Abstract
Language function in patients with impaired declarative memory presents a compelling opportunity to investigate the interdependence of memory and language in referential communication. We examined amnesic patients’ use of definite references during a referential communication task. Discursively, definite references can be used to mark a referent as situationally unique (e.g., “the game,” as in the case of a recently publicized game) or as shared information (e.g., “the game,” as in one discussed previously). We found that despite showing normal collaborative learning after repeated referring—as indexed by consistent and increasingly efficient descriptive labels for previously unfamiliar tangram figures—amnesic patients did not consistently use definite references in referring to those figures. The use of definite references seems to be critically dependent on declarative memory, and the engagement of such memory is signaled by language.
Keywords: memory, language, definite reference, discourse analysis
Consider the following utterances:
“Hey Dan, did you buy this [pointing to a cap] at the game?”
“Hey Dan, did you buy this [pointing to a cap] at a game?”
Both of these sentences display the three basic types of definite references—the proper noun Dan, the deictic referent this (pointing to a cap that both the speaker and the listener can see), and anaphora (you and the game). The sentences differ linguistically only in their use of two small words: the and a, but this difference confers a critical difference in meaning. The use in (a) of the definite article (the game), unlike the use in (b) of an indefinite article (a game), indicates that the speaker has in mind a particular referent that he or she believes the listener can uniquely identify from among other possibilities (Agha, 2007; Clark, 1992; Hanks, 1990). Such would be the case, for example, if the speaker were referring to a long-awaited football game completed earlier that day on Dan’s campus. Strikingly, even without analysis of the content or context of the utterance, the use of the definite article alone signals the referencing of shared knowledge and thus the critical use of some aspect of memory.
In our work on language-and-memory-in-use, we have drawn on a line of research by Clark and his colleagues that suggests specific predictions about the memory determinants of definite references (Clark, 1992; Clark & Marshall, 1981; Clark & Wilkes-Gibbs, 1986). This work has focused on common ground, that is, the mutually shared information between conversational partners that shapes the use and understanding of referents in their verbal interaction (Clark, 1992). The proposal is that conversational partners employ co-presence heuristics to infer common ground; for example, speakers may draw on explicit records of previous communicative exchanges and updates of these records with new events. A consistent finding from this work is that referring expressions are shortened and simplified when addressed to the same interlocutor to refer to the same material; this shorthand allows communication partners to successfully establish and resolve individual references in less time and with fewer communicative resources (number of words and turns) across repeated interactions (Clark & Wilkes-Gibbs, 1986). The use of a definite reference, according to this view, is an indication of the speaker’s confidence that the reference is part of shared knowledge and interactional histories. Relating this back to our example about Dan, this cap, and the game, the use of the definite article the depends on and signals the use of memory for a specific fact or event—either about an earlier conversation the speaker and listener had about some game or about a particular event that was highly publicized. From this perspective, it is declarative memory that supports and is signaled by the use of definite references.
There are some aspects of common ground, however, that do not seem to require declarative memory. In previous work, we used a collaborative referencing task to test the acquisition of referential labels for a set of cards displaying Chinese tangrams with no preestablished names across a series of interactions with familiar communication partners (Duff, Hengst, Tranel, & Cohen, 2006). We studied neurological patients with profound impairment of declarative memory following hippocampal damage. These patients had deficits in acquiring information about new facts and events and in consciously recollecting the events of their lives. In our version of the referential communication task, patients with hippocampal amnesia described the tangram cards to their partners, and the partners tried to place the cards where the amnesic patient directed.
We found that despite profound declarative amnesia, the patients showed robust learning across trials, as measured by their use of stable labels for each target card and the simplification of those labels across trials. Consistent with previous work with healthy college-age participants (Clark, 1992), our results showed that all amnesic pairs developed and used unique references for each card (e.g., “Viking ship”). Across trials, they produced these references in an increasingly concise and simplified form requiring fewer words and less time to complete the task.1 The labels for the tangrams also stabilized. For example, labels that were established in the early trials were then used consistently throughout the task (e.g., a description of a card as “someone sleeping” or “taking a siesta” became just “siesta man” on subsequent trials). The amnesic pairs’ use of specific labels developed and stabilized across trials at a rate comparable to that in healthy comparison pairs (Duff et al., 2006). Moreover, the observed learning was enduring; amnesic participants could generate as many of their final concise labels outside the context of the task as healthy comparison participants could at 30 min and 6-month follow-up interviews. One participant we had the opportunity to test 2 years later named the tangrams using 10 of the same 12 labels.
