Abstract
Fowl cholera is caused by Pasteurella multocida serovars A:1, A:3, and A:4. The 39-kDa cross-protective factor protein and four other membrane proteins of the membrane proteome of P. multocida were identified. We determined that the 39-kDa cross-protective protein was Pasteurella lipoprotein B, or PlpB.
Fowl cholera continues to be of major concern to the poultry industry, especially for turkey growers. Fowl cholera costs the turkey industry millions of dollars annually. The disease is caused primarily by three serotypes of Pasteurella multocida, A:1, A:3, and A:4. A live attenuated vaccine strain, strain M-9 (serotype 3,4), has been implicated in outbreaks associated with vaccination (3, 4, 6, 8, 14). Commercial bacterins prepared from serotypes A:1, A:3, and A:4 confer cross protection and are currently used to control outbreaks of disease in poultry flocks. Recently, Rimler (11) identified an outer membrane-associated protein with a molecular mass of approximately 39 kDa that was believed to be one of the cross-protective antigens. However, the identity of the cross-protective antigen expressed by the P. multocida serovars was not known (11). The objectives of this study were to (i) identify the 39-kDa protein by using trypsin digestion followed by peptide mass fingerprinting with matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) and (ii) identify the remaining proteins of the outer membrane proteome.
The outer membrane protein extract was prepared by Rimler (11) from in vitro-grown P. multocida serotype A:3 strain P-1059. Briefly, the bacterial pellet was suspended in a solution containing lysozyme, EDTA, and Triton X-100. The viscosity was reduced by incubating the suspension with DNase and hyaluronidase (12). The suspension was centrifuged at 100,000 × g for 1 h. The supernatant was removed, and the pellet was extracted with 0.5% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS). The extract was stored frozen at −80°C.
The CHAPS-solubilized proteins were denatured under reducing conditions (7) and applied to either a 10-well (10 μg of protein per well) or a two-dimensional-well (250 μg of protein) 4 to 12% bis-Tris gradient gel (Invitrogen, Carlsbad, Calif.) by using the 3-(N-morpholino)-2-hydroxypropanesulfonic acid (MOPS) buffer system (Invitrogen). Gels were stained with Coomassie brilliant blue R-250 and destained as described previously (7).
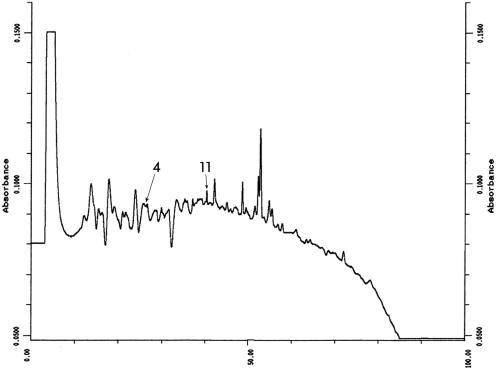
Gel plugs were removed from the stained bands of the gel by using a blunt-cut 16-gauge needle. The gel plugs were deposited into a 96-well microtiter plate and digested with trypsin by using the automated digester ProGest (Genomic Solutions, Ann Arbor, Mich.). The extracted peptides were desalted using a C18 ZipTip (Millipore, Bedford, Mass.), mixed with an equal volume of saturated α-cyano-hydroxy-cinnamic acid (Sigma Chemical Co., St. Louis, Mo.) in 30% acetonitrile-0.2% trifluoroacetic acid, and spotted onto the target plate. MALDI mass spectra were obtained by using a Voyager DE-PRO mass spectrometer (Applied Biosystems, Foster City, Calif.). We used the MS-Fit search engine of the Prospector website of the University of California at San Francisco (http://prospector.ucsf.edu/) to search the nonredundant database of the National Center for Biotechnology Information. The identity of the 39-kDa protein was confirmed by automated Edman degradation. Briefly, 15 gel plugs were treated with trypsin as described above and the extracted peptides (20 μl) in 1% formic acid were injected onto the C18 column (Vydac; 2.1 by 250 mm) equilibrated in 0.1% (vol/vol) trifluoroacetic acid-MilliQ water. The peptide fragments were eluted with a linear gradient of 0.08% (vol/vol) trifluoroacetic acid-80% (vol/vol) acetonitrile-MilliQ water for 100 min at a flow rate of 200 μl/min and monitored at 214 nm. Each peak was collected into a methanol-washed microfuge tube. A 0.5-μl sample was prepared for MALDI-TOF as described above and subjected to MALDI-TOF mass spectrometry using the Dynamo (ThermoBioanalysis, Hemel Hempstead, United Kingdom) mass spectrometer. Based on the MALDI spectra obtained (data not shown), two fractions containing one major peptide (peak 4 and peak 11) were selected for Edman degradation. Fractions were dried down, redissolved in 30% (vol/vol) acetonitrile-0.2% (vol/vol) trifluoroacetic acid, and analyzed by automated Edman degradation using an Applied Biosystems model 494 Procise sequencer (Iowa State University Protein Facility).
