Abstract
Using a neonatal rat meningitis model, we examined the involvement of three iron uptake systems, namely, the high-pathogenicity island, the hemin receptor ChuA, and the siderophore receptor IroN, in the pathogenesis of Escherichia coli neonatal meningitis. Only IroN appeared to play a major role during the bacteremic step of the disease.
Escherichia coli neonatal meningitis comprises three main pathophysiological steps, namely, (i) bacterial translocation from the intestinal lumen to the bloodstream, (ii) sustained high-level bacteremia, (iii) and bacterial passage through the blood-brain barrier (BBB), followed by invasion of and growth in the arachnoidal space (8, 18). Iron is an essential nutrient for strains of E. coli causing neonatal meningitis (ECNM) but, being tightly bound to proteins in eukaryotes, is largely unavailable for bacterial growth (19). Although several investigations have focused on determination of specific virulence factors involved in the resistance to the bactericidal activity of the serum or the invasiveness of the pathogen in brain capillary endothelial cells, the role of bacterial iron uptake systems in E. coli neonatal meningitis has not been explored.
E. coli has developed a variety of mechanisms to acquire iron from mammalian hosts. One major mechanism involves the synthesis and transport of siderophores—low-molecular-weight high-affinity iron chelators (13). Three siderophore systems are more prevalent in extraintestinal pathogenic E. coli (ExPEC) than in commensal strains, namely, (i) the aerobactin system (12, 22); (ii) the yersiniabactin system, synthesized by genes located on the high-pathogenicity island (HPI) (9, 12, 28); and (iii) the iro system, characterized by the siderophore receptor IroN (2, 24). The specific siderophore recognized by IroN has recently been identified as salmochelin, which is composed of 2,3-dihydroxybenzoylserine moieties linked to glucose (20). Most ExPEC strains can also use heme as an iron source by expressing a specific outer membrane receptor encoded by the chuA gene (11, 17, 29).
In a recent study of 134 French and North American ECNM isolates, we found that 74, 99, 62, and 76% of strains, respectively, possessed the aerobactin, yersiniabactin, salmochelin, and heme receptor systems (6). The corresponding rates are far lower in commensal strains (41, 32, 18, and 26%, respectively), suggesting that these iron uptake systems may have important roles in the pathogenesis of E. coli neonatal meningitis (2, 17, 22, 28). To investigate the roles of the HPI, iroN, and chuA present in the archetypal ECNM strain C5, we assessed the virulence of isogenic mutants in a rat model of neonatal meningitis. Strain C5, kindly provided by C. Bortolussi (8), is representative of the major American clonal group O18:K1:H7 and possesses one copy of the HPI, together with the iroN and chuA genes, but not the aerobactin genes (6, 7).
Before mutagenesis experiments, iron-regulated transcription of the genes fyuA (the yersiniabactin receptor gene located in the HPI), iroN, and chuA, encoding outer membrane receptors, was examined by quantitative reverse transcriptase PCR (RT-PCR) under various conditions of iron availability. Strain C5 was grown in Luria-Bertani (LB) medium with or without the iron chelator 2,2′-dipyridyl (Sigma, Saint Quentin Fallavier, France) at a final concentration of 200 μM. Cultures were then centrifuged for 5 min at 4,000 × g and 4°C. RNA was extracted using the Aquapure RNA isolation kit (Bio-Rad, Marnes la Coquette, France) according to the manufacturer's instructions. To avoid DNA contamination, amplification grade DNase I (0.1 IU/μl; Sigma) was added to the RNA extract for 15 min and was then inactivated by heating (65°C for 10 min). Each reverse transcription step was performed with 0.15 μg of RNA in the Platinum Quantitative RT-PCR Thermoscript One-Step system (Invitrogen, Cergy Pontoise, France) according to the manufacturer's instructions. The primers, designated by the suffixes RT1 and RT2, are shown in Table 1. SYBR green was added to the reaction mix, and amplification signals were analyzed at each cycle by the iCycler iQ thermal cycler (Bio-Rad). Transcripts of the three genes were detected by RT-PCR in standard LB medium; fyuA, iroN, and chuA mRNA levels were increased, respectively, 1,000-, 16-, and 64-fold in the presence of the iron chelator 2,2′-dipyridyl (results were observed in three different experiments). This showed that the three genes were expressed in strain C5 and, as expected, were regulated by the extracellular iron concentration.
