Abstract
Approximately 50,000 new cases of head and neck squamous cell carcinoma (HNSCC) will be diagnosed in the United States (US) in 2009. Whereas the gradual decline in smoking rates in the US is a highly favorable trend, future global HNSCC incidence will likely reflect the increased marketing and penetration of tobacco products across several of our most populous countries. Although modern surgery, radiation and conventional chemotherapy strategies for HNSCC continue to provide gradual improvement in outcome, the first molecular targeting approach to demonstrate a survival advantage for HNSCC patients has recently emerged in the context of EGFR biology. The scientific background and current challenges accompanying this recent advance are described in this article as are several additional promising molecular targets for HNSCC. There is cautious anticipation that the logical advancement of molecular targeting agents in conjunction with radiation may afford increased cure rates and diminished normal tissue toxicity profiles for HNSCC patients over the years to come.
EGFR as a therapeutic target in head and neck cancer
The loss of growth control in head and neck squamous cell carcinoma (HNSCC) is characterized by acquisition of an autocrine regulatory pathway involving the epidermal growth factor receptor (EGFR) (1-3). Several studies have demonstrated that EGFR and its autocrine ligand transforming growth factor alpha (TGF-ạ, are upregulated in HNSCC (4, 5). EGFR expression was first described in HNSCC cell lines in the early 1980's (Beguinot et al 1984). Soon after, Partridge et al reported that EGFR was expressed in HNSCC tumors. Elevated EGFR expression levels in the primary SCCHN tumor have consistently been correlated with decreased survival (6, 7). Increased expression of EGFR in HNSCC appears to be the result of gene amplification and transcriptional activation (8, 9). In addition to HNSCC, EGFR overexpression has also been described in premalignant dysplastic tissue that preceded the development of invasive cancer (10). The primary rationale for the design of EGFR targeting strategies has been based on the increased EGFR expression levels detected on tumor cells, although evidence suggests that constitutive EGFR activation can occur in the absence of increased expression (11). In addition to the importance of EGFR expression in human HNSCC, many studies have reported anti-tumor effects when EGFR targeting strategies were used in preclinical HNSCC models. Several therapeutic approaches have been developed including monoclonal antibodies, tyrosine kinase-specific inhibitors, ligand-linked immunotoxins, and antisense approaches (12).
EGFR inhibitors have been shown to abrogate the growth of HNSCC cell lines and xenografts when administered alone, or in combination with standard therapy such as chemotherapy and/or radiation (13). The EGFR monoclonal antibody cetuximab has been combined with cisplatin in platinum-refractory HNSCC patients in a phase III trial supported by the Eastern Cooperative Oncology Group (ECOG) that demonstrated enhanced response rates when subjects received the combined treatment regimen (14). The FDA approved the use of cetuximab for SCCHN in 2006 based on the results of a phase III trial showing prolonged survival when cetuximab was administered in conjunction with radiation (15). This was the first phase III trial to demonstrate a survival advantage using a molecular targeting agent combined with radiation. In addition, the combination of radiation and cetuximab did not significantly increase the toxicity profile or compromise the effective delivery of full course external beam radiation therapy. It is noteworthy that cetuximab was the first new drug approved for use in this cancer in 45 years. While the combination of cetuximab and radiation increased survival compared with radiation alone, cetuximab did not reduce the incidence of distant metastases nor did it completely prevent local-regional failure. These facts indicate the persistence of oncogenic signaling pathways.
EGFR-specific tyrosine kinase inhibitors such as erlotinib have also been explored as antitumor agents in SCCHN, although phase III data are lacking (16). Several ongoing US and international clinical trials are exploring the combination of chemoradiotherapy with EGFR targeting as a curative treatment strategy. Other questions that remain to be answered include the timing of radiation or chemotherapy delivery with EGFR targeted therapies and the role of other targets, in addition to EGFR. There is no evidence to date of an association between human papilloma virus (HPV) status of the tumor and response to EGFR targeting. An improved understanding of EGFR signaling interactions with other oncogenic pathways should facilitate the design of more effective targeting strategies by elucidating the critical proliferative and survival pathways that persist in the setting of EGFR blockade.
Challenges of EGFR targeted therapy
Despite the nearly ubiquitous expression of EGFR in HNSCC tumors, there is no evidence to date that expression levels can predict an individual patient's response to EGFR targeted therapy. Therefore, we still do not know how to identify those HNSCC patients who will respond to EGFR targeted therapy. There are several potential explanations for the modest efficacy of EGFR inhibitors in patients whose tumors express EGFR including: 1) heterogeneous EGFR expression with tumorigenic potential residing in EGFR-null cells; 2) persistent signaling through pathways that crosstalk with EGFR despite upstream blockade; and/or 3) mutations that confer resistance to EGFR inhibition. EGFR is a member of the receptor tyrosine kinase family (Type I) of cell surface receptors that also include HER2, HER3 and HER4. While there is little evidence that HER4 is expressed in HNSCC, the role of EGFR dimerization and signaling through HER2 or HER3 remains incompletely understood. Clinical studies to date using HER2 inhibitors including the monoclonal antibody trastuzumab or the small molecule inhibitor lapatinib in HNSCC patients have been disappointing (17). Further investigation is required to delineate the role of EGFR-HER2 heterodimers in oncogenic signaling pathways in HNSCC. Although HER3 upregulation has been implicated in the lack of response to EGFR tyrosine kinase inhibitors in lung cancer, the role of Her3 signaling in HNSCC is largely unexplored.
