Abstract
Region II of the 175-kDa erythrocyte-binding antigen (EBA-175RII) of Plasmodium falciparum is functionally important in sialic acid-dependent erythrocyte invasion and is considered a prime target for an invasion-blocking vaccine. The objectives of this study were to (i) determine the prevalence of anti-EBA-175RII antibodies in a naturally exposed population, (ii) determine whether naturally acquired antibodies have a functional role by inhibiting binding of EBA-175RII to erythrocytes, and (iii) determine whether antibodies against EBA-175RII correlate with immunity to clinical malaria. We treated 301 lifelong residents of an area of malaria holoendemicity in western Kenya for malaria, monitored them during a high-transmission season, and identified 33 individuals who were asymptomatic despite parasitemia (clinically immune). We also identified 50 clinically susceptible individuals to serve as controls. These 83 individuals were treated and monitored again during the subsequent low-transmission season. Anti-EBA-175RII antibodies were present in 98.7% of the individuals studied. The antibody levels were relatively stable between the beginning and end of the high-transmission season and correlated with the plasma EBA-175RII erythrocyte-binding-inhibitory activity. There was no difference in anti-EBA-175RII levels or plasma EBA-175RII erythrocyte-binding-inhibitory activity between clinically immune and clinically susceptible groups. However, these parameters were higher in nonparasitemic than in parasitemic individuals at enrollment. These results suggest that although antibodies against EBA-175RII may be effective in suppressing some of the wild parasite strains, EBA-175RII is unlikely to be effective as a monovalent vaccine against malaria, perhaps due to allelic heterogeneity and/or presence of sialic acid-independent strains.
A vaccine against Plasmodium falciparum that can prevent the morbidity and mortality (8, 9, 25) from this parasite is greatly needed. Among the antigens being considered for vaccine development is the erythrocyte-binding antigen 175 (EBA-175). This is a 175-kDa protein that is expressed in the micronemes of merozoites (24), the stage of the parasite that invades erythrocytes. Its potential as a malaria vaccine antigen is based on the fact that the majority of P. falciparum isolates use EBA-175 as a ligand for the invasion of erythrocytes (6, 18). EBA-175 binds to sialic acid-dependent epitopes on erythrocyte glycophorin A (11, 23) and is probably involved in the formation of a junction between the erythrocyte and the apical portion of the merozoite just before invagination (10). This step is a key part of the erythrocyte invasion process and provides a logical target for vaccine-mediated immunity (23).
EBA-175 is structurally divided into seven regions (1), and the cysteine-rich region II functions as the erythrocyte-binding ligand domain (23). Region II contains epitopes recognized by antibodies that block erythrocyte invasion (15, 19, 22) and by antibodies eluted from immune clusters of merozoites (21). Furthermore, although region II is relatively well conserved in P. falciparum laboratory clones and field isolates (13), the nucleotide polymorphisms that do occur in the EBA-175 ligand domain are biased towards nonsynonymous changes (2). One possible explanation of this observation is that escape mutants have a survival advantage in the context of an effective immune response against EBA-175. This provides further justification to consider EBA-175 an appealing antimalarial vaccine target.
While a large amount of work has been done on the characterization of EBA-175, only one previous study described the natural immune responses induced by this molecule in an area where malaria is endemic and their relationship to malaria immunity (17). With this in mind, the present study sought to characterize humoral immune responses to EBA-175 region II (EBA-175RII) among semi-immune residents of an area of holoendemicity for malaria in of western Kenya, to determine the role of these antibody responses in disrupting the binding to erythrocytes, and to determine whether these responses play a role in protection against clinical malaria.
MATERIALS AND METHODS
Study design and population.