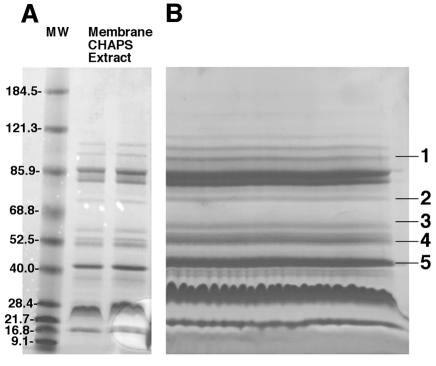
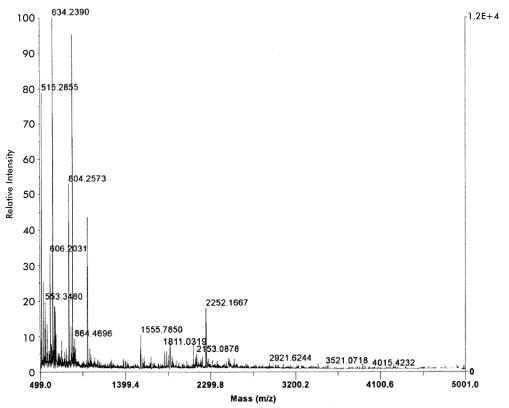
The protein profile of 10 μg of cross-protective CHAPS-solubilized fraction is shown in analytical format in Fig. 1A. The same fraction at a 25-fold-higher concentration is shown in preparative format in Fig. 1B. The latter gel shows 11 major proteins of which the 39-kDa protein and four other proteins (marked as bands 1 through 5) gave mass spectra with sufficient information for database searching. The lack of data from the remaining bands may indicate a lack of trypsin cleavage sites in these proteins. The identity of the 39-kDa protein (Fig. 1B, band 5) was consistent with the molecular masses of tryptic peptides of the Pasteurella lipoprotein B, PlpB, listed in the National Center for Biotechnology Information database (1) for P. multocida (Table 1 and Table 2). N-terminal sequencing of peptide peaks 4 and 11 (Fig. 2) showed amino acid sequences identical to those obtained by peptide mass fingerprinting and database searching using MS-Fit. Similarly, the molecular masses of the purified peptide fragments obtained by high-performance liquid chromatography (Fig. 3) and analyzed by MALDI-TOF (Fig. 4) were similar to the molecular masses observed for the unfractionated trypsin digest.
FIG. 1.
SDS-PAGE of CHAPS-solubilized P. multocida outer membrane fraction. An overnight culture of P. multocida A:3 strain P-1059 in a peptone-based medium was harvested by centrifugation, lysed with EDTA, lysozyme, and Triton X-100, treated with DNase and hyaluronidase, and centrifuged at 100,000 × g. The pellet was extracted with CHAPS, centrifuged at 100,000 × g, and analyzed by SDS-PAGE. (A) Ten micrograms of protein per lane. (B) Two hundred fifty micrograms of protein. MW, molecular weight.
TABLE 1.
