Abstract
Mice immunized with irradiated Onchocerca volvulus third-stage larvae developed protective immunity. Eosinophil levels were elevated in the parasite microenvironment at the time of larval killing, and measurements of total serum antibody levels revealed an increase in the immunoglobulin E (IgE) level in immunized mice. The goal of the present study was to identify the role of granulocytes and antibodies in the protective immune response to the larval stages of O. volvulus in mice immunized with irradiated larvae. Immunity did not develop in mice if granulocytes, including both neutrophils and eosinophils, were eliminated, nor did it develop if only eosinophils were eliminated. Moreover, larvae were killed in naïve interleukin-5 transgenic mice, and the killing coincided with an increase in the number of eosinophils and the eosinophil peroxidase (EPO) level in the animals. To determine if EPO was required for protective immunity, mice that were genetically deficient in EPO were immunized, and there were no differences in the rates of parasite recovery in EPO-deficient mice and wild-type mice. Two mouse strains were used to study B-cell function; μMT mice lacked all mature B cells, and Xid mice had deficiencies in the B-1 cell population. Immunity did not develop in the μMT mice but did develop in the Xid mice. Finally, protective immunity was abolished in mice treated to eliminate IgE from the blood. We therefore concluded that IgE and eosinophils are required for adaptive protective immunity to larval O. volvulus in mice.
Infection of humans with the filarial worm Onchocerca volvulus results in a spectrum of disease states ranging from mild to hyperreactive disease. In addition, there are individuals who are considered immunologically resistant to the infection, based on the fact that they live in an endemic area and are free of infection and disease. Individual immune responses appear to be responsible for the different disease states, and roles have been attributed to Th1 cells, Th2 cells, antibodies, and granulocytes in the different disease presentations (24). Specific mechanisms of protective immunity have been identified in vitro with human cells and serum, and it has been shown that eosinophils and neutrophils adhere to and kill larval O. volvulus in the presence of serum or complement (9, 27, 41).
O. volvulus has a very limited host range, infecting only humans and chimpanzees; therefore, other animal models have been utilized to study immunity to this infection. It has been observed that cattle immunized with irradiated Onchocerca ochengi third-stage larvae (L3) were protected against challenge infection, based on greatly reduced burdens of adult worms in the immunized animals (1). In order to generate a more practical way to study immunity to the larval stages of O. volvulus, a mouse model was developed. L3 were implanted subcutaneously in diffusion chambers to allow efficient recovery of parasites and to permit analysis of the parasite's microenvironment. Larvae survived and grew at the same rate when they were implanted in naïve susceptible chimpanzees and in normally resistant mice. It was clear, however, that the parasite survival rates in both hosts decreased over time, suggesting that protective immune responses were capable of eliminating a portion of the parasite population (37). Mice were immunized with irradiated O. volvulus L3 and then received challenge infections of L3 in diffusion chambers. Protective immunity developed, which required direct contact between host cells and the parasites for killing of larvae. The only cell type whose levels increased in diffusion chambers in immunized mice was eosinophils, and the maximal levels of eosinophils coincided with the time of parasite killing (38). The observation that the number of eosinophils increased in immunized animals suggested that immunity was dependent on a Th2 response. This hypothesis was confirmed in studies in which interleukin-5 (IL-5) or IL-4 was eliminated by monoclonal antibody (MAb) treatment (38) and in studies in which cytokine-deficient mice were used (28). Additionally, the finding that immunity was dependent on IL-4 suggested that the protective immune response depended on the antibody isotype immunoglobulin G1 (IgG1) or IgE. Measurement of total serum antibody levels and identification of specific antibody responses to surface antigens, internal antigens, and soluble antigens in Western blots revealed responses by IgM, IgE, and all the subclasses of IgG. However, the complex pattern of recognition of parasite antigens by antibodies found in immunized mice made it difficult to discern the protective antibody isotypes and their antigenic targets (58).
The goal of the present study was to identify the immune components required for the protective immune response to the larval stages of O. volvulus in mice immunized with irradiated L3. Specifically, the roles of granulocytes and antibodies were assessed by using either MAb to deplete the immune function or mice genetically deficient in the specific immune function. The following approaches were used. (i) Granulocytes, including both neutrophils and eosinophils, were eliminated by using MAb RB6-8C5, which recognizes a surface marker on mature murine granulocytes (23). In vivo treatment of mice with this MAb severely depresses blood and spleen granulocyte counts for up to 5 days (26). (ii) Eosinophils were eliminated in vivo with MAb 6S2-19-4 directed at the mouse eotaxin receptor CCR3, which has been shown to be effective at eliminating eosinophils in the blood and in tissues (11, 16). (iii) Mouse strains with genetically altered IL-5 expression were used to study the effect of having a severely deficient eosinophil response to the parasite, such as that seen in IL-5 knockout (KO) mice (34), compared to a massive eosinophil response, such as that seen in IL-5 transgenic (TG) mice (34). (iv) The role of eosinophil peroxidase (EPO) in larval killing was studied by utilizing EPO KO mice (11). (v) Two mouse strains were used to study B-cell function; μMT mice lacked all mature B cells in the lymphocyte pool (33), and Xid mice had deficiencies in the B-1 cell population (29, 30). (vi) MAb EM-95, which is directed at the Fcɛ portion of the murine H chain, was used to eliminate IgE from the blood. A single treatment with this MAb was shown to deplete serum and cell-bound IgE for several days (5, 54). Collectively, these experiments identified some of the essential components required for the mouse immune response to kill challenge larvae after immunization with irradiated L3.
MATERIALS AND METHODS
Source of parasites and mice.
Cryopreserved L3 were prepared in Cameroon by the following method. Black flies (Simulium damnosum) were fed on consenting donors infected with O. volvulus, and after 7 days the developed L3 were collected from dissected flies, cleaned, and cryopreserved in dimethyl sulfoxide and sucrose by using Biocool II computerized freezing equipment (FTS Systems Inc., Stone Ridge, N.Y.) as previously described (13). Cryopreserved L3 were thawed by placing tubes containing the L3 on dry ice for 15 min, followed by immersion in a 37°C water bath. The L3 were then washed in a 1:1 mixture of Iscove's modified Dulbecco's medium and NCTC-135 with 100 U of penicillin per ml, 100 μg of streptomycin per ml, 100 μg of gentamicin per ml, and 30 μg of chloramphenicol (Sigma Chemical Co., St. Louis, Mo.) per ml.
Male BALB/cByJ, C57BL/6J, CBA/J, 129/SvJ, Igh-6(−/−) (μMT), and CBA/CaN-Btk xid/J (Xid) mice that were 6 to 8 weeks old were obtained from Jackson Laboratory (Bar Harbor, Maine). The NJ.1638 IL-5 TG mouse line (39) and EPO KO mice (11) were obtained from our original animal stocks. IL-5 KO mice were a generous gift from Manfred Kopf of the Basel Institute for Immunology and have been described elsewhere (34). IL-5 TG, IL-5 KO, and EPO KO mice were bred in the Laboratory of Animal Sciences facility at Thomas Jefferson University. All mice were housed in Micro-Isolator boxes (Lab Products Inc., Maywood, N.J.) and were fed Autoclaveable Laboratory Rodent Chow (Ralston Purina, St. Louis, Mo.) ad libitum. The animal room was temperature, humidity, and light cycle controlled.