What is it that amnesic participants learn in the collaborative-referencing paradigm, and what memory systems support it? Participants were not required to learn a specific set of labels or predetermined, experimenter-generated labels, but rather, drew on preexisting mental representations, or semantic knowledge, to generate card labels. We suggested that what we observed behaviorally across the multiple interactions was a change in the linguistic expressions used by the participants across trials, and that the learning occurs across multiple levels, including linguistic, conceptual (or semantic), and perceptual levels (Duff et al., 2006). Given that the learning occurred gradually, and at a normal rate, it was likely achieved through nondeclarative memory systems, such as procedural memory, and more specifically through the tuning of cortical processors, including conceptual, semantic, and visual processors (Cohen & Eichenbaum, 1993; Eichenbaum & Cohen, 2001). The finding that patients with hippocampal amnesia can acquire and use common ground in their interactions suggests that procedural or nondeclarative forms of memory may, in part, support common ground.
A question not addressed in our previous work is whether amnesic patients use a definite reference to signal explicitly to the listener that they believe the referent to be part of shared knowledge. For example, do the patients say “a Viking ship” (an indefinite reference) or signal the definiteness of the reference by saying “the Viking ship” or simply “Viking ship”? In other words, would the patients (in contrast to healthy comparison participants) tend not to use definite references in referring to the very objects whose labels they arrived at in collaboration with their partner? Such a dissociation would be striking and would suggest a bifurcation of the memory systems supporting common ground—the patients would learn labels normally (a process supported by nondeclarative forms of memory) but would not mark this learned common ground through definite references for the same objects (a process supported by declarative memory). Such findings would also argue strongly that the use of definite references depends critically on and signals the use of declarative memory, a fact that would provide compelling evidence for language-memory interdependence.
Method
This study was a discourse analysis of definite-reference use, in which participant pairs completed a referential communication task. The data were collected as part of an earlier study focusing on the acquisition of the referential labels (Duff et al., 2006; Duff, Hengst, Tranel, & Cohen, 2008).
Participants
The participants included 6 amnesic patients and 6 healthy comparison participants. Each amnesic and comparison participant selected a familiar partner (e.g., a friend or family member) with whom they were paired to complete the task. Four of the amnesic pairs and 4 of the comparison pairs had been included in our previous study (Duff et al., 2006). For the current study, we added 2 comparison participants and 2 amnesic patients (along with their communication partners) who had memory profiles similar to the 4 patients in the earlier study.
The neuroanatomical and neuropsychological characteristics of the 6 amnesic participants have been published previously (Allen, Tranel, Bruss, & Damasio, 2006; Duff, Hengst, Tranel, & Cohen, 2008; Duff, Wszalek, Tranel, & Cohen, 2008). Structural MRI examinations completed on 5 of the 6 patients confirmed bilateral hippocampal damage. For Participant 2563 (who wears a pacemaker and was unable to undergo the MRI examination), anatomical analysis was based on computerized tomography, and damage confined to the hippocampal region was visible. Of the 6 participants with amnesia, 3 had sustained bilateral hippocampal damage from an anoxic (hypoxic) event, 2 had sustained more extensive bilateral medial temporal lobe damage (including hippocampus bilaterally) from herpes simplex encephalitis, and 1 had sustained shearing lesions in the white-matter tracts surrounding the hippocampus following a closed head injury (see Table 1 for demographic characteristics of the amnesic patients).
Table 1.
