Abstract
Bacille Calmette–Guerin (BCG) vaccination for protection against tuberculosis has been in use for long. Although the vaccine is safe, its administration can result in complications such as BCG adenitis. We report here a series of children with BCG adenitis with a view to recognise and manage this condition. It is hoped that this case series would encourage the increased identification of this condition.
Keywords: adverse effects, axilla, BCG, children, lymphadenitis, vaccines
Introduction
The incidence of smear-positive tuberculosis (TB) was 63.6 per 100 000 population in 1997 and 45 per 100 000 in 2007 (1). Bacille Calmette–Guerin (BCG) vaccination was introduced in the Malaysian Immunization Scheme since 1961 (2). Malaysian healthcare system practices BCG vaccination by giving a single intradermal injection of 0.05 mL Tokyo 172 BCG strain for children less than 1 year old at left deltoid soon after birth. A repeat dose of 0.1ml is given for children at 6 years of age if no scar was formed after the first injection (1). The incidence of suppurative lymphadenitis due to BCG vaccination is 100–1000 per million doses administered (3).
Subjects and methods
We reviewed the case notes of children with BCG adenitis attending the paediatric surgical clinic from January 2009 to January 2010. The children were followed-up to document the course of adenitis within the study period. The parents of these children were human immunodeficiency virus (HIV)-negative.
BCG adenitis was labeled based on the following criteria: isolated axillary or supraclavicular lymph node enlargement, BCG vaccination on the ipsilateral arm, and the absence of local or systemic signs of inflammation (4).
Results
Over the 1-year study period, 6 children presented with BCG adenitis to the paediatric surgical clinic. The age range was 2–5 months at presentation. Clinical presentation included left axillary nodes and BCG scar in all cases, with supraclavicular neck nodes and chest wall mass in case each. Three patients were referred from the emergency department, and the remaining 3 were referred by general practitioner, paediatric clinic, and district clinic.
Out of the 6 children, 3 children were subjected to needle aspiration in view of the node size and suppuration, and the outcomes were good. Acid-fast staining of the aspirated pus failed to show presence of acid-fast bacilli. Cultures of the aspirates were negative. Further tests, such as the polymerase chain reaction assay, were not available and hence not done. On follow-up, 4 children showed complete resolution of the nodes over a period of 4–6 months, and 2 children with partial resolution are currently under review as the follow-up is less than 2 months. None of the children required excision or anti-TB therapy. The results are summarized in the Table 1.
Table 1:
Summary of case series
| Case 1 | Site | Presenting symptom | Size |
| Age: 2 months | Left axilla | Painless firm mass | 2 × 2 cm |
| Referral | Management | Follow-Up | |
| General practitioner | Conservative | Complete resolution (4 months) | |
| Case 2 | Site | Presenting symptom | Size |
| Age: 3 months | Left axilla, left supra-clavicular | Suppurative mass | 3 × 2 cm, 1 × 1 cm |
| Referral | Management | Follow-Up | |
| Emergency department | Needle aspiration | Complete resolution (5 months) | |
| Case 3 | Site | Presenting symptom | Size |
| Age: 3 months | Left axilla | Fluctuant mass | 2 x 2 cm, 1 x 1 cm |
| Referral | Management | Follow-Up | |
| District clinic | Needle aspiration | Partial resolution, on follow-up (2 months) | |
| Case 4 | Site | Presenting symptom | Size |
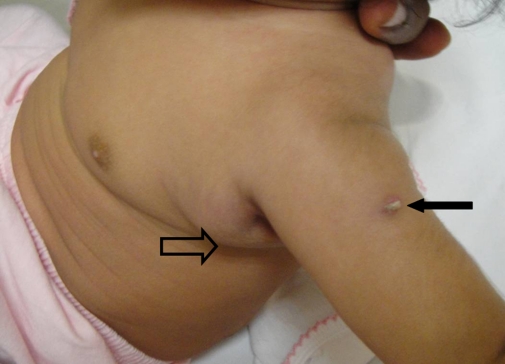
| Age: 3 months | Left axilla | Painless firm mass (Figure 1) | 2 × 2 cm |
| Referral | Management | Follow-Up | |
| Emergency department | Conservative | Complete resolution (4 months) | |
| Case 5 | Site | Presenting symptom | Size |
| Age: 4 months | Left axilla | Painless firm mass | 2 × 1 cm |
| Referral | Management | Follow-Up | |
| Paediatric clinic | Conservative | Partial resolution (4 months) | |
| Case 6 | Site | Presenting symptom | Size |
| Age: 5 months | Left chest, left axilla | Painless fluctuant chest mass, painless firm axillary mass | 2 × 1 cm, 1 × 1 cm |
| Referral | Management | Follow-Up | |
| Emergency department | Chest wall suppuration aspirated | Complete resolution (6 months) |
Discussion
BCG vaccine was introduced to the world in 1921. It was incorporated in the World Health Organization (WHO)’s Expanded Program on Immunization in 1974 to strengthen the fight against TB meningitis and disseminated TB in young children and infant of developing country. To date, BCG is the only TB vaccine available. Although it does not prevent the establishment of primary TB infection or the reactivation of latent TB, BCG vaccine is considered an important part of TB control measure in endemic areas. In 2004, WHO has recommended general BCG vaccination in countries with high burden of TB. At present, it is estimated that 100 million children receive BCG vaccination each year (5).