Immunization and challenge protocol.
Mice were immunized by two subcutaneous injections (in the nape of the neck) of O. volvulus L3 irradiated with 35 krads by using a cesium source. The primary immunization dose consisted of 50 irradiated L3, and this was followed 2 weeks later by a booster immunization consisting of 25 irradiated L3. Mice received challenge infections 1 week after the booster immunization.
Diffusion chambers were constructed from 14-mm-diameter Lucite rings and were covered with 5.0-μm-pore-size hydrophilic Durapore membranes (Millipore, Bedford, Mass.) as previously described (37). Twenty-five O. volvulus L3 in Iscove's modified Dulbecco's medium-NCTC-135 with antibiotics were inserted into each diffusion chamber prior to implantation in mice. The animals were anesthetized with inhaled isoflurane, and a subcutaneous pocket was formed in the rear flank of each mouse, into which a single diffusion chamber was inserted.
The diffusion chambers were recovered 21 days after challenge. At the time of diffusion chamber recovery, mice were anesthetized with ketamine (Fort Dodge Animal Health, Fort Dodge, Iowa) and acepromazine (Phoenix Pharmaceuticals, St. Joseph, Mo.) and then killed by exsanguination, and the serum was collected. Diffusion chamber contents were analyzed to assess larval survival and the nature of the cellular infiltration into the diffusion chamber. Larvae recovered from diffusion chambers were considered live if they exhibited motility. Cells found within the diffusion chambers were collected by centrifugation on slides with a Cytospin 3 (Shandon Inc., Pittsburgh, Pa.) and then were stained for differential counting with HEMA 3 (Fischer Diagnostics, Middletown, Va.).
MAbs
Granulocytes, including both neutrophils and eosinophils, were eliminated by using MAb RB6-8C5 (23) (cell lines contributed by R. Coffman, DNAX Corp., Palo Alto, Calif.). This MAb was injected by using the following protocol: 200 μg intraperitoneally on the day of challenge and 200 μg intraperitoneally on day 3 after challenge. Eosinophils were eliminated in vivo with MAb 6S2-19-4 (cell lines contributed by R. Coffman). This MAb was injected by using the following protocol: 150 μg intraperitoneally on days 2 and 4 after booster immunization, 50 μg subcutaneously on the day of challenge, and 150 μg intraperitoneally on days 3 and 5 after challenge. MAb EM-95 (cell lines contributed by Zelig Eshar, Rehoveth, Israel) was used to eliminate IgE from the blood (5). This MAb was injected by using the following protocol: 1 mg intraperitoneally 1 day prior to challenge and on the day of the challenge infection.
Measurement of EPO levels.
The method of Strath et al. (55), as modified by White et al. (57), was used for the EPO assay. Briefly, 50 μl of diffusion chamber fluid was combined with 75 μl of a 16 mM o-phenylenediamine solution in 100 mm Tris-HCl (pH 8.0) containing 0.1% Triton X-100 and 0.01% hydrogen peroxide. Polystyrene 96-well microtiter plates containing the reaction mixtures were kept at 37°C until a color change was detected, and absorbance at 492 nm was measured with a Dynatech MR 5000 microtiter plate reader (Dynatech Laboratories Inc., Chantilly, Va.). Horseradish peroxidase (Sigma Chemical Co.) was used as a standard.
Enzyme-linked immunosorbent assay for total serum IgE.
A standard enzyme-linked immunosorbent assay was used to determine the total serum IgE levels. Monoclonal rat anti-mouse IgE was used as the capture antibody, and biotinylated rat anti-mouse IgE (Pharmingen, San Diego, Calif.) was used as the secondary antibody. Biotin on the secondary antibody was then reacted with avidin-peroxidase (Sigma Chemical Co.). 2,2′-Azinobis(3-ethylbenzthiazolinesulfonic acid (ABTS) peroxidase substrate (one component; Kirkgaard and Perry Laboratories, Inc., Gaithersburg, Md.) was used as the substrate, and the reaction results were read at a wavelength of 405 nm. The amounts of IgE in the serum samples were calculated based on a series of IgE isotype standards (Pharmingen) run on the same plates.
Statistical analyses.
Data were analyzed by performing an MGLH multifactorial analysis of variance with Systat 5.2 (Systat, Inc., Evanston, Ill.). Probability values of <0.05 were considered significant. All experiments were performed a minimum of two times with five or six animals per group. The data from all of the repeated experiments are presented below.
RESULTS
Role of granulocytes in protective immunity.
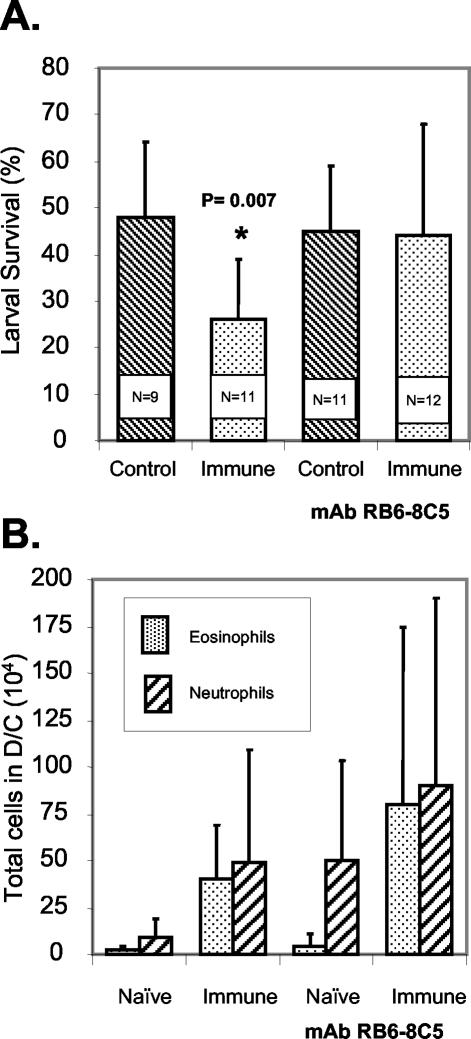
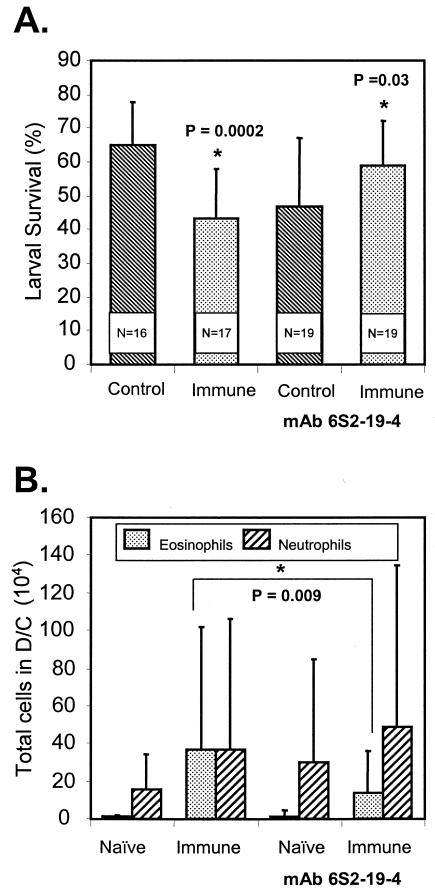
BALB/cByJ mice were immunized with irradiated O. volvulus L3, and on the day of the challenge infection and 3 days later they were treated with MAb RB6-8C5 to eliminate granulocytes. The protocol for treatment with the MAb was designed to ensure elimination of the cells during the first week of the challenge infection. Larvae were implanted for 3 weeks to allow adequate time for potentially slow killing processes to reach completion. Statistically significant levels of protective immunity developed in immunized untreated mice, whereas in mice treated to eliminate granulocytes there was no protective immunity (Fig. 1A). There was no statistically significant difference between the total numbers of cells found in the diffusion chambers recovered from control and immunized mice regardless of whether they received treatment with the MAb. There was, however, a statistically