TABLE 1.
Oligonucleotide primersa
| Primer designation | Primer sequence | Target |
|---|---|---|
| fyuA.P0 | 5′-ACGTCTCGTCTACTGTTGTCAGCGCGCCGGAATTAAGCGATGTGTAGGCTGGAGCTGCTT-3′ | fyuA |
| fyuA.P2 | 5′-GTACGGTAACGACGGTCGAACAGGTTATCGACATAGACGGCATATGAATATCCTCCTTAG-3′ | fyuA |
| HPI.P0 | 5′-GCTGGCTATCCTGAAATACGGAACGCAGAACTAGCGCCGCTGTGTAGGCTGGAGCTGCTT-3′ | ybtS |
| HPI.P2 | 5′-GTACGGTAACGACGGTCGAACAGGTTATCGACATAGACGGCATATGAATATCCTCCTTAG-3′ | fyuA |
| chuA.P0 | 5′-ATGTCACGTCCGCAATTTACCTCGTTGCGTTTGAGTTTGTTGTGTAGGCTGGAGCTGCTT-3′ | chuA |
| chuA.P2 | 5′-TTCCGTTACGACCATCCTGTGGGATGCCTTGCGGCGACCACATATGAATATCCTCCTTAG-3′ | chuA |
| iroN.P0 | 5′-AACTGTGCTCCTGGTTGGGTTGAATAGCCAGGTATCAGTATGTGTAGGCTGGAGCTGCTT-3′ | iroN |
| iroN.P2 | 5′-AAGCCCGGCCTGGCTCGTTATAGGTATTCGCCCCTTCAGACATATGAATATCCTCCTTAG-3′ | iroN |
| fyuA.FR1 | 5′-GGATTTGCGCGTTTATCATC-3′ | fyuA |
| fyuA.FR2 | 5′-AAGGAGAGAAAGGAACGAGAAA-3′ | fyuA |
| HPI.FR1 | 5′-TGAGTCATCAGGAGCGCAAT-3′ | HPI |
| HPI.FR2 | 5′-AAGGAGAGAAAGGAACGAGAAA-3′ | HPI |
| iroN.FR1 | 5′-GCGCATCTTGATGATGTCAG-3′ | iroN |
| iroN.FR2 | 5′-CAGGGTGATAACCGGGAAAC-3′ | iroN |
| ChuA.FR1 | 5′-AAAGCTGAGAACCCGGAGAT-3′ | chuA |
| ChuA.FR2 | 5′-CCGTTCAAATGCTGGTTTGT-3′ | chuA |
| C1 | 5′-TTATACGCAAGGCGACAAGG-3′ | cat |
| C2 | 5′-GATCTTCCGTCACAGGTAGG-3′ | cat |
| fyuA.RT1 | 5′-TGATTAACCCCGCGACGGGAA-3′ | fyuA |
| fyuA.RT2 | 5′-TGGGTGGCGCGGGTACATTC-3′ | fyuA |
| iroN.RT1 | 5′-GAAAGCTCTGGTGGACGGTA-3′ | iroN |
| iroN.RT2 | 5′-CGACAGAGGATTACCGGTGT-3′ | iroN |
| chuA.RT1 | 5′-GACGAACCAACGGTCAGGAT-3′ | chuA |
| chuA.RT2 | 5′-TGCCGCCAGTACCAAAGACA-3′ | chuA |
Oligonucleotide primers used for gene inactivation are designated by the suffixes P0 and P2; boldface characters in the primer sequences indicate the 20 nucleotides homologous to the cat gene sequence. The oligonucleotide primers used to control correct introduction and excision of the cat gene are designated by the suffixes FR1 and FR2 and flank the target gene or region. The oligonucleotide primers used for RT-PCR are designated by the suffixes RT1 and RT2.
