Abstract
The 104-kDa Listeria adhesion protein (LAP) in Listeria monocytogenes is involved in binding to various mammalian cell lines. However, the receptor that interacts with LAP in eukaryotic cells is unknown. In this study, scanning immunoelectron microscopy qualitatively demonstrated greater binding capacity of wild-type (WT) L. monocytogenes strain (F4244) than a LAP-deficient mutant strain (KB208) to Caco-2 cells. The goal of this study was identification of the host cell receptor for LAP. Using a Western blot ligand overlay assay, we identified a protein of 58 kDa to be the putative receptor for LAP from Caco-2 cells. N-terminal sequencing and subsequent database search identified this protein as heat shock protein 60 (Hsp60). Modified immunoseparation with protein A-Sepharose beads bound to the LAP-specific monoclonal antibody H7 (MAb-H7) and a sequential incubation with LAP preparation and Caco-2 lysate confirmed the receptor to be the same 58-kDa protein. Western blot analysis with anti-Hsp60 MAb of whole-cell adhesion between Caco-2 and WT also revealed the receptor protein to be a 58-kDa protein, thus corroborating the identification of Hsp60 as a host cell receptor for LAP. Furthermore, the anti-Hsp60 antibody also caused approximately 74% reduction in binding of L. monocytogenes WT to Caco-2 cells, whereas a control antibody, C11E9, had no effect on binding. The adhesion mechanism of L. monocytogenes to eukaryotic cells is a complex process, and identification of Hsp60 as a receptor for LAP adds to the list of previously discovered ligand-receptor modules that are essential to achieve successful adhesion.
The food-borne pathogen Listeria monocytogenes is a facultatively intracellular bacterium and causes health problems such as meningitis, brain-stem encephalitis, and abortion in humans (46). L. monocytogenes initially enters the human host via contaminated foods, penetrates the intestinal cell lining, and translocates into the liver, spleen, lymph nodes, brain, and, in pregnant women, the placenta. However, to gain entry, the bacterium must first adhere to the intestinal epithelial cells (46).
Multiple L. monocytogenes cell surface proteins are reported to mediate the attachment to epithelial cells by using specific receptors. For example, the listerial cell surface protein internalin A (InlA) initiates contact with eukaryotic cell receptor E-cadherin (33), whereas internalin B (InlB) binds to gC1q-R (6) or activates the tyrosine kinase MET receptor (41) prior to entry into cells. InlA has been implicated in the intestinal phase of infection (31), and InlB is involved in the hepatic phase of infection (10, 18). Another human adhesion protein receptor, fibronectin, has facilitated binding to L. monocytogenes (16), and the L. monocytogenes surface protein autolysin amidase (Ami) is also involved in bacterial adhesion to eukaryotic cells (34). A 104-kDa Listeria adhesion protein (LAP) was also shown to be responsible for adherence to numerous intestinal cultured cell lines, including Caco-2, HCT-8, and HT-29 cells (24, 36). Later, LAP was characterized as an alcohol/acetaldehyde dehydrogenase and shown to be involved in the intestinal phase of infection (K.-P. Kim, Z. W. Jaradat, and A. K. Bhunia, Abstr. 103rd Gen. Meet. Am. Soc. Microbiol., abstr. P-004, 2003).
The goals of the present study were to illustrate the morphological binding characteristics of LAP to the putative receptor and to identify the host cell receptor that interacts with the LAP molecule. This would help elucidate the binding characteristics of pathogenic L. monocytogenes in human hosts during food-borne infection. Using a ligand overlay assay and N-terminal sequencing, we identified Hsp60 as a LAP receptor from the Caco-2 cell line. Hsp60 is a chaperonin that assists in protein folding under both normal and stressful conditions (19) and is ubiquitously produced in mammalian cellular mitochondria (39). Surface expression of Hsp60 in the cellular membrane has been identified in antigen-presenting cells (APC) from murine liver and spleen in vivo (3) and in cultured mammalian cell lines in vitro (21, 26, 43). Furthermore, systemic stress or Crohn's disease can also increase Hsp60 expression, respectively, in rat or human intestinal mucosa (30, 37). Hsp60 has been identified as a ligand for fibronectin in Staphylococcus aureus (11) and the human immunodeficiency virus protein gp41 (44) in human cultured cell lines.
MATERIALS AND METHODS
Bacterial and mammalian cell growth.
L. monocytogenes F4244 wild type (WT; Emr) was grown in brain heart infusion broth (Difco) containing erythromycin (10 μg/ml; Fisher) for 18 to 24 h at 37°C to obtain maximum LAP expression in viable cells (40). Cultures of the LAP-deficient mutant L. monocytogenes strain KB208 (Kim et al., Abstr. 103rd Gen. Meet. Am. Soc. Microbiol.) were grown in medium containing erythromycin (5 μg/ml) for 24 h at 42°C.
Secondary human enterocyte-like Caco-2 cells (HTB37; American Type Culture Collection) were grown in Dulbecco modified Eagle medium containing 10% fetal bovine serum (Atlanta Biologicals, Norcross, Ga.). Cells were seeded in T-25 or T-75 flasks or on 12-mm etched glass coverslips (Electron Microscopy Supply, Ft. Washington, Pa.) and grown to confluence at 37°C under 7% CO2.
Antibodies.