EGFR is bound by a series of ligands that trigger receptor dimerization, internalization and downstream signaling. These ligands include TGF-α, amphiregulin, and EGF. Both TGF-α and amphiregulin are produced at relatively high levels by HNSCC tumors leading to the stimulation of autocrine growth pathways. In addition to direct activation by EGFR autocrine ligand, EGFR can also be activated indirectly by G-protein-coupled receptors (GPCR), a process known as transactivation. Ligands for GPCRs including prostaglandin E2 (PGE2) bradykinin (BK), lysophosphatidic acid (LPA), and gastrin-releasing peptide (GRP) have been implicated in the pathophysiology of multiple tumor lineages (18). For example, PGE2 is a downstream product of cyclooxygenase 2 (COX-2), and this pathway is upregulated in SCCHN where it correlates with tumor progression and survival (19, 20). BK is known to induce calcium influx and cause phosphorylation of EGFR in HNSCC (21, 22). We recently found upregulation of bradykinin 2 receptor (B2R) in HNSCC tumors implicating an autocrine loop for this peptide hormone in HNSCC (unpublished observations). Likewise, increased PGE2 levels have been described in many tumors including HNSCC (23).
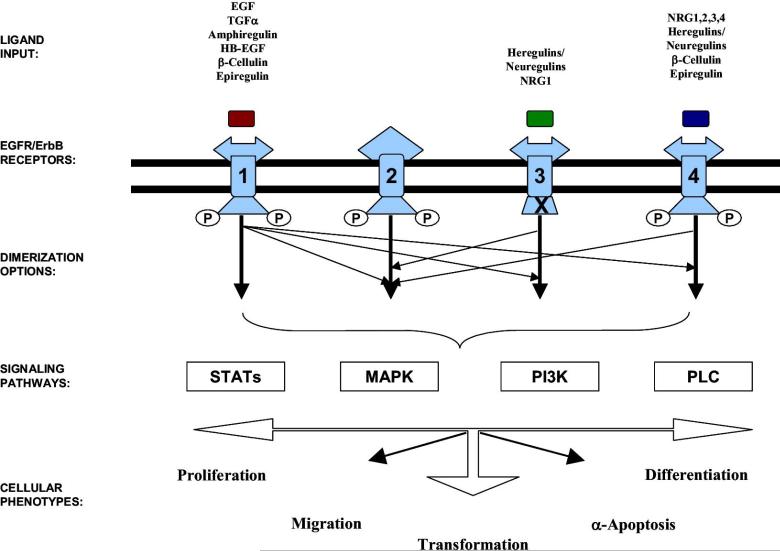
Activation of EGFR by direct ligand or transactivation by GPCR leads to stimulation of downstream signaling pathways including Src family kinases, PI3-Kinase/AKT, Signal Transducers and Activators of Transcription (STATs), phospholipase C-gamma (PLC-g) and mitogen activated protein kinase (MAPK). In general, activation of Src has been shown to mediate invasion, PI3-K/AKT stimulates growth and survival, STATs induce proliferation and survival, PLC-gmediates invasion and MAPK induces growth in HNSCC models (24) (Figure 1). The precise role of each pathway downstream of EGFR, or other receptors, in mediating the response to EGFR targeting is incompletely understood.
Figure 1. The diversity of the epidermal growth factor receptor-signaling network.
