Abstract
The diagnosis of infective endocarditis can be difficult, particularly with atypical presentation and negative blood cultures. A 61-year-old man with a porcine aortic valve presented with fever, intermittent confusion, diarrhea, and fatigue. In the community clinic setting, a colonoscopy performed for anemia demonstrated colitis. Symptoms progressed for months; elicitation of a history of significant kitten exposure and the finding of an axillary lymph node prompted testing for Bartonella henselae antibodies. High titer antibodies by indirect immunofluorescence assay indicated chronic B. henselae infection. Surgical valve replacement followed by prolonged doxycycline and rifampin led to cure. This case illustrates the complexities of infective endocarditis and is the first description B. henselae endocarditis associated with colitis in an immunocompetent adult.
Key Words: Bartonella henselae, Colitis, Polymerase chain reaction, Prosthetic valve endocarditis
Introduction
Late diagnosis and inconsistent isolation of etiologic organisms contribute to the difficulties in managing endocarditis (Cicalini et al. 2006, Paterick et al. 2007). However, careful attention to potential patient exposures, physical examination finding, knowledge of the clinical subtleties of infective endocarditis, and a systematic, syndromic approach to differential diagnosis are critical to prompt diagnosis even in the face of atypical presentations and lack of positive culture cultures. This report describes a patient with Bartonella henselae prosthetic valve endocarditis with a novel presentation. Despite classic risk factors, diagnosis was delayed because of atypical presentation (colitis) and culture negativity.
Case Description
A 61-year-old man with a porcine aortic valve presented with fever, intermittent confusion, diarrhea, and fatigue to his community-based physician. Initial laboratory evaluation revealed anemia prompting a bone marrow biopsy and colonoscopy. Examination of the bone marrow demonstrated increased iron stores and hypercellular marrow, whereas colonoscopy showed acute and chronic colitis with abnormal architecture consistent with inflammatory bowel disease (IBD). However, negative IBD serologies (anti-neutrophil cytoplasmic antibody, anti–Saccharomyces cerevisiae antibody, anti-OmpC, and anti-CBir1) discouraged treatment for IBD (Nakamura and Barry 2001). Blood cultures (Bactec® [BD, Franklin Lakes, NJ] bottles incubated 3 weeks, including weekly blind subcultures onto chocolate agar), a transthoracic echocardiogram, and radiographic imaging of the head and abdomen were negative. Coxiella burnetti serological results (phase 1 titer, 1:128; phase 2 titer, 1:256) obtained before our evaluation prompted therapy with doxycycline and hydroxychloroquine.
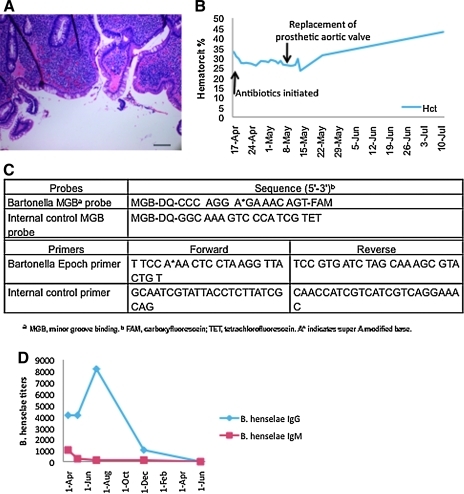
The patient continued with 4 months of unremitting symptoms before an infectious diseases consultant at our medical center elicited a history of cat scratches during work as a volunteer in a Mexican cat and dog rescue mission; the patient's two pet kittens that also had scratched him died 1 month before the onset of his symptoms. Physical examination findings on referral to our facility included fever, axillary lymphadenopathy, systolic and diastolic murmurs, and hepatosplenomegaly. A second colonoscopy showed neutrophil infiltration of the lamina propria, abnormal crypt architecture, and crypt abscesses consistent with IBD (Fig. 1A); polymerase chain reaction (PCR) for B. henselae of fixed colonic biopsy specimens was negative.
FIG. 1.
(A) Chronic inflammation of the colon lamina propria with architectural distortion of crypts (hematoxylin and eosin, 400×, scale bar 50 μM). (B) Response of hematocrit to antibiotics and surgery over time. (C) Sequences of primers and probes used in Bartonella henselae quantitative real-time polymerase chain reaction of cardiac valve. (D) Immunofluorescence assay titers against B. henselae (IgG and IgM) over the 13 months of doxycycline and rifampin treatment. (Color image available at www.liebertpub.com/vbz).