LAP-specific monoclonal antibody H7 (MAb-H7) was developed by immunizing mice with heat-killed L. monocytogenes cells (A. K. Bhunia, D. G. Westbrook, R. Story, and M. G. Johnson, Abstr. 95th Annu. Meet. Am. Soc. Microbiol., abstr. P-79, 1995) and purified from mouse ascites. Ascites fluid was diluted with 0.02 M phosphate-buffered saline at pH 7.4 (PBS; 0.01 M Na2HPO4, 0.01 M NaH2PO4, 0.5 M NaCl) and filtered by using a 0.45-μm-pore-size syringe filter (Nalgene, Rochester, N.Y.). Aliquots of 2 ml were passed over a 5-ml HiTrap Protein G column (Amersham Pharmacia Biotech, Inc., Piscataway, N.J.) and eluted with 0.1 M glycine-HCl (pH 2.7) by using the ÄKTAprime automated liquid chromatography system (Amersham Pharmacia Biotech, Inc.). The MAb-H7 antibody concentration was determined to be 2.2 mg/ml. An L. monocytogenes-reactive affinity-purified MAb, C11E9 (2.26 mg/ml), was used as an isotype control antibody (4). Hsp60 MAb (0.7 mg/ml) designed for eukaryotic Hsp60 identification only was purchased from StressGen Biotechnologies Corp. (Victoria, British Columbia, Canada).
Protein isolation and affinity purification of LAP.
WT cells were harvested from 6 liters of brain heart infusion broth by centrifugation (7,500 × g, 15 min), and the cell pellet was resuspended in 60 ml of 0.02 M PBS (pH 7.4) and washed twice. Crude surface proteins were extracted by using 60 ml of 4 M guanidine-HCl prepared in PBS and incubated in a shaker-incubator (New Brunswick Scientific Company, Inc., Edison, N.J.) at 37°C for 45 min. This preparation was centrifuged (7,500 × g, 15 min), and the supernatant was filtered (0.45-μm-pore-size Nuclepore filter; Whatman, Inc., Newton, Mass.) and dialyzed in 0.02 M PBS by using a 12- to 14-kDa-cutoff Spectra/Por membrane (Fisher). MAb-H7 (10.2 mg/ml of beads) was immobilized to cyanogen bromide-activated Sepharose 4B (Sigma), and LAP was purified from the crude preparation according to the method of Fang et al. (12). The concentration of purified LAP was determined to be 7.7 mg/ml.
Preparation of Caco-2 protein.
Crude Caco-2 protein extracts were obtained from T-75 flasks grown to confluence (15). Cells were detached with 2 ml of Triton X-100 lysis buffer (300 mM NaCl, 50 mM Tris-HCl [pH 7.6], 0.5% Triton X-100) (8) and 200 μl of 10× Protease Inhibitor Cocktail (S-2714; Sigma), with rocking of the flasks on ice for 45 min. The lysate was centrifuged at 10,000 × g for 15 min at 4°C, and the supernatant was stored at −80°C until used.
Analysis of expression of LAP in L. monocytogenes during ex vivo Caco-2 infection by scanning electron microscopy.
Caco-2 cells were grown on glass coverslips, and the monolayers were infected with WT or KB208 at a multiplicity of infection (MOI) of 10 to 100 bacteria or no bacteria at 37°C for 30 min. The cell fixation protocol of Gounon et al. (17) was followed with some modifications. Cells were washed three times with 0.1 M PBS (pH 7.4) and then fixed with glutaraldehyde buffer (1%) prepared in PBS for 30 min at 4°C. Cells were blocked for 5 min with 1% bovine serum albumin (BSA) prepared in PBS and then incubated facedown in 100 μl of MAb-H7 or a PBS control for 24 h at 4°C. Cells were washed twice in BSA solution (0.1%) for 5 min each and again incubated facedown in a 1:20 dilution of 15-nm colloidal gold-conjugated goat anti-mouse immunoglobulin G (IgG; Ted Pella, Inc., Reading, Calif.) for 1 h at room temperature. Cells were washed three times in PBS and then fixed with glutaraldehyde buffer for 5 min. Samples were rinsed with distilled water for 2 min, dehydrated in a graded series of ethanol exchanges until they reached 100%, dried in a Critical Point Dryer (Ladd Research Industries, Burlington, Vt.) mounted on metal stubs with adhesive carbon circles, and coated with a layer of carbon (NRC-3114 Vacuum Evaporator). Finally, the samples were examined under a scanning electron microscope (JSM 840; JEOL USA, Inc., Peabody, Mass.) at 10 kV by using both secondary electron imaging and backscattering.
Ligand overlay assay.
Caco-2 proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in 10% acrylamide gels and transferred to an Immobilon P (Millipore, Bradford, Mass.) membrane by using a Trans-Blot electrophoresis apparatus (Bio-Rad Laboratories, Hercules, Calif.). The following procedure was performed as described previously (23) with some modifications. Membranes were blocked with 3% gelatin (Sigma) prepared in Tris-buffered saline (TBS; 20 mM Tris, 500 mM NaCl) at pH 7.5 for 1.5 h. Membranes were washed twice with TBS-Tween 20 (0.5%) for 10 min each, incubated overnight on a shaker at room temperature with 0 or 25 μl of purified LAP (7.7 mg/ml) in 1 ml of TBS-Tween 20, and then washed as before. Membranes were next incubated with MAb-H7 (1:500) or MAb C11E9 (1:500) in 1 ml of TBS-Tween 20 overnight at room temperature, washed, incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (1:1,000; Bio-Rad Laboratories) for 1 h, and developed with an appropriate substrate system (22).
N-terminal sequencing.