The EGFR transduction cascade is a highly complex network, consisting of signaling options based on multiple layers. The ligand input and receptor engagement occurs in the extracellular layer. Receptor-specific ligands for ErbB1,ErbB3, and ErbB4 have been identified as shown. Note that no direct ligands for ErbB2 had been isolated to date. At the cell surface, receptor engagement leads to tyrosine phosphorylation and several receptor dimerization options (depicted by arrows: thick arrow denotes homodimerization and thin arrow denotes heterodimerization; the “X” in ErbB3 represents absence of the intrinsic tyrosine kinase activity). The selective activation of well-characterized signaling transduction pathways (shown in boxes), depends on the various arrangements of ligand-ErbB engagement, tyrosine phosphorylation, and subsequent receptor dimerization combinations beneath the cell surface. Finally, the output layer includes a variety of cell responses (shown in bold). J.R. Grandis, J.C. Sok / Pharmacology & Therapeutics 38 102 (2004) 37–46
Unlike non-small cell lung cancer, there is little evidence of activating mutations of the EGFR tyrosine kinase domain in HNSCC (25). A genetic variant of EGFR, EGFRvIII, was originally described in glioblastoma where up to two thirds of patients express this variant. EGFRvIII is a somatic alteration in wtEGFR leading to deletion of exons 2-7 (26, 27). This deletion results in the loss of the ligand-binding domain, yet the protein remains constitutively activated in the absence of ligand (28, 29). Similarly, HER2 truncation molecules are also constitutively active and resistant to the action of trastuzumab (30). EGFRvIII is expressed by nearly 40% of all HNSCC tumors (31). EGFRvIII is capable of constitutively activating downstream signal transduction cascades leading to increased cell proliferation and survival in vitro and tumorigenicity in vivo. Expression of EGFRvIII, which lacks the ligand-binding domain recognized by therapeutic antibodies such as cetuximab, confers significant drug resistance to HNSCC and other tumor cells to cisplatin and erlotinib in addition to cetuximab (31-34). The contribution of EGFRvIII in mediating resistance to these agents in HNSCC is unknown.
The precise signaling pathways induced by EGFRvIII are incompletely understood. Indirect evidence of preferential signaling of EGFRvIII through STATs was provided by a study demonstrating a correlation between EGFRvIII and pSTAT3 expression in a cohort of malignant gliomas (35). The potential role of Src in EGFRvIII signaling was demonstrated by the recent finding that the growth of EGFRvIII-expressing glioblastoma xenografts containing a dominant-negative (DN) mutant c-Src was inhibited to a greater degree compared with tumors that did not contain DN Src, (36). In gliomas, EGFRvIII has been reported to signal through PI3-kinase/Akt (37). Several strategies to target EGFRvIII are in active clinical development including the immunotoxin MR1-1, the antibody 806 and several irreversible EGFR/HER2 tyrosine kinase inhibitors that have selective activity against EGFRvIII (38-41). The contribution of EGFRvIII in mediating resistance to EGFR targeting in HNSCC requires further investigation.
Intrinsic and Acquired Resistance to EGFR Inhibitors
As described above, targeting the EGFR has been intensely pursued in the last decade as a cancer treatment strategy. Clinical trials to investigate the activity of EGFR inhibitors commonly identify 10-20% major response rates. Although highly valuable for those patients that respond, approximately 80% of patients tumors show no response (intrinsic resistance) to EGFR inhibition strategies. Further, as increasing numbers of patients are treated with EGFR inhibitors, the emergence of acquired resistance following initial favorable response has been identified. Preclinical models of resistance to EGFR inhibitors have been recently established and are providing new insights regarding mechanisms of response to EGFR agents.
One approach to inhibit the activity of the EGFR involves the use of small molecule tyrosine kinase inhibitors (TKIs) that bind to the ATP-binding site in the tyrosine kinase domain (TKD) of the EGFR. These agents inhibit EGFR autophosphorylation and ultimately lead to blockade of downstream signaling and cellular proliferation. To date, three anti-EGFR TKIs have been approved by the FDA for use in oncology; erlotinib (OSI-774, Tarceva), gefitinib (ZD1839, Iressa) and lapatinib (GW572016, Tykerb). The identification of mutations in the TKD of the EGFR that predict response to EGFR-TKIs in selected lung cancer patients represents a landmark development in the EGFR field (42). Mutation in exon 21 of the EGFR TKD, L858R, may predict increased sensitivity to TKIs, whereas the T790M mutation in exon 20 is associated with acquired resistance to TKI therapy (43). These findings suggest that patient selection may be critical for successful therapies using EGFR TKIs (44). Although TKD mutations have not been identified with high frequency in HNSCC, there may well emerge mutations in other key signaling pathways that prove more pertinent to HNSCC growth behavior.
Other receptor tyrosine kinases (RTKs) with overlapping signal transduction pathways with the EGFR can promote resistance in EGFR driven cancers (45). cMET (HGFR) is a RTK that regulates cell cycle progression, migration, angiogenesis and cell survival. Increasing evidence identifies cMET as an attractive target for molecular-targeted cancer therapy (46). Previous studies identified cross-talk between the EGFR and cMET in transformed cells (47). However, until recently there has been no clear evidence demonstrating that cMET is involved in regulating acquired resistance to EGFR targeting agents. Engelman et al recently reported that NSCLC HCC827 cells chronically exposed to gefitinib in vitro led to the amplification of cMET. This increased activity of cMET resulted in the constitutive activation of the HER3-Akt signaling pathway in gefitinib-resistant cells and was abrogated by the selective cMET inhibitor PHA665752 thus restoring the sensitivity of resistant cells to gefitinib (45). Taken together, these data indicate that mutations in the EGFR or altered signaling can lead to acquired resistance to EGFR TKIs.