significant increase in the number of cells found in diffusion chambers recovered from immunized mice that received the MAb treatment (2.6 × 106 ± 2.6 × 106 cells) compared to the number of cells found in diffusion chambers recovered from immunized mice that did not receive the treatment (1.2 × 106 ± 1.0 × 106 cells; P = 0.043). No decrease was observed in the number of eosinophils and neutrophils found in the diffusion chambers recovered from immunized animals at the time of parasite recovery after they were treated with MAb RB6-8C5 18 days previously (Fig. 1B). Immunized mice were next treated with MAb 6S2-19-4 to determine the role of eosinophils in killing of the larvae. Mice were treated with the antibody twice before challenge, on the day of challenge, and on days 3 and 5 after challenge. Treatment to specifically eliminate eosinophils resulted in blocking of protective immunity. Furthermore, it was observed that the level of parasite survival in immunized mice treated with the MAb was statistically higher than the level of parasite survival in the treated naïve mice (Fig. 2A). There was no effect on the total number of cells migrating into the diffusion chambers in the control and immunized mice based on whether they received MAb treatment. There was a statistically significant reduction in the number of eosinophils migrating into the diffusion chambers of immunized mice treated with MAb 6S2-19-4, whereas there was no effect on the recruitment of neutrophils (Fig. 2B).
FIG. 1.
(A) Effect of granulocyte depletion by MAb RB6-8C5 on the ability of immunized BALB/cByJ mice to kill larvae in a challenge infection. The data are means and standard deviations. N is the number of animals per group. An asterisk indicates that there is a statistically significant difference between the survival of larvae in naïve mice and the survival of larvae in immunized mice. (B) Effect of granulocyte depletion by MAb RB6-8C5 on the ability of eosinophils and neutrophils to migrate into diffusion chambers (D/C) implanted in immunized BALB/cByJ mice. The data are means and standard deviations.
FIG. 2.
(A) Effect of eosinophil depletion by MAb 6S2-19-4 on the ability of immunized BALB/cByJ mice to kill larvae in a challenge infection. The data are means and standard deviations. N is the number of animals per group. An asterisk indicates that there is a statistically significant difference between the survival of larvae in naïve mice and the survival of larvae in immunized mice. (B) Effect of eosinophil depletion by MAb 6S2-19-4 on the ability of eosinophils and neutrophils to migrate into diffusion chambers (D/C) implanted in immunized BALB/cByJ mice. The data are means and standard deviations. An asterisk indicates that there is a statistically significant difference between the eosinophil levels in treated mice and the eosinophil levels in untreated immunized mice.
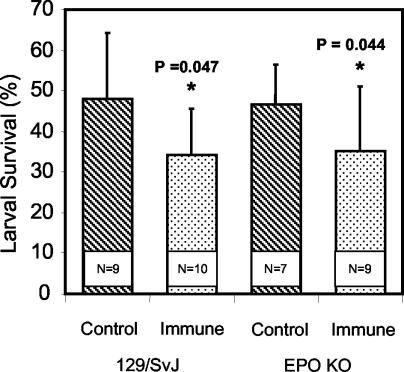
Naïve IL-5 TG and IL-5 KO mice were infected with O. volvulus L3 for 1 or 3 weeks to determine the effect of eosinophils on the survival of the parasites in the absence of a specific immune response. There was a significant decrease in parasite survival after 1 week in the IL-5 TG mice compared to the survival in the naïve C57BL/6J wild-type mice and the IL-5 KO mice, and the results after 3 weeks were essentially identical. Small numbers of eosinophils migrated into the diffusion chambers implanted in the naïve C57BL/6J wild-type and IL-5 KO mice, and there were low levels of EPO in the diffusion chamber fluids recovered from these mice. This was in marked contrast to the high numbers of eosinophils that migrated into the diffusion chambers and the elevated EPO levels in the fluid of diffusion chambers implanted in the IL-5 TG mice (Table 1). To determine if EPO was required for innate or adaptive immunity, naïve and immunized EPO KO mice received implanted diffusion chambers containing L3. There was no difference between the recovery rates after 3 weeks for the naïve wild-type 129/SvJ mice and the recovery rates for the naïve EPO KO mice, thus showing that innate antilarval immunity was not affected. Furthermore, equal levels of adaptive antilarval protective immunity developed in the immunized wild-type and EPO KO mice (Fig. 3).
TABLE 1.
Innate immune responses to larval O. volvulus at 1 and 3 weeks postinfection in C57BL/6J wild-type mice, IL-5 KO mice, and IL-5 TG micea
| Time postinfection (wk) | Mice | % L3 survival | % of eosinophils | No. of eosinophils (104) | EPO activity (mU/ml) |
|---|---|---|---|---|---|
| 1 | Wild type | 65 ± 8 | 4 ± 3 | 0.4 ± 0.3 | 3.6 ± 1.9 |
| IL-5 KO | 56 ± 8 | 2 ± 2 | 0.1 ± 0.1 | 2.2 ± 0.9 | |
| IL-5 TG | 35 ± 3* | 60 ± 27* | 110 ± 87* | 51 ± 43* | |
| 3 | Wild type | 62 ± 21 | 2 ± 2 | 0.03 ± 0.03 | ND |
| IL-5 KO | 63 ± 14 | 2 ± 2 | 0.01 ± 0.02 | ND | |
| IL-5 TG | 34 ± 9* | 34 ± 11* | 34 ± 32* | 5.1 ± 1.4* |
Larval survival, the percentages of the cells in the diffusion chambers that were eosinophils, the number of eosinophils found per diffusion chamber, and the levels of EPO found in the diffusion chamber fluid were determined. ND, not detectable. The data are means ± standard deviations for five mice per group from a single experiment. Duplicate experiments yielded equivalent results. *, statistically significant difference between results obtained for the IL-5 TG mice compared to those for the wild-type and IL-5 KO mice.