To investigate the putative role of the three iron uptake systems, C5 mutants were obtained with the PCR-based method of Datsenko and Wanner (14) by using plasmids kindly provided by Lionello and Nara Bossi (Centre de Genetique Moleculaire, CNRS, Gif sur Yvette, France). Briefly, plasmid pKD46, a temperature-sensitive replicon that carries the bacteriophage λ Red system (λ, β, and exo genes) under the control of an arabinose-inducible promoter, was introduced by electroporation into strain C5 in order to render the bacterium directly transformable with linear DNA. The chloramphenicol acetyltransferase (cat) gene carried by plasmid pKD3 (14) was amplified by PCR with primers bearing extensions of 40 nucleotides (Proligo, Paris, France) homologous to the initial and final portions of the target gene (primers listed with suffixes P0 and P2, respectively, in Table 1). Transformation by electroporation of strain C5 expressing bacteriophage λ Red functions with the PCR product yielded recombinants carrying the target gene fused to the cat gene. To obtain double mutants, the cat gene introduced into the first target gene was eliminated by using plasmid pCP20 expressing FLP recombinase (14), which acts on the directly repeated FLP recognition target (FRT) sites flanking the resistance gene in plasmid pKD3. Correct introduction and excision of the cat gene were controlled by PCR by using primers flanking the initial and final portions of the target gene or region and primers homologous to the cat gene as previously described (14) and illustrated in Fig. 1. All the primers used are shown in Table 1. The growth rates of all the mutants were shown to be identical to that of the parental C5 strain. Expression of the capsule and O18 antigens was also controlled for in the mutants by using specific antisera as previously described (6).
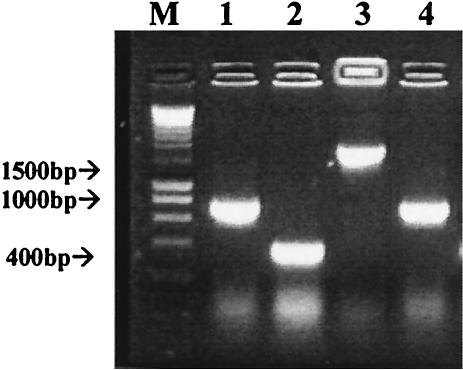
FIG. 1.
Agarose gel of PCR products from strain VL1 showing introduction of the cat gene and deletion of the HPI. Lane 1, amplification between primers HPI.FR1 and C1; lane 2, amplification between primers C2 and HPI.FR2; lane 3, amplification between primers HPI.FR1 and HPI.FR2. Lane 4 corresponds to the amplification between primers HPI.FR1 and HPI.FR2 after excision of the cat gene. M, molecular mass marker.
E. coli bacteremia and meningitis were induced in newborn rats as described elsewhere (21). Briefly, 5-day-old pathogen-free Sprague-Dawley rats (Janvier Laboratories, Le Genest St. Isle, France) were inoculated intraperitoneally with 300 CFU of strain C5 or its mutants in physiological saline. This inoculum was prepared from a culture in brain heart infusion broth as previously described (21). Blood and cerebrospinal fluid (CSF) were quantitatively cultured after 18 h. Competition experiments were performed to obtain a sensitive measurement of the virulence attenuation of some mutants. Two hundred CFU of strain C5 and its isogenic mutant were inoculated simultaneously into each animal. Quantitative culture was performed by plating serial dilutions onto LB agar with and without the appropriate antibiotic, yielding differential counts for the wild-type strain and the mutant. A competitive index was calculated for each rat as follows: the ratio of the amount of output mutant to that of wild-type bacteria recovered from blood was divided by the ratio of the amount of input mutant to that of wild-type bacteria inoculated into the rat. A competitive index not significantly different from 1.0 indicates a lack of virulence attenuation (5). Bacterial counts obtained in the animal model were compared by using a two-sample unpaired t test. Data are expressed as means ± standard deviations. Groupwise comparisons of proportions were based on the Pearson chi-square test or Fisher's exact test, as appropriate. P values below 0.05 were considered to denote significant differences.