MS-Fit analysis of five P. multocida membrane proteins
| Band | Gel molecular weight | Molecular weight/pI | % Match of peptides | Identitya (accession no.) |
|---|---|---|---|---|
| 1 | 117 | 117/5.6 | 4 | Put Vir Fact (15603684) |
| 2 | 79 | 79.7/9.2 | 11 | LipA/PhyA (15602638) |
| 3 | 68 | 47.8/5.9 | 1 | HemY (15603680) |
| 4 | 52 | 37.4/7.7 | 7 | Unkn lipoprot (15602915) |
| 5 | 39 | 30/5.2 | 7 | PlpB (15603595) |
Put Vir Fact, putative virulence factor by homology to Pseudomonas syringae and Yersinia pestis proteins (10); LipA/PhyA, lipase A, replaces phospholipid in hyaluronic acid (2, 10, 15); HemY, protoporphyrinogen oxidase by homology to Haemophilus influenzae protein (10); Unkn lipoprot, unknown lipoprotein of membrane biogenesis by homology to H. influenzae protein (10).
TABLE 2.
Peptide masses and peptide sequences predicted for the tryptic digest of the 39-kDa protein by using MS-Fita
| Peptide | Predicted mi-m/zb | Predicted av-m/zc | Observed mi-m/zb | Start position | End position | No. of missed cleavages | Database sequence |
|---|---|---|---|---|---|---|---|
| 11 | 1811.0107 | 1812.1761 | 1811.0319 | 110 | 126 | 0 | (K)LAIVGNTFVPIAAYSK(R) |
| 4 | 2252.1271 | 2253.4490 | 2252.1667 | 130 | 151 | 0 | (K)NVSELQDGATVAVNNPSNLG(R) |
Predicted peptide masses and predicted peptide sequences were determined from the mass spectrometry data obtained by MALI-TOF mass spectrometry using the MS-Fit algorithm of the University of California at San Francisco Prospector database (http://prospector.ucsf.edu) as described in the text. Only the peptide masses for the two sequenced peptides are indicated.
mi-m/z, monoisotopic mass/charge ratio.
av-m/z, average mass/charge ratio.
FIG. 2.
N-terminal sequence of the isolated tryptic peptides and protein sequence of the lipoprotein B. (A) Amino acid sequences of peptides 4 and 11. (B) Protein sequence of lipoprotein B, PlpB. Sequences corresponding to those of peptides 4 and 11 are underlined. The notation at the top of panel B indicates the database search result and accession numbers.
FIG. 3.
Results of C18 high-performance liquid chromatography of a trypsin digest of band 5 protein. Multiple gel plugs were digested with trypsin using the automated digester. Twenty microliters of extracted and concentrated peptide was loaded onto a C18 column and eluted with a linear gradient of acetonitrile in aqueous trifluoroacetic acid. The absorbance was monitored at 214 nm. Each peak was collected into a methanol-washed microfuge tube and analyzed by MALDI-TOF mass spectrometry. Peptide peaks 4 and 11 were analyzed by N-terminal sequencing.
FIG. 4.
MALDI-TOF mass spectrum of a trypsin digest of band 5 protein. Protein plugs from the gel were treated with trypsin by using an automated digester. The extracted peptides were desalted, mixed with an equal volume of saturated α-cyano-hydroxy-cinnamic acid in 30% acetonitrile-0.2% trifluoroacetic acid, and analyzed by MALDI-TOF mass spectrometry. The peak with an m/z of 2252.1667 is clearly visible. Relative intensities are given as percentages.
Table 1 shows the MS-Fit results with the mass spectra of tryptic digests prepared from proteins of bands 1, 2, 3, and 4 of the preparative sodium dodecyl sulfate (SDS) gel (Fig. 1B). These proteins include two membrane biogenesis proteins and two virulence-related proteins. The reason for analyzing these proteins is that often improved protection was observed with the cruder fraction compared to that with the purified 39-kDa protein (11) and it is likely that more than one protein is necessary to afford complete protection.