Caco-2 proteins were separated by SDS-PAGE (10% acrylamide), transferred to an Immobilon P membrane, and stained with Coomassie blue solution (42). The desired protein bands at approximately 58 kDa (as previously identified by ligand overlay) were identified, excised, and microsequenced (Procise 492; Applied Biosystems, Foster City, Calif.) at the Purdue Protein Sequencing Laboratory. The BLASTP program (1) at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov:80/BLAST/) was used for a sequence homology search.
Confirmation of receptor. (i) Immunoprecipitation assay.
Immunoprecipitation of the receptor protein was carried out as previously described (11) with modification. A 400-μl aliquot of protein A-Sepharose beads (Sigma) was washed in 0.02 M phosphate buffer (pH 7.2) (PB) and incubated at room temperature with 200 μl of MAb-H7 for 1 h. Unbound antibody was removed by extensive washing. Antibody-bound beads were incubated overnight with 1.5 ml of crude LAP protein and then washed twice for 5 min each. Beads were incubated overnight with 1.5 ml of crude Caco-2 lysate, washed twice, resuspended in 200 μl of double-strength SDS-PAGE sample buffer (42), heated at 60°C for 15 min, and centrifuged (10,000 × g, 15 min). The supernatant was removed, resolved by SDS-PAGE (10% acrylamide), and transferred to an Immobilon membrane for Western blotting with anti-Hsp60 MAb (1:500).
(ii) Whole-host-cell binding assay.
A whole-cell binding assay was performed according to the method of Dziewanowska et al. (11) with some modifications. Confluent Caco-2 monolayers in T-25 flasks were inoculated with WT or KB208 cells at an MOI of 100 or with no bacteria and then incubated at 37°C for 30 min. Caco-2 monolayers were washed three times with 0.02 M PBS (pH 7.2) and lysed by the addition of 1 ml of lysing solution (50 mM HEPES buffer with 1% Triton X-100, 1% sodium deoxycholate, 1 mM EDTA, and 100 μl of protease inhibitor cocktail [Sigma] per ml of lysing solution). The lysate was centrifuged (10,000 × g, 10 min) at 4°C, and the sample pellet was washed three times with lysing solution prior to the addition of SDS-PAGE sample buffer (200 μl) and heating at 60°C for 15 min. Samples were immediately resolved by SDS-PAGE, followed by Western blotting with anti-LAP MAb-H7 or anti-Hsp60 MAb.
Adherence-inhibition assay with anti-Hsp60 antibody.
Caco-2 cells on glass coverslips were incubated for 1 h with 0, 1.0, or 10 μg of anti-Hsp60 or C11E9 MAb/ml, washed once with culture medium, and infected with WT or KB208 at an MOI of 100 or with no bacteria at 37°C for 30 min. Caco-2 cells were washed four times with 0.02 M PBS (pH 7.2) and then immersed in PBS for 30 s. Cells were immersed in 1.0 ml of 100% ethanol for 5 min, air dried, and immersed in 1.0 ml of Giemsa staining solution (2.5 ml of KaryoMAX Giemsa staining solution [Invitrogen Corp., Carlsbad, Calif.] and 48.5 ml of 10 mM potassium phosphate buffer [20 mM KH2PO4, 20 mM K2HPO4; pH 6.8]), washed twice with distilled water, air dried again, and examined under a Leica DAS Mikroskop (Leica Mikroskopie und Systeme GmbH, Wetzlar, Germany) at a magnification of ×1,000. Counts of bacterial adhesion were taken at four to five random locations for a total of at least 50 cells, averaged, and statistically analyzed by the Duncan test by using SAS software (SAS Institute, Cary, N.C.).
RESULTS
Ex vivo expression of LAP during adhesion to Caco-2 cells.
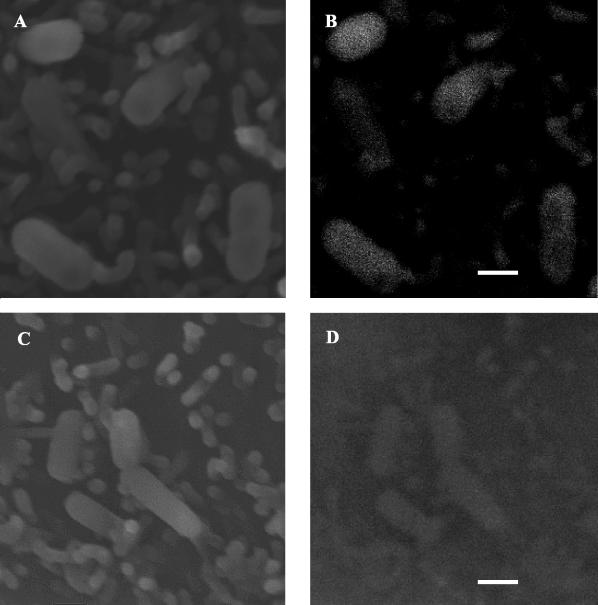
Secondary electron imaging of Caco-2 monolayers infected with WT cells indicated surface attachment of bacteria to eukaryotic cells (Fig. 1A). Evaluation of this attachment by using the backscattered electron imaging mode indicated a uniform distribution of LAP expression evidenced by gold particles on the surfaces of WT cells (Fig. 1B). The LAP mutant (KB-208) demonstrated some attachment to Caco-2 cells (Fig. 1C), but with significantly reduced LAP expression on cells (Fig. 1D).
FIG. 1.