The very high proportion (99%) of ECNM strains bearing the HPI compared to that of strains with other iron uptake systems suggests that the HPI may play a key role in virulence. To test this possibility, we first disrupted the fyuA gene encoding the yersiniabactin receptor in strain C5 and found that the level of bacteremia and the frequency of meningitis in the rat model did not differ significantly for the wild-type strain and the isogenic mutant (VL4) thus obtained (Table 2). Then, to investigate the role of the HPI in its globality, we disrupted the entire island, from the first gene (ybtS) to the last (fyuA), a region of almost 30 kb. Surprisingly, this did not affect the results obtained with the rat model (Table 2). To maximize the sensitivity for identifying potential differences between strain C5 and the HPI deletion strain VL1, we used a dual experimental approach. The mean competitive index (19 animals) was 1.01 ± 0.1, indicating a lack of virulence attenuation of VL1. Nine cases of meningitis occurred after competitive inoculation, five with pure CSF cultures of strain C5, three with pure CSF cultures of strain VL1, and one with a mixed CSF culture of strains C5 and VL1. Finally, as HPI-encoded functions form a complex system and as yersiniabactin production may be altered in HPI-positive E. coli strains (27), we examined whether the lack of influence of the HPI in our model was related to defective yersiniabactin production in strain C5. For this investigation, the yersiniabactin biosynthesis of the strains was evaluated by detecting yersiniabactin in the cell-free supernatants of iron-starved bacteria by using both an indicator system based on promoter fusion to the reporter gene luciferase (26) and yersiniabactin-specific cross-feeding assays. As expected, synthesis and release of yersiniabactin were observed only in the E. coli wild-type strain C5 and in the mutant strain VL4 (deletion of fyuA does not affect yersiniabactin synthesis) and not in mutant strain VL1 carrying a deletion of the entire core of the HPI. Accordingly, only the supernatants of wild-type strain C5 and mutant strain VL4 supported growth of the yersiniabactin indicator strain Yersinia enterocolitica WA-irp in cross-feeding assays. Taken together, these results demonstrated that the HPI had no discernible influence on the virulence of strain C5 in our meningitis model.
TABLE 2.
Mean bacterial counts in blood, and proportion of culture-positive CSF specimens, in a neonatal rat model of meningitis with strain C5 and its mutants
| Strain or mutant | Relevant property(ies) | No. of infected animals | Mean level of bacteremia, in log CFU/ml (SD) | % Of culture-positive CSF specimens |
|---|---|---|---|---|
| C5 | C5 wild type | 33 | 5.35 (0.65) | 45 |
| VL4 | C5 (fyuA::cat) | 36 | 5.37 (0.79)a | 28a |
| VL1 | C5 (HPI::cat) | 17 | 5.3 (0.73)a | 41a |
| VL24 | C5 (HPI::FRT chuA::cat) | 13 | 5.23 (0.81)a | 38a |
| VL36 | C5 (HPI::FRT iroN::cat) | 10 | 3.64 (1.23)b | 10b |
| VL37 | C5 (iroN::cat) | 30 | 3.81 (0.81)b | 7b |
No significant difference versus strain C5.
P < 0.05 versus strain C5.
We then examined whether the other iron and/or heme uptake systems in strain C5 could compensate for the loss of yersiniabactin production and thereby mask the contribution of this siderophore to C5 pathogenicity. Two double mutants were constructed, one (VL24) lacking the HPI and chuA and the other (VL36) lacking the HPI and iroN. Mutant VL24 behaved identically to strain C5 in the meningitis model. In contrast, the double mutant VL36 (lacking the HPI and iroN) was markedly less virulent, resulting in a significantly lower level of bacteremia (3.64 ± 1.23 log CFU/ml compared to 5.35 ± 0.65 log CFU/ml with the wild-type strain) (Table 2) and a significantly lower rate of meningitis (10% versus 45%) (Table 2). To examine the role of iroN in the virulence of strain C5, we constructed a single mutant lacking iroN (VL37). This single mutant behaved like the double mutant VL36 (lacking the HPI and iroN) (Table 2), indicating that the observed attenuation of virulence was due to the lack of iroN.