The original BCG vaccine is a live-attenuated form of Mycobacterium bovis. Four main BCG vaccine strains, namely Pasteur strain 1173, Danish strain 1331, Glaxo strain 1077, and Tokyo strain 172, account for almost 90% vaccination worldwide. According to the different strains and their production, the concentration ranges from 50 thousand to 3 million live particles per dosage (3). Among those BCG strains available, Tokyo 172 strain was registered as an International Reference Strain in 1965 by WHO (6). According to Smith et al. (7), animal immunogenicity study showed that Glaxo 1077 and Tokyo 172 are “weak” strains, whereas Pasteur 1173 and Danish 1331 are “strong” strains; the BCG vaccination complication rate differs between “strong” and “weak” strains. Hooi et al. (8) reported a series of 638 BCG-related lymphadenitis in infants due to the change in the vaccine strain from Tokyo-172 to Pasteur-1173. The incidence of lymphadenitis declined after the Tokyo strain was reintroduced. The BCG strain, immunization technique, dose, age, and physique of the vaccinees influence the incidence of complications (4,6).
Complications due to BCG vaccination can be classified into mild and severe. Mild complications are usually localized, and the most commonly seen complication is regional lymphadenitis. Cutaneous complications, such as lupoid reaction and eczema vaccinatum, form part of the mild spectrum of complications. The incidence of mild complication due to BCG is less than 1 per 1000 cases (5). Severe complications caused by BCG are suppurative lymphadenitis, osteitis/osteomyelitis, and disseminated BCG infection. Their incidence rates are 100–1000 cases, 1–700 cases, and 2 cases per 1 million vaccinations, respectively (3). In our series, true incidence could not be determined as all post-BCG children were not seen routinely in the surgical clinic.
Mild complications due to BCG usually heal spontaneously and require no treatment (1). In the natural course of BCG lymphadenitis, it could develop into simple, non-suppurative form and suppurative form. Diagnosis of BCG lymphadenitis is clinical, and its criteria are as listed in the Subjects and Methods section. Simple BCG lymphadenitis will regress spontaneously over a period of a few weeks without the need for anti-TB therapy. Treatment with oral erythromycin and anti-TB drugs do not hasten the regression or prevent progression into suppuration (4,9). Reassurance and follow-up until resolution is recommended. In our series, all of the children responded well to the management of drainage or needle aspiration without the need for excision.
The suppurative form of BCG lymphadenitis is considered as a severe complication. Its incidence is declining due to better inoculations technique by well-trained staff, using a standardized freeze-dried vaccine (3). It is distinguished by the fluctuant consistency of the swelling, with erythema and oedema of the overlying skin. The suppuration could develop into spontaneous rupture, sinus formation, and later, healing by cicatrization. The sinus may persist for a few months. To prevent these complications and to hasten healing process, needle aspiration is recommended. It is safe, and usually, one aspiration is adequate, but some patients might need repeated aspiration. Surgical excision is recommended in cases with draining sinus or failed needle aspiration due to multiloculated abscess or matted lymph node (4).
We ascertained that the children in the series were vaccinated by trained staff, though we could not confirm the technique or dosage.
The incidence of chest wall abscess associated with BCG vaccination is unknown due to its rarity. Although the effort on isolating BCG from the drained pus is futile, diagnosis could be confirmed either by demonstration of characteristic features in histopathology of the lesion or through genetic analysis with polymerase chain reaction assay (10). Confirmed cases can be treated surgically and followed by a course of anti-TB therapy for 6 to 12 months based on the culture and sensitivity result (11). A recent review states that node size of more than 3 cm warrants surgical therapy and does not respond to medical treatment (12). None of the children in our series required additional treatment, such as node excision or anti-TB therapy.
