Abstract
Recent reports have suggested that oral vaccination of mice against Helicobacter pylori is dependent on a Th1-mediated immune response. However, oral vaccination in mice neither induces sterilizing immunity nor leads to complete protection from disease. Therefore, in this study we investigated whether a systemic subcutaneous immunization against H. pylori by using CpG oligodeoxynucleotides as a Th1 adjuvant could achieve protection in a mouse model of H. pylori infection. CpG oligodeoxynucleotides are known for their ability to induce nearly entirely Th1-biased immune responses and may be approved for human use in future. Immunization of mice with H. pylori lysate and CpG induced a strong local and systemic Th1 immune response. Despite this strong Th1 response, mice were not protected from infection with H. pylori yet had a 10-fold reduction in the number of H. pylori in the gastric mucosa compared to nonimmunized mice. Of note, reduction of the bacterial density in immunized mice was accompanied by a significantly enhanced gastritis. Hence, systemic Th1 immunization of mice, even though being able to reduce the bacterial load in the stomach, is associated with aggravated pathology.
Infection with Helicobacter pylori is thought to represent one of the most common bacterial infections, with about half of the worldwide population being infected. H. pylori is a gram-negative bacterium that resides in the mucosa of the human stomach (21). Colonization of the stomach by H. pylori is associated with the risk of different gastroduodenal diseases including atrophic gastritis, duodenal ulcer, gastric cancer, and mucosa-associated lymphoid tissue lymphoma. The pathogenesis of these diseases is not yet fully understood; however, virulence factors of H. pylori as well as the immune response of the host are thought to play a role. H. pylori infection can be treated with a combination of antibiotics and proton pump inhibitors, with eradication rates above 80%. However, side effects of such a therapy as well as increasing bacterial resistance have raised the question whether H. pylori infection can be prevented or cured by vaccination.
To analyze the efficacy and mechanisms of vaccination, mouse models of H. pylori infection have been extensively used. These studies have focused primarily on oral and intranasal vaccination protocols to achieve mucosal immunity. Although an attractive concept, it is not yet clear whether mucosal immunization can protect from H. pylori infection or from H. pylori-mediated diseases in humans (18, 22). Therefore, the idea of systemic immunization against H. pylori has also been promoted (11, 12). Since recent reports proposed that the protective effects seen after mucosal vaccination are dependent on a Th1-type response, systemic application of a Th1-vaccine may be a suggestive approach. So far, only one study has addressed the question of Th1-biased systemic immunization against H. pylori. In that study, complete Freund's adjuvant was used and applied intraperitoneally, together with a Helicobacter lysate, to achieve complete protection from infection (11).
Even though it showed that a Th1 response may lead to protection, this vaccination protocol is not applicable in humans. Therefore, we wondered whether a Th1 vaccination protocol with the chance for approval in humans would also be able to achieve protection in the mouse model. For this reason, a CpG oligodeoxynucleotide was chosen as an adjuvant, since studies using CpGs as adjuvants in humans are under way and because of their outstanding activity of inducing Th1-biased immune responses. CpGs are synthetic oligodeoxynucleotides which contain cytosine-guanosine dinucleotide motifs and therefore can mimic the immunostimulatory capacities of bacterial DNA (14). In the present study, mice were vaccinated subcutaneously with CpGs plus a bacterial whole-cell lysate of H. pylori and the effect of this vaccination on the course of the disease was investigated.
MATERIALS AND METHODS
Oligonucleotides.
CpG oligonucleotide 1668 (TCCATGACGTTCCTGATGCT) was obtained from MWG-Biotech as complete phosphothioate-modified oligonucleotide. All oligonucleotides used for quantitative reverse transcriptase PCR (RT-PCR) were synthesized by Applied Biosystems (OligoFactory, Weiterstadt, Germany). The sequences were as follows: gamma interferon (IFN-γ) forward primer, GCAACAGCAAGGCGAAAAAG; IFN-γ reverse primer, TTCCTGAGGCTGGATTCCG; IFN-γ TaqMan probe, 6-FAM-ATGCATTCATGAGTATTGCCAAGTTTGAGGTC-TAMRA; interleukin-4 (IL-4) forward primer, GGCATTTTGAACGAGGTCAC; IL-4 reverse primer, GCATGGAGTTTTCCCATGTT; IL-4 TaqMan probe, 6-FAM-TCCTCACAGCAACGAAGAACACCACA-TAMRA; IL-10 forward primer, GTTGCCAAGCCTTATCGGAA; IL-10 reverse primer, CCGCATCCTGAGGGTCTTC; IL-10 TaqMan probe, 6-FAM-CAGTTTTACCTGGTAGAAGTGATGCCCCAGG-TAMRA; hypoxanthine phosphoribosyltransferase (HPRT) forward primer, CTGGTGAAAAGGACCTCTCG; HPRT reverse primer, TGAAGTACTCATTATAGTCAAGGG; HPRT TaqMan probe, 6-FAM-TGTTGGATACAGGCCAGACTTTGTTGGAT-TAMRA; tumor necrosis factor alpha (TNF-α) forward primer, AAAATTCGAGTGACAAGCCTGTAG, TNF-α reverse primer, CCCTTGAAGAGAACCTGGGAGTAG; TNF-α TaqMan probe, 6-FAM-CACGTCGTAGCAAACCACCAAGTGGA-TAMRA.
Mice.