In a recent study of 134 ECNM isolates, we found that 99% possessed the HPI, including those belonging to the nonpathogenic phylogenetic groups A and B1, which are usually devoid of virulence genes (6). Interestingly, the HPI has also been found in 100% of strains of Citrobacter koseri (1, 26), the second most frequent gram-negative bacterium causing meningitis and abscesses in neonates (31). It is noteworthy that Yersinia pestis, one of the Yersinia species in which the HPI was originally described, is one of the rare Enterobacteriaceae capable of causing a disease (plague) which may be complicated in its septicemic forms by meningitis (4). All these data suggest that the HPI may play an important role in the development of meningitis. Although we used a sensitive approach by deleting all of the pathogenicity island and inducing competitive infections, no significant virulence contribution of the HPI was observed in our animal model. Our results appear to conflict with those of a recent study of irp1 deletion E. coli mutants in an adult mouse model of subcutaneous infection (27), in which the mutants were less lethal than the wild-type strains (IAI51 and IAI52). Several factors may explain these differences, such as the ages of the animals (adults versus newborns) and the pathovars of the strains (uropathogenic versus meningitis strains). However, one of the main reasons for the discrepancy observed may be the route of inoculation. Indeed, it has been shown that the HPI has an impact on the virulence of Yersinia species inoculated subcutaneously but not on the virulence of those inoculated intravenously (10). Therefore, the route of inoculation may also be a crucial determinant of HPI-mediated pathogenicity in E. coli. It must be further noticed that the HPI encodes a complex system for synthesis of the siderophore yersiniabactin which may not be entirely expressed within the course of infection demonstrated by our meningitis model. This suggestion is supported by in vitro experiments that revealed detection of yersiniabactin only by prolonged incubation under strong iron starvation conditions (26). Thus, the presence of different iron uptake systems found in ExPEC strains may reflect the necessity to respond to different extents of iron depletion during the course of the infection. Finally, it must be underlined that the model we used allows both the sustained bacteremic phase and BBB passage to be investigated, and our results therefore suggest that the HPI is not essential for either of these key steps. The possible contribution of the HPI to the first step in meningitis—bacterial translocation from the intestinal lumen—remains to be determined.
The redundancy of iron uptake systems in most pathogenic bacteria both offers multiple iron sources and compensates for the loss or inhibition of one or more systems during the infectious process. We hypothesized that the chu heme transport system might represent a good candidate to compensate for the loss of the HPI. Indeed, we have shown that the chuA gene is present in all E. coli strains belonging to phylogenetic groups B2 and D, which encompass most ExPEC strains. Moreover, strain C5 expresses a hemolysin that may act synergistically with the chu system by inducing the release of heme or hemoglobin from host cells. However, we found that the double mutant VL24 (lacking the HPI and chuA) was as virulent as the wild-type strain, suggesting that the heme uptake system does not play a significant role, at least in our experimental model (Table 2). This lack of influence of chuA in our model is in keeping with results from Torres et al., who found only a modest contribution of chuA to kidney infection in an animal model of pyelonephritis (30). Taken together, these results suggest that the heme transport system may be simply an auxiliary system in ExPEC virulence.
Contrary to the chuA gene, we found that the iroN gene itself played a key role in the virulence of E. coli strain C5, possibly by contributing to the sustained high-level bacteremia that precedes meningitis. Although the frequencies of meningitis were significantly different for the IroN− mutant and the wild-type strain (Table 2), the involvement of IroN in BBB passage cannot be affirmed, as the latter step is highly dependent on the level of bacteremia (15, 23). Finally, considering that IroN is the product of a unique transcript (3, 24), the phenotype observed could be attributed to the deletion of the gene rather than to a polar effect. IroN, the siderophore receptor initially found in the bacteremic E. coli strain CP9, has been shown by Russo and colleagues to contribute to the urovirulence of this strain (25). However, in the same study, the authors found that iroN deletion had no impact in a rat granuloma pouch model (25). Therefore, our study is the first to provide experimental evidence for the contribution of iroN to a model of human extraintestinal infection other than urinary tract infection. Although iron acquisition is the most likely mechanism explaining IroN-associated virulence, the markedly lower level of bacteremia obtained with the IroN− mutant VL37 prompted us to investigate the possible role of IroN in resistance to serum bactericidal activity. Bacterial survival rates, studied in the sera of 5-day-old neonatal rats as previously described (21), were not significantly different between strain C5 and mutant VL37 (data not shown).