FIG. 3.
Effect of immunization of EPO KO mice and control 129/SvJ mice on the ability of the mice to kill larvae in a challenge infection. N is the number of animals per group. An asterisk indicates that there is a statistically significant difference between the survival of larvae in naïve mice and the survival of larvae in immunized mice.
Role of B cells in protective immunity.
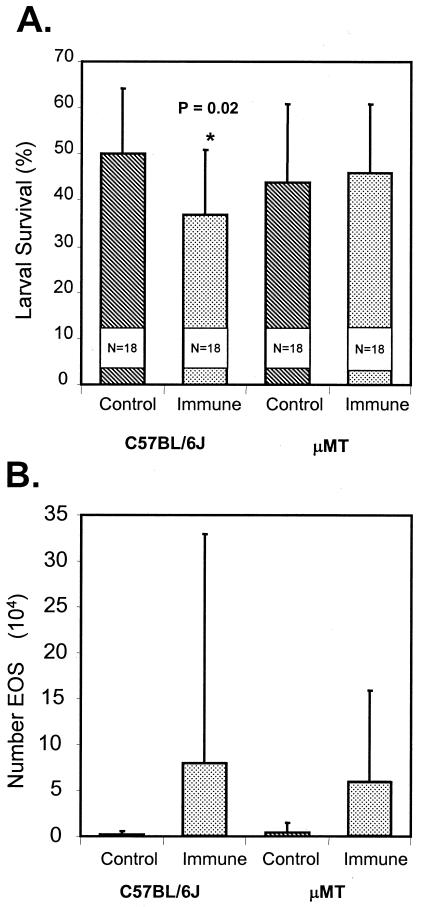
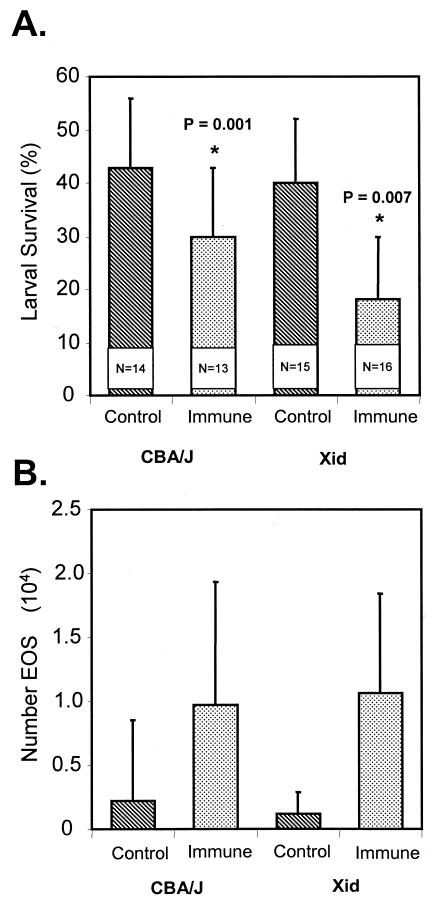
μMT mice, which are deficient in all B cells, were immunized with irradiated L3 and then received challenge infections. Immunity developed in the wild-type C57/BL6J mice but not in the B-cell-deficient mice (Fig. 4A), even though they had equal numbers of infiltrating eosinophils (Fig. 4B). Xid mice, which are deficient in B-1 cells, were immunized with irradiated L3 and then received challenge infections. Immunity developed in the B-1-cell-deficient mice as it did in the control CBA/J mice (Fig. 5A). The eosinophil levels in the immunized Xid mice were elevated and were equal to the levels in the immunized wild-type counterparts (Fig. 5B).
FIG. 4.
(A) Effect of immunization of B-cell-deficient μMT mice and control C57BL/6J mice on the ability of the mice to kill larvae in a challenge infection. The data are means and standard deviations. N is the number of animals per group. An asterisk indicates that there is a statistically significant difference between the survival of larvae in naïve mice and the survival of larvae in immunized mice. (B) Eosinophil (EOS) migration into diffusion chambers implanted in immunized B-cell-deficient μMT mice and immunized control C57BL/6J mice. The data are means and standard deviations.
FIG. 5.
(A) Effect of immunization of B-1-cell deficient Xid mice and control CBA/J mice on the ability of the mice to kill larvae in a challenge infection. The data are means and standard deviations. N is the number of animals per group. An asterisk indicates that there is a statistically significant difference between the survival of larvae in naïve mice and the survival of larvae in immunized mice. (B) Eosinophil (EOS) migration into diffusion chambers implanted in immunized B-1-cell-deficient Xid mice and immunized control CBA/J mice. The data are means and standard deviations.
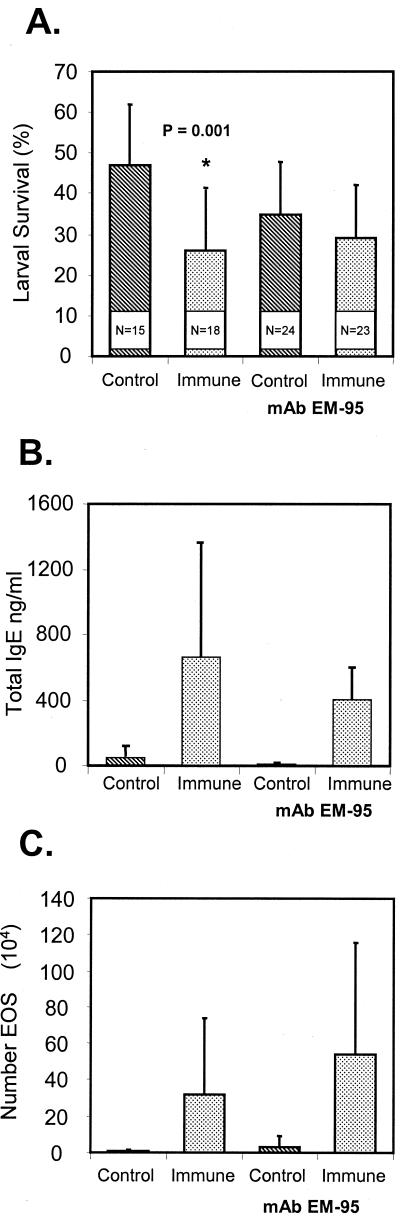
BALB/cByJ mice were treated with MAb EM-95 to eliminate IgE 1 day before challenge and on the day of challenge. Protective immunity was abolished in mice that were treated to eliminate IgE (Fig. 6A). The total IgE serum levels measured at the time of larval recovery were reduced in mice treated with EM-95, but the reductions were not statistically significant (Fig. 6B). The eosinophil levels were the same in the two groups of immunized mice (Fig. 6C).
FIG. 6.
