Abstract
We have previously shown that Salmonella enterica serovar Typhimurium infection perturbs the host cholesterol biosynthetic pathway. Here we show that inhibiting the first step of this pathway (3-hydroxy-3-methylglutaryl coenzyme A reductase) reduces the growth of intracellular S. enterica serovar Typhimurium and has no effect on extracellular bacterial growth. Selectively inhibiting synthesis of downstream sterol components has no effect on infection, suggesting that the effect of statins on host nonsterol intermediates is detrimental to bacterial growth. Furthermore, statins also reduce bacterial proliferation in the S. enterica serovar Typhimurium mouse model. This suggests that blocking the production of nonsterol precursors in the host cell can be used to reduce infection.
Salmonella enterica serovar Typhimurium is a well-characterized model system for the study of host-pathogen interactions. It possesses two functionally distinct type III secretion systems, both of which are encoded by pathogenicity islands. Salmonella pathogenicity island 1 (SPI-1) is required for invasion of epithelial cells, as it secretes effector proteins that induce cytoskeletal polymerization and membrane ruffling in the target host cell (44). SPI-2 is required for intracellular replication and systemic disease in mice (8, 15). Upon entering host cells, Salmonella resides in a membrane-bound vacuole, and expression of bacterial genes from SPI-2 requires signals from the vacuolar environment (40). SPI-2 effector proteins are secreted across the vacuolar membrane, where they modify the structural integrity of the vacuole (3, 32) and interfere with endocytic trafficking (38). While S. enterica serovar Typhimurium can survive in both epithelial cells and professional phagocytes, in order to disseminate and cause systemic infection, it must infect phagocytes that migrate to peripheral tissues, such as the spleen and liver (34, 41). Multiple bacterially encoded virulence determinants required for intracellular proliferation in both macrophages and epithelial cells have been identified as primary targets for disease intervention. In contrast, much less is known regarding host cell pathways required for intracellular bacterial survival. Our previous studies, as well as those of other workers (13), have demonstrated that S. enterica serovar Typhimurium infection of RAW 264.7 murine macrophages and epithelial cells results in high levels of cholesterol accumulation in the Salmonella-containing vacuole (SCV). S. enterica serovar Typhimurium also perturbs levels of host cholesterol biosynthetic intermediates (5), suggesting that the bacteria interact with the host sterol biosynthetic pathway. We therefore investigated the requirement of this pathway for the survival of S. enterica serovar Typhimurium in in vitro culture and with regard to bacterial proliferation in mice.
MATERIALS AND METHODS
Materials.
Lovastatin was a gift from Merck and was also purchased from Sigma. Prescription formulation atorvastatin was obtained from Pfizer Inc. 4,4,10β-Trimethyl-trans-decal-3β-ol (TMD) was a gift from T. A. Spencer (Dartmouth College). Mevalonate, gliotoxin, and antibiotics were obtained from Sigma.
Growth conditions for bacteria and mammalian cells.
Visualization and selection of S. enterica serovar Typhimurium wild-type strain SL1344 were done with plasmid pFVP25.1, which contains gfpmut3A under a constitutive promoter, as well as an ampicillin resistance marker (39). The orgA and ssaT mutants have been described previously (12, 18). For macrophage infection, S. enterica serovar Typhimurium cultures were grown in Luria-Bertani broth in a 37°C shaking incubator to the late log or early stationary phase. For mouse infection, bacteria were grown overnight in a 37°C shaking incubator. Antibiotics were used at the following concentrations: ampicillin, 100 μg/ml; streptomycin, 50 μg/ml; and kanamycin, 50 μg/ml. RAW 264.7 murine macrophages (American Type Culture Collection [ATCC], Rockville, Md.) were maintained in Dulbecco's modified Eagle medium (DMEM) (ATCC) with 10% fetal bovine serum (ATCC) at 37°C in the presence of 5% CO2 without antibiotics.
Gentamicin protection assays.
RAW 264.7 macrophages were seeded at a concentration of 5 × 105 cells/well in 24-well plates. Bacteria were opsonized in DMEM containing 20% mouse serum for 30 min at 37°C and then added to macrophages at a multiplicity of infection of 10 bacteria/cell. Plates were centrifuged at 200 × g (Beckman centrifuge with a GH3.8 rotor) for 5 min and incubated for 15 min at 37°C. After infection, cells were washed three times with serum-free medium, incubated with 100 μg of gentamicin per ml for 90 min, and then maintained in the presence of 10 μg of gentamicin per ml for the remainder of the experiment. At various times postinfection, cells were lysed with 1% Triton X-100, and intracellular bacteria were counted on selective media. When inhibitors were used to study the effects of host cell pathways on bacteria, cells were incubated in the presence of inhibitors for 4 h prior to infection and then maintained in the presence of these inhibitors throughout the experiment. The results of intracellular growth assays were expressed as fold changes in growth, which were determined by dividing the number of bacterial CFU recovered at 20 h by the number of CFU recovered at 2 h.
Drug treatments.
In experiments with TMD, 12 μg of the compound per ml in dimethyl sulfoxide was added to the medium, while the vehicle alone was added to control wells. Lovastatin was solubilized in 0.1 N NaOH at 60°C, and then the pH was adjusted to 7.4 with HCl. Lovastatin (30 μM) was added to the medium, and an equal volume of water was added to control wells. For experiments with a nanomolar concentration of lovastatin (50 nM), cell monolayers were treated for 3 days prior to infection. The medium and drug were replaced every 24 h.
Macrophage viability assays.
The cytopathic effects of lovastatin on RAW 264.7 macrophages were measured by reduction of Alamar blue (Biosource International, Vacaville, Calif.). Cells were treated for 20 h with lovastatin, washed, and then incubated with DMEM containing Alamar blue (diluted 1:10) at 37°C for 3 h. Dye reduction was measured by determining the fluorescence with excitation at 540 nm and emission at 590 nm.
Mouse studies.