Characteristics and Scores of the Amnesic Participants
| Participant | Demographic characteristics
|
Neuropsychological scores
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Etiology | Sex | Handedness | Education (years) | Age at testing (years) | WAIS-III FSIQ | WMS-III GMI | AVLT Trial 5/Delay | CFT Delay | Boston Naming Test | MAE Token Test | |
| 2563 | Anoxia | M | Left | 16 | 47 | 102 | 75 | 7/1 | 7 | 52 | 44 |
| 2363 | Anoxia | M | Right | 16 | 45 | 98 | 73 | 9/0 | 5 | 58 | 44 |
| 0001 | Anoxia | F | Right | 9 | 54 | 90 | 54 | 7/0 | 2 | 56 | 44 |
| 1951 | HSE | M | Right | 16 | 51 | 106 | 59 | 6/2 | 4 | 49 | 44 |
| 2308 | HSE | M | Left | 18 | 46 | 87 | 45 | 5/0 | 0 | 52 | 44 |
| 0002 | CHI | F | Right | 20 | 45 | 126 | 49 | 3/0 | 0 | 59 | 44 |
| Mean | 15.8 (3.7) | 48.0 (3.68) | 101.5 (13.4) | 59.2 (12.4) | 6.2 (2)/0.5 (0.1) | 3 (2.8) | 54.3 (3.9) | 44.0 (0) | |||
Note: Standard deviations are given in parentheses. Neuropsychological data include Full Scale IQ (FSIQ) from the Wechsler Adult Intelligence Scale—Third Edition (WAIS-III; Wechsler, 1997a), General Memory Index (GMI) score from the Wechsler Memory Scale—Third Edition (WMS-III; Wechsler, 1997b), the Trial 5 and Delay scores from the Rey Auditory and Verbal Learning Test (AVLT; Schmidt, 1996), Delay score from the Complex Figure Test (CFT; Meyers & Meyers, 1995), Boston Naming Test score (Goodglass & Kaplan, 2000), and Token Test score from the Multilingual Aphasia Examination (MAE; Benton, Hamsher, & Sivan, 1994). HSE = herpes simplex encephalitis; CHI = closed head injury; M = male; F = female.
All amnesic participants had a selective and severe memory impairment, in the context of generally preserved intelligence, cognition (e.g., language), and nondeclarative memory (see Cavaco, Anderson, Allen, Castro-Caldas, & Damasio, 2004). General Memory Index score from the Wechsler Memory Scale—Third Edition (Wechsler, 1997b) was at least 25 points lower than Full Scale IQ from the Wechsler Adult Intelligence Scale—Third Edition (Wechsler, 1997a; mean Full Scale IQ – mean General Memory Index score = 42.3). Table 1 displays the neuropsychological scores of the participants with amnesia. Healthy comparison participants were matched, pair-wise, with amnesic participants on age, sex, education, and handedness. Familiar partners of the amnesic and comparison participants were all nonamnesic and of similar age (amnesic partners: 57.6 years; comparison partners: 56.5 years) and education (amnesic partners: 14.0 years; comparison partners: 13.7 years).
Materials and procedure
Participant pairs were instructed to complete a matching task, in which they sat at a table facing each other. Each member of the pair had a playing board with 12 numbered spaces and identical sets of 12 playing cards displaying Chinese tangrams with no descriptive labels (see Fig. 1). A partial barrier obscured each player’s view of the other’s board and cards but allowed pairs to see each other’s facial expressions and gestures. In each pair, the amnesic patient or the matched comparison participant was always the director, and the familiar partners were always the matchers. The director began with his or her cards on the board and told the matcher which cards to place in which numbered spaces. Thus, at the end of the trial, both boards looked alike. Pairs were allowed to communicate freely throughout the task. There were 24 trials (six trials in each of four sessions, with two sessions daily).
Fig. 1.
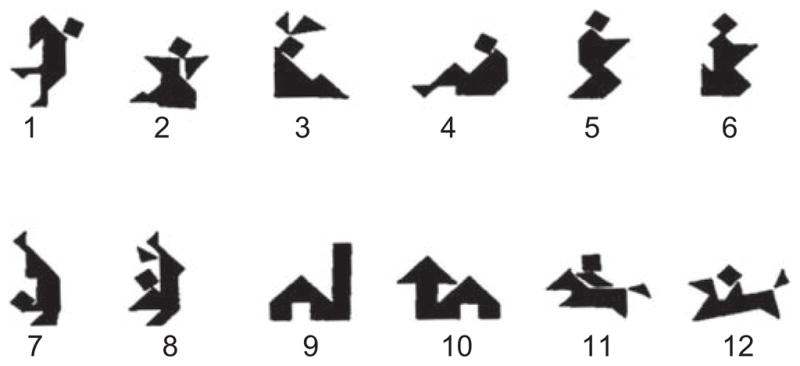
Tangrams used as stimuli in the experiment.