Female C57BL/6 mice were obtained from Charles River Breeding Laboratories (Sulzfeld, Germany) and were infected at 8 to 12 weeks of age.
Culture of H. pylori and preparation of whole-cell lysates.
The Sydney strain of H. pylori (19) was kindly provided by A. Lee (University of New South Wales, Sydney, Australia) and was used throughout these experiments. H. pylori was cultured microaerobically at 37°C on Columbia agar plates containing 10% horse serum. For the preparation of a whole-cell lysate, H. pylori was harvested from the agar plates with a cotton swab and suspended in phosphate-buffered saline (PBS). The ice-cold suspension was subjected to four sonication steps (30 s at 4°C). After each sonication step, the suspension was cooled on ice again for 1 min. This whole-cell lysate was used for the immunization of mice. Protein concentrations of the lysates were measured after low-speed centrifugation in the supernatant by a standard protein assay (Sigma).
Infection of mice with H. pylori.
Female C57BL/6 mice were infected three times at 48-h time intervals with 200 μl of a bacterial suspension in PBS containing approximately 109 CFU of the H. pylori Sydney strain. The bacterial suspension was applied directly into the stomach through a feeding needle. Control mice received 200 μl of PBS.
Immunization of mice.
Female C57BL/6 mice were immunized with 200 μg of H. pylori whole-cell lysate in 100 μl of PBS containing 5 nmol of CpG oligonucleotide 1668. The vaccine was injected subcutaneously in the back of each mouse, to the left or right of the spinal column, on days 0 and 14. Control mice received 200 μl of PBS or 200 μl of PBS containing CpG oligonucleotide 1668. The mice were challenged three times with viable H. pylori on days 30, 32, and 34.
Assessment of bacterial load in the stomach.
H. pylori was recovered from the stomach of mice as described previously (29). In brief, the stomach of each mouse was divided longitudinally into three parts. One-third of the stomach was fixed in 4% formaldehyde, and one-third was immediately frozen in liquid nitrogen for RT-PCR analysis. Another third was mechanically disrupted in an Eppendorf Cap containing 100 μl of PBS, and 1:10, 1:100, and 1:1,000 dilutions of this suspension in PBS were prepared. A 50-μl volume of each dilution was spread on Columbia agar plates containing 10% horse serum, Dent's supplement (Oxoid), bacitracin, and nalidixic acid. At 5 days later, numbers of H. pylori colonies on the plates were counted. H. pylori was identified by positive urease and oxidase reaction and the Gram's reaction.
Determination of cytokines by ELISA.
Single-cell suspensions were prepared from the spleens of the mice. Spleen cells from individual mice were cultured in vitro (106 per well) together with irradiated (20 Gy) syngeneic spleen cells (2 × 106 per well) and with or without H. pylori lysate (1 and 10 μg/ml) in a total volume of 1 ml. Supernatants were harvested after 48 h and tested for IFN-γ and IL-4 by using commercial enzyme-linked immunosorbent assay (ELISAs) (Pharmingen, Hamburg, Germany). The values were standardized against recombinant IL-4 and IFN-γ.
Determination of H. pylori-specific antibodies in serum.
Sera from individual mice were tested for antibodies by ELISAs. Microtiter plates were coated with H. pylori lysate (10 μg/ml) in coating buffer (0.1 M NAHCO3 [pH 8.2]) overnight at 4°C. After being washed, the plates were blocked for 2 h with sample buffer (PBS containing 10% fetal calf serum). The plates were washed again, and 50 μl of serum, diluted in sample buffer, was added. Binding of mouse antibodies to the plates was detected using peroxidase-conjugated anti-mouse antibodies and 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) as a substrate. Isotype-specific antibodies (immunoglobulin G1 [IgG1] and IgG2a) were detected using a kit purchased from Southern Biotechnology Associates (Birmingham, Ala.)
Histopathological evaluation.
One-third of the longitudinally bisected murine stomach was fixed in 4% formalin solution. The specimens were dehydrated and paraffin embedded by using routine procedures. Blocks were cut into 3-μm sections and stained with hematoxylin-eosin. The slides were histologically examined by one experienced pathologist. Alterations in the gastric mucosa were classified according to the updated Sydney classification system (4). The grade and activity of gastritis were scored with respect to the density of the lymphocytic or polymorphonuclear infiltrate, respectively, from grade 0 (no gastritis) to grade 3 (severe gastritis). All histological analyses were performed blinded, i.e., without knowing the experimental protocol used for each tissue sample.
Immunohistochemistry.
Sections 3 μm thick were deparaffinized and hydrated with graded ethanols by using routine procedures. For antigen retrieval, sections for CD3 immunostaining were heated at 120°C and 15 lb/in2 for 30 s in 10 mM citrate buffer (pH 6.0). The sections were then washed with 0.5 M Tris buffer (pH 7.4) and incubated overnight with primary rat anti-CD3 antibody (Serotec, Duesseldorf, Germany), diluted 1:50, to detect T cells and with rat anti-CD45R/B220 (BD Biosciences Pharmingen, San Diego, Calif.), diluted 1:500 to detect B cells. After the sections were rinsed in Tris-buffer, detection was carried out with biotinylated secondary rabbit anti-rat antibodies (Vektor/Linaris, Wertheim, Germany), diluted 1:50, for 30 min at room temperature. The sections were washed as above in Tris buffer and then incubated with streptavidin-biotin-alkaline phosphatase (Dako, Hamburg, Germany) in 10 ml of Tris buffer. Immunohistochemical staining was visualized with Fast Red (2 mg of 3-hydroxy-2-naphthoic acid-2,4-dimethylanilide-phosphate [naphthol-As-MX-phosphate], 10 μl of 1 M levamisole, and 10 mg of Fast Red [Sigma] in 0.2 ml of N,N-dimethylformamide [Merck, Darmstadt, Germany] and 9.8 ml of 0.1 M Tris-HCl buffer at pH 8.6). The sections were rinsed in double-distilled H2O and counterstained with Mayer's hemalaun (Merck).