(A) Effect of IgE depletion by MAbEM-95 on the ability of immunized BALB/cByJ mice to kill larvae in a challenge infection. The data are means and standard deviations. N is thenumber of animals per group. An asterisk indicates that there is a statistically significant difference between the survival of larvae in naïve mice and the survival of larvae in immunized mice.(B) Total serum IgE levels in immunized BALB/cByJ mice depleted of IgE by MAbEM-95. The data are means and standard deviations.(C) Effect of IgE depletion by MAbEM-95 on the ability of eosinophils (EOS) to migrate into diffusion chambers implanted in immunized BALB/cByJ mice. The data are means and standard deviations.
DISCUSSION
Immunization of mice with irradiated L3 resulted in development of protective immunity to larval O. volvulus. Four different strains of wild-type mice (BALB/cByJ, C57BL/6J, CBA/J, and 129/SvJ) were used in this study, and the immune response eliminated statistically significant numbers of the challenge larvae regardless of the strain of mouse. Immunity, however, has been shown to be dependent on the host mouse strain for the nematodes Trichuris muris (12), Litmosoides sigmodontis (25), and Brugia malayi (50). These observations suggest that O. volvulus, which does not develop normally in mice, might be an easier and more consistent target for the immune response to attack because of the absence of any evolved escape mechanisms devised specifically for murine hosts.
Granulocytes, particularly eosinophils, were found to be required for the protective immune response. The basis for this conclusion was that elimination of granulocytes by MAb treatment blocked protective immunity. The association between helminth infections and eosinophils has been widely recognized; however, the function of the cells in immunity remains controversial (6, 47). The results of the present study demonstrate that blocking eosinophil recruitment in vivo blocked protective immunity. Furthermore, treatment of mice with an MAb against CCR3, the eotaxin receptor, not only eliminated protective immunity but actually significantly increased the survival of the challenge parasites in immunized mice compared to the survival of the parasites in naïve mice that received the same MAb treatment. A similar observation was made when resistance to Trichinella spiralis in CCR3 KO mice was examined; in this case there was an absence of eosinophil recruitment in the mice and there was a concomitant increase in survival of the larval parasite (18). These two models thus confirm the observations that eosinophils are effective at killing nematode larvae and that blocking the recruitment of eosinophils enhances parasite survival.
Further confirmation of the ability of eosinophils to kill O. volvulus L3 came from studies in which L3 were implanted in naïve IL-5 KO and IL-5 TG mice for 1 or 3 weeks. It was observed that there was a significant reduction in parasite survival when the larvae were implanted in IL-5 TG mice for 1 week and that the level of survival did not decrease at 3 weeks after implantation. The level of protection seen in the naïve IL-5 TG mice was comparable to the level seen in the immunized wild-type mice, while the larvae survived at the same rate in the naïve IL-5 KO and wild-type mice. When the diffusion chamber contents were studied to determine some of the factors found in the parasite's microenvironment, it was observed that there was an influx of eosinophils into diffusion chambers implanted in IL-5 TG mice. This was observed after 1 week and to a lesser degree after 3 weeks. There were no significant differences in the eosinophil recruitment rates in the IL-5 KO mice and the wild-type mice. EPO was measured in the diffusion chamber fluid, and elevated levels coincided with elevated numbers of eosinophils present. This finding suggested that the eosinophils degranulated and that EPO might be the toxic agent released from eosinophils responsible for killing the larvae. Eosinophil degranulation has been observed in mice immunized against L. sigmodontis (45) and in jirds immunized against Acanthocheilonema viteae (7). It was determined in those studies that the larvae were not killed even if eosinophils were present, if the eosinophils did not degranulate (45). In the present study, correlations were found between the elevated numbers of eosinophils and the EPO levels found in the diffusion chambers in IL-5 TG mice and the decrease in parasite survival observed in these mice. The eosinophils apparently degranulated, and the results suggested that EPO might be associated with larval killing. The results of this study show that degranulation may be required for immunity to O. volvulus in mice; however, it was clear from the EPO KO mouse studies that EPO is not required for killing to occur in both the innate immune response and the adaptive immune response. Immunization of EPO KO mice revealed that the levels of immunity that developed in these mice were comparable to the levels that developed in immunized wild-type mice. It is possible that one of the other cationic proteins, such as the major basic protein found in the eosinophil secondary granule, or a combination of two or more granule proteins may be required for effective killing of the larvae. Alternatively, it is possible that EPO may be required to control the infection in strains of mice other than 129/SvJ, as it has been shown that inbred strains of mice have different susceptibilities to infection with filarial worms (17).
Several nematode parasites have been shown to have decreased survival rates in IL-5 TG mice. These include L. sigmodontis (44), Nippostrongylus brasiliensis (10), Strongyloides venezuelensis (13), and Strongyloides stercoralis (21). Interestingly, there was no difference between the survival of O. volvulus L3 in IL-5 KO mice and the survival of O. volvulus L3 in wild-type mice, which has also been reported for L. sigmodontis (40). This is in contrast to S. stercoralis, whose survival was enhanced in IL-5 KO mice compared to wild-type mice. In the case of S. stercoralis there was active and efficient recruitment of eosinophils to the larvae in wild-type mice that resulted in killing of the larvae, a phenomenon that did not occur in the IL-5 KO mice (21). Larval O. volvulus is susceptible to killing by eosinophils in the innate immune response, as seen in the IL-5 TG mice. However, the larvae do not induce the recruitment of large numbers of eosinophils in naïve wild-type mice, which could explain why these parasites survive for long periods of time in mice compared to the survival of the larvae of S. stercoralis.
The role of antibody in the protective immune response was initially investigated by using mice deficient in B cells. μMT mice lack all mature B cells (33), which leaves T-cell responses against a variety of antigens unaltered (14). Xid mice lack B-1 cells, which are characterized by their primary localization within the peritoneal cavity and their production of natural antibodies of the isotypes IgM, IgA, and IgG that bind to a variety of self and foreign antigens (20). B-1 cells also release IL-10, which promotes Th2 responses and suppresses Th1 responses (15). It was observed that immunity did not develop in μMT mice lacking B cells but did develop in Xid mice which were missing B-1 cells. Similar to the results of this study, μMT mice were shown to be more permissive to primary infections with B. malayi (4) and to suppress the development of adaptive immunity to L. sigmodontis (45). Furthermore, B cells were required for clearance of both primary and challenge infections with Brugia pahangi (49). In comparison to the results of the present study, B-1 cells were shown to be required for immunity to B. malayi (48), L. sigmodontis (2), and S. stercoralis (22), thereby confirming that different protective mechanisms operate in these different nematode infections in mice. The observation that B-2 cells were required in immunity to larval O. volvulus suggested that immunity might be dependent on an antibody response. It was observed in previous studies of antibody responses in mice immunized against infection with O. volvulus that IgE was the only antibody isotype for which the total amount of antibody increased in the serum of the immunized mice (58). MAb EM-95 was used to eliminate IgE from the blood of immunized mice (5, 54), and it was observed that protective immunity to larval O. volvulus was blocked in these animals. The level of recovery of parasites from the control mice treated with the anti-IgE MAb was lower than that from the untreated controls; the difference was not statistically significant and may have been the result of the reported mild anaphylaxis caused by the MAb in the control animals (54). Treatment of immunized mice with the MAb did not result in a reduction in the level of larval survival compared to that in the treated controls. Therefore, IgE was required for protective immunity to O. volvulus larvae in mice. Similar results were obtained with IgE KO mice, in which the levels of survival after primary infections with B. malayi (53) and Schistosoma mansoni (31) were increased in mice deficient in IgE. Furthermore, elevated IgE responses against recombinant B. malayi γ-glutamyl transpeptidase were associated with resistance to infection with lymphatic filariasis in patients (42). This is in contrast to several studies performed with the parasites N. brasiliensis (43), S. mansoni (3), Paragonimus westermani (52), and Strongyloides ratti (35), in which it was shown that elimination of IgE by MAb treatment either had no effect or was beneficial for survival of the parasites.