Data analyses
All interactions between each participant pair on each trial were transcribed, and referential expressions were analyzed by two coders who were blind to the hypothesis of the study. The coders first identified and marked the initiating references in the transcripts. The initiating reference was the director’s first attempt during each trial at describing or labeling each of the 12 cards (e.g., “Number one kinda looks like a dragon reading a book”). Across all 24 trials for all 12 participant pairs, there were 16 occasions (4 in amnesic pairs, 12 in comparison pairs) in which an initiating reference was not explicitly produced (e.g., the matcher terminated the trial before the director referenced the last card). This yielded a total of 3,440 initiating references (1,724 for amnesic pairs and 1,716 for comparison pairs).
The 3,440 initiating references were coded as definite (e.g., “the camel,” “the dragon reading a book,” “the one reclining in the chair with his feet stickin’ out, against the tree”) or indefinite (e.g., “looks like a couple of hills,” “it has a diamond at the top of the figure”; see Clark & Wilkes-Gibbs, 1992; Hengst, 2003). As these examples suggest, definite references were often marked linguistically with definite articles (the), but this was not always the case. Indeed, particularly on later trials, when labels had become increasingly concise and streamlined, it was not uncommon for pairs to produce a label that functioned more like a proper noun. The frequency of use of a noun phrase without an article (e.g., “siesta man” instead of “the siesta man”) was higher for comparison pairs (734/1,716 = 43%) than for amnesic pairs (576/1,724 = 33%). When the definite article was omitted from a reference that would have otherwise been understood as definite (e.g., “kicker”), we coded the reference as definite (following Wilkes-Gibbs & Clark, 1992). References produced with an indefinite article (a, an), however, even if the referent was concise (e.g., “an angel,” “a windmill”) were coded as indefinite. Inter- and intrarater reliability of the coding were 96% and 90%, respectively.
Results
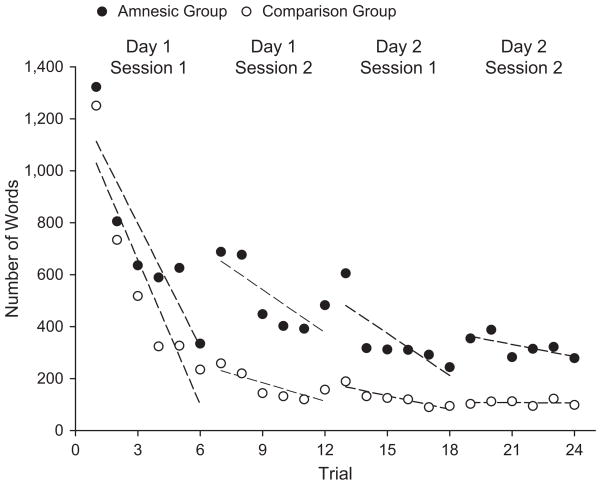
The six amnesic and six comparison pairs displayed robust learning (results of four of these pairs were reported in the Duff et al., 2006, study). Mean time to completion dropped across trials from 12 min 57 s (Trial 1) to 2 min 35 s (Trial 24) for amnesic pairs and from 9 min 8 s (Trial 1) to 0 min 48 s (Trial 24) for comparison pairs. There was a strong linear trend across trials, F(1, 10) = 17.099, p = .002. Mean total words spoken decreased across trials from 1,321.83 (Trial 1) to 278.50 (Trial 24) for amnesic pairs and from 1,250.16 (Trial 1) to 97.83 (Trial 24) for comparison pairs (Fig. 2). There was a significant linear trend across trials, F(1, 10) = 18.549, p < .002. Relative to the comparison pairs, the amnesic pairs were slower overall, F(1, 10) = 8.004, p = .018; they also required more words, F(1, 10) = 8.356, p < .016. However, this effect was seen from the earliest trial, and there was no interaction of group with the learning for time, F(5, 50) = 0.503, p > .773, or words, F(5, 50) = 1.569, p > .18. Overall card-placement accuracy was high for amnesic pairs (93.8%) and comparison pairs (97.6%), and both groups reached ceiling levels on accuracy by Trial 7.
Fig. 2.