This study received ethical clearance from both the Kenya Medical Research Institute Ethical Review Committee and the Human Subjects Research Review Board of the Office of the Surgeon General, U.S. Army. The study site was in Kombewa Division, Nyanza Province, western Kenya. Malaria is holoendemic in this region, occurring throughout the year and with peak seasons during the long rains (March thru August) and during the short rains (October thru December) (4). Malaria infections are predominantly due to P. falciparum. The entomological inoculation rate in this area ranges between 237 and 300 infectious bites per person per year (4). The major malaria vectors are Anopheles gambiae, Anopheles arabiensis, and Anopheles funestus. Over 90% of the residents are of the Luo ethnic group, and most depend on farming and fishing for subsistence. Inclusion criteria included an age between 18 and 35 years and lifelong residency in the study area. Subjects were excluded if they were not in good general health, had clinical evidence of chronic illness, or had a hemoglobin level of <10 g/dl. In addition, women were tested for pregnancy and excluded if positive. All volunteers signed an informed consent agreement before entry into the study. After a physical examination, EDTA-anticoagulated blood was collected for plasma storage and preparation of Giemsa-stained thick and thin blood smears. Prior to the start of the study, during the last week of April 1998, each volunteer was treated with 7 days of quinine (10 mg/kg of body weight twice daily) and 7 days of doxycycline (100 mg twice daily) for radical malaria cure. This was done to allow us to measure the time to the first episode of clinical malaria from a baseline of no parasitemia for all participants. Clinical malaria was defined as the presence of asexual P. falciparum parasites in a thick or thin Giemsa-stained blood smear from an individual with an oral temperature of >37.5°C or, in the absence of the latter, two of the following symptoms: headache, myalgia, nausea or vomiting, or diarrhea. Active follow-up consisted of daily visits to the volunteer's home by a field worker, during which he or she measured the oral temperature and asked the participant questions about his or her general wellness. Thick and thin Giemsa-stained blood smears were prepared weekly by finger prick from each volunteer. Symptomatic volunteers were referred to the Walter Reed Project Kombewa Clinic for evaluation. Passive follow-up consisted of self-initiated visits to the Walter Reed Project Kombewa Clinic. After a 4-month follow-up period, volunteers who had had no episodes of clinical malaria (clinically immune [CI]) were identified. For each CI individual, we identified one to three individuals with clinical malaria (clinically susceptible [CS]) who matched his or her age (within ±2 years) and gender to serve as controls. Cases and controls were retreated for malaria in October 1998 and monitored again for an additional 16-week period as before. A minimum of 200 high-power fields of a Giemsa-stained thick blood smear were scanned microscopically before it was declared negative. The number of asexual parasites per 500 white blood cells was recorded. The number of parasites per microliter was obtained by assuming a white blood cell count of 8 × 103/μl.
Antibody enzyme-linked immunosorbent assay.
Recombinant EBA-175RII of the 3D7 clone of P. falciparum was expressed as a His6-tagged polypeptide spanning residues 144 through 753, using a baculovirus-derived vector in Sf9 insect cells (16). Immulon II HB 96-well flat-bottom plates (Thermo Labsystems, Helsinki, Finland) were coated with 50 μl of recombinant EBA-175RII (15.6 ng/ml) in 10 mM phosphate-buffered saline (PBS) (pH 7.4) (Sigma-Aldrich, St. Louis, Mo.). After a 60-min incubation at room temperature (RT), the plates were inverted, tapped onto paper towels to remove unbound antigen and immediately blocked with 200 μl of 0.5% boiled casein-1% Tween 20 (Sigma) per well in PBS at RT for 1 h. Plasma samples were diluted twofold serially starting at 1:50 in 0.5% boiled casein-0.025% Tween 20 in PBS (dilution buffer) and plated at 50 μl/well in triplicate wells. Serum pooled from two malaria-naive North American residents was used as the negative control, and the positive control was a high-titer plasma sample from a malaria-immune resident of western Kenya. Sera from another 27 North American residents were used as an additional comparison group. Unbound antibodies were washed six times with 10 mM PBS containing 0.05% Tween 20. Peroxidase-labeled goat anti-human immunoglobulin G (IgG) (Kirkegaard and Perry Laboratories, Gaithersburg, Md.) was diluted 1:1,000 in dilution buffer and 50 μl was added to each well, followed by a 1-h incubation at RT. For determination of IgG isotypes and IgM levels, peroxidase-labeled mouse monoclonal antibodies against IgG1, IgG2, IgG3, IgG4, or IgM (Kirkegaard and Perry) were used instead in duplicate wells. The bound antibodies were detected by incubation with 100 μl of ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] peroxidase substrate (Kirkegaard and Perry) per well for 1 h, and the reaction was stopped with 10 μl of 20% sodium dodecyl sulfate (Sigma) per well, followed by measurement of the optical density (OD) at 415 nm. The results were calculated by first obtaining the weighted means for the OD of 1.0 for each sample, which was defined as the mean of the product of three ODs (one above, one near, and one below 1.0) by their respective dilutions. To correct for plate-to-plate and day-to-day variation, values were normalized first to the weighted mean of the positive control within each plate and then for all the plates.