In conclusion, this study provides evidence that, of the three iron uptake systems frequently present in ExPEC strains, IroN may represent one of the major systems contributing to the virulence of ECNM. Our results and the recent finding of the contribution of IroN to the virulence of human uropathogenic E. coli (25) and also avian pathogenic E. coli (16) suggest that IroN may represent a key virulence factor in ExPEC in general. Furthermore, the presence of a homologous gene in Salmonella enterica serotype Typhi (3) makes IroN a good potential target for broad vaccinal or antipathogenicity strategies.
Acknowledgments
We thank Lionello and Nara Bossi (Centre de Génétique Moleculaire, CNRS, Gif sur Yvette, France) for providing plasmids and technical assistance and Elisabeth Carniel (Centre National de Référence des Yersinia, Institut Pasteur, Paris, France) for enlightening discussions.
Editor: J. N. Weiser
REFERENCES
- 1.Bach, S., A. de Almeida, and E. Carniel. 2000. The Yersinia high-pathogenicity island is present in different members of the family Enterobacteriaceae. FEMS Microbiol. Lett. 183:289-294. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, R. J., L. Zhang, B. Foxman, A. Siitonen, M. E. Jantunen, H. Saxen, and C. F. Marrs. 2002. Molecular epidemiology of 3 putative virulence genes for Escherichia coli urinary tract infection—usp, iha, and iroNE. coli. J. Infect. Dis. 185:1521-1524. [DOI] [PubMed] [Google Scholar]
- 3.Baumler, A. J., T. L. Norris, T. Lasco, W. Voight, R. Reissbrodt, W. Rabsch, and F. Heffron. 1998. IroN, a novel outer membrane siderophore receptor characteristic of Salmonella enterica. J. Bacteriol. 180:1446-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker, T. M., J. D. Poland, T. J. Quan, M. E. White, J. M. Mann, and A. M. Barnes. 1987. Plague meningitis, a retrospective analysis of cases reported in the United States, 1970-1979. West. J. Med. 147:554-557. [PMC free article] [PubMed] [Google Scholar]
- 5.Beuzon, C. R., and D. W. Holden. 2001. Use of mixed infections with Salmonella strains to study virulence genes and their interactions in vivo. Microbes Infect. 3:1345-1352. [DOI] [PubMed] [Google Scholar]
- 6.Bonacorsi, S. P., O. Clermont, V. Houduoin, C. Cordevant, N. Brahimi, A. Marecat, C. Tinsley, X. Nassif, M. Lange, and E. Bingen. 2003. Molecular analysis and experimental virulence of French and North American Escherichia coli neonatal meningitis isolates: identification of new virulent clone. J. Infect. Dis. 187:1895-1906. [DOI] [PubMed] [Google Scholar]
- 7.Bonacorsi, S. P., O. Clermont, C. Tinsley, I. Le Gall, J. C. Beaudoin, J. Elion, X. Nassif, and E. Bingen. 2000. Identification of regions of the Escherichia coli chromosome specific for neonatal meningitis-associated strains. Infect. Immun. 68:2096-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bortolussi, R., P. Ferrieri, and L. W. Wannamaker. 1978. Dynamics of Escherichia coli infection and meningitis in infant rats. Infect. Immun. 22:480-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carniel, E. 2001. The Yersinia high-pathogenicity island: an iron-uptake island. Microbes Infect. 3:561-569. [DOI] [PubMed] [Google Scholar]
- 10.Carniel, E., A. Guyol, O. Mercereau-Puijalon, and H. Mollaret. 1991. Chromosomal marker from high pathogenicity phenotype in Yersinia. Contrib. Microbiol. Immunol. 12:192-197. [PubMed] [Google Scholar]
- 11.Clermont, O., S. Bonacorsi, and E. Bingen. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66:4555-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clermont, O., S. Bonacorsi, and E. Bingen. 2001. The Yersinia high-pathogenicity island is highly predominant in virulence-associated phylogenetic groups of Escherichia coli. FEMS Microbiol. Lett. 196:153-157. [DOI] [PubMed] [Google Scholar]
- 13.Crosa, J. H. 1989. Genetics and molecular biology of siderophore-mediated iron transport in bacteria. Microbiol. Rev. 53:517-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietzman, D. E., G. W. Fischer, and F. D. Schoenknecht. 1974. Neonatal Escherichia coli septicemia: bacterial counts in blood. J. Pediatr. 85:128-130. [DOI] [PubMed] [Google Scholar]
- 16.Dozois, C. M., F. Daigle, and R. Curtiss III. 2003. Identification of pathogen-specific and conserved genes expressed in vivo by an avian pathogenic Escherichia coli strain. Proc. Natl. Acad. Sci. USA 100:247-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duriez, P., O. Clermont, S. Bonacorsi, E. Bingen, A. Chaventre, J. Elion, B. Picard, and E. Denamur. 2001. Commensal Escherichia coli isolates are phylogenetically distributed among geographically distinct human populations. Microbiology 147:1671-1676. [DOI] [PubMed] [Google Scholar]
- 18.Glode, M. P., A. Sutton, E. R. Moxon, and J. B. Robbins. 1977. Pathogenesis of neonatal Escherichia coli meningitis: induction of bacteremia and meningitis in infant rats fed Escherichia coli K1. Infect. Immun. 16:75-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffiths, E. 1999. Iron in biological systems, p. 1-26. In J. Bullen and E. Griffiths (ed.), Iron and infection. Wiley, Chichester, United Kingdom.