Analysis of LAP expression in L. monocytogenes during adhesion to Caco-2 cells by scanning electron microcopy. LAP was probed by using MAb-H7, followed by labeling with goat anti-mouse-15-nm-gold conjugate cells. Secondary electron emission and the backscattered electron imaging mode were used at each location to establish bacterial attachment and LAP expression. Adhesion of LAP-positive WT cells was established (A), with clearly visible immunogold labeling of MAb-H7 in the same field (B). The LAP mutant KB208 also displayed some attachment (C), but no apparent immungold labeling was observed by using backscatter imaging (D). Bar, 1 μm.
Identification and confirmation of Hsp60 as a receptor for LAP.
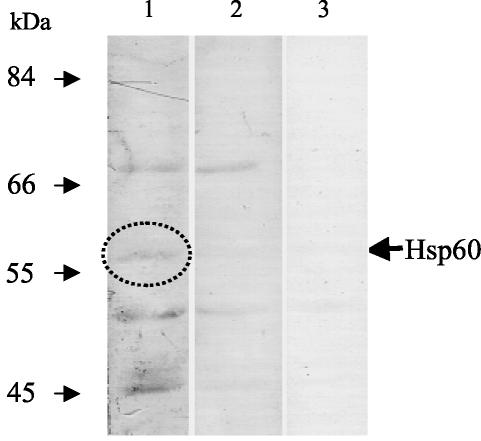
In the ligand overlay assay, Caco-2 proteins in the membrane were sequentially reacted with LAP, anti-LAP MAb-H7, and MAb C11E9 (control, data not shown) and developed. This resulted in four bands of proteins that could possibly act as receptors for LAP (Fig. 2, lane 2). However, when LAP preparation was omitted from the reaction (lane 3), three of those bands showed a positive reaction, indicating that anti-LAP MAb-H7 reacted nonspecifically with these proteins and that they were not likely to be a receptor candidate for LAP. Thus, the remaining protein band of 58 kDa could possibly be the potential receptor for LAP. The N-terminal sequence of this band (58 kDa) provided 10 residues, AKDVKFGADA, which showed exact matches with the bovine and human heat shock protein 60 (Hsp60). This suggests that Hsp60 could be the potential receptor for LAP. An antibody against Hsp60 was used to conduct additional experiments to confirm the role of Hsp60 as a receptor for LAP.
FIG. 2.
Identification of LAP-specific receptor by ligand overlay assay in Caco-2 cells. Caco-2 proteins were separated by SDS-PAGE (10% acrylamide), transferred to an Immobilon membrane, and probed with affinity chromatography-purified LAP and ant-LAP MAb-H7. A 58-kDa protein band (arrow) was identified to be the putative receptor by the subtraction method. Lanes: 1, Caco-2 protein plus LAP plus MAb-H7 plus peroxidase-conjugated anti-mouse IgG plus substrate; 2, Caco-2 protein plus MAb-H7 plus peroxidase-conjugated anti-mouse IgG plus substrate; 3, Caco-2 protein plus peroxidase-conjugated anti-mouse IgG plus substrate.
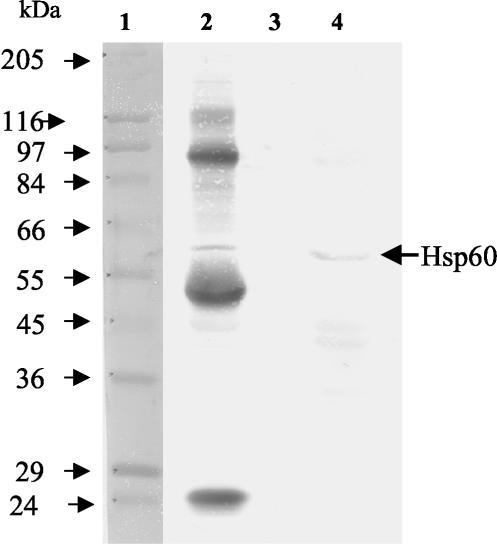
In the immunoprecipitation assay, MAb-H7-coated Sepharose beads bound to LAP captured an apparent 58-kDa protein from a Caco-2 lysate. A subsequent Western blot with anti-Hsp60 antibody showed a positive reaction with the 58-kDa band (Fig. 3, lane 2) and confirms this protein to be Hsp60 and a receptor for LAP. Other extraneous bands present in lane 2 were derived from MAb-H7 (intact antibody, 150 kDa; antibody heavy chain, 55 kDa; antibody light chain, 24 kDa), LAP molecules (104 kDa), and protein A (45 kDa) initially bound to the bead to capture the LAP-specific receptor. Control beads, without MAb-H7, showed no reaction with anti-Hsp60 antibody (Fig. 3, lane 3) when incubated sequentially with LAP and Caco-2 protein preparations. In another control lane, Caco-2 protein preparations, as expected, reacted with anti-Hsp60 antibody (Fig. 3, lane 4).
FIG. 3.
Western blot analysis of LAP receptor in Caco-2 lysates by immunoprecipitation assay. Sepharose 4B beads linked to MAb-H7 bound to LAP from a crude preparation captured Hsp60 (arrow) from a Caco-2 protein lysate (lane 2). Control Sepharose beads not linked with MAb-H7 captured few, if any, nonspecific proteins when incubated with LAP and Caco-2 proteins (lane 3). Control Caco-2 protein preparations reacted with anti-Hsp60 antibody showed reaction with Hsp60 (lane 4). Lane 1 contains molecular mass standards.