A second approach to inhibit the activity of the EGFR uses monoclonal antibodies (mAbs) that target the extracellular domain of the EGFR. Cetuximab (IMC-C225, Erbitux) blocks natural ligand binding (48), prevents receptor activation and dimerization and ultimately induces receptor internalization and downregulation (49). Cetuximab exhibits promising anti-tumor activity as monotherapy or in combination with chemotherapy and/or radiation (50, 51). A series of clinical trials demonstrating clinical benefit led to the FDA approval of cetuximab for use in patients with HNSCC and in metastatic colorectal cancer (52, 53). Another anti-EGFR monoclonal antibody, panitumumab, has also gained recent FDA approval for use in the metastatic colorectal cancer setting (54). Although EGFR TKD mutations appear to correlate with response to the TKIs erlotinib and gefitinib, no such correlation exists for cetuximab response (55). This indicates that other molecular based mechanisms exist for resistance to cetuximab therapy.
One of the first reports on acquired-resistance to cetuximab suggested that altered control of angiogenesis could be one mechanism responsible for resistance to EGFR targeting agents. Ciardiello et al found a 5- to 10-fold increase in VEGF production and secretion in cetuximab-resistant cell lines established from GEO colon cancer xenograft. Growth of EGFR inhibitor-resistant tumors could be inhibited by ZD6474, a dual EGFR/VEGFR TKI. (56). In addition, using A431 xenograft, Viloria-Petit et al generated six variant cell lines resistant to anti-EGFR antibody and found that these cells produced significant amounts of VEGF when compared to parental cells (57). Collectively these findings indicate that receptor ligands play a critical role in EGFR inhibitor resistance.
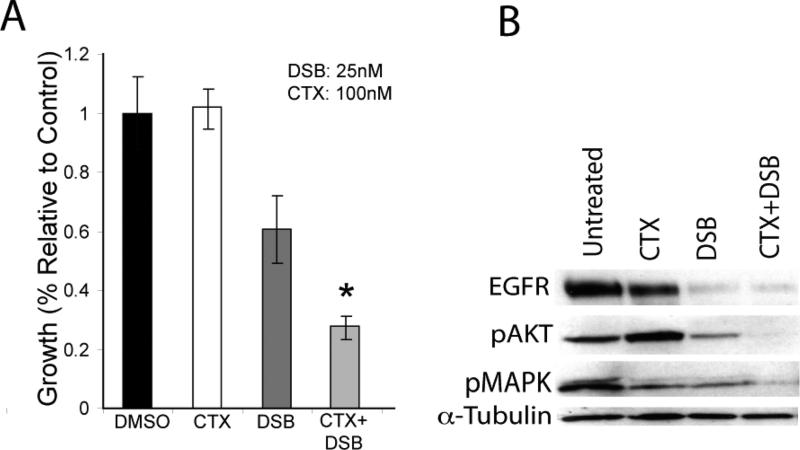
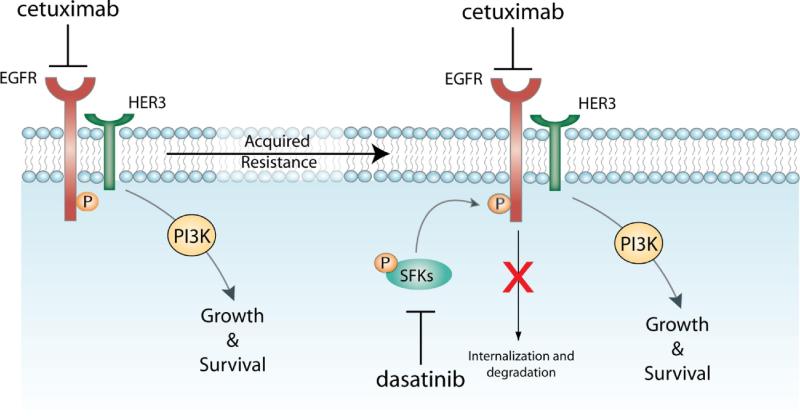
Another pre-clinical study addressing acquired-resistance to cetuximab in vitro utilized high-throughput screening to examine the activity of RTKs in cetuximab-resistant tumor cells following chronic exposure to cetuximab (58). The findings suggested that cells developing acquired-resistance to cetuximab exhibited increased steady-state EGFR expression secondary to alterations in trafficking and degradation. EGFR upregulation promoted increased dimerization with HER2 and HER3 leading to their transactivation and subsequent activation of the PI(3)K/Akt pathway. Blockade of EGFR and HER2 led to loss of HER3 and PI(3)K/Akt activity. These data suggest that acquired-resistance to cetuximab is accompanied by dysregulation of EGFR internalization/degradation and subsequent EGFR-dependent activation of HER3. These findings suggest a rationale for the clinical evaluation of combinatorial anti-HER targeting approaches in tumors manifesting acquired resistance to cetuximab (58). Further investigations of this model of acquired resistance to cetuximab indicated that Src family kinases (SFKs) are highly activated in cetuximab-resistant cells and enhance EGFR activation of HER3 and PI(3)K/Akt. Studies using the FDA approved Src kinase inhibitor dasatinib decreased HER3 activity followed by loss of PI(3)K/Akt (unpublished data). In addition, dasatinib therapy re-sensitized cetuximab resistant cells to cetuximab therapy (Figure 2). These results indicate that SFKs and the EGFR cooperate in acquired-resistance to cetuximab and suggest that dasatinib therapy in combination with cetuximab may have strong clinical benefit (Figure 3).