TaqMan Analysis.
One-third of the murine stomach was immediately frozen in liquid nitrogen and stored at −80°C. The tissue was homogenized in TRIZOL reagent (Gibco-BRL, Eggenstein, Germany), and RNA was prepared from these specimens as specified by the manufacturer. DNA was digested at 37°C for 30 min in 100 μl of transcription-optimized buffer (Promega, Madison, Wis.) containing DNase I (10 U/μl; Roche, Basel, Switzerland) and RNasin (40 U/μl; Promega). Then the RNA was further purified by using the RNeasy minikit (Qiagen, Hilden, Germany) and the protocol for RNA cleanup (Qiagen). DNA (final volume, 25 μl) was synthesized from 2 μg of purified RNA with the Revert Aid first-strand cDNA synthesis kit (MBI Fermentas, St. Leon-Rot, Germany). A 2.5-μl volume of each DNA sample was transferred into PCR buffer (Perkin-Elmer) containing 6 mM MgCl2, 10 mM each dATP, dCTP, and dGTP, 20 mM dUTP, 50 pmol of each primer, 150 nM TaqMan probe, 0.25 μl of AmpErase, and 2.5 U of AmpliTaq in a final volume of 25 μl. Amplification was performed in an ABI Prism 7700 sequence detector programmed to hold at 50°C for 2 min, hold at 95°C for 10 min, and complete 45 cycles of 95°C for 15 s and 60°C for 1 min. The data were analyzed in the real-time mode. All PCR amplifications were performed at least in duplicate and with a no-reverse transcriptase (no-RT) control run for each sample. Baseline values and thresholds were set manually. The values are given as difference in threshold cycle (ΔCT), which was calculated as ΔCT = CT(cytokine) − CT(HPRT).
RESULTS
Systemic immunization with CpG oligonucleotides and H. pylori lysate induces high titers of Helicobacter-specific antibodies.
Systemic immunization of mice against H. pylori has been described to induce immunity that protects mice to different degrees from infection (11, 12, 33). However, it is not yet clear whether Th1 cells, Th2 cells or a combination of the two should be used to achieve complete protection. To gain more insight into the question of the extent to which Th1 or Th2 cells might contribute to protection after systemic immunization, we set up a study in which C57BL/6 mice were immunized twice subcutaneously with a whole-cell lysate of H. pylori with CpG as adjuvant. CpG is known for its strong ability to exclusively induce Th1-biased immune responses and has potential applications in humans. Control mice received CpG in PBS or PBS alone. At 4 weeks after the first immunization, mice were challenged three times with viable H. pylori. At 12 to 14 weeks after challenge, the mice were sacrificed and immunological parameters as well as pathology and bacterial load of the stomach were assessed.
To test whether this immunization protocol efficiently elicits a serological response, levels of Helicobacter-specific IgG in the sera of infected and/or immunized mice were determined by ELISA 3 months after challenge. As depicted in Table 1, immunized mice had high levels of Helicobacter-specific IgG regardless of whether they were challenged with viable H. pylori. In contrast, mice that were infected but not immunized had lower levels of Helicobacter-specific IgG. Mice that were injected with CpG alone (without H. pylori lysate) and challenged had a serological response comparable to that of nonimmunized, infected mice (data not shown). No Helicobacter-specific antibodies could be detected in uninfected animals or in mice that received CpG only (data not shown). Hence, systemic immunization with Helicobacter lysate and CpG leads to a humoral response that is significantly stronger than the antibody response during infection alone.
TABLE 1.
Helicobacter-specific IgG in immunized and/or infected mice
| Treatmenta | OD of Helicobacter-specific IgGb | IgG1/IgG2ac |
|---|---|---|
| Nonimmunized, infected | 204 ± 177 | 1.58 ± 0.62 |
| Immunized, infected | 1,354 ± 511 | 0.85 ± 0.6 |
| Immunized, noninfected | 1,200 ± 125 | NDd |
Mice were immunized with CpG and H. pylori lysate (immunized) or injected with PBS (nonimmunized) and challenged with H. pylori (infected) or PBS (noninfected). At 3 months after the challenge, sera were taken from the mice and tested for H. pylori-specific IgG1, IgG2a, or total IgG by ELISA.
Mean optical density (OD) of specific IgG for at least 12 mice in each group.
The IgG1/IgG2a ratio was calculated from the ODs of Helicobacter-specific IgG1 and IgG2a of 12 mice in each group.
ND, not done.
CpG immunization prior to infection with H. pylori leads to an enhanced Th1 response.