Scatter plot showing the mean number of words spoken by each of the two participant groups across the six trials of each session. Best-fitting regression lines are shown for each session and each group.
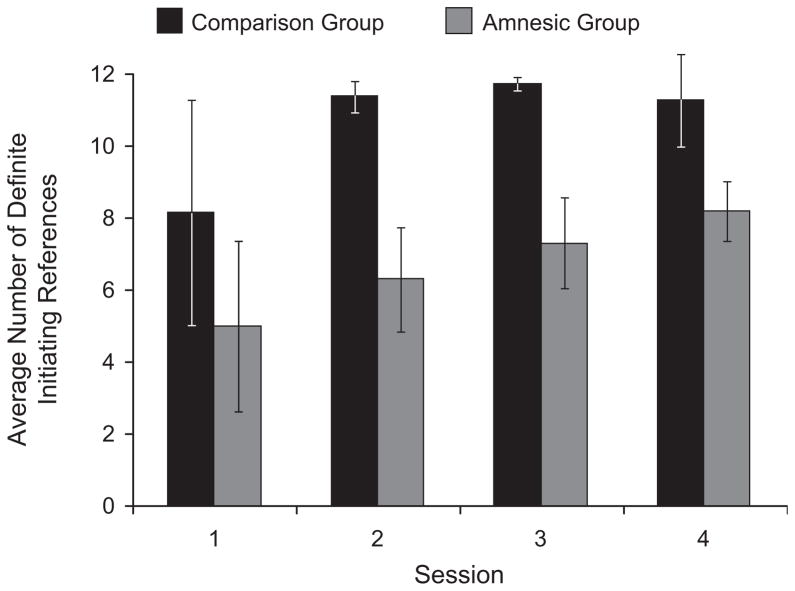
Given this context of robust learning, did amnesic patients explicitly signal shared knowledge with their partners by using a definite reference? Across all trials and participants, 73.1% of initiating references were definite. Comparison pairs produced significantly more definite initiating references (1,549; i.e., 90% of all initiating references) than amnesic pairs did (967; i.e., 56% of all initiating references), t(10) = 3.176, p = .01, d = 1.74. Figure 3 displays the average use of definite initiating references by the two groups for each of the four sessions. The difference in the use of definite references between amnesic and comparison pairs was evident on the first trial: Comparison pairs used twice as many definite initiating references (20% of expressions) than did amnesic pairs (10% of expressions) on Trial 1. The same difference between groups was apparent for the last card in each trial: Whereas comparison participants nearly always used a definite reference to describe each trial’s final card (92.6% of trials), amnesic patients used a definite reference to describe trial-final cards on only 59.7% of trials.
Fig. 3.
Average number of definite initiating references generated by amnesic and comparison participants across the six trials of each session. Error bars show standard deviations.
The reduced use of definite references by amnesic pairs is especially conspicuous in light of how similar the labels were for the two groups. Table 2 shows examples of the referential expressions used by one of the amnesic participants and the matched comparison participant on the final trial, after each had shown substantial collaborative learning with their respective partners. Although this amnesic pair arrived at linguistically concise labels for all 12 abstract tangrams (labels that were semantically similar to those produced by the comparison pair), in this last trial, 4 of the amnesic pair’s initiating references were indefinite (e.g., “a dog,” “a camel”). In contrast, only 1 of the 12 referential expressions produced by the comparison pair during the final trial were coded as indefinite (i.e., their final concise labels were definite references). Recall that repeated occurrences of a streamlined noun phrase produced without any article (e.g., “siesta man”) were coded as definite because we assumed that such phrases had taken on the status of a proper noun or nickname. However, to ensure that such proper-noun-like references were not driving our results, we performed an analysis of only the referents produced with an article (e.g., “the barn”) on the final trial. The analysis revealed that our results do not depend on the inclusion of referents with an omitted article: Comparison participants used a definite reference for 97% (33 of 34) of the labels with articles, whereas amnesic participants used a definite reference for only 44% (16 of 36) of the labels with an article.
Table 2.