Figure 2. Dasatinib re-sensitizes cetuximab-resistant cells to cetuximab therapy.
A: Dasatinib re-sensitizes cetuximab-resistant cells to cetuximab therapy. Cetuximab-resistant cells were treated with DMSO, cetuximab (100nM, CTX), dasatinib (25nM, DSB) or the combination for 72 hours. Growth was measured at 72 hours after drug treatment using the growth proliferation assay as described in the experimental procedures and plotted as a percentage of growth relative to the untreated control cells. Data points are represented as mean ±SEM (n=3). *, P<0.05
B: Dual blockade of SFKs and EGFR have additive effects on Akt and MAPK activity. HC4 cells were plated and treated with the vehicle DMSO (control), 100nM cetuximab, 25nM dasatinib or the combination for 48 hours. Cells were harvested and protein was collected, fractionated by SDS-PAGE and immunoblotted for the indicated proteins. a-tubulin was used as a loading control.
Figure 3. Potential mechanisms of acquired-resistance to cetuximab.
Cells that acquire resistance to chronic cetuximab therapy dysregulate the Cbl/ubiquitination of the EGFR(58). This modulation of internalization and degradation of the receptor results in increased steady-expression of EGFR followed by increased cooperation and activation of Src Family Kinases (SFKs). This cooperation between SFKs and the EGFR results in resistance to cetuximab therapy. Blockade using the SFK inhibitor dasatinib re-sensitizes cetuximab-resistant cells to cetuximab therapy.
Promising molecular targets for HNSCC
Looking beyond EGFR, many promising molecular targets are emerging in HNSCC. One highly promising molecule that resides within the HER family is HER3. Themes have emerged in the last two years that escape from therapies targeting the EGFR involve equilibrium shifts in HER family signaling via HER3 (45, 58, 59). Several laboratories are designing antibodies that will block HER3 hetero-dimerization with EGFR or HER2 to prevent signals transduced to PI(3)K/Akt.
The mammalian target of rapamycin (mTOR) is a serine/threonine protein kinase that regulates cell growth, cell proliferation, cell motility, cell survival, protein synthesis, and transcription. Further, it has recently been implicated in the pathogenesis of HNSCC and therefore serves as a promising molecular target (60). mTOR is activated by Akt and ultimately blocks apoptosis and increases proliferative potential of cancer cells. Several mTOR inhibitors are being investigated ( Rapamycin, CI-779, RAD001 and AP23753).
Signaling through the insulin-like growth factor-I receptor (IGF-IR) leads to cellular proliferation, apoptosis, metastasis, and resistance to cytotoxic cancer therapies. Recent work combining the fully humanized anti–IGF-IR monoclonal antibody A12 (ImClone Systems, Inc., New York, NY) with radiation resulted in pronounced anti-tumor growth than either agent alone (61). In addition, TKIs directed against the IGF-IR are now in clinical development. These results suggest IGF-IR signal transduction blockade as a promising strategy to improve radiation therapy efficacy in human tumors, forming a basis for future clinical trials in HNSCC.
Aurora kinases are serine/threonine kinases that are essential for cell proliferation by playing a crucial role in cell division by controlling spindle formation during mitosis. Studies investigation the relationship between Aurora kinases and HNSCC found a strong correlation between the up-regulation of aurora kinase (A) mRNA and distant metastases, poor disease-free and overall survival (62). Several aurora kinase A inhibitors (MP529, MLN8054) and aurora kinase B (AZD1152) are in clinical development.
Conclusions
HNSCC provides a rich environment for preclinical and clinical study in light of the relative accessibility of tumor and normal tissue for controlled investigation. The recent incorporation of EGFR inhibitors into the available armamentarium for HNSCC therapy is a landmark development for a highly complex cancer. Several additional molecular targets of potential interest for HNSCC therapy are described in this article. Strategies that combine radiation with molecular targeting agents offer high promise for achieving meaningful improvements in HNSCC therapy. The radiation/EGFR story represents only the beginning of many successful future steps.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Paul M. Harari, Department of Human Oncology, University of Wisconsin School of Medicine and Public Health, 600 Highland Ave, K4/336 CSC, Madison, WI 53792, USA.