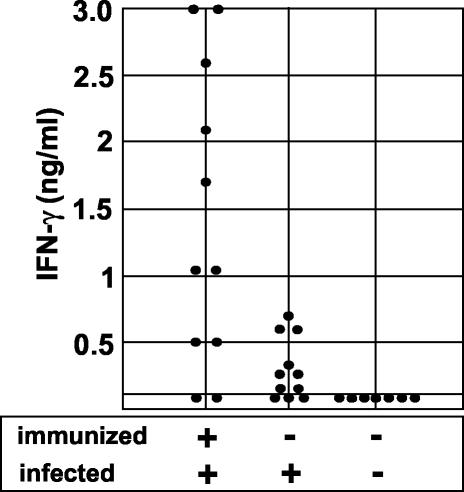
As mentioned above, CpG is known to be an adjuvant that triggers primarily a Th1-biased immune response. However, H. pylori infection itself is thought to elicit a Th1 rather than a Th2 response. To test whether immunization with CpG prior to infection is still able to modulate the immune response, IFN-γ production by splenic cells in response to H. pylori antigens was analyzed. In addition, the mRNA levels of IFN-γ, TNF-α, IL-4, and IL-10 in the gastric mucosa were determined by quantitative RT-PCR. As shown in Fig. 1, splenic cells of mice that were immunized and challenged produced significantly more IFN-γ than did cells of nonimmunized and infected mice. The application of CpG alone (without H. pylori lysate) prior to infection did not enhance IFN-γ production by splenocytes (data not shown). Splenic cells from control mice (nonimmunized and not infected) did not secrete IFN-γ in the supernatant. IL-4, as a marker of Th2 responses, was detectable neither in the supernatants from infected mice nor in the supernatants from immunized and challenged mice (data not shown).
FIG. 1.
IFN-γ production by splenic cells from immunized and/or infected mice. Mice were immunized with CpG and H. pylori lysate or injected with PBS and challenged with H. pylori or PBS. At 3 months after the challenge, the mice were killed and the spleens were removed. Single-cell suspensions from the spleens of individual mice were restimulated in vitro with or without H. pylori lysate (10 μg/ml). Supernatants were harvested after 48 h and tested for IFN-γ and IL-4 by ELISA.
Analysis of gastric mucosal mRNA levels for the Th1 cytokines IFN-γ and TNF-α revealed that expression of both cytokines was higher in infected than in noninfected mice 3 months after challenge (P < 0.05 [Fig. 2]). Immunization prior to infection led to an additional increase in the level of mucosal mRNA for IFN-γ and TNF-α (P < 0.05). Immunization and challenge, but not infection alone, led to only a slight increase in the mucosal levels of the Th2 cytokine IL-10 over those in noninfected control mice. IL-4 was found to be only slightly expressed in the gastric mucosa, with no statistically significant differences between control mice and infected and/or immunized mice (Fig. 2).
FIG. 2.
Levels of mRNA for IFN-γ, TNF-α, IL-4, and IL-10 in the stomach mucosa of immunized and/or infected mice as assessed by quantitative RT-PCR. One-third of the stomach of each of the mice described in Fig. 1 was immediately frozen in liquid nitrogen and stored at −80°C. Quantitative RT-PCR was performed to determine the amount of mRNA for IFN-γ, TNF-α, IL-4, IL-10, and HPRT (as a housekeeping gene) in the stomach. The values are given as ΔCT.
Therefore, immunization with CpG and H. pylori lysate prior to infection leads to an enhanced systemic as well as local immune response, which is predominantly of a Th1 type. This enhanced Th1 response was also reflected by a decrease in the ratio of H. pylori-specific IgG1/IgG2a (Table 1).
Th1 immunization against H. pylori with CpG oligonucleotides does not protect mice from infection but enhances gastritis.
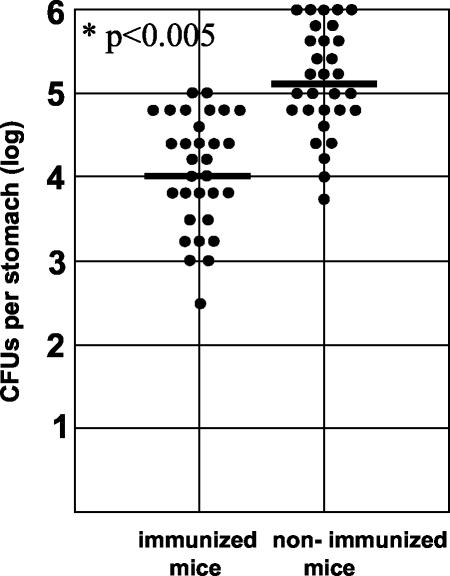
Of note, despite Th1 immunization, the mice were not protected from infection by H. pylori, because H. pylori could be recovered from the stomachs of nearly all immunized mice (Fig. 3). However, the bacterial load in the stomach was 10 times lower than that in nonimmunized animals. Thus, Th1 immunization of mice against H. pylori did not protect mice from infection but did reduce the bacterial load in the stomach. To analyze whether the reduction of the bacterial load in the immunized mice was associated with a change in the severity of the pathology, gastritis scores for immunized mice were compared to those for nonimmunized mice. The histopathological evaluation was performed blinded by one experienced pathologist. The grade and activity of gastritis were assessed with respect to the density of the lymphocytic (grade [Fig. 4A]) or polymorphonuclear (activity [Fig. 4B]) infiltrate on the basis of the updated Sydney classification system. As expected, nonimmunized mice had developed gastritis 12 weeks after infection (Fig. 4 and 5A). Gastritis in these mice was corpus and cardia predominant, with an average gastritis grade and activity of 0.7. Nonimmunized control mice that had received PBS instead of H. pylori did not show signs of gastritis (data not shown).