Animal experiments were performed with approval and authorization from the institutional review board and the Animal Care and Use Committee of Northwestern University. Female BALB/c mice (8 to 10 weeks old; Charles River, Portage, Mich.) were used. Atorvastatin was resuspended in phosphate-buffered saline (PBS) containing 5% ethanol and administered to mice subcutaneously at a dose of 10 mg/kg/day for 9 days. Bacteria from overnight cultures were pelleted by centrifugation for 5 min at 3,800 × g and were resuspended in PBS. Mice were given 1 × 105 bacteria by intraperitoneal injection on day 7. Serial dilutions of inoculants were plated on selective media to determine the actual doses. On day 9 (2 days postinfection), mice were sacrificed. Spleens and livers were removed, weighed, homogenized, and then plated on selective media to determine the number of bacterial CFU. Serum cholesterol levels were measured with an Infinity cholesterol reagent kit from Sigma.
Statistical analyses.
Statistical analyses for CFU comparisons were performed by using Student's t test with the Microsoft Excel data analysis add-in package. Differences were considered significant when P values were <0.05.
TUNEL assays.
Macrophages were seeded at a concentration of 3 × 105 cells/well on coverslips in 24-well plates. The cells were washed with PBS at 13 h postinfection, fixed in 4% formaldehyde in PBS for 30 min, and then stained with terminal deoxytransferase end-labeling (TUNEL) reagents by using the protocol provided by the manufacturer (Roche Diagnostics). Nuclei were stained with 10 μg of Hoechst dye (Molecular Probes) per ml. Coverslips on glass slides were examined by fluorescence microscopy. A 3-h treatment with gliotoxin (1 μg/ml) was used as a positive control for cell death. For three-dimensional models, infected macrophages were stained with TUNEL reagents and filipin (100 μg/ml), photographed with a fluorescent microscope as previously described (5), and imported into Volocity (Improvision) for three-dimensional rendering.
Flow cytometry.
Rhodamine-conjugated beads (diameter, 1 μm; Molecular Probes) were diluted in DMEM and then added to macrophages (10 beads/cell) for 15 min at 37°C. The cells were washed, fixed in 4% formaldehyde for 15 min, and scraped from the plates in PBS-3% bovine serum albumin. Samples were analyzed with a Becton Dickinson FACScan (FACSCalibur). Data for 10,000 light-scattered events were collected and analyzed with CELLQuest software (BD Biosciences).
HPLC.
Cells were labeled by using [3H]mevalonolactone or [3H]acetate, and cellular lipids were extracted and prepared for high-performance liquid chromatography (HPLC) as previously described (5). Briefly, radiolabel (25 μCi/well) was added to the cells at 2 h postinfection. At various times thereafter, cells were harvested by scraping, centrifugation, and water lysis. Lipids were extracted in chloroform-methanol (2:1) at a ratio of 5:1 (vol/vol). The samples were dried under nitrogen and dissolved in acetonitrile-isopropyl alcohol (90:10, vol/vol) for analysis by HPLC and scintillation counting. HPLC was performed at 30 or 50°C
RESULTS
Inhibition of the host sterol biosynthetic pathway by lovastatin blocks intracellular proliferation of S. enterica serovar Typhimurium in macrophages.
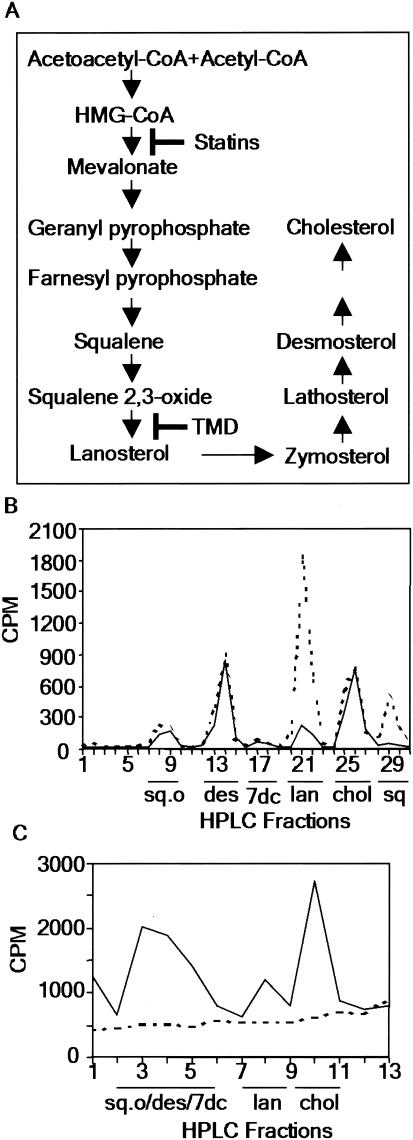
Previous studies demonstrated that S. enterica serovar Typhimurium infection caused accumulation of cholesterol mass, as well as lanosterol (5), a biosynthetic cholesterol precursor, in macrophages (Fig. 1A). We also found that the levels of the nonsterol precursors squalene and squalene oxide were elevated in infected cells (Fig. 1B). Therefore, we examined whether inhibiting the cholesterol biosynthetic pathway had any effect on the intracellular proliferation of S. enterica serovar Typhimurium. We used lovastatin, a drug that inhibits 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, a rate-limiting enzyme of the mammalian sterol biosynthetic pathway (Fig. 1A). As expected, lovastatin treatment of RAW 264.7 macrophages blocked the sterol biosynthetic pathway (Fig. 1C); it also reduced the intracellular proliferation of S. enterica serovar Typhimurium 6- to 10-fold (Fig. 2A). The inhibition could be reversed by addition of mevalonate, indicating that there was a specific requirement for a product of HMG-CoA reductase. S. enterica serovar Typhimurium does not have the HMG-CoA reductase gene (25), suggesting that the decreased intracellular bacterial proliferation resulted from inhibition of the host pathway. These data indicate that one or more components of the host cholesterol biosynthetic pathway are required for S. enterica serovar Typhimurium intracellular proliferation. Furthermore, targeting this pathway resulted in clearance of intracellular bacteria and/or infected cells in culture.