Expression of EBA-175RII as a GFP construct.
EBA-175RII of the 3D7 clone of P. falciparum was cloned into the plasmid pRE4 in frame with the extracellular domain of the herpes simplex virus (HSV) glycoprotein D1, a type I integral membrane protein, at the C terminus (23). The plasmid construct targeted expression of EBA-175RII to the surface of transiently transfected COS-7 cells in vitro. To improve expression levels and to use green fluorescent protein (GFP) as a marker of transfected cells, we used the plasmid pEGFP (BD Biosciences Clontech, Palo Alto, Calif.) to express the EBA-175RII in a manner similar to that described for the Plasmodium vivax Duffy binding protein (DBP) (14). The EBA-175RII/HSV gD1 coding sequence was PCR amplified from pRE4 by using primer pairs 5′-ATA AAG CTT CAG CGC GAA CGA CC-3′ and 5′-ATA GGA TCC TTG TAA AAC AAG GGC TGG TGC GA-3′. After hot start denaturation at 94°C for 5 min, we carried out 35 cycles of annealing at 59°C for 30 s, extension at 72°C for 1 min, and denaturation at 94°C for 15 s. The 2.5-kb product was cloned into a TOPO TA vector (Invitrogen Life Technologies, Carlsbad, Calif.). The EBA-175RII/HSV gD1 coding sequence was excised from TOPO TA by cutting with both BamHI and EcoRI and was ligated into the plasmid pEGFP. The ligation site in pEGFP is upstream from the gene for GFP, and the inserted protein was expressed as a GFP fusion. DNA for transfection was purified with an endotoxin-free DNA purification system (Qiagen Inc., San Diego, Calif.), and the resulting purified DNA was reconstituted into endotoxin-free water to be used for transfection assays.
COS-7 culture and rosette inhibition assay.
Green monkey kidney cells (COS-7) (American Type Culture Collection, Manassas, Va.) were grown to confluency in T-25 flasks (Corning Costar, Cambridge, Mass.) containing 8 ml of Dulbecco's minimal Eagle's medium (DMEM) (Sigma) plus 10% fetal bovine serum (Invitrogen Life Technologies) at 37°C in a 5% CO2 atmosphere. Just before each rosette inhibition assay, confluent cells were detached with trypsin-EDTA (Sigma) and seeded at a 1:1 dilution in six-well tissue culture plates (Corning Inc. Life Sciences, Acton, Mass.) containing complete medium as described above. After the first 2 h, the cells were checked for adherence and confluency and used for transfection. Prior to transfection, PLUS reagent (Invitrogen Life Technologies) was added to pEGFP-EBARII DNA at a ratio of 6 μl per each 1 μg of DNA (10 μg/ml) in endotoxin-free water and allowed to stand at RT for 15 min. Lipofectamine (Invitrogen Life Technologies) was diluted 1:33 in endotoxin-free water and added to the precomplexed DNA at a 1:1 (vol/vol) ratio by gentle mixing over 30 s in a polypropylene tube. The mixture was allowed to stand for an additional 15 min at RT. As complexes formed, the plated COS-7 cells were washed twice with 2 ml of plain DMEM. The DNA-PLUS-Lipofectamine was diluted 1:5 in plain DMEM, and 1 ml of transfection medium was transferred into each well of the six-well plate containing adherent COS-7 cells. The plate was swirled 30 times and then incubated at 37°C in a 5% CO2 atmosphere. After a 24-h incubation, the medium containing complexes was replaced with fresh complete medium and the cells were incubated for an additional 24 h. Over 95% of the cells demonstrated green fluorescence, indicating that they were successfully transfected.
For the rosette inhibition assay, medium was replaced with test plasma diluted 1:80 in DMEM or with 10% fetal bovine serum in DMEM (negative control). A dilution of 1:80 was chosen because in preliminary experiments this dilution provided a wide range of inhibitory activity among different samples. After a 1-h incubation at 37°C in a 5% CO2 atmosphere, 200 μl of washed O+ erythrocytes at 10% hematocrit in DMEM was added to each well of the six-well culture plate, followed by incubation at 37°C for an additional 3 h. Unbound erythrocytes were removed with five 2-ml washes of 10 mM PBS (pH 7.4). The number of rosettes around GFP-positive cells within 30 fields at a magnification of ×20 was counted in each well. The percent inhibition was calculated as 100 × (Rc − Rs)/Rc, where Rc is the number of rosettes in the control well and Rs is the number of rosettes in the test wells.