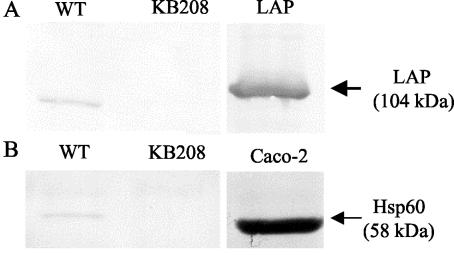
Again to confirm surface Hsp60 as a receptor for LAP, a whole-host-cell binding assay was performed. Caco-2 monolayers were incubated with WT cells, with LAP-deficient KB208 cells, or without bacteria and washed extensively to remove any unbound bacteria. Cell monolayers were then lysed and washed several times to remove any nonspecific Caco-2 proteins not bound by bacteria. The bacterial pellet was incubated with SDS sample solvent to release mammalian proteins bound by the bacteria. In Western blotting assays of proteins captured by WT cells, the 58-kDa band was observed after reaction with anti-Hsp60 MAb (Fig. 4B), and subsequently LAP was detected by using MAb-H7 (Fig. 4A), confirming the involvement of LAP in the interaction. In KB208 protein preparations, no Hsp60 band (Fig. 4A) or LAP band (Fig. 4B) was detected. As expected, lysates from control monolayers without bacteria showed no reaction with anti-Hsp60 antibody, suggesting that proteins were removed in the absence of bacteria. The present study again confirms the specific capture of receptor-Hsp60 protein from Caco-2 cells by LAP molecules.
FIG. 4.
Confirmation of Hsp60 as a receptor for LAP by whole-cell binding assay. Caco-2 monolayers were coincubated for 30 min with L. monocytogenes WT (lane 1) or KB208 (lane 2). Proteins were analyzed by Western blotting and probed with MAb-H7 (A) or anti-Hsp60 MAb (B). Appropriate controls for LAP or Hsp60 were included in each blot to verify the experimental reactions (lane 3).
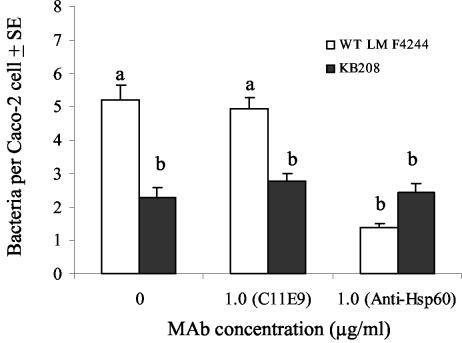
Inhibition of adhesion by using anti-Hsp60 antibody.
Preincubation of Caco-2 monolayers with 1.0 μg of anti-Hsp60 MAb/μl significantly (P < 0.05) reduced WT adhesion compared to that of control monolayers with no anti-Hsp60 MAb treatment (Fig. 5), whereas an equivalent concentration of MAb C11E9 (an isotype control) did not have any effect on WT adhesion to Caco-2 cells (Fig. 5). Anti-Hsp60 antibody at 10 μg/μl also significantly reduced WT binding to Caco-2 cells (data not shown). Mutant KB208 adhesion to Caco-2 cells was decreased by approximately 50% compared to the WT control and remained unchanged regardless of incubation with anti-Hsp60 or the C11E9 MAb. This again confirms the significance of Hsp60 as a receptor and its role as a ligand for LAP-mediated adhesion of L. monocytogenes to Caco-2 cells.
FIG. 5.
Giemsa staining analysis of inhibition of L. monocytogenes WT or mutant KB208 adhesion to Caco-2 cells by anti-Hsp60 or MAb C11E9 antibody. The data are the average count of at least 50 Caco-2 cells generated from a single representative experiment and presented as mean ± the standard error of mean. Counts for WT/Caco-2 cells in the presence of 0 or 1.0 μg of anti-Hsp60/ml were significantly different at P < 0.05. A control MAb C11E9 had no effect on WT binding to Caco-2 cells. As expected, neither anti-Hsp60 MAb nor C11E9 affected the binding of mutant strain KB208 to Caco-2 monolayers. Means marked with the same letters are not significantly different at a P of <0.05.
DISCUSSION
We have previously demonstrated that the 104-kDa LAP acts as an adhesion protein and that blocking with anti-LAP MAb-H7 caused a 50% reduction in WT binding to Caco-2 cells (36). LAP is mostly cytosolic, with only 10 to 15% expression on the cell surface (24), and expression is temperature, growth phase (40), and nutrient dependent (22). However, LAP when in contact with mammalian cells was not examined. Here scanning electron microscopy analysis indicated the expression of LAP when in contact with Caco-2 cells and uniform surface distribution (Fig. 1). Although no comparative study was made between in vitro and in vivo expression, the present study qualitatively determines expression in the WT but not in the mutant KB208 strain. It has been shown before that Clostridium difficile in contact with eukaryotic Vero cells also induced a two- to fivefold increase in expression of a GroEL adhesion molecule (20).
The present study was undertaken to identify the receptor for LAP in enterocyte-like Caco-2 cells. Interestingly, Hsp60 was identified as a receptor for LAP, and its interaction with LAP was confirmed by using immunoprecipitation and a whole-cell binding assay. Furthermore, using an adherence inhibition assay, the specificity of LAP-Hsp60 interaction was confirmed and quantified compared to the LAP mutant KB208. Previously, interaction of eukaryotic Hsp60 with staphylococcal fibronectin-binding protein had also been established in both human and bovine epithelial cell lines (11). Surface Hsp60 extracted from Raji B cells also interacted with the human immunodeficiency virus transmembrane glycoprotein gp41 (44).