Deric L. Wheeler, University of Wisconsin School of Medicine and Public Health, Department of Human Oncology, 600 Highland Ave, K4/319 CSC, Madison, WI 53792, USA; Phone: (608) 265-3716; fax: (608) 263-9947; dlwheeler@wisc.edu.
Jennifer R. Grandis, University of Pittsburgh School of Medicine, The Eye and Ear Institute Building, Suite 500, 200 Lothrop St, Pittsburgh, PA 15213, USA; Phone: 412-647-5280; Fax: 412-647-0108; jgrandis@pitt.edu.
References
- 1.Grandis JR, Chakraborty A, Zeng Q. Downmodulation of TGF-alpha protein expression with antisense oligonucleotides inhibits proliferation of head and neck squamous carcinoma but not normal mucosal epithelial cells. J Cell Biochem. 1998;69:55–62. [PubMed] [Google Scholar]
- 2.Grandis JR, Chakraborty A, Melhem MF. Inhibition of epidermal growth factor receptor gene expression and function decreases proliferation of head and neck squamous carcinoma but not normal mucosal epithelial cells. Oncogene. 1997;15:409–416. doi: 10.1038/sj.onc.1201188. [DOI] [PubMed] [Google Scholar]
- 3.Grandis JR, Tweardy DJ, Melhem MF. Asynchronous modulation of transforming growth factor alpha and epidermal growth factor receptor protein expression in progression of premalignant lesions to head and neck squamous cell carcinoma. Clin Cancer Res. 1998;4:13–20. [PubMed] [Google Scholar]
- 4.Grandis JR, Sok JC. Signaling through the epidermal growth factor receptor during the development of malignancy. Pharmacol Ther. 2004;102:37–46. doi: 10.1016/j.pharmthera.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Egloff AM, Grandis J. Epidermal growth factor receptor--targeted molecular therapeutics for head and neck squamous cell carcinoma. Expert Opin Ther Targets. 2006;10:639–647. doi: 10.1517/14728222.10.5.639. [DOI] [PubMed] [Google Scholar]
- 6.Grandis JR, Melhem MF, Gooding WE. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90:824–832. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 7.Ang KK, Berkey BA, Tu X. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–7356. [PubMed] [Google Scholar]
- 8.Chung CH, Ely K, McGavran L. Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. J Clin Oncol. 2006;24:4170–4176. doi: 10.1200/JCO.2006.07.2587. [DOI] [PubMed] [Google Scholar]
- 9.Grandis JR, Zeng Q, Tweardy DJ. Retinoic acid normalizes the increased gene transcription rate of TGF- alpha and EGFR in head and neck cancer cell lines. Nat Med. 1996;2:237–240. doi: 10.1038/nm0296-237. [DOI] [PubMed] [Google Scholar]
- 10.Rubin Grandis J, Tweardy DJ, Melhem MF. Asynchronous modulation of transforming growth factor alpha and epidermal growth factor receptor protein expression in progression of premalignant lesions to head and neck squamous cell carcinoma. Clin Cancer Res. 1998;4:13–20. [PubMed] [Google Scholar]
- 11.Lui VW, Grandis JR. EGFR-mediated cell cycle regulation. Anticancer Res. 2002;22:1–11. [PubMed] [Google Scholar]
- 12.Thomas SM, Grandis JR. Pharmacokinetic and pharmacodynamic properties of EGFR inhibitors under clinical investigation. Cancer Treat Rev. 2004;30:255–268. doi: 10.1016/j.ctrv.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Huang SM, Bock JM, Harari PM. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59:1935–1940. [PubMed] [Google Scholar]
- 14.Burtness B, Goldwasser MA, Flood W. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23:8646–8654. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 15.Bonner JA, Harari PM, Giralt J. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 16.Soulieres D, Senzer NN, Vokes EE. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol. 2004;22:77–85. doi: 10.1200/JCO.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 17.Agulnik M, Cohen EW, Cohen RB. Phase II study of lapatinib in recurrent or metastatic epidermal growth factor receptor and/or erbB2 expressing adenoid cystic carcinoma and non adenoid cystic carcinoma malignant tumors of the salivary glands. J Clin Oncol. 2007;25:3978–3984. doi: 10.1200/JCO.2007.11.8612. [DOI] [PubMed] [Google Scholar]
- 18.Siegfried JM, Krishnamachary N, Gaither Davis A. Evidence for autocrine actions of neuromedin B and gastrin-releasing peptide in non-small cell lung cancer. Pulm Pharmacol Ther. 1999;12:291–302. doi: 10.1006/pupt.1999.0210. [DOI] [PubMed] [Google Scholar]
- 19.Gallo O, Franchi A, Magnelli L. Cyclooxygenase-2 pathway correlates with VEGF expression in head and neck cancer. Implications for tumor angiogenesis and metastasis. Neoplasia. 2001;3:53–61. doi: 10.1038/sj.neo.7900127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masferrer JL, Leahy KM, Koki AT. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–1311. [PubMed] [Google Scholar]