FIG. 3.
Density of H. pylori in the stomachs of immunized and/or infected mice. Mice were immunized with CpG and H. pylori lysate or injected with PBS and challenged with H. pylori or PBS. At 3 months after the challenge, the mice were killed and the numbers of H. pylori bacteria per stomach were assessed by agar dilution as described in Materials and Methods.
FIG. 4.
Gastritis scores for immunized and/or infected mice. Mice were immunized with CpG and H. pylori lysate or injected with PBS and challenged with H. pylori or PBS. At 3 months after the challenge, the mice were killed and one-third of the stomach of each mouse was subjected to histopathological analysis. The grade and activity of gastritis was scored with respect to the density of the lymphocytic or polymorphonuclear infiltrate from grade 0 (no gastritis) to grade 3 (severe gastritis).
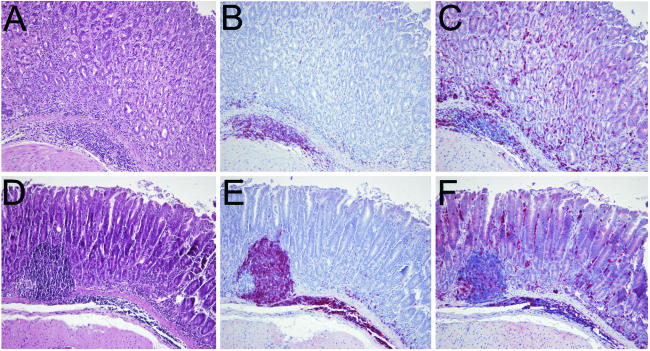
FIG. 5.
Histopathology and immunohistochemistry in a nonimmunized, infected mouse (A to C) and an immunized and challenged mouse (D to F). (A and D) Representative stainings with hematoxylin-eosin of a nonimmunized, infected mouse (A) and an immunized and challenged mouse (D). (B and E) Staining of B cells with anti-B220 antibodies in a nonimmunized, infected mouse (B) and an immunized and challenged mouse (E). B lymphocytes assembled mainly in follicle-like aggregates, and only a few were detectable in more luminal areas of the mucosa. (C and F) Staining of T cells with anti-CD3 antibodies in a nonimmunized, infected mouse (C) and an immunized and challenged mouse (F). In contrast to B cells, T cells were detected predominantly in the upper parts of the mucosa between gastric foveoli and glands. Magnification, ×10.
Interestingly, mice that had been immunized against H. pylori and challenged had significantly higher gastritis scores, i.e., had increased lymphocytic (score, 1.5) and polymorphonuclear (score, 1.3) infiltration (Fig. 4 and 5D). Thus, despite a reduction of the bacterial density in the stomach of immunized mice, gastric inflammation and pathology in these mice was enhanced (P < 0.005).
To check whether immunization with CpG leads to an alteration in the composition of the lymphocytic infiltrate, immunohistochemical stainings for B and T cells were performed. In animals which developed gastritis, mixed T- and B-lymphocytic infiltrates were detected in the mucosa and submucosa (Fig. 5). Lymphocytes were partly assembled in follicle-like aggregates, which consisted mainly of B cells. These aggregates were located in the basal part of the mucosa or in the submucosa. Only a few B cells were detectable in the more luminal areas of the mucosa (Fig. 5B and E). In contrast, T cells were detected predominantly in the upper parts of the mucosa between gastric foveoli and glands (Fig. 5C and F). However, the composition and distribution of inflammatory cells were not different in immunized and nonimmunized animals with gastritis. Taken together, the results show that a strong Th1 response to H. pylori can be achieved by systemic immunization with H. pylori lysate and CpG. This Th1 response may be considered a two-edged sword: it is able to reduce the bacterial load at the expense of enhanced gastritis.
DISCUSSION
A couple of earlier studies have addressed the role of T cells in H. pylori infection by using mouse models. Most of these models have unequivocally demonstrated that development of gastritis and/or pathology is dependent on Th1 cells and Th1 cytokines (23, 27, 28, 29). Hence, the Th1-cell response to H. pylori is thought to be essential for the development of gastritis during H. pylori infection in mice. In contrast, the role of T cells in protection after immunization of mice against H. pylori is far less clear, even though it is generally accepted that T cells play a decisive role in the immunization process (5, 7). This has been elegantly shown in a study by Ermak et al. (7), where a 100- to 1,000-fold reduction in the density of H. pylori in the stomach mucosa could be achieved by intranasal immunization with Helicobacter-derived urease. This effect was dependent on the presence of CD4+ T cells, since major histocompatibility complex class II-deficient mice, which lack CD4+ T cells, could not be protected. However, so far it is not clear whether particular subtypes of CD4+ T cells, such as Th1 or Th2 cells, contribute to protection in murine vaccination models. In a mouse model using H. felis, the adoptive transfer of H. felis-specific Th2 cell lines resulted in a reduction of the bacterial load in the stomach (24). Accordingly, it was reported that oral immunization with urease is associated with an enhanced IL-4 production by splenocytes (26). Thus, oral immunization of mice may lead to the induction of Helicobacter-specific Th2 cells and these cells may exert protective effects. However, in mouse models using H. pylori, it was demonstrated that transfer of T cells from immunized animals can lead to partial protection independent of IL-4 or IL-13 receptor signaling (20). Thus, if Th2 cells indeed play a role in protection, their effects may be mediated by other cytokines than IL-4, for example IL-10, a cytokine that has been associated with protection in H. pylori gastritis (2, 15, 31). In view of these findings, it has been suggested that in H. pylori infection, Th1 cells cause disease whereas Th2 cells might mediate protection, i.e., are able to reduce the bacterial density in the stomach.