Statistical analysis.
Total IgG and IgM anti-EBA-175RII antibody levels in CI and CS groups were compared by analysis of variance (ANOVA) that also took into account their matching. Since the total anti-EBA-175RII antibody levels showed a large variance relative to their mean, they were log10 transformed. Unmatched logistic regression was used to study the relationship between the presence or absence of parasitemia at the start of the study and clinical immunity. The independent-sample t test was used to compare the rosette-inhibitory activity and the anti-EBA-175RII levels between parasitemic and nonparasitemic individuals at the start of the study.
RESULTS
Demographic and clinical characteristics of the study population.
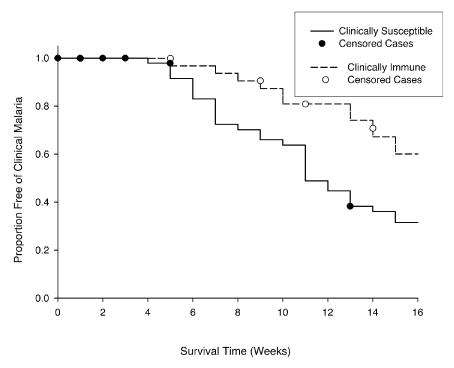
Three hundred ninety-nine subjects were screened, and 301 were enrolled for the initial longitudinal cohort study. The mean age of the enrollees ± standard deviation (SD) was 24.4 ± 5.4 years. Of these, 185 (61.5%) were male and 116 (38.5%) were female. Thirty-three individuals were found to be asymptomatic during the first follow-up period from May through August 1998, in most cases despite evidence of parasitemia. These individuals composed the CI group. For each individual in the CI group we identified one to three CS individuals (n = 50) from the original 301 enrollees who matched both his or her age (within ±2 years) and his or her gender. The mean age ± SD of the CI group was 25.1 ± 5.6 years, and the mean age ± SD of the CS group was 24.4 ± 5.4 years. There were 27 males (82%) among the CI individuals and 43 males (86%) among the CS individuals. The mean parasite density ± SD at the first episode of parasitemia, regardless of symptoms, during the first follow-up period was 90 ± 159 parasites/μl for the CI group (28 of 33 individuals) and 1,106 ± 3,653 parasites/μl (50 of 50 individuals) for the CS group (ANOVA for matched samples with log-transformed data, P = 0.04). During the second follow-up period from October 1998 to February 1999, the CI group still had a significantly more prolonged clinical malaria-free survival than the CS group (Fig. 1) (P < 0.01 by log rank test). The mean parasite density ± SD at the first episode of parasitemia during this second follow-up period was 168 ± 209 parasites/μl for the CI group (30 of 33 individuals) and 262 ± 732 parasites/μl for the CS group (48 of 50 individuals) (ANOVA for matched samples with log-transformed data, P = 0.92).
FIG. 1.
Clinical malaria-free survival of individuals in the CI and CS groups from October 1998 to February 1999. Individuals were treated with quinine and doxycycline in October 1998 and monitored daily for symptoms and weekly for malaria smears.
Antibody levels.
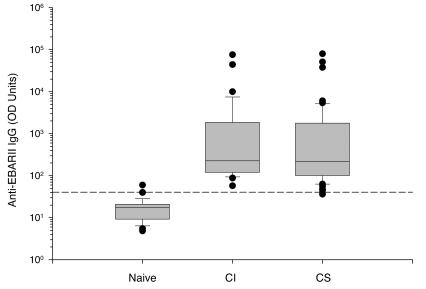
In order to determine the specificity of the anti-EBA-175RII enzyme-linked immunosorbent assay, we compared the total IgG antibody levels of the CI and CS groups to those in a sample of 27 North American naive donors (Fig. 2). The levels in all but one of the 83 African samples were more than 2 SDs above the mean of the levels the North American samples (98.7% antibody prevalence). Table 1 summarizes anti-EBA-175RII antibody levels in the CI and CS groups. Both populations had high antibody levels against EBA-175RII, and there were no significant differences in total anti-EBA-175RII IgG antibody levels, IgG isotypes, or IgM between the two groups. There was also no significant change in mean antibody levels within each group from April to October 1998 (Table 1).
FIG. 2.
Comparison of anti-EBA-175RII total IgG antibody levels between 83 western Kenya samples and 27 North American naive samples. CI, n = 33; CS, n = 50. The dashed line represents the mean value plus 2 SDs for the naive samples. The lines in the boxes represent medians.