- 21.Gschwind A, Prenzel N, Ullrich A. Lysophosphatidic acid-induced squamous cell carcinoma cell proliferation and motility involves epidermal growth factor receptor signal transactivation. Cancer Res. 2002;62:6329–6336. [PubMed] [Google Scholar]
- 22.Klingmuller U, Wu H, Hsiao JG. Identification of a novel pathway important for proliferation and differentiation of primary erythroid progenitors. Proc Natl Acad Sci U S A. 1997;94:3016–3021. doi: 10.1073/pnas.94.7.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altorki NK, Keresztes RS, Port JL. Celecoxib, a selective cyclo-oxygenase-2 inhibitor, enhances the response to preoperative paclitaxel and carboplatin in early-stage non-small-cell lung cancer. J Clin Oncol. 2003;21:2645–2650. doi: 10.1200/JCO.2003.07.127. [DOI] [PubMed] [Google Scholar]
- 24.Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006;24:2666–2672. doi: 10.1200/JCO.2005.04.8306. [DOI] [PubMed] [Google Scholar]
- 25.Temam S, Kawaguchi H, El-Naggar AK. Epidermal growth factor receptor copy number alterations correlate with poor clinical outcome in patients with head and neck squamous cancer. J Clin Oncol. 2007;25:2164–2170. doi: 10.1200/JCO.2006.06.6605. [DOI] [PubMed] [Google Scholar]
- 26.Sugawa N, Ekstrand AJ, James CD. Identical splicing of aberrant epidermal growth factor receptor transcripts from amplified rearranged genes in human glioblastomas. Proc Natl Acad Sci U S A. 1990;87:8602–8606. doi: 10.1073/pnas.87.21.8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ekstrand AJ, James CD, Cavenee WK. Genes for epidermal growth factor receptor, transforming growth factor alpha, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res. 1991;51:2164–2172. [PubMed] [Google Scholar]
- 28.Huang HS, Nagane M, Klingbeil CK. The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J Biol Chem. 1997;272:2927–2935. doi: 10.1074/jbc.272.5.2927. [DOI] [PubMed] [Google Scholar]
- 29.Moscatello DK, Montgomery RB, Sundareshan P. Transformational and altered signal transduction by a naturally occurring mutant EGF receptor. Oncogene. 1996;13:85–96. [PubMed] [Google Scholar]
- 30.Scaltriti M, Rojo F, Ocana A. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99:628–638. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- 31.Sok JC, Coppelli FM, Thomas SM. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin Cancer Res. 2006;12:5064–5073. doi: 10.1158/1078-0432.CCR-06-0913. [DOI] [PubMed] [Google Scholar]
- 32.Mellinghoff IK, Wang MY, Vivanco I. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 33.Friedman HS, Bigner DD. Glioblastoma multiforme and the epidermal growth factor receptor. N Engl J Med. 2005;353:1997–1999. doi: 10.1056/NEJMp058186. [DOI] [PubMed] [Google Scholar]
- 34.Learn CA, Hartzell TL, Wikstrand CJ. Resistance to tyrosine kinase inhibition by mutant epidermal growth factor receptor variant III contributes to the neoplastic phenotype of glioblastoma multiforme. Clin Cancer Res. 2004;10:3216–3224. doi: 10.1158/1078-0432.ccr-03-0521. [DOI] [PubMed] [Google Scholar]
- 35.Mizoguchi M, Betensky RA, Batchelor TT. Activation of STAT3, MAPK, and AKT in malignant astrocytic gliomas: correlation with EGFR status, tumor grade, and survival. J Neuropathol Exp Neurol. 2006;65:1181–1188. doi: 10.1097/01.jnen.0000248549.14962.b2. [DOI] [PubMed] [Google Scholar]
- 36.Johns TG, Perera RM, Vernes SC. The efficacy of epidermal growth factor receptor-specific antibodies against glioma xenografts is influenced by receptor levels, activation status, and heterodimerization. Clin Cancer Res. 2007;13:1911–1925. doi: 10.1158/1078-0432.CCR-06-1453. [DOI] [PubMed] [Google Scholar]
- 37.Li B, Yuan M, Kim IA. Mutant epidermal growth factor receptor displays increased signaling through the phosphatidylinositol-3 kinase/AKT pathway and promotes radioresistance in cells of astrocytic origin. Oncogene. 2004;23:4594–4602. doi: 10.1038/sj.onc.1207602. [DOI] [PubMed] [Google Scholar]