However, this view has been challenged by a couple of recent studies. In a mouse model of H. felis infection, coinfection of mice with a replication-defective adenovirus led to a significant decrease in the density of H. felis colonization (16). Interestingly, this effect was not present in IL-12- or IFN-γ-deficient mice. In line with the results of this study, two recent reports demonstrated that oral immunization of mice with H. pylori lysate and cholera toxin had protective effects in IL-4-deficient mice but not in IL-12-deficient mice (1, 8). Controversial data were obtained in those studies regarding IFN-γ: whereas IFN-γ was essential for protection in one study (1), it was not required in the other (8). Thus, in contrast to the above-mentioned hypothesis, induction of a Th1-type response by IL-12 seems to be essential for protective effects after mucosal immunization, but it is unclear whether these protective effects are dependent on IFN-γ.
Mucosal immunization of mice has been shown in most studies to significantly reduce the bacterial load but not to induce sterilizing immunity. In addition, studies of mucosal vaccination against H. pylori in humans have so far not been successful (18, 22). Given that Th1 immunity might be necessary for protection, one could hypothesize that mucosal vaccination against H. pylori is not effective because it does not sufficiently activate Th1 cells. For this reason, systemic application of H. pylori antigens together with Th1 adjuvants has also been considered as an alternative strategy for vaccination. In mice, systemic immunization induced partially protective immunity (7, 12, 33). However, these studies did not address the effect of adjuvants that exclusively activate Th1 cells. One recent study achieved sterilizing immunity by systemic Th1 immunization with complete Freund's adjuvant (12). Even though the results are interesting, the protocol for Th1 vaccination in that study is not applicable in humans due to the adjuvant and the intraperitoneal route of immunization.
In the present study we asked whether systemic subcutaneous immunization with an adjuvant that triggers primarily Th1 cells and that has a chance to be approved for human use in future would be protective in mice. CpG oligodeoxynucleotide 1668 was used as the adjuvant because of its outstanding capacity to induce almost entirely a Th1-type response (14). A whole-cell lysate of H. pylori was chosen as the antigen, since lysates of H. pylori are relatively effective oral vaccines (24, 32). It should be mentioned that whole-cell lysates of H. pylori have previously been shown to induce Th2 responses in vitro (13). However, in our experiments, mice that were immunized (and not challenged) with CpG and H. pylori lysate clearly mounted a Th1 response, since splenic cells produced IFN-γ, but not IL-4, in response to H. pylori antigens in vitro (data not shown). Thus, the strong Th1-inducing effect of CpG may override the Th2-inducing effect of a whole-cell H. pylori lysate.
One important result of our study is that despite the induction of a strong local and systemic Th1 response to H. pylori by CpG vaccination, mice were not protected from colonization or from disease. The reasons for the difference from the study cited above, which achieved complete protection by systemic Th1 immunization, is so far unknown but could reside in the adjuvants used (e.g. complete Freund's adjuvant versus CpG) or the route of application. Independent of those factors, the efficacy of immunization in different models may also be dependent on the H. pylori strain used for challenge (17).
Furthermore, we observed, that Th1 vaccination by CpG led to a 10-fold decrease in bacterial density but to significantly enhanced gastritis. This inverse relationship between the number of H. pylori cells and the grade of inflammation has already been noted in many vaccination studies that used the oral/mucosal route (6, 9, 10, 25, 30). Increased gastric inflammation after oral vaccination has been referred to as postimmunization gastritis. The extent and duration of postimmunization gastritis after oral vaccination varied among different studies (6, 9, 10, 25, 30). Our study extends this inverse relationship between gastritis and bacterial density to a systemic model of Th1 immunization. For this reason, our data would support the idea that Th1 responses are able to exert protective effects only if protection is defined as a decrease in bacterial density and not as prevention of gastritis. In a kinetic study with both H. pylori and H. felis models, postimmunization gastritis has been described to be a transient phenomenon (9, 30). Therefore, the question whether systemic immunization with CpG has a long-term benefit (i.e. leads to disappearance of gastritis in vaccinated animals at later time points), is important. Even though, compared to other studies, a late time point of assessment of gastritis (3 months after infection) was chosen in our study, a long-term benefit of CpG immunization cannot be ruled out. In one study using oral immunization, gastritis was resolved in vaccinated mice 1 year after infection (9). However, in a similar investigation using H. felis instead of H. pylori, immunized mice had gastritis scores equal to those for nonimmunized mice 1 year after infection, despite experiencing severe postimmunization gastritis (30).
The pathomechanism of how immunization with CpG as an adjuvant leads to enhanced gastritis is so far unknown, but one could hypothesize that an enhanced systemic and local Th1 response to H. pylori is the decisive factor. Since mice are still colonized by H. pylori despite vaccination, these remaining bacteria may maintain this strong Th1 response, as has already been suggested on the basis of the results of oral vaccination studies (6). Alternatively, immunization with CpG could lead to the development of autoreactivity against gastric antigens, thus enhancing gastritis. This would be of interest, since autoimmunity has been discussed as a possible pathomechanism in H. pylori gastritis in humans (3).