Initiating References Used by an Amnesic and Matched Comparison Director on Trial 24
| Card | Amnesic participant | Comparison participant |
|---|---|---|
| 6 | praying | a kneeling per-, uh, attached kneelera |
| 8 | the tall dude | Viking ship |
| 4 | Gary | the recliner |
| 7 | reading a book | the reader |
| 1 | kicking | the kicker |
| 3 | a windmilla | the Indian |
| 2 | an anglea | the mess |
| 11 | a doga | the horse |
| 12 | a camela | the camel |
| 10 | the pine tree and barn | the arrow |
| 9 | the barn and silo | the chimney |
| 5 | bending the knee | the detached |
This reference was coded as indefinite.
Although amnesic pairs used significantly fewer definite references than comparison pairs did overall, both groups showed an increase in definite-reference use across trials. We examined the individual trajectories of definite-initiating- reference use for each card by each participant to determine whether there might be different trends in the use of definite references across trials for amnesic versus comparison pairs. Comparison pairs quickly and consistently moved to definite references for the cards (> 90% after Trial 3; 99% on Trial 24), a result consistent with findings in college-age participants by Clark (1992). For amnesic pairs, this transition was inconsistent and more gradual. Amnesic pairs increased their use of definite references across trials in general (53% during the second session; 75% by Trial 24). But there was no point at which amnesic pairs consistently used a definite initiating reference for any given card across trials. This pattern is illustrated in Table 3, which lists the initiating references used by an amnesic director for the same card on all trials in Sessions 3 and 4; despite inclusion of a stable label (“barn”), the amnesic participant’s use of a definite reference was strikingly inconsistent. This is most apparent in the use of the indefinite article (“a barn”) in five trials. In contrast, the matched comparison participant produced a definite reference 100% of the time.
Table 3.
Initiating References Used for Card 10 by an Amnesic Director During Sessions 3 and 4
| Session and trial | Referential expression | Coding |
|---|---|---|
| Session 3 | ||
| Trial 1 | the barn | Definite |
| Trial 2 | barn | Definite |
| Trial 3 | barn with a roof | Definite |
| Trial 4 | a barn with a top | Indefinite |
| Trial 5 | a barn with a roof on the silo | Indefinite |
| Trial 6 | a barn with a silo | Indefinite |
| Session 4 | ||
| Trial 1 | the roof on the barn | Definite |
| Trial 2 | the barn with a roof | Definite |
| Trial 3 | a barn with a top | Indefinite |
| Trial 4 | barn with a top | Definite |
| Trial 5 | barn with a roof | Definite |
| Trial 6 | a barn with a roof on the silo | Indefinite |
To analyze whether the use of a definite reference for a particular card on a given trial could be predicted by card repetition across trials, memory status, or use of a definite reference for the same card on the prior trial, we fitted a logistic hierarchical linear model. In this model, pairs of participants and repetition were treated as random effects (intercept and slope, respectively), and all other effects were fixed. The fitted model indicated that all participants were more likely to use definite references as the experiment continued, but amnesic patients were not more likely to use definite references after using a definite reference with the same card on the previous trial overall (z = 1.13, p > .25). This effect was no more than marginally reliable when interacting with item repetition (z = 1.65, p = .10). In contrast, comparison participants were more likely to use a definite reference for a card after using a definite reference for the same card during the previous trial (z = 3.57, p < .01).
Discussion
The critical finding in this study was the dissociation in amnesic patients between successful learning of labels and the reduced and inconsistent use of referential forms that would mark these labels as part of the knowledge they shared with their partners. This disruption in the use of definite references (even on the final card of a trial and even during the final trial) to refer to tangrams that had been described multiple times, and for which increasingly concise labels had been developed, was striking; it seemed as though the patients were encountering the shapes and deriving the descriptors for the first time. By contrast, comparison directors nearly always used definite references to refer to the tangrams. These findings argue strongly that definite-reference use depends on declarative memory and signals its engagement.