FIG. 1.
S. enterica serovar Typhimurium perturbs intermediates of the host sterol biosynthetic pathway. (A) Mammalian sterol biosynthetic pathway, showing steps inhibited by statins and TMD. (B) Control (solid line) and infected (dashed line) macrophages were incubated with [3H]mevalonate to label the host sterol biosynthetic pathway. At 12 h postinfection, cellular lipids were extracted and analyzed by HPLC (30°C) and scintillation counting. The data are representative of the data from at least three independent experiments. (C) Macrophages were grown in control (solid line) or 30 μM lovastatin-treated (dashed line) medium with [3H]acetate to label the host sterol biosynthetic pathway. At 20 h postinfection, cellular lipids were extracted and analyzed by HPLC (50°C) and scintillation counting. Abbreviations: sq.o, squalene oxide; des, desmosterol; 7dc, 7-dehydrocholesterol; lan, lanosterol; chol, cholesterol; sq, squalene.
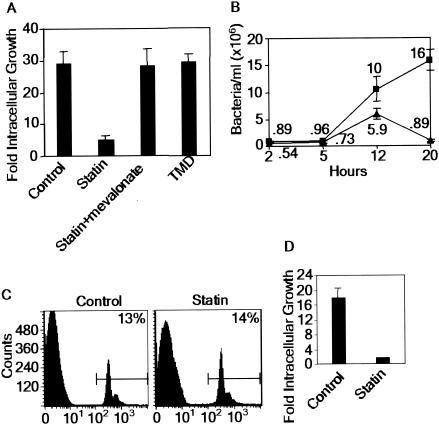
FIG. 2.
S. enterica serovar Typhimurium requires nonsterol intermediates of host sterol biosynthesis. (A) Intracellular growth of S. enterica serovar Typhimurium in RAW 264.7 macrophages treated with various media and supplements. Growth is expressed as the number of bacteria recovered at 20 h relative to the number of bacteria recovered at 2 h. (B) Intracellular growth curve for S. enterica serovar Typhimurium infection in control (▪) and 30 μM lovastatin-treated (▴) RAW 264.7 macrophages. (C) Phagocytosis of fluorescent latex beads in control and 30 μM lovastatin-treated macrophages. The percentages indicate the percentages of bead-containing cells relative to the total number of cells. (D) Intracellular growth of nonopsonized S. enterica serovar Typhimurium in control or 30 μM lovastatin-treated RAW 264.7 macrophages. The data are means ± standard errors of the means for three replicates.
To elucidate the nature of the intermediates required, we used the inhibitor TMD, which blocks the conversion of squalene oxide to lanosterol (31) (Fig. 1A). TMD blocked synthesis of all sterols in both infected and uninfected macrophages (5). However, it had no effect on S. enterica serovar Typhimurium growth in macrophages (Fig. 2A). This finding suggests that biosynthetic sterols are not essential for intracellular proliferation of S. enterica serovar Typhimurium. Rather, one or more prelanosterol intermediates must play a key role.
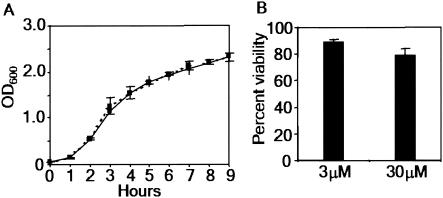
Next, we examined the effect of lovastatin on the time course of infection. At 2 h postinfection, the numbers of bacteria in treated cells were ∼60% of the numbers of bacteria in control cells (0.54 × 106 and 0.89 × 106 bacteria/cell, respectively) (Fig. 2B). By 20 h, the numbers of bacteria in treated cells were more than 10-fold lower than the numbers of bacteria recovered from untreated monolayers. This strongly suggests that lovastatin has a minimal effect on bacterial entry. Rather, the prominent inhibitory effect of this compound occurs during intracellular growth. Furthermore, treatment with lovastatin had no effect on the uptake of latex beads by macrophages (Fig. 2C), suggesting that the drug does not affect endocytosis, which is consistent with the lack of an effect on bacterial entry into macrophages (Fig. 2B). Finally, even if the initial bacterial inoculants were not opsonized, lovastatin blocked intracellular bacterial replication (Fig. 2D), suggesting that the route of bacterial entry into macrophages did not influence the inhibitory effect of the drug. To further characterize the action of lovastatin on bacteria and host cells, we examined its effect on extracellular bacterial replication and the viability of uninfected macrophages. First, lovastatin had no effect on extracellular bacterial growth in liquid broth culture (Fig. 3A). Second, more than 80% of uninfected, lovastatin-treated host cells remained viable, as measured by cellular respiration (Fig. 3B). These data suggest that lovastatin is selectively detrimental to infected cells and specifically blocks intracellular proliferation of S. enterica serovar Typhimurium in macrophages.
FIG. 3.
Statins specifically target the interaction between intracellular bacteria and macrophages. (A) Extracellular growth curve for S. enterica serovar Typhimurium in control (solid line) or 30 μM lovastatin-treated (dashed line) broth cultures. OD600, optical density at 600 nm. (B) Viability of uninfected RAW 264.7 macrophages treated with 3 or 30 μM lovastatin, as measured by Alamar blue dye reduction. The data are means ± standard errors of the means for three replicates.
Lovastatin induces host cell death in S. enterica serovar Typhimurium-infected macrophages.
The observation that lovastatin blocked bacterial proliferation at late stages of intracellular growth prompted us to look at host cell death as a potential mechanism of inhibition. This is because S. enterica serovar Typhimurium induces replication-dependent, late host cell death in macrophages (27, 29). Early cell death is associated with bacterial invasion and is linked to the SPI-1 secreted protein SipB (16). Late cell death (18 to 24 h postinfection) is linked to SPI-2, since a SPI-2 null mutation largely reduces the cytotoxicity caused by late-stage infection (27). While both kinds of cell death caused by S. enterica serovar Typhimurium are largely dependent on caspase-1, SPI-2 cell death can also be independent of caspase-1 (27).
