga S. Maric I. Whitby D. Tosato G. Little RF. Yarchoan R. An interleukin-6-related systemic inflammatory syndrome in patients co-infected with Kaposi sarcoma-associated herpesvirus and HIV but without Multicentric Castleman disease. Clin Infect Dis. 2010;51(3):350–358. doi: 10.1086/654798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J. Huang ML. Koelle DM. Corey L. Transmissible Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in saliva of men with a history of Kaposi's sarcoma. J Virol. 1997;71(9):7083–7087. doi: 10.1128/jvi.71.9.7083-7087.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J. O'Hearn P. Kimball L. Chandran B. Corey L. Activation of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) lytic replication by human cytomegalovirus. J Virol. 2001;75(3):1378–1386. doi: 10.1128/JVI.75.3.1378-1386.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X. Wang H. Nicholas J. Human herpesvirus 8 interleukin-6 (vIL-6) signals through gp130 but has structural and receptor-binding properties distinct from those of human IL-6. J Virol. 1999;73(10):8268–8278. doi: 10.1128/jvi.73.10.8268-8278.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterston A. Bower M. Fifty years of multicentric Castleman's disease. Acta Oncol. 2004;43(8):698–704. doi: 10.1080/02841860410002752. [DOI] [PubMed] [Google Scholar]
- Wen Z. Zhong Z. Darnell JE., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82(2):241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- West JT. Wood C. The role of Kaposi's sarcoma-associated herpesvirus/human herpesvirus-8 regulator of transcription activation (RTA) in control of gene expression. Oncogene. 2003;22(33):5150–5163. doi: 10.1038/sj.onc.1206555. [DOI] [PubMed] [Google Scholar]
- Woodberry T. Suscovich TJ. Henry LM. Martin JN. Dollard S. O'Connor PG. Davis JK. Osmond D. Lee TH. Kedes DH. Khatri A. Lee J. Walker BD. Scadden DT. Brander C. Impact of Kaposi sarcoma-associated herpesvirus (KSHV) burden and HIV coinfection on the detection of T cell responses to KSHV ORF73 and ORF65 proteins. J Infect Dis. 2005;192(4):622–629. doi: 10.1086/432103. [DOI] [PubMed] [Google Scholar]
- Yamasaki K. Taga T. Hirata Y. Yawata H. Kawanishi Y. Seed B. Taniguchi T. Hirano T. Kishimoto T. Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor. Science. 1988;241(4867):825–828. doi: 10.1126/science.3136546. [DOI] [PubMed] [Google Scholar]
- Yarchoan R. Tosato G. Little RF. Therapy insight: AIDS-related malignancies—the influence of antiviral therapy on pathogenesis and management. Nat Clin Pract Oncol. 2005;2(8):406–415. doi: 10.1038/ncponc0253. quiz 423. [DOI] [PubMed] [Google Scholar]
- Yin T. Taga T. Tsang ML. Yasukawa K. Kishimoto T. Yang YC. Involvement of IL-6 signal transducer gp130 in IL-11-mediated signal transduction. J Immunol. 1993;151(5):2555–2561. [PubMed] [Google Scholar]
- Zhong Z. Wen Z. Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264(5155):95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- Zilberstein A. Ruggieri R. Korn JH. Revel M. Structure and expression of cDNA and genes for human interferon-beta-2, a distinct species inducible by growth-stimulatory cytokines. EMBO J. 1986;5(10):2529–2537. doi: 10.1002/j.1460-2075.1986.tb04531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]