It appears from the present study that larval killing in immunized mice occurred within the first 7 days after implantation of the diffusion chambers containing the challenge larvae. This conclusion is based on two sets of data. The first set of data comes from the experiments in which MAbs were used to eliminate granulocytes, eosinophils, and IgE. The three MAbs were administered only at the time of the challenge infection, and all three MAbs had limited times of efficacy. This was confirmed by the fact that at the time of parasite recovery the levels of granulocytes, eosinophils, and IgE had all essentially returned to normal. The fact that the three MAbs had an effect on blocking protective immunity and the fact that they were functional only during the early part of the immune response suggest that the larvae which were susceptible to the killing process were killed within the first few days. The immune response rebounded after the MAbs had dissipated, as shown by the elevated cell and IgE levels, yet no additional larvae were killed. The second set of data supporting the conclusion that larval killing occurred within the first 7 days came from the innate killing reactions seen in the IL-5 TG mice. The larvae were killed within the first 7 days, and no additional killing was observed at 3 weeks. The fact that larvae were killed within 7 days was previously observed in mice immunized against O. volvulus (38). In addition, it was reported that human eosinophils bind to the surface of the L3 but not to the fourth-stage larvae in vitro (9, 56). The observations with human eosinophils support the concept that the larvae are killed by eosinophils early in larval development; the larvae that survive develop into a stage that is resistant to killing by eosinophils.
In conclusion, this study provides evidence that immunity to larval O. volvulus in mice is dependent on both eosinophils and IgE. However, the mechanism for how these two immune components cooperate to kill the larvae is unclear. Mouse eosinophils lack IgE receptors, so therefore a classic antibody-dependent cellular reaction in which the effector cell binds to the antibody on the surface of the target apparently cannot occur (32). In place of antibody, other ligands have been proposed for eosinophil binding to the targets. Eosinophil binding to the larvae of N. brasiliensis was blocked with MAb to CD11b or VLA-4, suggesting that the binding was dependent on their ligands complement and fibronectin (51). Fibronectin has also been found on the surface of O. volvulus L3, which was shown to interact with activated human eosinophils (8). In studies with A. viteae it was shown that parasite killing was independent of antibody but dependent on eosinophils. If mast cells were reduced, there was a decrease in killing of the larvae by the eosinophils (19). In nodules from patients containing adult and microfilarial stages of O. volvulus, hyperreactivity was associated with mast cells carrying IgE and the recruitment of eosinophils into the tissues (36). Therefore, a possible mechanism used by IgE and eosinophils to kill O. volvulus L3 in mice may involve parasite-specific IgE interacting with mast cells, resulting in the release from the mast cells of inflammatory mediators capable of recruiting eosinophils (46). The recruited eosinophils accumulate around L3 and bind to the parasite through a receptor, such as complement. The eosinophils then degranulate, as they apparently do in IL-5 TG mice, and the released products kill the L3. Specificity in this proposed mechanism comes from IgE, while actual killing is eosinophil mediated and antibody independent.
Acknowledgments
We acknowledge support from NIH grants AI 47189 and AI 42328 and from The Edna McConnel Clark and Mayo Foundations.
We also thank Jessica Ligas and Shalom Leon for expert and enthusiastic technical assistance and Juergen Landmann for editorial assistance.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Abraham, D., R. Lucius, and A. J. Trees. 2002. Immunity to Onchocerca spp. in animal hosts. Trends Parasitol. 18:164-171. [DOI] [PubMed] [Google Scholar]
- 2.Al-Qaoud, K. M., A. Taubert, H. Zahner, B. Fleischer, and A. Hoerauf. 1997. Infection of BALB/c mice with the filarial nematode Litomosoides sigmodontis: role of CD4+ T cells in controlling larval development. Infect. Immun. 65:2457-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amiri, P., M. Haak-Frendscho, K. Robbins, J. H. McKerrow, T. Stewart, and P. Jardieu. 1994. Anti-immunoglobulin E treatment decreases worm burden and egg production in Schistosoma mansoni-infected normal and interferon gamma knockout mice. J. Exp. Med. 180:43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babu, S., L. D. Shultz, T. R. Klei, and T. V. Rajan. 1999. Immunity in experimental murine filariasis: roles of T and B cells revisited. Infect. Immun. 67:3166-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baniyash, M., and Z. Eshhar. 1984. Inhibition of IgE binding to mast cells and basophils by monoclonal antibodies to murine IgE. Eur. J. Immunol. 14:799-807. [DOI] [PubMed] [Google Scholar]
- 6.Behm, C. A., and K. S. Ovington. 2000. The role of eosinophils in parasitic helminth infections: insights from genetically modified mice. Parasitol. Today 16:202-209. [DOI] [PubMed] [Google Scholar]
- 7.Bleiss, W., U. Oberlander, S. Hartmann, R. Adam, A. Marko, A. Schonemeyer, and R. Lucius. 2002. Protective immunity induced by irradiated third-stage larvae of the filaria Acanthocheilonema viteae is directed against challenge third-stage larvae before molting. J. Parasitol. 88:264-270. [DOI] [PubMed] [Google Scholar]
- 8.Brattig, N. W., A. Z. Abakar, F. Geisinger, and T. F. Kruppa. 1995. Cell-adhesion molecules expressed by activated eosinophils in Onchocerca volvulus infection. Parasitol. Res. 81:398-402. [DOI] [PubMed] [Google Scholar]
- 9.Brattig, N. W., F. W. Tischendorf, G. Strote, and C. E. Medina-de la Garza. 1991. Eosinophil-larval interaction in onchocerciasis: heterogeneity of in vitro adherence of eosinophils to infective third and fourth stage larvae and microfilariae of Onchocerca volvulus. Parasite Immunol. 13:13-22. [DOI] [PubMed] [Google Scholar]