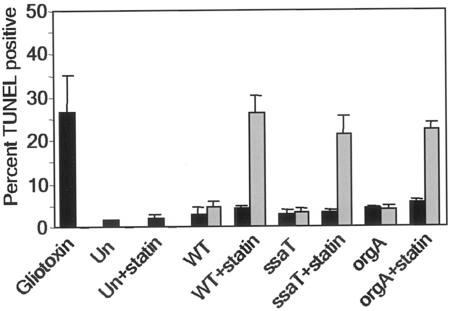
We used TUNEL to determine whether lovastatin induced higher levels of DNA double-stranded cuts in Salmonella-infected macrophages. Cells were infected with S. enterica serovar Typhimurium expressing green fluorescent protein (GFP) and then were fixed and processed for TUNEL staining at 13 h postinfection. We chose 13 h postinfection in order to minimize the late-stage TUNEL-positive cell death linked to SPI-2 of S. enterica serovar Typhimurium and because we observed a significant reduction in the number of bacteria recovered at this time (Fig. 2B). Lovastatin caused a fivefold increase in the number of TUNEL-positive, S. enterica serovar Typhimurium-infected cells (25%) compared to the number of untreated cells (5%) (Fig. 4), and 63% of the TUNEL-positive macrophages were infected. Uninfected cells showed insignificant levels of TUNEL staining in the presence of lovastatin. Furthermore, the lovastatin-induced cell death in infected cells was approximately the same as the cell death seen in a gliotoxin-treated positive control. These results imply that host cell death is a potential mechanism for lovastatin's inhibition of intracellular bacterial proliferation. This is consistent with our finding that lovastatin targets a host cell pathway that is perturbed and utilized by intracellular bacteria.
FIG. 4.
Statins induce host cell death in S. enterica serovar Typhimurium-infected macrophages. Macrophages were plated, treated with 30 μM lovastatin, and infected with various strains of GFP-expressing S. enterica serovar Typhimurium. At 13 h postinfection TUNEL-stained cells were analyzed by fluorescence microscopy. The bars indicate the results for a minimum of 100 uninfected cells (solid bars) and 100 infected cells (gray bars) obtained from counting TUNEL-positive cells in each monolayer. The data are means ± standard errors of the means for three slides. Abbreviations: Un, uninfected monolayers; WT, wild-type bacterium-infected monolayers.
To address whether the TUNEL-positive cell death induced by lovastatin required SPI-1 or SPI-2 genetic loci, we infected control and lovastatin-treated cells with orgA or ssaT mutant bacteria. The orgA mutant was deficient in SPI-1 genes (18), while the ssaT mutant lacked a structural component of the SPI-2-dependent type III secretion system and could not secrete any of the SPI-2 bacterial effector proteins (12). Lovastatin induced the same rate of TUNEL-positive cell death in orgA and ssaT mutant-infected macrophages that it induced in cells infected with wild-type S. enterica serovar Typhimurium, suggesting that the effect of this compound is independent of both SPI-1 and SPI-2 (Fig. 4).
Physiologically relevant concentrations of lovastatin inhibit proliferation of intracellular S. enterica serovar Typhimurium.
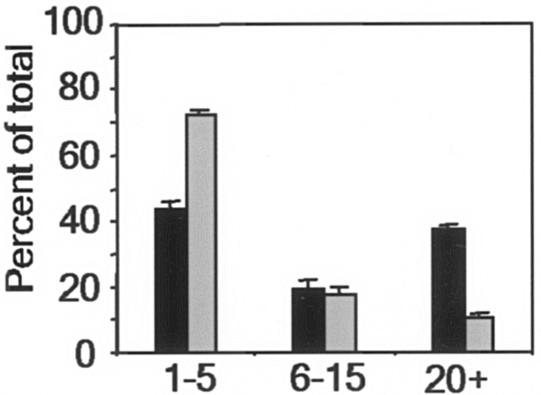
Most in vitro experiments with statins are done with concentrations of the drugs in the micromolar range (11, 21). However, treatment of hypercholesterolemia is effective when nanomolar concentrations of a drug are achieved in human plasma (26). To determine whether lovastatin is effective against S. enterica serovar Typhimurium at concentrations normally achieved in plasma, we treated RAW 264.7 macrophages for 3 days with 50 nM lovastatin, replacing the medium and drug every 24 h. After 3 days, control and lovastatin-treated macrophages were infected with S. enterica serovar Typhimurium (expressing GFP) and then examined after 20 h. To directly monitor bacterial growth in the presence of lovastatin, we evaluated infected monolayers by fluorescence microscopy. After 20 h, only 10% of infected cells in the lovastatin-treated sample had large vacuoles (20 to 200 bacteria), compared to 40% of the untreated control cells (Fig. 5). Small vacuoles (one to five bacteria) were predominant in lovastatin-treated cells. Thus, even at a nanomolar concentration, lovastatin reduced proliferation of intracellular S. enterica serovar Typhimurium. In addition, intracellular growth assays (data not shown) confirmed that treatment of macrophages with 50 nM lovastatin resulted in 6- to 10-fold reductions in the number of bacteria at 20 h, which were comparable to the reductions obtained with 30 μM lovastatin (Fig. 2A and B).
FIG. 5.
Statins inhibit bacterial infection at physiologically relevant concentrations. RAW 264.7 macrophages were treated with 50 nM lovastatin (gray bars) or the vehicle control (solid bars) for 3 days prior to infection with GFP-expressing S. enterica serovar Typhimurium for 20 h and then were examined by fluorescence microscopy. For each sample, 100 infected cells were counted, and the numbers of bacteria in individual cells were assigned to one of three categories: 1 to 5 bacteria, 6 to 15 bacteria, or 20 to 200 bacteria. The data are means ± standard errors of the means for three slides.