BCG osteitis/osteomyelitis and disseminated BCG infection are rare but potentially lethal conditions (10). They are associated with immunodeficient vaccinees with primary immunodeficiency disorder such as severe combined immunodeficiency, chronic granulomatous disease, DiGeorge syndrome, type 1 cytokine axis defects, and HIV infection (3,5,13,14). Treatment with anti-TB drugs, including isoniazid and rifampicin, has been shown to be effective (13). As a preventive measure, BCG vaccine is contraindicated in patients with known impaired immunity or undergoing immunosuppressive therapy (3,5).
A preliminary survey among paramedical personnel at our institution revealed that only 60% acknowledged the entity of BCG adenitis.
Conclusion
Simple BCG lymphadenitis is best managed conservatively, but suppuration should be aspirated to enhance recovery and prevent sinus formation. Surgical excision, but not incision, is recommended if needle aspiration fails. Other complications, such as chest wall abscess, might necessitate surgical intervention followed by a course of anti-TB therapy. Screening of immunodeficiency disorder needs to be considered in children who develop severe complication due to BCG vaccination. Parental education and awareness among paramedical personnel, including general practitioners, is essential so that prompt recognition and management of BCG adenitis can be ensured.
Figure 1:
The hollow arrow points to the BCG adenitis. The solid arrow indicates the BCG scar.
Footnotes
Authors’ Contributions
Conception and design, drafting of the article: FYC
Critical revision and final approval of the article: KKG
References
- 1.Clinical practice guidelines: Childhood immunisation. Kuala Lumpur: Ministry of Health Malaysia, Academy of Medicine; 2004. BCG vaccine; pp. 3–4. [Google Scholar]
- 2.Clinical practice guidelines: Childhood immunisation. Kuala Lumpur: Ministry of Health Malaysia, Academy of Medicine; 2004. Childhood immunisation; pp. 1–2. [Google Scholar]
- 3.Department of Vaccines and Biologicals, World Health Organization . In: Supplementary information on vaccine safety: Part 2: Background and rates of adverse events following immunization. Clements CJ, editor. Geneva: World Health Organization; 2000. [Google Scholar]
- 4.Goraya JS, Virdi VS. Baccile Calmette-Guerin lymphadenitis. Postgrad Med J. 2002;78(920):327–329. doi: 10.1136/pmj.78.920.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization BCG vaccine. WHO position paper. Wkly Epidemiol Rec. 2004;79(4):27–38. [PubMed] [Google Scholar]
- 6.Yamamoto S, Yamamoto T. Historical review of BCG vaccine in Japan. Jpn J Infect Dis. 2007;60(6):331–336. [PubMed] [Google Scholar]
- 7.Smith D, Harding C, Chan J, Edwards M, Hank J, Muller D, et al. Potency of 10 BCG vaccines as evaluated by their influence on the bacillemic phase of experimental airborne tuberculosis in guinea-pigs. J Biol Stand. 1979;7(3):179–197. doi: 10.1016/s0092-1157(79)80021-9. [DOI] [PubMed] [Google Scholar]
- 8.Hooi LN, Athiyah SO. An outbreak of BCG related lymphadenitis in Malaysian infants. Med J Malaysia. 1994;49(4):327–335. [PubMed] [Google Scholar]
- 9.Goraya JS, Virdi VS. Treatment of Calmette-Guerin bacillus adenitis: A metaanalysis. Pediatr Infect Dis J. 2001;20(6):632–634. doi: 10.1097/00006454-200106000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Su WJ, Huang CY, Huang CY, Perng RP. Utility of PCR assays for rapid diagnosis of BCG infection in children. Int J Tuberc Lung Dis. 2001;5(4):380–384. [PubMed] [Google Scholar]
- 11.Aribas OK, Kanat F, Gormus N, Turk E. Cold abscess of the chest wall as an unusual complication of BCG vaccination. Eur J Cardiothorac Surg. 2002;21(2):352–354. doi: 10.1016/s1010-7940(01)01103-4. [DOI] [PubMed] [Google Scholar]
- 12.Nazir Z, Qazi SH. Bacillus Calmette-Guerin (BCG) lymphadenitis-changing trends and management. J Ayub Med Coll Abbottabad. 2005;17(4):16–18. [PubMed] [Google Scholar]
- 13.Rezai MS, Khotaei G, Mamishi S, Kheirkhah M, Parvaneh N. Disseminated Bacillus Calmette-Guerin infection after BCG vaccination. J Trop Pediatr. 2008;54(6):413–416. doi: 10.1093/tropej/fmn053. [DOI] [PubMed] [Google Scholar]
- 14.Ottenhoff TH, Verreck FA, Hoeve MA, van de Vosse E. Control of human host immunity to mycobacteria. Tuberculosis. 2005;85(1–2):53–64. doi: 10.1016/j.tube.2004.09.011. [DOI] [PubMed] [Google Scholar]