A repeat transthoracic echocardiogram revealed a prosthetic aortic valve vegetation, but blood cultures, incubated for 7 days, were again negative. Serology (immunofluorescence assay) for B. henselae (B. henselae immunoglobulin G [IgG] 1:4096, IgM 1:1024; B. quintana IgG 1:256, IgM <1:16) was consistent with endocarditis. Chlamydophila serology was also positive (IgG 1:2048; IgM <1:20), reflecting cross-reaction with the Bartonella spp.
Two weeks of treatment with intravenous gentamicin and oral doxycycline and rifampin did not lead to clinical response or change in hematocrit (Fig. 1B), reflecting ongoing infection and systemic inflammatory response despite maximal medical therapy. The prosthetic valve was surgically removed. While no organisms were observed on Warthin-Starry stain of the removed aortic valve prosthesis, real-time PCR demonstrated the presence of B. henselae DNA (Fig. 1C). A prolonged course of oral doxycycline and rifampin was associated with clinical improvement; this treatment was continued for ∼12 months until two consecutive serum samples were found to be negative for B. henselae titers (Fig. 1D). Clinical follow-up 2 years after stopping antibiotics found the patient symptom free and with a normal colonscopy.
Discussion
This report is the first to document B. henselae prosthetic valve endocarditis associated with colitis, identifying this complication as a new manifestation of chronic B. henselae infection. The first case of B. henselae endocarditis was reported by Spach et al. (1993), followed by a number of additional reports (reviewed in ref. Houpikian and Raoult 2005). Three previous cases of prosthetic cardiac valve-associated endocarditis have been reported (Lesprit et al. 2003, Hoffman et al. 2007, Vikram et al. 2007), one of which described cure without valve replacement (Lesprit et al. 2003). In the present case, valve replacement was clearly key to clinical cure in contrast to this previous case (Lesprit et al. 2003). The initial mistaken diagnosis of IBD delayed the identification of endocarditis; a directed history based on the syndromic diagnosis of culture-negative endocarditis led to the etiological identification of the patient's disease.
One other report of B. henselae infection describes association of this organism with small/large bowel involvement (Massei et al. 2000). An immunocompetent 13-year-old boy who had complained of 3 weeks of fever, fatigue, and right lower quadrant pain (Massei et al. 2000) had terminal ileal thickening demonstrated by ultrasonography that was considered consistent with Crohn's disease manifesting as ileitis; confirmatory biopsy was not performed. History in this case was pertinent for potential exposure to a kitten and a neighbor recently found to have Parinaud's oculoglandular syndrome; B. henselae serologies were positive and a repeat ultrasonography after treatment with ciprofloxacin and azithromycin revealed resolution of bowel wall thickening. More widespread colonic involvement was not demonstrated in this case, as was present in the patient discussed herein.
Bartonella spp. were first described as a cause of endocarditis in 1993 (Maurin and Raoult 1998). Despite advances in PCR diagnosis, Bartonella spp. remain uncommonly reported as agents of endocarditis (<5%) with B. quintana and B. henselae representing 95% of these cases. Homelessness, alcoholism, and body lice have been associated with disease caused by B. quintana, whereas pre-existing valvular disease and cat contact are risk factors for B. henselae (Raoult et al. 1996, Maurin and Raoult 1998, Fournier et al. 2001, Houpikian and Raoult 2005).
The diagnosis of Bartonella endocarditis is difficult because the organism is fastidious, only growing from blood cultures in a minority of cases (Raoult et al. 1996, La Scola and Raoult 1999). The diagnosis of systemic Bartonella endocarditis generally depends on serology, but cross-reactivity exists among Bartonella species and with other organisms. Chlamydophila species and Coxiella burnetti cross-react with Bartonella, but at lower titers (Raoult et al. 1996). Understanding this relationship allows practitioners to use these serological tests as surrogate biomarkers of infection while awaiting confirmatory Bartonella spp. immunofluorescence assay titers (Fournier et al. 2002). Culture and PCR of cardiac tissue can provide a definitive diagnosis (Maurin and Raoult 1998, Houpikian and Raoult 2005).
This is the first reported case of B. henselae endocarditis in an immunocompetent adult associated with colitis that mimicked IBD. To the best of our knowledge, this is the only reported case of infectious endocarditis demonstrated to present as colitis. Whether the clinical picture consistent with IBD was due to the direct effect of the pathogen or immune triggering of an IBD-like colitis remains unclear. Infection with B. henselae in the immunocompetent person prompts a strong cellular-mediated immune response, as evident by the historical cat-scratch disease skin test (Resto-Ruiz et al. 2003). Recent studies in vitro and ex vivo of B. henselae–infected A/J murine splenocytes highlight the importance of the Th-1 pathway in the pathogenesis of Bartonella spp., but a complete understanding of host–bacterial interactions in thius infection remain elusive (Resto-Ruiz et al. 2003). Our patient's negative blood and explanted aortic prosthesis culture, Warthin-Starry staining, and PCR of the colon biopsy suggest that immunopathogenesis was the central mechanism responsible for the IBD-like colitis. Confirmation of immune system driving clinical manifestations of Bartonella infection will require future in vivo work with mouse models and ex vivo studies with chronically infected patients. Understanding the microbiological differential diagnosis of culture-negative endocarditis in patients with prosthetic valves requires a good history and physical examination, which, combined with focused approaches to establish the specific etiology, allows for timely and appropriate management.