Statins limit bacterial proliferation in the S. enterica serovar Typhimurium mouse model.
The S. enterica serovar Typhimurium mouse model is a well-characterized animal system with which to examine bacterial virulence. A variety of bacterial virulence determinants have already been characterized by using this model, including SPI-1 and SPI-2 secretion mutants (19, 32). SPI-2 mutants have reduced virulence in macrophages and are severely deficient in dissemination to peripheral tissues, such as the spleen and liver, after oral and intraperitoneal infection (3, 8). These findings emphasize the importance of intramacrophage survival in systemic spread of S. enterica serovar Typhimurium and the ability of this organism to cause disease.
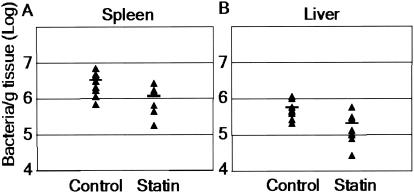
Since statins inhibited bacterial infection within cultured macrophages, we decided to examine the ability of these agents to reduce S. enterica serovar Typhimurium growth in mice. We used atorvastatin as a second statin for these studies because it is the most commonly prescribed statin and is well tolerated in animals (2, 7). While a common dose of atorvastatin in humans is 80 mg/day (about 1 mg/kg/day), previous data have shown that due to an alteration in the kinetics of HMG-CoA reductase, higher doses of statins are required in rodents (7, 10). In previous studies workers have used a 10-mg/kg/day dose of atorvastatin to reduce stroke (23) and autoimmune disease (42) in mice. Toxicity studies with rodents have indicated that the minimum toxic doses are more than 100 mg/kg/day. Toxicity has not been associated with atorvastatin administered to rodents at a dose of 1 or 10 mg/kg/day (6, 9). Therefore, we used a dose of 10 mg/kg/day. Mice were treated subcutaneously with atorvastatin or a vehicle control for 7 days prior to infection with S. enterica serovar Typhimurium by intraperitoneal injection. The intraperitoneal route of infection allowed S. enterica serovar Typhimurium to bypass the innate immune defenses in the gut-associated lymphoid tissue and gave the bacteria immediate access to peritoneal macrophages. No drug toxicity was observed during the course of the experiments. Treatment was continued for 2 days postinfection, the mice were sacrificed, and specific organs were plated on selective media for enumeration of bacteria. The spleens of atorvastatin-treated mice contained 65% less bacteria than the spleens of controls contained (1.1 × 107 and 3.2 × 107 bacteria/g of tissue, respectively; P < 0.02), while the livers contained 62% less bacteria than the control livers contained (2.1 × 106 and 5.5 × 106 bacteria/g tissue, respectively; P < 0.02) (Fig. 6). Even with the relatively short 48-h infection, we observed a more-than-60% reduction in the number of bacteria in the spleens and livers of statin-treated mice. Although we used a larger infectious dose (1 × 105 bacteria), our results are comparable to the ∼80% reduction in S. enterica serovar Typhimurium levels seen in the spleens of mice inoculated with 2 × 104 bacteria and treated with the antibiotic ciprofloxacin at a dose of 6 mg/kg/day (4). For a parallel mouse infection in the absence of pretreatment we detected no significant difference in the number of bacteria recovered between control and drug-treated animals (data not shown). This suggests that at this dose of drug and bacteria, pretreatment with statin is necessary for a reduction in bacterial growth in vivo.
FIG. 6.
Statins reduce S. enterica serovar Typhimurium growth in vivo. Female BALB/c mice were given 10 mg of atorvastatin per kg per day or the vehicle control by subcutaneous injection for 9 days. The mice were infected intraperitoneally with 1 × 105 bacteria on day 7 and then sacrificed on day 9. Spleens (A) and livers (B) were plated on selective media to determine the number of bacteria per gram of tissue. The horizontal lines indicate the means (nine mice per group; P < 0.02).
Blood analysis indicated that serum cholesterol levels were not significantly altered in vehicle-treated mice compared with atorvastatin-treated mice (165 ± 30 and 154 ± 16 mg/dl, respectively). This is in agreement with the finding that statins do not reduce overall plasma cholesterol levels in mice, as they do in humans (due to very low levels of low-density lipoproteins in rodents) (22). However, they do block host HMG-CoA reductase and the sterol biosynthetic pathway, which is presumably the basis of the statins' inhibitory effect on bacterial proliferation. In addition, previous studies have demonstrated that administration of statin to mice blocks the sterol biosynthetic pathway in peritoneal macrophages (20). These data validate our findings obtained with lovastatin and cultured macrophages and suggest that statins can reduce S. enterica serovar Typhimurium growth in animals independent of lowering cholesterol levels.
DISCUSSION
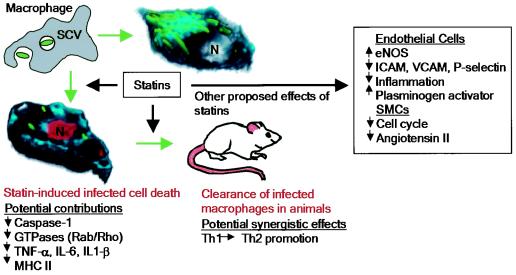
Here we show that intracellular infection by S. enterica serovar Typhimurium results in disruption of the host sterol biosynthetic pathway. Statins, which block this pathway by inhibition of HMG-CoA reductase, reduce the intracellular growth of S. enterica serovar Typhimurium in cultured macrophages and in a mouse model (Fig. 7). The in vivo effect of statins on proliferation suggests that they may target bacterial dissemination by macrophages, a key step in systemic disease. To our knowledge, this is the first example of the use of a host metabolic pathway as a drug target to block intracellular bacterial proliferation.
FIG. 7.