TABLE 1.
Anti-EBA-175RII antibody levels measured with no episodes of clinical malaria (CI group) and in matched individuals (CS group) who had been diagnosed with clinical malaria between May and August 1998
| Parameter | Mean OD
|
SEDa | P | |
|---|---|---|---|---|
| CI group (n = 33) | CS group (n = 50) | |||
| Log10 total anti-EBA175RII IgG, April 1998b | 2.7 (501) | 2.6 (398) | 0.19 | 0.65 |
| Log10 Total anti-EBA175RII IgG, October 1998b | 2.7 (501) | 2.7 (501) | 0.21 | 0.90 |
| Anti-EBA175RII IgG1, April 1998 | 23.2 | 21.9 | 2.4 | 0.58 |
| Anti-EBA-175RII IgG2, April 1998 | 16.0 | 15.1 | 1.1 | 0.40 |
| Anti-EBA-175RII IgG3, April 1998 | 26.2 | 21.8 | 4.2 | 0.30 |
| Anti-EBA-175RII IgG4, April 1998 | 14.1 | 13.2 | 0.61 | 0.13 |
| Anti-EBA-175RII IgM, April 1998 | 95.9 | 93.1 | 7.74 | 0.71 |
Standard error of difference between means.
This variable was log10 transformed due to its large variance relative to its mean; hence, the P value represents the probability level resulting from the analysis of the transformed data. The values in parentheses are the antilogs.
Rosette inhibition studies.
In an attempt to define the functional role of anti-EBA-175RII antibodies, we studied the ability of individual plasma samples to inhibit binding of erythrocytes to COS-7 cells expressing EBA-175RII. While there was a trend for the mean percent inhibition for the CI group (44.2) to be higher than that of the CS group (32.7), the difference was not significant (standard error of the difference = 11.5; P = 0.32). Interestingly, about 25% of the samples tested appeared to enhance erythrocyte binding.
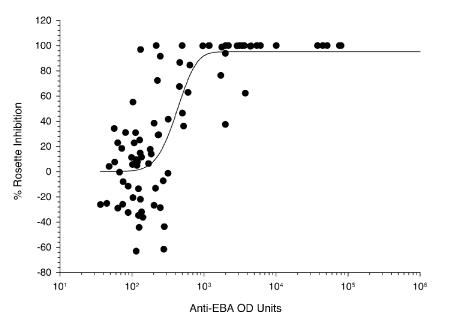
We also studied the relationship between anti-EBA-175RII total IgG and the rosette-inhibitory activity (Fig. 3). The relationship between these two variables could be best described by a sigmoidal curve where y = 95(1 − e−0.005*logx)5.4 (r2 = 0.64; P < 0.001). There is a progressive increase in the inhibition of binding as the level of anti-EBA-175RII specific antibodies rises. However, this reaches a saturation point beyond which inhibition is complete. The rosette-enhancing activity was limited to plasma from individuals with very low anti-EBA-175RII antibody levels.
FIG. 3.
Scatter plot of rosette-inhibitory activity versus total anti-EBA-175RII IgG from 33 CI and 50 CS individuals at the time of initial enrollment in April 1998.
Relationship between malaria status at enrollment and anti-EBA-175RII antibodies, rosette inhibition, and clinical immunity to malaria.