- 38.Kuan CT, Wikstrand CJ, Archer G. Increased binding affinity enhances targeting of glioma xenografts by EGFRvIII-specific scFv. Int J Cancer. 2000;88:962–969. doi: 10.1002/1097-0215(20001215)88:6<962::aid-ijc20>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 39.Scott AM, Lee FT, Tebbutt N. A phase I clinical trial with monoclonal antibody ch806 targeting transitional state and mutant epidermal growth factor receptors. Proc Natl Acad Sci U S A. 2007;104:4071–4076. doi: 10.1073/pnas.0611693104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ji H, Zhao X, Yuza Y. Epidermal growth factor receptor variant III mutations in lung tumorigenesis and sensitivity to tyrosine kinase inhibitors. Proc Natl Acad Sci U S A. 2006;103:7817–7822. doi: 10.1073/pnas.0510284103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reid A, Vidal L, Shaw H. Dual inhibition of ErbB1 (EGFR/HER1) and ErbB2 (HER2/neu). Eur J Cancer. 2007;43:481–489. doi: 10.1016/j.ejca.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Sequist LV, Bell DW, Lynch TJ. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007;25:587–595. doi: 10.1200/JCO.2006.07.3585. [DOI] [PubMed] [Google Scholar]
- 43.Riely GJ, Politi KA, Miller VA. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res. 2006;12:7232–7241. doi: 10.1158/1078-0432.CCR-06-0658. [DOI] [PubMed] [Google Scholar]
- 44.Arteaga CL, Baselga J. Tyrosine kinase inhibitors: why does the current process of clinical development not apply to them? Cancer Cell. 2004;5:525–531. doi: 10.1016/j.ccr.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 45.Engelman JA, Zejnullahu K, Mitsudomi T. MET Amplification Leads to Gefitinib Resistance in Lung Cancer by Activating ERBB3 Signaling. Science. 2007 doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 46.Peruzzi B, Bottaro DP. Targeting the c-Met Signaling Pathway in Cancer. Clin Cancer Res. 2006;12:3657–3660. doi: 10.1158/1078-0432.CCR-06-0818. [DOI] [PubMed] [Google Scholar]
- 47.Jo M, Stolz DB, Esplen JE. Cross-talk between epidermal growth factor receptor and c-Met signal pathways in transformed cells. J Biol Chem. 2000;275:8806–8811. doi: 10.1074/jbc.275.12.8806. [DOI] [PubMed] [Google Scholar]
- 48.Mendelsohn J. Antibody-mediated EGF receptor blockade as an anticancer therapy: from the laboratory to the clinic. Cancer Immunology & Immunotherapy. 2003;52:342–346. doi: 10.1007/s00262-002-0354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sunada H, Magun BE, Mendelsohn J. Monoclonal antibody against epidermal growth factor receptor is internalized without stimulating receptor phosphorylation. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:3825–3829. doi: 10.1073/pnas.83.11.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baselga J, Arteaga CL. Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J Clin Oncol. 2005;23:2445–2459. doi: 10.1200/JCO.2005.11.890. [DOI] [PubMed] [Google Scholar]
- 51.Mendelsohn J, Baselga J. Epidermal growth factor receptor targeting in cancer. Semin Oncol. 2006;33:369–385. doi: 10.1053/j.seminoncol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 52.Bonner JA, Harari PM, Giralt J. Radiotherapy plus Cetuximab for Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 53.Cunningham D, Humblet Y, Siena S. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 54.Van Cutsem E, Peeters M, Siena S. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 55.Mukohara T, Engelman JA, Hanna NH. Differential effects of gefitinib and cetuximab on non-small-cell lung cancers bearing epidermal growth factor receptor mutations. J Natl Cancer Inst. 2005;97:1185–1194. doi: 10.1093/jnci/dji238. [DOI] [PubMed] [Google Scholar]
- 56.Ciardiello F, Bianco R, Caputo R. Antitumor activity of ZD6474, a vascular endothelial growth factor receptor tyrosine kinase inhibitor, in human cancer cells with acquired resistance to antiepidermal growth factor receptor therapy. Clin Cancer Res. 2004;10:784–793. doi: 10.1158/1078-0432.ccr-1100-03. [DOI] [PubMed] [Google Scholar]
- 57.Viloria-Petit A, Crombet T, Jothy S. Acquired resistance to the antitumor effect of epidermal growth factor receptor-blocking antibodies in vivo: a role for altered tumor angiogenesis. Cancer Research. 2001;61:5090–5101. [PubMed] [Google Scholar]
- 58.Wheeler DL, Huang S, Kruser TJ. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008 doi: 10.1038/onc.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sergina NV, Rausch M, Wang D. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437–441. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 61.Allen GW, Saba C, Armstrong EA. Insulin-like growth factor-I receptor signaling blockade combined with radiation. Cancer Res. 2007;67:1155–1162. doi: 10.1158/0008-5472.CAN-06-2000. [DOI] [PubMed] [Google Scholar]
- 62.Reiter R, Gais P, Jutting U. Aurora kinase A messenger RNA overexpression is correlated with tumor progression and shortened survival in head and neck squamous cell carcinoma. Clin Cancer Res. 2006;12:5136–5141. doi: 10.1158/1078-0432.CCR-05-1650. [DOI] [PubMed] [Google Scholar]