The gel molecular weights of proteins in bands 1 and 2 correspond to the molecular weights of the proteins determined based on amino acid composition as identified in the database, whereas those of the proteins in bands 3, 4, and 5 do not. For these three proteins (HemY, the unknown lipoprotein, and PlpB), the gel molecular weights do not correspond to the actual molecular weights based on amino acid composition. This is not uncommon for membrane proteins, which may migrate anomalously in SDS-polyacrylamide gel electrophoresis (PAGE) (5, 9, 13). The percentages of matched peptides for the identified proteins varied from 11 to 1% (Table 1), and this is not uncommon for membrane proteins.
Identification of the 39-kDa outer membrane protein as the Pasteurella lipoprotein B, or PlpB, a member of a family of virulence-related proteins, should now permit cloning and expression of PlpB for vaccine experiments.
Editor: J. T. Barbieri
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyce, J. D., J. Y. Chung, and B. Adler. 2000. Genetic organisation of the capsule biosynthetic locus of Pasteurella multocida M1404 (B:2). Vet. Microbiol. 72:121-134. [DOI] [PubMed] [Google Scholar]
- 3.Christensen, J. P., and M. Bisgaard. 2000. Fowl cholera. Rev. Sci. Tech. 19:626-637. [DOI] [PubMed] [Google Scholar]
- 4.Christiansen, K. H., K. P. Snipes, and D. W. Hird. 1991. Transmission of Pasteurella multocida on California turkey premises in 1988-1989. Avian Dis. 36:262-271. [PubMed] [Google Scholar]
- 5.Diedrich, D. L., M. A. Stein, and C. A. Schnaitman. 1990. Association of Escherichia coli K-12 OmpF trimers with rough and smooth lipopolysaccharides. J. Bacteriol. 172:5307-5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hird, D. W., T. E. Carpenter, K. P. Snipes, D. C. Hirsch, and R. H. McCapes. 1991. Case control study of fowl cholera outbreaks in meat turkeys in California August 1985-July 1986. Am. J. Vet. Res. 52:212-216. [PubMed] [Google Scholar]
- 7.Laemmli, U. K. 1970. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature (London) 227:680-685. [DOI] [PubMed] [Google Scholar]
- 8.Lee, M. D., R. E. Wooley, J. R. Glissen, and J. Brown. 1988. Comparison of Pasteurella multocida serotype 3,4 isolates from turkeys with fowl cholera. Avian Dis. 32:501-508. [PubMed] [Google Scholar]
- 9.Lobos, S. R., and G. C. Mora. 1991. Alteration in the electrophoretic mobility of OmpC to variations in the ammonium persulfate concentration in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Electrophoresis 12:448-450. [DOI] [PubMed] [Google Scholar]
- 10.May, B. J., Q Zhang, L. L. Li, M. L. Paustian, T. S. Whittam, and V. Kapur. 2001. Complete genomic sequence of Pasteurella multocida, Pm70. Proc. Natl. Acad. Sci. USA 98:3460-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rimler, R. B. 2001. Purification of a cross-protective antigen from Pasteurella multocida grown in vitro and in vivo. Avian Dis. 45:572-580. [PubMed] [Google Scholar]
- 12.Rimler, R. B., and K. R. Rhoades. 1981. Lysates of turkey-grown Pasteurella multocida: protection against homologous and heterologous serotype challenge exposures. Am. J. Vet. Res. 42:2117-2121. [PubMed] [Google Scholar]
- 13.Rosenbusch, J. P. 1976. Characterization of the major envelope protein from Escherichia coli. J. Biol. Chem. 249:8019-8029. [PubMed] [Google Scholar]
- 14.Snipes, K. P., D. C. Hirsch, R. W. Kasten, T. E. Carpenter, D. W. Hird, and R. H. McCapes. 1990. Differentiation of field isolates of Pasteurella multocida serotype 3,4 from live vaccine strain by genotypic characterization. Avian Dis. 34:419-424. [PubMed] [Google Scholar]
- 15.Townsend, K. M., J. D. Boyce, J. Y. Chung, A. J. Frost, and B. Adler. 2001. Genetic organization of Pasteurella multocida cap loci and development of a multiplex capsular PCR typing system. J. Clin. Microbiol. 39:924-929. [DOI] [PMC free article] [PubMed] [Google Scholar]