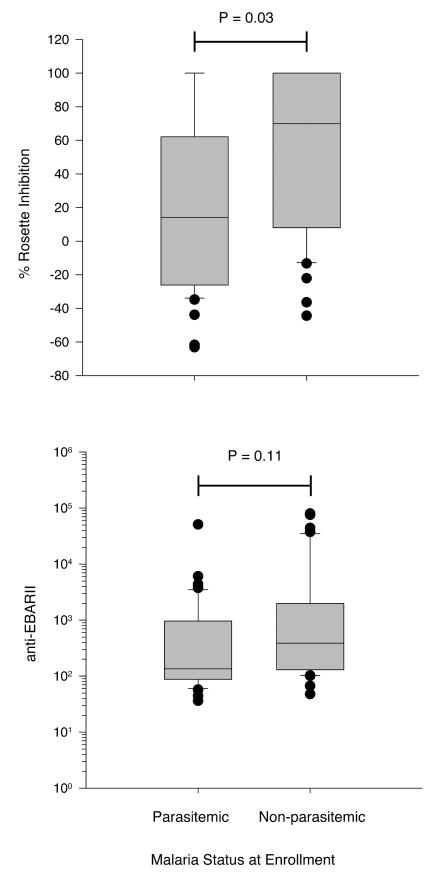
As indicated in Table 1, there was no difference in anti-EBA-175RII antibody levels between the CI and CS groups at enrollment. Because of the significant difference in parasite density between the CI and CS groups during the first follow-up period, we explored the possibility that this may be a better predictor of clinical immunity in the original cohort. The original cohort of 301 enrollees was coded for the presence or absence of parasitemia at enrollment. Using logistic regression for these 301 individuals, an individual with no parasitemia was more likely to be CI than CS (odds ratio, 2.3; 95% confidence interval, 1.1 to 4.8; P = 0.03). Of the 83 subjects studied in the second follow-up period, 43 (52%) were parasitemic at enrollment and 40 were not. Figure 4 shows the distributions of anti-EBA-175RII antibody levels and percent rosette-inhibitory activity for these individuals. Both rosette-inhibitory activity and anti-EBA-175RII levels were higher in nonparasitemic individuals, but the difference was statistically significant only for rosette-inhibitory activity (P = 0.03).
FIG. 4.
Rosette-inhibitory activity and total anti-EBA-175RII IgG antibody levels at enrollment in April 1998 in 83 individuals who were originally selected on the basis of presence or absence of susceptibility to clinical malaria but divided according to their parasitemia status at enrollment. Parasitemic, n = 43; nonparasitemic, n = 40.
DISCUSSION
A clear understanding of the role of EBA-175 as a target of naturally acquired immune responses is a prerequisite for the design of a vaccine aimed at inhibiting erythrocyte invasion by merozoites. However, the prevalence and functional role of naturally acquired antibodies against the important region II portion of EBA-175 are not well characterized. We demonstrate here that anti-EBA-175RII antibodies are highly prevalent and are present at high levels in individuals at our study site. Further, these antibodies have a functional role in inhibiting the interaction of EBA-175RII with erythrocytes. However, naturally acquired anti-EBA-175RII antibodies do not appear to play a role by themselves in conferring immunity to clinical malaria.
We found that 98.7% of samples tested had detectable total IgG against EBA-175RII. By contrast, in two areas of West Africa the proportion of individuals with antibodies against the same molecule was 60 to 70% (17). These differences may be attributable to the higher transmission intensity and holoendemicity at our site compared to the West African sites. The level of anti-EBA-175RII antibodies was relatively stable between the start and the end of the high-transmission season. Since the start of the high-transmission season is in effect the end of the low-transmission season, we can say that there were minimal seasonal changes. This observation suggests that in the study area, vaccine-induced immune responses, if boosted by natural exposure, could remain stable throughout the year.
A second objective of our study was to determine whether the naturally acquired antibodies against EBA-175RII have a functional role in vitro by inhibiting erythrocyte binding. We observed a wide range of rosette-inhibitory activity among the study participants. In general, the ability to inhibit binding correlated with the observed total anti-EBA-175RII antibody level. This is consistent with the results of Michon et al. (14), who, using region II of the DBP of P. vivax, demonstrated that the titer of naturally acquired antibodies against DBP region II correlated with their rosette-inhibitory activity. Of great interest in our studies is the fact that a substantial number of samples with low anti-EBA-175RII enhanced rosetting. This observation is consistent with other studies that have shown that purified IgG from semi-immune individuals can enhance parasite growth in vitro (5, 20). Whatever the explanation, this observation may offer clues to alternative mechanisms by which EBA-175 interacts with the erythrocyte surface and promotes invasion.