Statins and bacterial infection: model illustrating the effects of statins on S. enterica serovar Typhimurium infection in vitro and in vivo. Control (top right) or statin-treated (bottom left) macrophages were infected with bacteria (green) for 13 h, stained for cholesterol (cyan) and TUNEL-positive nuclei (red), and used to create three-dimensional models. Nuclei (N) are indicated. Statin-dependent effects that may contribute to bacterial clearance are also indicated (TNF-α, tumor necrosis factor alpha; MHC II, major histocompatibility class II). In addition, proposed effects of statins on the vasculature are indicated (eNOS, endothelial nitric oxide synthase; SMCs, smooth muscle cells; ICAM, intercellular adhesion molecule; VCAM, vascular cell adhesion molecule).
In addition to their cholesterol-lowering effects, statins have received attention for their abilities to promote vascular health in the areas of atherosclerosis (1), stroke (17), and coronary artery disease (33) (Fig. 7). In one study the workers reported a significant reduction in mortality due to bacteremia in patients taking statins (24). However, the mechanisms underlying these effects were not elucidated. Our studies suggest that statins' effects on bacteria may reflect a need for nonsterol intermediates in critical host-pathogen interactions in vitro and in vivo.
Cholesterol and other sterols from the host biosynthetic pathway are not required by S. enterica serovar Typhimurium during intracellular infection, as demonstrated by the experiments with TMD. Furthermore, cholesterol is recruited to the SCV in TMD-treated cells (5), suggesting that cholesterol delivered to the vacuole must come from other sources, such as the plasma membrane (where 90% of cholesterol resides) or low-density lipoprotein. The requirement for prelanosterol intermediates implicates other major branches of the host cholesterol biosynthetic pathway. One such branch involves the generation of isoprenoid compounds from farnesyl and geranyl pyrophosphate (Fig. 1A). S. enterica serovar Typhimurium has the genes for isoprenoid synthesis (via the 1-deoxy-d-xylulose 5-phosphate pathway), but it is unclear whether they and/or their products are exported or even expressed during intracellular growth. Host isoprenyl groups can be transferred to a variety of proteins that contain the appropriate carboxy-terminal CAAX motif, including Ras, Rho, Rac, and Rab proteins, which bind GTP and modulate a wide range of host signaling pathways (35, 36). Several of these proteins, including Cdc42, Rac, Rab5, and Rab7, are known to interact with Salmonella or Salmonella effectors during invasion and intracellular growth (14, 43). Modulation of small GTPases is a common strategy employed by a variety of bacterial pathogens, and statin inhibition of their prenylation may block critical host-pathogen interactions, thereby reducing infection.
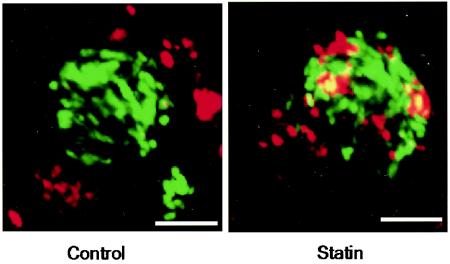
It was shown recently that a geranylgeranyltransferase inhibitor could reduce the disruption of polarized epithelial barriers caused by S. enterica serovar Typhimurium, thus decreasing SPI-1-dependent bacterial invasion in vitro (37). In contrast, the primary inhibitory effect which we observed with statins occurs late during intracellular growth and is prominent in macrophages, which engulf bacteria by an SPI-1-independent mechanism. We observed a high level of association between cathepsin D and the SCV in infected macrophages treated with lovastatin (Fig. 8), suggesting that the treatment may compromise the trafficking status of the vacuole. In addition, the antibacterial effects of statins may be linked to induction of apoptosis in infected cells, although the underlying mechanisms remain unknown. The finding that statin-induced cell death is independent of SPI-1 and SPI-2 suggests that it may be regulated by bacterial effectors from other pathogenicity islands or by the host cell itself. Finally, statins reduce bacterial growth with respect to spread to the liver and spleen in an acute mouse model of infection. This suggests that they have the capacity to act beyond the gut epithelium and influence systemic infection.
FIG. 8.
Lovastatin increases the association between the SCV and cathepsin D. Control macrophages or macrophages treated with lovastatin (30 μM) were infected with S. enterica serovar Typhimurium. At 13 h postinfection, cells were processed for immunofluorescence microscopy and stained with an antibody to cathepsin D. Representative cells are shown. Scale bars = 5 μm.
While the in vivo experiments with atorvastatin-treated mice validated the effects which we observed with cultured macrophages, they did not preclude the possibility that other mechanisms may also contribute to the protective effect of statins during bacterial infection. Recently, Youssef et al. demonstrated that treatment of mice with atorvastatin promoted a Th2 immune response, resulting in reversal of paralysis in a central nervous system autoimmune disease (42). This finding is particularly interesting considering that Salmonella initiates a Th1-like inflammatory response to cause disease in the mouse model. Salmonella infection of macrophages promotes the release of the inflammatory cytokines interleukin 1β (IL-1β) and IL-18, a caspase-1-dependent process that is underscored by the reduction in bacterial virulence in caspase-1-deficient mice (28). In addition, it has been shown that lovastatin blocks lipopolysaccharide-induced expression of tumor necrosis factor alpha, IL-1β, IL-6, and inducible nitric oxide synthase in rat primary macrophages (30). Thus, in vivo, statins may target several mechanisms to reduce bacterial growth.
Acknowledgments
We thank members of the Cianciotto and Haldar laboratories at Northwestern University for helpful discussions of this work; J. Lomasney for advice; and T. Akompong, E. Roark, T. Harrison, and B. Myles for help with mouse experiments.
This work was supported in part by National Institutes of Health predoctoral training grant 5T32GM08061 to D.M.C. and by grants from the National Institutes of Health to K.H. (grant AI054529) and Y.L. (grant HL28448).