- 10.Daly, C. M., G. Mayrhofer, and L. A. Dent. 1999. Trapping and immobilization of Nippostrongylus brasiliensis larvae at the site of inoculation in primary infections of interleukin-5 transgenic mice. Infect. Immun. 67:5315-5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denzler, K. L., M. T. Borchers, J. R. Crosby, G. Cieslewicz, E. M. Hines, J. P. Justice, S. A. Cormier, K. A. Lindenberger, W. Song, W. Wu, S. L. Hazen, G. J. Gleich, J. J. Lee, and N. A. Lee. 2001. Extensive eosinophil degranulation and peroxidase-mediated oxidation of airway proteins do not occur in a mouse ovalbumin-challenge model of pulmonary inflammation. J. Immunol. 167:1672-1682. [DOI] [PubMed] [Google Scholar]
- 12.Deschoolmeester, M. L., and K. J. Else. 2002. Cytokine and chemokine responses underlying acute and chronic Trichuris muris infection. Int. Rev. Immunol. 21:439-467. [DOI] [PubMed] [Google Scholar]
- 13.El-Malky, M., H. Maruyama, Y. Hirabayashi, S. Shimada, A. Yoshida, T. Amano, A. Tominaga, K. Takatsu, and N. Ohta. 2003. Intraepithelial infiltration of eosinophils and their contribution to the elimination of adult intestinal nematode, Strongyloides venezuelensis, in mice. Parasitol. Int. 52:71-79. [DOI] [PubMed] [Google Scholar]
- 14.Epstein, M. M., F. Di Rosa, D. Jankovic, A. Sher, and P. Matzinger. 1995. Successful T cell priming in B cell-deficient mice. J. Exp. Med. 182:915-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaubert, S., A. Viana da Costa, C. A. Maurage, E. C. Lima, J. Fontaine, S. Lafitte, P. Minoprio, A. Capron, and J. M. Grzych. 1999. X-linked immunodeficiency affects the outcome of Schistosoma mansoni infection in the murine model. Parasite Immunol. 21:89-101. [DOI] [PubMed] [Google Scholar]
- 16.Grimaldi, J. C., N. X. Yu, G. Grunig, B. W. Seymour, F. Cottrez, D. S. Robinson, N. Hosken, W. G. Ferlin, X. Wu, H. Soto, A. O'Garra, M. C. Howard, and R. L. Coffman. 1999. Depletion of eosinophils in mice through the use of antibodies specific for C-C chemokine receptor 3 (CCR3). J. Leukoc. Biol. 65:846-853. [DOI] [PubMed] [Google Scholar]
- 17.Gupta, R., K. Tyagi, S. K. Jain, and S. Misra-Bhattacharya. 2003. Brugia malayi: establishment in inbred and outbred strains of mice. Exp. Parasitol. 103:57-60. [DOI] [PubMed] [Google Scholar]
- 18.Gurish, M. F., A. Humbles, H. Tao, S. Finkelstein, J. A. Boyce, C. Gerard, D. S. Friend, and K. F. Austen. 2002. CCR3 is required for tissue eosinophilia and larval cytotoxicity after infection with Trichinella spiralis. J. Immunol. 168:5730-5736. [DOI] [PubMed] [Google Scholar]
- 19.Haque, A., A. Ouaissi, F. Santoro, I. des Moutis, and A. Capron. 1982. Complement-mediated leukocyte adherence to infective larvae of Dipetalonema viteae (Filarioidea): requirement for eosinophils or eosinophil products in effecting macrophage adherence. J. Immunol. 129:2219-2225. [PubMed] [Google Scholar]
- 20.Hayakawa, K., R. R. Hardy, M. Honda, L. A. Herzenberg, and A. D. Steinberg. 1984. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc. Natl. Acad. Sci. 81:2494-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herbert, D. R., J. J. Lee, N. A. Lee, T. J. Nolan, G. A. Schad, and D. Abraham. 2000. Role of IL-5 in innate and adaptive immunity to larval Strongyloides stercoralis in mice. J. Immunol. 165:4544-4551. [DOI] [PubMed] [Google Scholar]
- 22.Herbert, D. R., T. J. Nolan, G. A. Schad, and D. Abraham. 2002. The role of B cells in immunity against larval Strongyloides stercoralis in mice. Parasite Immunol. 24:95-101. [DOI] [PubMed] [Google Scholar]
- 23.Hestdal, K., F. W. Ruscetti, J. N. Ihle, S. E. Jacobsen, C. M. Dubois, W. C. Kopp, D. L. Longo, and J. R. Keller. 1991. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J. Immunol. 147:22-28. [PubMed] [Google Scholar]
- 24.Hoerauf, A., and N. Brattig. 2002. Resistance and susceptibility in human onchocerciasis—beyond Th1 vs. Th2. Trends Parasitol. 18:25-31. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann, W., G. Petit, H. Schulz-Key, D. Taylor, O. Bain, and L. Le Goff. 2000. Litomosoides sigmodontis in mice: reappraisal of an old model for filarial research. Parasitol. Today 16:387-389. [DOI] [PubMed] [Google Scholar]
- 26.Jensen, J., T. Warner, and E. Balish. 1993. Resistance of SCID mice to Candida albicans administered intravenously or colonizing the gut: role of polymorphonuclear leukocytes and macrophages. J. Infect. Dis. 167:912-919. [DOI] [PubMed] [Google Scholar]
- 27.Johnson, E. H., M. Irvine, P. H. Kass, J. Browne, M. Abdullai, A. M. Prince, and S. Lustigman. 1994. Onchocerca volvulus: in vitro cytotoxic effects of human neutrophils and serum on third-stage larvae. Trop. Med. Parasitol. 45:331-335. [PubMed] [Google Scholar]
- 28.Johnson, E. H., S. Schynder-Candrian, T. V. Rajan, F. K. Nelson, S. Lustigman, and D. Abraham. 1998. Immune responses to third stage larvae of Onchocerca volvulus in interferon-gamma and interleukin-4 knockout mice. Parasite Immunol. 20:319-324. [DOI] [PubMed] [Google Scholar]
- 29.Khan, W. N., F. W. Alt, R. M. Gerstein, B. A. Malynn, I. Larsson, G. Rathbun, L. Davidson, S. Muller, A. B. Kantor, L. A. Herzenberg, et al. 1995. Defective B cell development and function in Btk-deficient mice. Immunity 3:283-299. [DOI] [PubMed] [Google Scholar]
- 30.Khan, W. N., P. Sideras, F. S. Rosen, and F. W. Alt. 1995. The role of Bruton's tyrosine kinase in B-cell development and function in mice and man. Ann. N. Y. Acad. Sci. 764:27-38. [DOI] [PubMed] [Google Scholar]
- 31.King, C. L., J. Xianli, I. Malhotra, S. Liu, A. A. Mahmoud, and H. C. Oettgen. 1997. Mice with a targeted deletion of the IgE gene have increased worm burdens and reduced granulomatous inflammation following primary infection with Schistosoma mansoni. J. Immunol. 158:294-300. [PubMed] [Google Scholar]
- 32.Kita, H., and C. R. Adolphson. 2002. Eosinophil IgE receptors, p. 87-101. In R. B. Fick, Jr., and P. M. Jardieu (ed.), IgE and anti-IgE therapy in asthma and allergic disease. Marcel Dekker, New York, N.Y.