Taken together, our results show that systemic immunization with CpG and H. pylori lysate in a murine model of H. pylori gastritis is able to elicit a strong local and systemic Th1 response to H. pylori but does not confer protection. Thus, systemic immunization with adjuvants that preferentially induce Th1 responses may be associated with the risk of aggravated pathology in humans. However, we cannot exclude the possibility that other protocols, using different antigens and/or different adjuvants, would be able to prevent the development of gastritis in mice after systemic Th1 immunization.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (grant So-452).
We gratefully acknowledge the perfect technical assistance of Annette Schmitt, Christine Barett, and Britta Körner. In addition, we thank Alexander Dalpke for advice in the RT-PCR experiments.
Editor: F. C. Fang
REFERENCES
- 1.Akhiani, A. A., J. Pappo, Z. Kabok, K. Schon, W. Gao, L. E. Franzen, and N. Lycke. 2002. Protection against Helicobacter pylori infection following immunization is IL-12-dependent and mediated by Th1 cells. J. Immunol. 169:6977-6984. [DOI] [PubMed] [Google Scholar]
- 2.Berg, D. J., N. A. Lynch, R. G. Lynch, and D. M. Lauricella. 1998. Rapid development of severe hyperplastic gastritis with gastric epithelial dedifferentiation in Helicobacter felis-infected IL-10(−/−) mice. Am. J. Pathol. 152:1377-1386. [PMC free article] [PubMed] [Google Scholar]
- 3.Claeys, D., G. Faller, B. J. Appelmelk, R. Negrini, and T. Kirchner. 1998. The gastric H+, K+-ATPase is a major autoantigen in chronic Helicobacter pylori gastritis with body mucosa atrophy. Gastroenterology 115:340-347. [DOI] [PubMed] [Google Scholar]
- 4.Dixon, M. F., R. M. Genta, J. H. Yardley, and P. Correa. 1996. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 20:1161-1181. [DOI] [PubMed] [Google Scholar]
- 5.Eaton, K. A., and M. E. Mefford. 2001. Cure of Helicobacter pylori infection and resolution of gastritis by adoptive transfer of splenocytes in mice. Infect. Immun. 69:1025-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ermak, T. H., R. Ding, B. Ekstein, J. Hill, G. A. Myers, C. K. Lee, J. Pappo, H. K. Kleanthous, and T. P. Monath. 1997. Gastritis in urease-immunized mice after Helicobacter felis challenge may be due to residual bacteria. Gastroenterology 113:1118-1128. [DOI] [PubMed] [Google Scholar]
- 7.Ermak, T. H., P. J. Giannasca, R. Nichols, G. A. Myers, J. Nedrud, R. Weltzin, C. K. Lee, H. Kleanthous, and T. P. Monath. 1998. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J. Exp. Med. 188:2277-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garhart, C. A., F. P. Heinzel, S. J. Czinn, and J. G. Nedrud. 2003. Vaccine-induced reduction of Helicobacter pylori colonization in mice is interleukin-12 dependent but gamma interferon and inducible nitric oxide synthase independent. Infect. Immun. 71:910-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garhart, C. A., R. W. Redline, J. G. Nedrud, and S. J. Czinn. 2002. Clearance of Helicobacter pylori infection and resolution of postimmunization gastritis in a kinetic study of prophylactically immunized mice. Infect. Immun. 70:3529-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goto, T., A. Nishizono, T. Fujioka, J. Ikewaki, K. Mifune, and M. Nasu. 1999. Local secretory immunoglobulin A and postimmunization gastritis correlate with protection against Helicobacter pylori infection after oral vaccination of mice. Infect. Immun. 67:2531-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottwein, J. M., T. G. Blanchard, O. S. Targoni, J. C. Eisenberg, B. M. Zagorski, R. W. Redline, J. G. Nedrud, M. Tary-Lehmann, P. V. Lehmann, and S. J. Czinn. 2001. Protective anti-Helicobacter immunity is induced with aluminum hydroxide or complete Freund's adjuvant by systemic immunization. J. Infect. Dis. 184:308-314. [DOI] [PubMed] [Google Scholar]
- 12.Guy, B., C. Hessler, S. Fourage, J. Haensler, E. Vialon-Lafay, B. Rokbi, and M. J. Millet. 1998. Systemic immunization with urease protects mice against Helicobacter pylori infection. Vaccine 16:850-856. [DOI] [PubMed] [Google Scholar]
- 13.Haeberle, H. A., M. Kubin, K. B. Bamford, R. Garofalo, Y. D. Graham, F. El-Zaatari, R. Karttunen, S. E. Crowe, V. E. Reyes, and P. B. Ernst. 1997. Differential stimulation of interleukin-12 (IL-12) and IL-10 by live and killed Helicobacter pylori in vitro and association of IL-12 production with gamma interferon-producing T cells in the human gastric mucosa. Infect. Immun. 65:4229-4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heeg, K., and S. Zimmermann. 2000. CpG DNA as a Th1 trigger. Int. Arch. Allergy Immunol. 121:87-97. [DOI] [PubMed] [Google Scholar]
- 15.Ismail, H. F., P. Fick, J. Zhang, R. G. Lynch, and D. J. Berg. 2003. Depletion of neutrophils in IL-10(−/−) mice delays clearance of gastric Helicobacter infection and decreases the Th1 immune response to Helicobacter. J. Immunol. 170:3782-3789. [DOI] [PubMed] [Google Scholar]