Editor: J. T. Barbieri
REFERENCES
- 1.Bellosta, S., N. Ferri, L. Arnaboldi, F. Bernini, R. Paoletti, and A. Corsini. 2000. Pleiotropic effects of statins in atherosclerosis and diabetes. Diabetes Care 23(Suppl. 2):B72-B78. [PubMed] [Google Scholar]
- 2.Bernini, F., A. Poli, and R. Paoletti. 2001. Safety of HMG-CoA reductase inhibitors: focus on atorvastatin. Cardiovasc. Drugs Ther. 15:211-218. [DOI] [PubMed] [Google Scholar]
- 3.Beuzon, C. R., S. Meresse, K. E. Unsworth, J. Ruiz-Albert, S. Garvis, S. R. Waterman, T. A. Ryder, E. Boucrot, and D. W. Holden. 2000. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J. 19:3235-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler, T., and A. E. Girard. 1993. Comparative efficacies of azithromycin and ciprofloxacin against experimental Salmonella typhimurium infection in mice. J. Antimicrob. Chemother. 31:313-319. [DOI] [PubMed] [Google Scholar]
- 5.Catron, D. M., M. D. Sylvester, Y. Lange, M. Kadekoppala, B. D. Jones, D. M. Monack, S. Falkow, and K. Haldar. 2002. The Salmonella-containing vacuole is a major site of intracellular cholesterol accumulation and recruits the GPI-anchored protein CD55. Cell Microbiol. 4:315-328. [DOI] [PubMed] [Google Scholar]
- 6.Ciaravino, V., M. L. Kropko, C. E. Rothwell, C. A. Hovey, and J. C. Theiss. 1995. The genotoxicity profile of atorvastatin, a new drug in the treatment of hypercholesterolemia. Mutat. Res. 343:95-107. [DOI] [PubMed] [Google Scholar]
- 7.Cilla, D. D., Jr., L. R. Whitfield, D. M. Gibson, A. J. Sedman, and E. L. Posvar. 1996. Multiple-dose pharmacokinetics, pharmacodynamics, and safety of atorvastatin, an inhibitor of HMG-CoA reductase, in healthy subjects. Clin. Pharmacol. Ther. 60:687-695. [DOI] [PubMed] [Google Scholar]
- 8.Cirillo, D. M., R. H. Valdivia, D. M. Monack, and S. Falkow. 1998. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol. Microbiol. 30:175-188. [DOI] [PubMed] [Google Scholar]
- 9.Dostal, L. A., J. L. Schardein, and J. A. Anderson. 1994. Developmental toxicity of the HMG-CoA reductase inhibitor, atorvastatin, in rats and rabbits. Teratology 50:387-394. [DOI] [PubMed] [Google Scholar]
- 10.Dostal, L. A., L. R. Whitfield, and J. A. Anderson. 1996. Fertility and general reproduction studies in rats with the HMG-CoA reductase inhibitor, atorvastatin. Fundam. Appl. Toxicol. 32:285-292. [DOI] [PubMed] [Google Scholar]
- 11.Farina, H. G., D. R. Bublik, D. F. Alonso, and D. E. Gomez. 2002. Lovastatin alters cytoskeleton organization and inhibits experimental metastasis of mammary carcinoma cells. Clin. Exp. Metastasis. 19:551-559. [DOI] [PubMed] [Google Scholar]
- 12.Gallois, A., J. R. Klein, L. A. Allen, B. D. Jones, and W. M. Nauseef. 2001. Salmonella pathogenicity island 2-encoded type III secretion system mediates exclusion of NADPH oxidase assembly from the phagosomal membrane. J. Immunol. 166:5741-5748. [DOI] [PubMed] [Google Scholar]
- 13.Garner, M. J., R. D. Hayward, and V. Koronakis. 2002. The Salmonella pathogenicity island 1 secretion system directs cellular cholesterol redistribution during mammalian cell entry and intracellular trafficking. Cell Microbiol. 4:153-165. [DOI] [PubMed] [Google Scholar]
- 14.Hashim, S., K. Mukherjee, M. Raje, S. K. Basu, and A. Mukhopadhyay. 2000. Live Salmonella modulate expression of Rab proteins to persist in a specialized compartment and escape transport to lysosomes. J. Biol. Chem. 275:16281-16288. [DOI] [PubMed] [Google Scholar]
- 15.Hensel, M., J. E. Shea, C. Gleeson, M. D. Jones, E. Dalton, and D. W. Holden. 1995. Simultaneous identification of bacterial virulence genes by negative selection. Science 269:400-403. [DOI] [PubMed] [Google Scholar]
- 16.Hersh, D., D. M. Monack, M. R. Smith, N. Ghori, S. Falkow, and A. Zychlinsky. 1999. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. 96:2396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hess, D. C., A. M. Demchuk, L. M. Brass, and F. M. Yatsu. 2000. HMG-CoA reductase inhibitors (statins): a promising approach to stroke prevention. Neurology 54:790-796. [DOI] [PubMed] [Google Scholar]
- 18.Jones, B. D., and S. Falkow. 1994. Identification and characterization of a Salmonella typhimurium oxygen-regulated gene required for bacterial internalization. Infect. Immun. 62:3745-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones, B. D., N. Ghori, and S. Falkow. 1994. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J. Exp. Med. 180:15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keidar, S., M. Aviram, I. Maor, J. Oiknine, and J. G. Brook. 1994. Pravastatin inhibits cellular cholesterol synthesis and increases low density lipoprotein receptor activity in macrophages: in vitro and in vivo studies. Br. J. Clin. Pharmacol. 38:513-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelynack, K. J., T. D. Hewitson, M. Martic, S. McTaggart, and G. J. Becker. 2002. Lovastatin downregulates renal myofibroblast function in vitro. Nephron 91:701-707. (Discussion, 91:708-709.) [DOI] [PubMed]
- 22.Krause, B. R., and H. M. Princen. 1998. Lack of predictability of classical animal models for hypolipidemic activity: a good time for mice? Atherosclerosis 140:15-24. [DOI] [PubMed] [Google Scholar]
- 23.Laufs, U., M. Endres, F. Custodis, K. Gertz, G. Nickenig, J. K. Liao, and M. Bohm. 2000. Suppression of endothelial nitric oxide production after withdrawal of statin treatment is mediated by negative feedback regulation of rho GTPase gene transcription. Circulation 102:3104-3110. [DOI] [PubMed] [Google Scholar]
- 24.Liappis, A. P., V. L. Kan, C. G. Rochester, and G. L. Simon. 2001. The effect of statins on mortality in patients with bacteremia. Clin. Infect. Dis. 33:1352-1357. [DOI] [PubMed] [Google Scholar]
- 25.McClelland, M., K. E. Sanderson, J. Spieth, S. W. Clifton, P. Latreille, L. Courtney, S. Porwollik, J. Ali, M. Dante, F. Du, S. Hou, D. Layman, S. Leonard, C. Nguyen, K. Scott, A. Holmes, N. Grewal, E. Mulvaney, E. Ryan, H. Sun, L. Florea, W. Miller, T. Stoneking, M. Nhan, R. Waterston, and R. K. Wilson. 2001. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413:852-856. [DOI] [PubMed] [Google Scholar]
- 26.Medical Economics Co. 2000. Physicians' desk reference, 54th ed. Medical Economics Co., Montvale, N.J.