Lastly, we were interested in determining whether antibodies against EBA-175RII correlated with immunity to clinical malaria. For this purpose, we identified a group of individuals who met a set of criteria for immunity to clinical malaria and compared them to age- and gender-matched controls who did not meet these criteria. These two groups of individuals differed in the way they responded to malaria infection, as demonstrated by the significant differences in their levels of parasitemia at the time of first infection following malaria treatment and differences in their susceptibility to clinical malaria in a subsequent follow-up period. We did not observe any significant differences in anti-EBA-175RII antibody levels, in agreement with a previous study (17), or in rosette-inhibitory activity between the two groups. Perhaps this is not too surprising given the complexity of factors that may play a role in determining clinical immunity to malaria (7, 12). Despite the lack of differences in antibody levels between the two groups, we found that if the two groups were divided according to the presence or absence of parasitemia at enrollment, nonparasitemic individuals tended to have higher rosette-inhibitory activity and higher total IgG against EBA-175RII, although the latter did not reach statistical significance. The lack of correlation between anti-EBA-175RII levels and clinical immunity is consistent with the notion that a substantial proportion of wild isolates can invade erythrocytes in a sialic acid-independent manner that is less susceptible to inhibition by anti-EBA-175 antibodies (3, 18; B. Guyah, E. Ohas, and J. A. Stoute, unpublished data). At any one time, such as we saw at enrollment, the predominant sialic acid-dependent parasites may be suppressible by antibodies against EBA-175RII, and these may correlate with presence or absence of parasitemia. However, over a period of follow-up such as during our studies, immunity to clinical malaria is determined by the ability of the host to suppress both sialic acid-dependent and -independent strains. In all, our results suggest that EBA-175RII, as a monovalent vaccine, is unlikely to provide protection against clinical malaria.
Acknowledgments
We are grateful to the study participants for their willingness to take part in this study and to the staff of the Walter Reed Project Kombewa Clinic, without whose hard work and dedication these studies would not have been possible. We also thank and acknowledge the statistical support of John Rowlands, Mamadou Diedhiou, and Sonal Nagda of the Biometrics Unit, International Livestock Research Institute, Nairobi, Kenya.
This work was supported by the Military Infectious Disease Research Program (MIDRP). E.A.O. was supported by a WHO/TDR training grant, The Burroughs Wellcome Fund, and The Graduate School of the University of Notre Dame.
The views of the authors do not purport to reflect the position of the Department of the Army or the Department of Defense. This work is published with the permission of the Office of the Director, the Kenya Medical Research Institute.
Editor: W. A. Petri, Jr.
Footnotes
This work is dedicated to the memory of Eunita Ohas, a bright star whose light shined too briefly but will be missed by many colleagues and friends.
REFERENCES
- 1.Adams, J. H., B. K. Sim, S. A. Dolan, X. Fang, D. C. Kaslow, and L. H. Miller. 1992. A family of erythrocyte binding proteins of malaria parasites. Proc. Natl. Acad. Sci. USA 89:7085-7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baum, J., A. W. Thomas, and D. J. Conway. 2003. Evidence for diversifying selection on erythrocyte-binding antigens of Plasmodium falciparum and P. vivax. Genetics 163:1327-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baum, J., M. Pinder, and D. J. Conway. 2003. Erythrocyte invasion phenotypes of Plasmodium falciparum in The Gambia. Infect. Immun. 71:1856-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beier, J. C., P. V. Perkins, F. K. Onyango, T. P. Gargan, C. N. Oster, R. E. Whitmire, D. K. Koech, and C. R. Roberts. 1990. Characterization of malaria transmission by Anopheles (Diptera: Culicidae) in western Kenya in preparation for malaria vaccine trials. J. Med. Entomol. 27:570-577. [DOI] [PubMed] [Google Scholar]
- 5.Bouharoun-Tayoun, H., P. Attanath, A. Sabchareon, T. Chongsuphajaisiddhi, and P. Druilhe. 1990. Antibodies that protect humans against P. falciparum blood stages do not on their own inhibit parasite growth in-vitro but act in cooperation with monocytes. J. Exp. Med. 172:1633-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camus, D., and T. J. Hadley. 1985. A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science 230:553-556. [DOI] [PubMed] [Google Scholar]