- 33.Kitamura, D., J. Roes, R. Kuhn, and K. Rajewsky. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350:423-426. [DOI] [PubMed] [Google Scholar]
- 34.Kopf, M., F. Brombacher, P. D. Hodgkin, A. J. Ramsay, E. A. Milbourne, W. J. Dai, K. S. Ovington, C. A. Behm, G. Kohler, I. G. Young, and K. I. Matthaei. 1996. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity 4:15-24. [DOI] [PubMed] [Google Scholar]
- 35.Korenaga, M., N. Watanabe, and I. Tada. 1991. Effects of anti-IgE monoclonal antibody on a primary infection of Strongyloides ratti in mice. Parasitol. Res. 77:362-363. [DOI] [PubMed] [Google Scholar]
- 36.Korten, S., G. Wildenburg, K. Darge, and D. W. Buttner. 1998. Mast cells in onchocercomas from patients with hyperreactive onchocerciasis (sowda). Acta Trop. 70:217-231. [DOI] [PubMed] [Google Scholar]
- 37.Lange, A. M., W. Yutanawiboonchai, J. B. Lok, M. Trpis, and D. Abraham. 1993. Induction of protective immunity against larval Onchocerca volvulus in a mouse model. Am. J. Trop. Med. Hyg. 49:783-788. [DOI] [PubMed] [Google Scholar]
- 38.Lange, A. M., W. Yutanawiboonchai, P. Scott, and D. Abraham. 1994. IL-4- and IL-5-dependent protective immunity to Onchocerca volvulus infective larvae in BALB/cBYJ mice. J. Immunol. 153:205-211. [PubMed] [Google Scholar]
- 39.Lee, N. A., M. P. McGarry, K. A. Larson, M. A. Horton, A. B. Kristensen, and J. J. Lee. 1997. Expression of IL-5 in thymocytes/T cells leads to the development of a massive eosinophilia, extramedullary eosinophilopoiesis, and unique histopathologies. J. Immunol. 158:1332-1344. [PubMed] [Google Scholar]
- 40.Le Goff, L., P. Loke, H. F. Ali, D. W. Taylor, and J. E. Allen. 2000. Interleukin-5 is essential for vaccine-mediated immunity but not innate resistance to a filarial parasite. Infect. Immun. 68:2513-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leke, R. G., W. M. Boto, G. Lando, and J. L. Ngu. 1989. Immunity to Onchocerca volvulus. Serum mediated leucocyte adherence to infective larvae in vitro. Trop. Med. Parasitol. 40:39-41. [PubMed] [Google Scholar]
- 42.Lobos, E., T. B. Nutman, J. S. Hothersall, and S. Moncada. 2003. Elevated immunoglobulin E against recombinant Brugia malayi gamma-glutamyl transpeptidase in patients with bancroftian filariasis: association with tropical pulmonary eosinophilia or putative immunity. Infect. Immun. 71:747-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marshall, J. S., P. D. Wells, and E. B. Bell. 1987. Accelerated elimination of N. brasiliensis from the small intestine after auto-anti-IgE induction. Immunology 60:303-308. [PMC free article] [PubMed] [Google Scholar]
- 44.Martin, C., L. Le Goff, M. N. Ungeheuer, P. N. Vuong, and O. Bain. 2000. Drastic reduction of a filarial infection in eosinophilic interleukin-5 transgenic mice. Infect. Immun. 68:3651-3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin, C., M. Saeftel, P. N. Vuong, S. Babayan, K. Fischer, O. Bain, and A. Hoerauf. 2001. B-cell deficiency suppresses vaccine-induced protection against murine filariasis but does not increase the recovery rate for primary infection. Infect. Immun. 69:7067-7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayr, S. I., R. I. Zuberi, M. Zhang, J. de Sousa-Hitzler, K. Ngo, Y. Kuwabara, L. Yu, W. P. Fung-Leung, and F. T. Liu. 2002. IgE-dependent mast cell activation potentiates airway responses in murine asthma models. J. Immunol. 169:2061-2068. [DOI] [PubMed] [Google Scholar]
- 47.Meeusen, E. N., and A. Balic. 2000. Do eosinophils have a role in the killing of helminth parasites? Parasitol. Today 16:95-101. [DOI] [PubMed] [Google Scholar]
- 48.Paciorkowski, N., P. Porte, L. D. Shultz, and T. V. Rajan. 2000. B1 B lymphocytes play a critical role in host protection against lymphatic filarial parasites. J. Exp. Med. 191:731-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paciorkowski, N., L. D. Shultz, and T. V. Rajan. 2003. Primed peritoneal B lymphocytes are sufficient to transfer protection against Brugia pahangi infection in mice. Infect. Immun. 71:1370-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rajan, T. V., L. Ganley, N. Paciorkowski, L. Spencer, T. R. Klei, and L. D. Shultz. 2002. Brugian infections in the peritoneal cavities of laboratory mice: kinetics of infection and cellular responses. Exp. Parasitol. 100:235-247. [DOI] [PubMed] [Google Scholar]
- 51.Shin, E. H., Y. Osada, H. Sagara, K. Takatsu, and S. Kojima. 2001. Involvement of complement and fibronectin in eosinophil-mediated damage to Nippostrongylus brasiliensis larvae. Parasite Immunol. 23:27-37. [DOI] [PubMed] [Google Scholar]
- 52.Shin, M. H., and H. K. Min. 1997. Effects of anti-IgE mAb on serum IgE, Fc epsilon RII/CD23 expression on splenic B cells and worm burden in mice infected with Paragonimus westermani. Korean J. Parasitol. 35:47-54. [DOI] [PubMed] [Google Scholar]
- 53.Spencer, L., L. Shultz, and T. V. Rajan. 2001. Interleukin-4 receptor-Stat6 signaling in murine infections with a tissue-dwelling nematode parasite. Infect. Immun. 69:7743-7752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strait, R. T., S. C. Morris, M. Yang, X. W. Qu, and F. D. Finkelman. 2002. Pathways of anaphylaxis in the mouse. J. Allergy Clin. Immunol. 109:658-668. [DOI] [PubMed] [Google Scholar]
- 55.Strath, M., D. J. Warren, and C. J. Sanderson. 1985. Detection of eosinophils using an eosinophil peroxidase assay. Its use as an assay for eosinophil differentiation factors. J. Immunol. Methods 83:209-215. [DOI] [PubMed] [Google Scholar]
- 56.Strote, G., N. W. Brattig, and F. W. Tischendorf. 1990. Ultrastructural study of the interaction between eosinophilic granulocytes and third and fourth stage larvae of Onchocerca volvulus. Acta Trop. 48:1-8. [DOI] [PubMed] [Google Scholar]
- 57.White, S. R., G. V. Kulp, S. M. Spaethe, E. Van Alstyne, and A. R. Leff. 1991. A kinetic assay for eosinophil peroxidase activity in eosinophils and eosinophil conditioned media. J. Immunol. Methods 144:257-263. [DOI] [PubMed] [Google Scholar]
- 58.Yutanawiboonchai, W., R. A. Brigandi, H. L. Rotman, and D. Abraham. 1996. Structural and molecular specificity of antibody responses in mice immune to third stage larvae of Onchocerca volvulus. Parasite Immunol. 18:95-102. [DOI] [PubMed] [Google Scholar]