Hsp60, a member of a class of conserved protein structures that act as molecular chaperones, regulates folding of mitochondrial proteins and proteolytic degradation of misfolded and damaged proteins. For this reason, initially heat shock proteins were thought to be present only in the mitochondria of cells; however, these proteins have been found in the plasma membranes of Chinese hamster ovary (CHO) cells (26), with an estimated 1 to 10% of total cellular Hsp60 being located on the cell surface (43). This could be explained by the strong affinity of Hsp60 for insertion into lipid bilayers (45). Hsp60 is also present on the surfaces of Burkitt's lymphoma cells (13, 14, 27), human and rat hepatocytes, and cultured macrophages (RAW264 and J774) and in epithelial cell lines (Caco-2 and HeLa) (5), thereby providing possible access for LAP-mediated interaction of L. monocytogenes.
In some cases, upregulation of expression of Hsp60 may indicate cell stress, and this could serve as a warning signal to the host immune system (39, 47). Due to the similarity in human and bacterial Hsp60s, they may be responsible for autoimmune diseases through cross-reactivity and molecular mimicry (25) and could also activate APC (29, 35). Significant increases in gamma interferon production were also noticed after the addition of Hsp60 to purified T cells and macrophages (7). Hsp60 and anti-Hsp60 antibodies have been found in healthy (38) or atherosclerotic (49) individuals.
In the present study, Hsp60 was detected from Caco-2 cells in the presence or absence of L. monocytogenes, suggesting that Hsp60 could be produced constitutively on the surface of cells. However, in an in vivo study of intravenous murine L. monocytogenes infection, a significant increase in surface Hsp60 expression was noticed on APC from the liver and spleen (3). This study suggested that upregulation of Hsp60 expression in APC possibly increases the T-cell-mediated immunity against L. monocytogenes infection (3).
Eukaryotic Hsp60 has been cited as a receptor for many different ligands, with possible further implications for roles in amino acid transport, chaperone activities, and signal transduction. Surface Hsp60 upregulation positively correlated with expression of amino acid transporters in CHO cells (26) and B cells (48) and may act as a chaperone for a cell surface histone protein found on CD4+ T cells (28). Surface Hsp60 may activate and modulate the binding activity of mature α3β1 integrin on breast cancer cells during their metastasis to lymph nodes and osteoblasts (2). In addition, Hsp60 enhanced the in vivo GTPase activity of surface p21ras, a signal transduction protein, in rat fibroblasts (9, 21). Similarly, interaction of the L. monocytogenes protein InlB with its receptor, the complement protein gC1q-R, is associated with phosphoinositide 3-kinase (6), whose activation may affect host cell actin polymerization, a key factor in L. monocytogenes uptake and virulence (32). This evidence suggests that the interaction of LAP with Hsp60 may also play a fundamental role in signal transduction during L. monocytogenes virulence. Conversely, since expression of surface Hsp60 is known to activate APC (29, 35), perhaps this interaction could trigger a cascade of reactions that could benefit the host cell by increasing immunity against L. monocytogenes infection (6).
Overall, interaction of L. monocytogenes with the eukaryotic cell is intricate and multifaceted; however, identification of Hsp60 as a receptor for LAP is yet another piece of information lending increased insight into the L. monocytogenes adhesion in the infection process.
Acknowledgments
We thank Michael G. Johnson for critical review of the manuscript.
Editor: B. B. Finlay
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barazi, H. O., L. G. Zhou, N. S. Templeton, H. C. Krutzsch, and D. D. Roberts. 2002. Identification of heat shock protein 60 as a molecular mediator of α3β1 integrin activation. Cancer Res. 62:1541-1548. [PubMed] [Google Scholar]
- 3.Belles, C., A. Kuhl, R. Nosheny, and S. R. Carding. 1999. Plasma membrane expression of heat shock protein 60 in vivo in response to infection. Infect. Immun. 67:4191-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhunia, A. K., P. H. Ball, A. T. Fuad, B. W. Kurz, J. W. Emerson, and M. G. Johnson. 1991. Development and characterization of a monoclonal antibody specific for Listeria monocytogenes and Listeria innocua. Infect. Immun. 59:3176-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bocharov, A. V., T. G. Vishnyakova, I. N. Baranova, A. T. Remaley, A. P. Patterson, and T. L. Eggerman. 2000. Heat shock protein 60 is a high-affinity high-density lipoprotein binding protein. Biochem. Biophys. Res. Commun. 277:228-235. [DOI] [PubMed] [Google Scholar]
- 6.Braun, L., B. Ghebrehiwet, and P. Cossart. 2000. gC1q-R/p32, a C1q-binding protein, is a receptor for the InlB invasion protein of Listeria monocytogenes. EMBO J. 19:1458-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breloer, M., B. Dorner, S. H. More, T. Roderian, B. Fleischer, and A. von Bonin. 2001. Heat shock proteins as “danger signals”: eukaryotic Hsp60 enhances and accelerates antigen-specific IFN-gamma production in T cells. Eur. J. Immunol. 31:2051-2059. [DOI] [PubMed] [Google Scholar]
- 8.Coligan, J. 1991. Isolation and analysis of proteins, p. 8.1.1-8.1.9. In J. Coligan (ed.), Current protocols in immunology. Greene Publishing Associates/Wiley Interscience, New York, N.Y.