- 27.Monack, D. M., C. S. Detweiler, and S. Falkow. 2001. Salmonella pathogenicity island 2-dependent macrophage death is mediated in part by the host cysteine protease caspase-1. Cell Microbiol. 3:825-837. [DOI] [PubMed] [Google Scholar]
- 28.Monack, D. M., W. W. Navarre, and S. Falkow. 2001. Salmonella-induced macrophage death: the role of caspase-1 in death and inflammation. Microbes Infect. 3:1201-1212. [DOI] [PubMed] [Google Scholar]
- 29.Monack, D. M., B. Raupach, A. E. Hromockyj, and S. Falkow. 1996. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc. Natl. Acad. Sci. 93:9833-9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pahan, K., F. G. Sheikh, A. M. Namboodiri, and I. Singh. 1997. Lovastatin and phenylacetate inhibit the induction of nitric oxide synthase and cytokines in rat primary astrocytes, microglia, and macrophages. J. Clin. Investig. 100:2671-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panini, S. R., G. T. Everson, and T. A. Spencer. 1991. Effects of specific inhibition of sterol biosynthesis on the uptake and utilization of low density lipoprotein cholesterol by HepG2 cells. J. Lipid Res. 32:1657-1665. [PubMed] [Google Scholar]
- 32.Ruiz-Albert, J., X. J. Yu, C. R. Beuzon, A. N. Blakey, E. E. Galyov, and D. W. Holden. 2002. Complementary activities of SseJ and SifA regulate dynamics of the Salmonella typhimurium vacuolar membrane. Mol. Microbiol. 44:645-661. [DOI] [PubMed] [Google Scholar]
- 33.Sacks, F. M., M. A. Pfeffer, L. A. Moye, J. L. Rouleau, J. D. Rutherford, T. G. Cole, L. Brown, J. W. Warnica, J. M. Arnold, C. C. Wun, B. R. Davis, and E. Braunwald. 1996. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N. Engl. J. Med. 335:1001-1009. [DOI] [PubMed] [Google Scholar]
- 34.Salcedo, S. P., M. Noursadeghi, J. Cohen, and D. W. Holden. 2001. Intracellular replication of Salmonella typhimurium strains in specific subsets of splenic macrophages in vivo. Cell Microbiol. 3:587-597. [DOI] [PubMed] [Google Scholar]
- 35.Scita, G., P. Tenca, E. Frittoli, A. Tocchetti, M. Innocenti, G. Giardina, and P. P. Di Fiore. 2000. Signaling from Ras to Rac and beyond: not just a matter of GEFs. EMBO J. 19:2393-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Somsel Rodman, J., and A. Wandinger-Ness. 2000. Rab GTPases coordinate endocytosis. J. Cell Sci. 113:183-192. [DOI] [PubMed] [Google Scholar]
- 37.Tafazoli, F., K. E. Magnusson, and L. Zheng. 2003. Disruption of epithelial barrier integrity by Salmonella enterica serovar Typhimurium requires geranylgeranylated proteins. Infect. Immun. 71:872-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uchiya, K., M. A. Barbieri, K. Funato, A. H. Shah, P. D. Stahl, and E. A. Groisman. 1999. A Salmonella virulence protein that inhibits cellular trafficking. EMBO J. 18:3924-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valdivia, R. H., and S. Falkow. 1996. Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol. Microbiol. 22:367-378. [DOI] [PubMed] [Google Scholar]
- 40.Valdivia, R. H., and S. Falkow. 1997. Fluorescence-based isolation of bacterial genes expressed within host cells. Science 277:2007-2011. [DOI] [PubMed] [Google Scholar]
- 41.Vazquez-Torres, A., J. Jones-Carson, A. J. Baumler, S. Falkow, R. Valdivia, W. Brown, M. Le, R. Berggren, W. T. Parks, and F. C. Fang. 1999. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 401:804-808. [DOI] [PubMed] [Google Scholar]
- 42.Youssef, S., O. Stuve, J. C. Patarroyo, P. J. Ruiz, J. L. Radosevich, E. M. Hur, M. Bravo, D. J. Mitchell, R. A. Sobel, L. Steinman, and S. S. Zamvil. 2002. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature 420:78-84. [DOI] [PubMed] [Google Scholar]
- 43.Zhou, D., and J. Galan. 2001. Salmonella entry into host cells: the work in concert of type III secreted effector proteins. Microbes Infect. 3:1293-1298. [DOI] [PubMed] [Google Scholar]
- 44.Zhou, D., M. S. Mooseker, and J. E. Galan. 1999. Role of the S. typhimurium actin-binding protein SipA in bacterial internalization. Science 283:2092-2095. [DOI] [PubMed] [Google Scholar]