- 7.Day, K. P., and K. Marsh. 1991. Naturally acquired immunity to Plasmodium falciparum. Immunol. Today 12:A68-A71. [DOI] [PubMed] [Google Scholar]
- 8.Greenwood, B., and T. Mutabingwa. 2002. Malaria in 2002. Nature 415:670-672. [DOI] [PubMed] [Google Scholar]
- 9.Guyatt, H. L., and R. W. Snow. 2001. The epidemiology and burden of Plasmodium falciparum-related anemia among pregnant women in sub-Saharan Africa. Am. J. Trop. Med. Hyg. 64:36-44. [DOI] [PubMed] [Google Scholar]
- 10.Hadley, T. J. 1986. Invasion of erythrocytes by malaria parasites: a cellular and molecular overview. Annu. Rev. Microbiol. 40:451-477. [DOI] [PubMed] [Google Scholar]
- 11.Klotz, F. W., P. A. Orlandi, G. Reuler, S. J. Cohen, D. J. Haynes, R. Schauer, R. J. Howard, P. Palese, and L. H. Miller. 1992. Binding of Plasmodium falciparum 175-kilodalton erythrocyte binding antigen and invasion of murine erythrocytes requires N-acetylneuraminic acid but not its O-acetylated form. Mol. Biochem. Parasitol. 51:49-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwiatkowski, D. 2000. Genetic susceptibility to malaria getting complex. Curr. Opin. Genet. Dev. 10:320-324. [DOI] [PubMed] [Google Scholar]
- 13.Liang, H., and B. K. Sim. 1997. Conservation of structure and function of the erythrocyte binding domain of Plasmodium falciparum EBA-175. Mol. Biochem. Parasitol. 84:241-245. [DOI] [PubMed] [Google Scholar]
- 14.Michon, P. T. Fraser, and J. H. Adams. 2000. Naturally acquired and vaccine-elicited antibodies that block erythrocyte cytoadherence of the Plasmodium vivax Duffy binding protein. Infect. Immun. 68:3164-3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narum, D. L., J. D. Haynes, S. Fuhrmann, K. Moch, H. Liang, S. L. Hoffman, and B. K. Sim. 2000. Antibodies against the Plasmodium falciparum receptor binding domain of EBA-175 block invasion pathways that do not involve sialic acid. Infect. Immun. 68:1964-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ockenhouse, C. F., A. Barbosa, D. P. Blackall, C. I. Murphy, O. Kashala, S. Dutta, D. E. Lanar, and J. R. Daugherty. 2001. Sialic acid-dependent binding of baculovirus-expressed recombinant antigens from Plasmodium falciparum EBA-175 to glycophorin A. Mol. Biochem. Parasitol. 113:9-21. [DOI] [PubMed] [Google Scholar]
- 17.Okenu, D. M., E. M. Riley, Q. D. Bickle, P. U. Agomo, A. Barbosa, J. R. Daugherty, D. E. Lanar, and D. J. Conway. 2000. Analysis of human antibodies to erythrocyte binding antigen 175 of Plasmodium falciparum. Infect. Immun. 68:5559-5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okoyeh, J. N., C. R. Pillai, and C. E. Chitnis. 1999. Plasmodium falciparum field isolates commonly use erythrocyte invasion pathways that are independent of sialic acid residues of glycophorin A. Infect. Immun. 67:5784-5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandey, K. C., S. Singh, P. Pattnaik, C. R. Pillai, U. Pillai, A. Lynn, S. K. Jain, and C. E. Chitnis. 2002. Bacterially expressed and refolded receptor binding domain of Plasmodium falciparum EBA-175 elicits invasion inhibitory antibodies. Mol. Biochem. Parasitol. 123:23-33. [DOI] [PubMed] [Google Scholar]
- 20.Shi, Y. P., V. Udhayakumar, A. J. Oloo, B. L. Nahlen, and A. A. Lal. 1999. Differential effect and interaction of monocytes, hyperimmune sera and immunoglobulin G on the growth of asexual stage Plasmodium falciparum parasites. Am. J. Trop. Med Hyg. 60:135-141. [DOI] [PubMed] [Google Scholar]
- 21.Sim, B. K. 1998. Delineation of functional regions on Plasmodium falciparum EBA-175 by antibodies eluted from immune complexes. Mol. Biochem. Parasitol. 95:183-192. [DOI] [PubMed] [Google Scholar]
- 22.Sim, B. K. L., P. A. Orlandi, J. D. Haynes, F. W. Klotz, J. M. Carter, D. Camus, M E. Zegans, and J. D. Chulay. 1990. Primary structure of the 175K Plasmodium falciparum erythrocyte binding antigen and identification of a peptide which elicits antibodies that inhibit malaria merozoite invasion. Cell Biol. 111:1877-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sim, B. K., C. E. Chitnis, K. Wasniowska, T. J. Hadley, and L. H. Miller. 1994. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science 264:1941-1944. [DOI] [PubMed] [Google Scholar]
- 24.Sim, B. K., T. Toyoshima, J. D. Haynes, and M. Aikawa. 1992. Localization of the 175-kilodalton erythrocyte binding antigen in micronemes of Plasmodium falciparum merozoites. Mol. Biochem. Parasitol. 51:157-159. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. 2002. World health report. World Health Organization, Geneva, Switzerland.