- 9.De Gunzburg, J., R. Riehl, and R. A. Weinberg. 1989. Identification of a protein associated with P21ras by chemical crosslinking. Proc. Natl. Acad. Sci. USA 86:4007-4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dramsi, S., I. Biswas, E. Maguin, L. Braun, P. Mastroeni, and P. Cossart. 1995. Entry of Listeria monocytogenes into hepatocytes requires expression of InlB, a surface protein of the internalin multigene family. Mol. Microbiol. 16:251-261. [DOI] [PubMed] [Google Scholar]
- 11.Dziewanowska, K., A. R. Carson, J. M. Patti, C. F. Deobald, K. W. Bayles, and G. A. Bohach. 2000. Staphylococcal fibronectin binding protein interacts with heat shock protein 60 and integrins: role in internalization by epithelial cells. Infect. Immun. 68:6321-6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang, L., Z. B. Gan, and R. R. Marquardt. 2000. Isolation, affinity purification, and identification of piglet small intestine mucosa receptor for enterotoxigenic Escherichia coli K88ac+ fimbriae. Infect. Immun. 68:564-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferm, M. T., K. Soderstrom, S. Jindal, A. Gronberg, J. Ivanyi, R. Young, and R. Kiessling. 1992. Induction of human Hsp60 expression in monocytic cell lines. Int. Immunol. 4:305-311. [DOI] [PubMed] [Google Scholar]
- 14.Fisch, P., M. Malkovsky, S. Kovats, E. Sturm, E. Braakman, B. S. Klein, S. D. Voss, L. W. Morrissey, R. Demars, W. J. Welch, R. L. H. Bolhuis, and P. M. Sondel. 1990. Recognition by human V-gamma-9/V-delta-2 T cells of a GroEL homolog on Daudi-Burkitt lymphoma cells. Science 250:1269-1273. [DOI] [PubMed] [Google Scholar]
- 15.Gaillard, J. L., and B. B. Finlay. 1996. Effect of cell polarization and differentiation on entry of Listeria monocytogenes into the enterocyte-like Caco-2 cell line. Infect. Immun. 64:1299-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilot, P., P. Andre, and J. Content. 1999. Listeria monocytogenes possesses adhesins for fibronectin. Infect. Immun. 67:6698-6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gounon, P., M. Jouve, and C. Le Bouguenec. 2000. Immunocytochemistry of the AfaE adhesin and AfaD invasin produced by pathogenic Escherichia coli strains during interaction of the bacteria with HeLa cells by high-resolution scanning electron microscopy. Microbes Infect. 2:359-365. [DOI] [PubMed] [Google Scholar]
- 18.Gregory, S. H., A. J. Sagnimeni, and E. J. Wing. 1997. Internalin B promotes the replication of Listeria monocytogenes in mouse hepatocytes. Infect. Immun. 65:5137-5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartl, F. U. 1996. Molecular chaperones in cellular protein folding. Nature 381:571-580. [DOI] [PubMed] [Google Scholar]
- 20.Hennequin, C., F. Porcheray, A. J. Waligora-Dupriet, A. Collignon, M. C. Bare, P. Bourlioux, and T. Karjalainen. 2001. GroEL (Hsp60) of Clostridium difficile is involved in cell adherence. Microbiology 147:87-96. [DOI] [PubMed] [Google Scholar]
- 21.Ikawa, S., and R. A. Weinberg. 1992. An interaction between P21ras and heat shock protein Hsp60, a chaperonin. Proc. Natl. Acad. Sci. USA 89:2012-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaradat, Z. W., and A. K. Bhunia. 2002. Glucose and nutrient concentrations affect the expression of a 104-kilodalton Listeria adhesion protein in Listeria monocytogenes. Appl. Environ. Microbiol. 68:4876-4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaradat, Z. W., and R. R. Marquardt. 2001. Identification of Escherichia coli K88 receptor in porcine intestinal mucus using anti-idiotypic antibodies. Food Agric. Immunol. 13:241-253. [Google Scholar]
- 24.Jaradat, Z. W., J. L. Wampler, and A. K. Bhunia. 2003. A Listeria adhesion protein-deficient Listeria monocytogenes shows reduced adhesion primarily to the cells of intestinal origin. Med. Microbiol. Immunol. 192:85-91. [DOI] [PubMed] [Google Scholar]
- 25.Jones, D. B., A. F. W. Coulson, and G. W. Duff. 1993. Sequence homologies between Hsp60 and autoantigens. Immunol. Today 14:115-118. [DOI] [PubMed] [Google Scholar]
- 26.Jones, M., R. S. Gupta, and E. Englesberg. 1994. Enhancement in amount of P1 (Hsp60) in mutants of Chinese hamster ovary (Cho-K1) cells exhibiting increases in the A-system of amino acid transport. Proc. Natl. Acad. Sci. USA 91:858-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaur, I., S. D. Voss, R. S. Gupta, K. Schell, P. Fisch, and P. M. Sondel. 1993. Human peripheral gamma-delta T cells recognize Hsp60 molecules on Daudi-Burkitts lymphoma cells. J. Immunol. 150:2046-2055. [PubMed] [Google Scholar]
- 28.Khan, I. U., R. Wallin, R. S. Gupta, and G. M. Kammer. 1998. Protein kinase A-catalyzed phosphorylation of heat shock protein 60 chaperone regulates its attachment to histone 2B in the T lymphocyte plasma membrane. Proc. Natl. Acad. Sci. USA 95:10425-10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kol, A., A. H. Lichtman, R. W. Finberg, P. Libby, and E. A. Kurt-Jones. 2000. Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J. Immunol. 164:13-17. [DOI] [PubMed] [Google Scholar]