Acknowledgments
This work was supported in part by U.S. Public Health Services grants from the National Institute of Allergy and Infectious Diseases, National Institutes of Health 5K24AI068903 (J.M.V.) and 5T32AI007384 (M.Y.K.).
Disclosure Statement
No competing financial interests exist.
References
- Cicalini S. Puro V. Angeletti C. Chinello P, et al. Profile of infective endocarditis in a referral hospital over the last 24 years. J Infect. 2006;52:140–146. doi: 10.1016/j.jinf.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Fournier PE. Lelievre H. Eykyn SJ, et al. Epidemiologic and clinical characteristics of Bartonella quintana and Bartonella henselae endocarditis: a study of 48 patients. Medicine (Baltimore) 2001;80:245–251. doi: 10.1097/00005792-200107000-00003. [DOI] [PubMed] [Google Scholar]
- Fournier PE. Mainardi JL. Raoult D. Value of microimmunofluorescence for diagnosis and follow-up of Bartonella endocarditis. Clin Diagn Lab Immunol. 2002;9:795–801. doi: 10.1128/CDLI.9.4.795-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman RM. AboulHosn J. Child JS. Pegues DA. Bartonella endocarditis in complex congenital heart disease. Congenit Heart Dis. 2007;2:79–84. doi: 10.1111/j.1747-0803.2007.00077.x. [DOI] [PubMed] [Google Scholar]
- Houpikian P. Raoult D. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine (Baltimore) 2005;84:162–173. doi: 10.1097/01.md.0000165658.82869.17. [DOI] [PubMed] [Google Scholar]
- La Scola B. Raoult D. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998) J Clin Microbiol. 1999;37:1899–1905. doi: 10.1128/jcm.37.6.1899-1905.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesprit P. Noel V. Chazouilleres P. Brun-Buisson C. Deforges L. Cure of Bartonella endocarditis of a prosthetic aortic valve without surgery: value of serologic follow-up. Clin Microbiol Infect. 2003;9:239–241. doi: 10.1046/j.1469-0691.2003.00509.x. [DOI] [PubMed] [Google Scholar]
- Massei F. Massimetti M. Messina F. Macchia P. Maggiore G. Bartonella henselae and inflammatory bowel disease. Lancet. 2000;356:1245–1246. doi: 10.1016/S0140-6736(00)02796-3. [DOI] [PubMed] [Google Scholar]
- Maurin M. Raoult D. Bartonella infections: diagnostic and management issues. Curr Opin Infect Dis. 1998;11:189–193. [PubMed] [Google Scholar]
- Nakamura RM. Barry M. Serologic markers in inflammatory bowel disease (IBD) MLO Med Lab Obs. 2001;33:8–15. quiz 6–9. [PubMed] [Google Scholar]
- Paterick TE. Paterick TJ. Nishimura RA. Steckelberg JM. Complexity and subtlety of infective endocarditis. Mayo Clin Proc. 2007;82:615–621. doi: 10.4065/82.5.615. [DOI] [PubMed] [Google Scholar]
- Raoult D. Fournier PE. Drancourt M, et al. Diagnosis of 22 new cases of Bartonella endocarditis. Ann Intern Med. 1996;125:646–652. doi: 10.7326/0003-4819-125-8-199610150-00004. [DOI] [PubMed] [Google Scholar]
- Resto-Ruiz S. Burgess A. Anderson BE. The role of the host immune response in pathogenesis of Bartonella henselae. DNA Cell Biol. 2003;22:431–440. doi: 10.1089/104454903767650694. [DOI] [PubMed] [Google Scholar]
- Spach DH. Callis KP. Paauw DS, et al. Endocarditis caused by Rochalimaea quintana in a patient infected with human immunodeficiency virus. J Clin Microbiol. 1993;31:692–694. doi: 10.1128/jcm.31.3.692-694.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikram HR. Bacani AK. DeValeria PA. Cunningham SA. Cockerill FR., 3rd Bivalvular Bartonella henselae prosthetic valve endocarditis. J Clin Microbiol. 2007;45:4081–4084. doi: 10.1128/JCM.01095-07. [DOI] [PMC free article] [PubMed] [Google Scholar]