The findings here are important in four key regards. First, this referential communication task was chosen to give the amnesic patients the best possible chance of success; their performance could be assessed in the context of intact learning of referential labels for the tangrams. Second, amnesia has classically been viewed as a pure memory deficit, leaving basic linguistic (and other cognitive) capacities unaffected. The ability to choose a specific class of linguistic elements (such as definite articles) to fill a particular grammatical role could be viewed as a purely linguistic capacity. Yet, we found that the use of definite references in patients with declarative-memory impairment deviated markedly from healthy comparison participants’ use of definite references. Third, these findings support recent views that memory and language are interdependent, particularly in real-world use. In previous studies, we and other researchers (e.g., MacKay, Burke, & Stewart, 1998) have found that hippocampal amnesia impairs various aspects of normal discourse, including reported speech, procedural discourse, and verbal play (Duff, Hengst, Tengshe, et al. 2008; Duff, Hengst, Tranel, & Cohen, 2007; Duff, Hengst, Tranel, & Cohen, 2009). Finally, the current study demonstrates that the effects of declarative-memory impairments on interactional aspects of language use reach into territory typically treated as basic syntactic functioning (e.g., using articles to mark noun phrases as definite or indefinite).
That memory should be critical for certain aspects of language, and hence that memory deficits would lead to certain disruptions of language use, should perhaps not come as a surprise. Many cognitive processes, including language, rely on aspects of memory for their effective operation—in general, memory can be seen as one of the most fundamental operations of the brain (e.g., Milner, Squire, & Kandel, 1998; Tranel & Damasio, 2002). That the effective use of definite references would require declarative memory, specifically, makes sense given the nature of that form of memory. The declarative-memory system creates relational representations of relations among the constituent elements of experience. These representations include information about the co-occurrences of people, places, and objects along with the spatial, temporal, and interactional relations among them, as well as representations of higher-order relationships among various events. Thus, these relational representations provide the larger record of people’s experiences over time (Cohen & Eichenbaum, 1993; Eichenbaum & Cohen, 2001). In the absence of such a declarative record of previous events and communicative exchanges to draw on, as in amnesia, the use of definite references is significantly disrupted.
Finally, these findings also suggest a more complex bifurcated model of common ground. Although Clark (1992) analyzed the collaborative work of referencing in terms of a single shared common ground, the current study and our previous work with individuals with amnesia (Duff et al., 2006, 2009; Duff, Hengst, Tranel, & Cohen 2008) suggest that common ground has multiple forms and determinants dependent on the contributions of different memory systems in the brain. On the one hand, the amnesic pairs displayed common ground in their accurate and successful performance of card placements and the increasingly succinct labels they developed. On the other hand, the amnesic participants displayed disruptions in the discursive representations of such shared knowledge through their inconsistent use of definite references. Procedural forms of memory (which are intact in amnesia) seem to support the gradual acquisition of linguistic, conceptual, and perceptual information necessary to arrive at concise labels in this task, and in contrast, declarative memory (which is impaired in amnesia) seems critical for the use of high-level discourse practices, such as managing shared perspectives and verbal play (Duff et al., 2009; Duff, Hengst, Tranel, & Cohen, 2008).
Amnesic patients showed the same benefit from their repeated experiences in this task as did comparison participants; however, in arriving at concise descriptors for the tangrams, it was the smallest of words—the articles a, an, and the—that revealed amnesic participants’ memory impairment. We suggest that the use of definite references (e.g., the ability to talk meaningfully about “the game”) depends critically on declarative memory and signals its engagement.
Acknowledgments
We thank David Warren for assistance with the analyses.
Funding
This research was supported by the National Institute on Deafness and Other Communication Disorders (Grant 1F32DC008825), National Institute of Neurological Disorders and Stroke (Grant P50 NS19632), and National Institute of Mental Health (Grant R01 MH062500).
Footnotes
Aspects of this type of observed learning have also been referred to as entrainment and alignment in the literature (e.g., Brennan & Clark, 1996; Garrod & Pickering, 2004).