- 30.Kuwabara, T., M. Otaka, H. Itoh, A. Zeniya, S. Fujimori, S. Otani, Y. Tashima, and O. Masamune. 1994. Regulation of 60-kDa heat shock protein expression by systemic stress and 5-hydroxytryptamine in rat colonic mucosa. J. Gastroenterol. 29:721-726. [DOI] [PubMed] [Google Scholar]
- 31.Lecuit, M., S. Vandormael-Pournin, J. Lefort, M. Huerre, P. Gounon, C. Dupuy, C. Babinet, and P. Cossart. 2001. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science 292:1722-1725. [DOI] [PubMed] [Google Scholar]
- 32.Marino, M., L. Braun, P. Cossart, and P. Ghosh. 2000. A framework for interpreting the leucine-rich repeats of the Listeria internalins. Proc. Natl. Acad. Sci. USA 97:8784-8788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mengaud, J., H. Ohayon, P. Gounon, R. M. Mege, and P. Cossart. 1996. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 84:923-932. [DOI] [PubMed] [Google Scholar]
- 34.Milohanic, E., R. Jonquieres, P. Cossart, P. Berche, and J. L. Gaillard. 2001. The autolysin Ami contributes to the adhesion of Listeria monocytogenes to eukaryotic cells via its cell wall anchor. Mol. Microbiol. 39:1212-1224. [DOI] [PubMed] [Google Scholar]
- 35.Ohashi, K., V. Burkart, S. Flohe, and H. Kolb. 2000. Heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J. Immunol. 164:558-561. [DOI] [PubMed] [Google Scholar]
- 36.Pandiripally, V. K., D. G. Westbrook, G. R. Sunki, and A. K. Bhunia. 1999. Surface protein p104 is involved in adhesion of Listeria monocytogenes to human intestinal cell line, Caco-2. J. Med. Microbiol. 48:117-124. [DOI] [PubMed] [Google Scholar]
- 37.Peetermans, W. E., G. R. Dhaens, J. L. Ceuppens, P. Rutgeerts, and K. Geboes. 1995. Mucosal expression by B7-positive cells of the 60-kilodalton heat shock protein in inflammatory bowel disease. Gastroenterology 108:75-82. [DOI] [PubMed] [Google Scholar]
- 38.Pockley, A. G., J. Bulmer, B. M. Hanks, and B. H. Wright. 1999. Identification of human heat shock protein 60 (Hsp60) and anti-Hsp60 antibodies in the peripheral circulation of normal individuals. Cell Stress Chaperones 4:29-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ranford, J. C., and B. Henderson. 2002. Chaperonins in disease: mechanisms, models, and treatments. J. Clin. Pathol. Mol. Pathol. 55:209-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santiago, N. I., A. Zipf, and A. K. Bhunia. 1999. Influence of temperature and growth phase on expression of a 104-kilodalton Listeria adhesion protein in Listeria monocytogenes. Appl. Environ. Microbiol. 65:2765-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen, Y., K. Naujokas, M. Park, and K. Ireton. 2000. InlB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell 103:501-510. [DOI] [PubMed] [Google Scholar]
- 42.Smith, B. J. 1994. SDS polyacrylamide gel electrophoresis of proteins, p. 23-34. In J. M. Walker (ed.), Basic protein and peptide protocols, vol. 32. Humana Press, Inc., Totowa, N.J.
- 43.Soltys, B. J., and R. S. Gupta. 1997. Cell surface localization of the 60 kDa heat shock chaperonin protein (Hsp60) in mammalian cells. Cell Biol. Int. 21:315-320. [DOI] [PubMed] [Google Scholar]
- 44.Speth, C., Z. Prohaszka, M. Mair, G. Stockl, X. J. Zhu, B. Jobstl, G. Fust, and M. P. Dierich. 1999. A 60 kD heat-shock protein-like molecule interacts with the HIV transmembrane glycoprotein gp41. Mol. Immunol. 36:619-628. [DOI] [PubMed] [Google Scholar]
- 45.Torok, Z., I. Horvath, P. Goloubinoff, E. Kovacs, A. Glatz, G. Balogh, and L. Vigh. 1997. Evidence for a lipochaperonin: association of active protein-folding GroESL oligomers with lipids can stabilize membranes under heat shock conditions. Proc. Natl. Acad. Sci. USA 94:2192-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vazquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, B. Gonzalez-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wallin, R. P. A., A. Lundqvist, S. H. More, A. von Bonin, R. Kiessling, and H. G. Ljunggren. 2002. Heat-shock proteins as activators of the innate immune system. Trends Immunol. 23:130-135. [DOI] [PubMed] [Google Scholar]
- 48.Woodlock, T. J., X. X. Chen, D. A. Young, G. Bethlendy, M. A. Lichtman, and G. B. Segel. 1997. Association of HSP60-like proteins with the L-system amino acid transporter. Arch. Biochem. Biophys. 338:50-56. [DOI] [PubMed] [Google Scholar]
- 49.Xu, Q. B., G. Schett, H. Perschinka, M. Mayr, G. Egger, F. Oberhollenzer, J. Willeit, S. Kiechl, and G. Wick. 2000. Serum soluble heat shock protein 60 is elevated in subjects with atherosclerosis in a general population. Circulation 102:14-20. [DOI] [PubMed] [Google Scholar]