- 20.Hantke, K., G. Nicholson, W. Rabsch, and G. Winkelmann. 2003. Salmochelins, siderophores of Salmonella enterica and uropathogenic Escherichia coli strains, are recognized by the outer membrane receptor IroN. Proc. Natl. Acad. Sci. USA 100:3677-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houdouin, V., S. Bonacorsi, N. Brahimi, O. Clermont, X. Nassif, and E. Bingen. 2002. A uropathogenicity island contributes to the pathogenicity of Escherichia coli strains that cause neonatal meningitis. Infect. Immun. 70:5865-5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson, J. R. 1991. Virulence factors in Escherichia coli urinary tract infection. Clin. Microbiol. Rev. 4:80-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim, K. S., H. Itabashi, P. Gemski, J. Sadoff, R. L. Warren, and A. S. Cross. 1992. The K1 capsule is the critical determinant in the development of Escherichia coli meningitis in the rat. J. Clin. Investig. 90:897-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russo, T. A., U. B. Carlino, A. Mong, and S. T. Jodush. 1999. Identification of genes in an extraintestinal isolate of Escherichia coli with increased expression after exposure to human urine. Infect. Immun. 67:5306-5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russo, T. A., C. D. McFadden, U. B. Carlino-MacDonald, J. M. Beanan, T. J. Barnard, and J. R. Johnson. 2002. IroN functions as a siderophore receptor and is a urovirulence factor in an extraintestinal pathogenic isolate of Escherichia coli. Infect. Immun. 70:7156-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schubert, S., S. Cuenca, D. Fischer, and J. Heesemann. 2000. High-pathogenicity island of Yersinia pestis in enterobacteriaceae isolated from blood cultures and urine samples: prevalence and functional expression. J. Infect. Dis. 182:1268-1271. [DOI] [PubMed] [Google Scholar]
- 27.Schubert, S., B. Picard, S. Gouriou, J. Heesemann, and E. Denamur. 2002. Yersinia high-pathogenicity island contributes to virulence in Escherichia coli causing extraintestinal infections. Infect. Immun. 70:5335-5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schubert, S., A. Rakin, H. Karch, E. Carniel, and J. Heesemann. 1998. Prevalence of the “high-pathogenicity island” of Yersinia species among Escherichia coli strains that are pathogenic to humans. Infect. Immun. 66:480-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torres, A. G., and S. M. Payne. 1997. Haem iron-transport system in enterohaemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 23:825-833. [DOI] [PubMed] [Google Scholar]
- 30.Torres, A. G., P. Redford, R. A. Welch, and S. M. Payne. 2001. TonB-dependent systems of uropathogenic Escherichia coli: aerobactin and heme transport and TonB are required for virulence in the mouse. Infect. Immun. 69:6179-6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Unhanand, M., M. M. Mustafa, G. H. McCracken, Jr., and J. D. Nelson. 1993. Gram-negative enteric bacillary meningitis: a twenty-one-year experience. J. Pediatr. 122:15-21. [DOI] [PubMed] [Google Scholar]