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
References
- Agha A. Language and social relations. New York, NY: Cambridge University Press; 2007. [Google Scholar]
- Allen JS, Tranel D, Bruss J, Damasio H. Correlations between regional brain volumes and memory performance in anoxia. Journal of Clinical and Experimental Neuropsychology. 2006;28:457–476. doi: 10.1080/13803390590949287. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K, Sivan AB. Multilingual Aphasia Examination. 3. Iowa City; AJA: 1994. [Google Scholar]
- Brennan SE, Clark HH. Conceptual pacts and lexical choice in conversation. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1996;22:1482–1493. doi: 10.1037//0278-7393.22.6.1482. [DOI] [PubMed] [Google Scholar]
- Cavaco S, Anderson SW, Allen JS, Castro-Caldas A, Damasio H. The scope of preserved procedural memory in amnesia. Brain. 2004;127:1853–1867. doi: 10.1093/brain/awh208. [DOI] [PubMed] [Google Scholar]
- Clark HH. Arenas of language use. Chicago, IL: University of Chicago Press; 1992. [Google Scholar]
- Clark HH, Marshall CR. Definite reference and mutual knowledge. In: Joshi AK, Webber BL, Sag IA, editors. Elements of discourse understanding. Cambridge, England: Cambridge University Press; 1981. pp. 10–63. [Google Scholar]
- Clark HH, Wilkes-Gibbs D. Referring as a collaborative process. Cognition. 1986;22:1–39. doi: 10.1016/0010-0277(86)90010-7. [DOI] [PubMed] [Google Scholar]
- Clark HH, Wilkes-Gibbs D. Referring as a collaborative process. In: Clark HH, editor. Arenas of language use. Chicago, IL: University of Chicago Press; 1992. pp. 107–143. [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia and the hippocampal system. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- Duff MC, Hengst J, Tengshe C, Krema A, Tranel D, Cohen NJ. Hippocampal amnesia disrupts the flexible use of procedural discourse in social interaction. Aphasiology. 2008;22:866–880. doi: 10.1080/02687030701844196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff MC, Hengst J, Tranel D, Cohen NJ. Development of shared information in communication despite hippocampal amnesia. Nature Neuroscience. 2006;9:140–146. doi: 10.1038/nn1601. [DOI] [PubMed] [Google Scholar]
- Duff MC, Hengst J, Tranel D, Cohen NJ. Talking across time: Using reported speech as a communicative resource in amnesia. Aphasiology. 2007;21:702–716. doi: 10.1080/02687030701192265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff MC, Hengst J, Tranel D, Cohen NJ. Collaborative discourse facilitates efficient communication and new semantic learning in amnesia. Brain & Language. 2008;106:41–54. doi: 10.1016/j.bandl.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff MC, Hengst J, Tranel D, Cohen NJ. Hippocampal amnesia disrupts verbal play and the creative use of language in social interaction. Aphasiology. 2009;23:926–939. doi: 10.1080/02687030802533748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff MC, Wszalek T, Tranel D, Cohen NJ. Successful life outcome and management of real-world memory demands despite profound anterograde amnesia. Journal of Clinical and Experimental Neuropsychology. 2008;30:931–945. doi: 10.1080/13803390801894681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From conditioning to conscious recollection: Memory systems of the brain. New York, NY: Oxford University Press; 2001. [Google Scholar]
- Garrod S, Pickering MJ. Why is conversation so easy? Trends in Cognitive Sciences. 2004;8:8–11. doi: 10.1016/j.tics.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. Boston Naming Test. Philadelphia, PA: Lippincott, Williams, & Wilkins; 2000. [Google Scholar]
- Hanks WF. Referential practice. Chicago, IL: University of Chicago Press; 1990. [Google Scholar]
- Hengst J. Collaborative referencing between individuals with aphasia and routine communication partners. Journal of Speech, Language, and Hearing Research. 2003;46:831–848. doi: 10.1044/1092-4388(2003/065). [DOI] [PubMed] [Google Scholar]
- MacKay DG, Burke DM, Stewart R. H.M.’s language production deficits: Implications for relations between memory, semantic binding, and the hippocampal system. Journal of Memory and Language. 1998;38:28–69. [Google Scholar]
- Meyers J, Meyers K. Rey Complex Figure Test and Recognition Trial: Professional manual. Odessa, FL: Psychological Assessment Resources; 1995. [Google Scholar]
- Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Rey Auditory and Verbal Learning Test: A handbook. Los Angeles, CA: Western Psychological Services; 1996. [Google Scholar]
- Tranel D, Damasio A. Neurobiological foundations of human memory. In: Baddeley AD, Kopelman MD, Wilson BA, editors. The handbook of memory disorders. 2. Chichester, England: Wiley; 2002. pp. 17–56. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio, TX: Psychological Corp; 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale. 3. San Antonio, TX: Psychological Corp; 1997b. [Google Scholar]
- Wilkes-Gibbs D, Clark HH. Coordinating beliefs in conversation. Journal of Memory and Language. 1992;31:183–194. [Google Scholar]