- 16.Jiang, B., M. Jordana, Z. Xing, F. Smaill, D. P. Snider, R. Borojevic, D. Steele-Norwood, R. H. Hunt, and K. Croitoru. 1999. Replication-defective adenovirus infection reduces Helicobacter felis colonization in the mouse in a gamma interferon- and interleukin-12-dependent manner. Infect. Immun. 67:4539-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleanthous, H., T. J. Tibbitts, H. L. Gray, G. A. Myers, C. K. Lee, T. H. Ermak, and T. P. Monath. 2001. Sterilizing immunity against experimental Helicobacter pylori infection is challenge-strain dependent. Vaccine 19:4883-4895. [DOI] [PubMed] [Google Scholar]
- 18.Kotloff, K. L., M. B. Sztein, S. S. Wasserman, G. A. Losonsky, S. C. DiLorenzo, and R. I. Walker. 2001. Safety and immunogenicity of oral inactivated whole-cell Helicobacter pylori vaccine with adjuvant among volunteers with or without subclinical infection. Infect. Immun. 69:3581-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, A., J. O'Rourke, M. C. De Ungria, B. Robertson, G. Daskalopoulos, and M. F. Dixon. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 20.Lucas, B., D. Bumann, A. Walduck, J. Koesling, L. Develioglu, T. F. Meyer, and T. Aebischer. 2001. Adoptive transfer of CD4+ T cells specific for subunit A of Helicobacter pylori urease reduces H. pylori stomach colonization in mice in the absence of interleukin-4 (IL-4)/IL-13 receptor signaling. Infect. Immun. 69:1714-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall, B. J., and J. R. Warren. 1984. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet i:1311-1315. [DOI] [PubMed] [Google Scholar]
- 22.Michetti, P., C. Kreiss, K. L. Kotloff, N. Porta, J. L. Blanco, D. Bachmann, M. Herranz, P. F. Saldinger, I. Corthesy-Theulaz, G. Losonsky, R. Nichols, J. Simon, M. Stolte, S. Ackerman, T. P. Monath, and A. L. Blum. 1999. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in Helicobacter pylori-infected adults. Gastroenterology 116:804-812. [DOI] [PubMed] [Google Scholar]
- 23.Mohammadi, M., S. Czinn, R. Redline, and J. Nedrud. 1996. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J. Immunol. 156:4729-4738. [PubMed] [Google Scholar]
- 24.Mohammadi, M., J. Nedrud, R. Redline, N. Lycke, and S. J. Czinn. 1997. Murine CD4 T-cell response to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology 113:1848-1857. [DOI] [PubMed] [Google Scholar]
- 25.Raghavan, S., M. Hjulstrom, J. Holmgren, and A. M. Svennerholm. 2002. Protection against experimental Helicobacter pylori infection after immunization with inactivated H. pylori whole-cell vaccines. Infect. Immun. 70:6383-6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saldinger, P. F., N. Porta, P. Launois, J. A. Louis, G. A. Waanders, H. Bouzourene, P. Michetti, A. L. Blum, and I. E. Corthesy-Theulaz. 1998. Immunization of BALB/c mice with Helicobacter urease B induces a T helper 2 response absent in Helicobacter infection. Gastroenterology 115:891-897. [DOI] [PubMed] [Google Scholar]
- 27.Sawai, N., M. Kita, T. Kodama, T. Tanahashi, Y. Yamaoka, Y. Tagawa, Y. Iwakura, and J. Imanishi. 1999. Role of gamma interferon in Helicobacter pylori-induced gastric inflammatory responses in a mouse model. Infect. Immun. 67:279-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smythies, L. E., K. B. Waites, J. R. Lindsey, P. R. Harris, P. Ghiara, and P. D. Smith. 2000. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-gamma, gene-deficient mice. J. Immunol. 165:1022-1029. [DOI] [PubMed] [Google Scholar]
- 29.Sommer, F., G. Faller, M. Rollinghoff, T. Kirchner, T. W. Mak, and M. Lohoff. 2001. Lack of gastritis and of an adaptive immune response in interferon regulatory factor-1-deficient mice infected with Helicobacter pylori. Eur. J. Immunol. 31:396-402. [DOI] [PubMed] [Google Scholar]
- 30.Sutton, P., S. J. Danon, M. Walker, L. J. Thompson, J. Wilson, T. Kosaka, and A. Lee. 2001. Post-immunisation gastritis and Helicobacter infection in the mouse: a long term study. Gut 49:467-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutton, P., T. Kolesnikow, S. Danon, J. Wilson, and A. Lee. 2000. Dominant nonresponsiveness to Helicobacter pylori infection is associated with production of interleukin 10 but not gamma interferon. Infect. Immun. 68:4802-4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutton, P., J. Wilson, T. Kosaka, I. Wolowczuk, and A. Lee. 2000. Therapeutic immunization against Helicobacter pylori infection in the absence of antibodies. Immunol. Cell Biol. 78:28-30. [DOI] [PubMed] [Google Scholar]
- 33.Weltzin, R., B. Guy, W. D. Thomas, Jr., P. J. Giannasca, and T. P. Monath. 2000. Parenteral adjuvant activities of Escherichia coli heat-labile toxin and its B subunit for immunization of mice against gastric Helicobacter pylori infection. Infect. Immun. 68:2775-2782. [DOI] [PMC free article] [PubMed] [Google Scholar]