Abstract
The usual agent of visceral leishmaniasis in the Old World is Leishmania donovani, which typically produces systemic diseases in humans and mice. L. donovani has developed efficient strategies to infect and persist in macrophages from spleen and liver. Dendritic cells (DC) are sentinels of the immune system. Following recognition of evolutionary conserved microbial products, DC undergo a maturation process and activate antigen-specific naïve T cells. In the present report we provide new insights into how DC detect Leishmania in vivo. We demonstrate that in both C57BL/6 and BALB/c mice, systemic injection of L. donovani induced the migration of splenic DC from marginal zones to T-cell areas. During migration, DC upregulated the expression of major histocompatibility complex II and costimulatory receptors (such as CD40, CD80, and CD86). Leishmania-induced maturation requires live parasites and is not restricted to L. donovani, as L. braziliensis, L. major, and L. mexicana induced a similar process. Using a green fluorescent protein-expressing parasite, we demonstrate that DC undergoing maturation in vivo display no parasite internalization. We also show that L. donovani-induced DC maturation was partially abolished in MyD88-deficient mice. Taken together, our data suggest that Leishmania-induced DC maturation results from direct recognition of Leishmania by DC, and not from DC infection, and that MyD88-dependent receptors are implicated in this process.
Leishmaniases result from infection with Trypanosomatidae protozoa of the genus Leishmania which are transmitted to mammals by the bite of an infected phlebotomine sand fly (reviewed in references 5 and 32). Leishmania parasites induce a large spectrum of diseases in humans, from cutaneous leishmaniasis to progressive fatal visceralizing leishmaniasis (VL) or kala azar. Active VL resulting from infection with members of the L. donovani complex (such as L. donovani) is characterized by fever and hepatosplenomegaly and usually leads to the death of the infected individual. Although anti-Leishmania drugs are available, treatments are toxic and are not always successful. The development of a safe, effective, and affordable vaccine should offer a good solution to control VL.
Several candidate vaccines have already been evaluated in the mouse model of leishmaniases. Narrow-spectrum vaccines used live attenuated or killed parasites (6, 19, 29). They induced low levels of protective immunity in vaccinated patients who displayed a previous positive intradermal reaction to Leishmania (19). Expanded-spectrum vaccines used (i) genetically modified live Leishmania strains causing abortive infection in humans, (ii) recombinant bacteria or viruses carrying Leishmania antigen genes (21), and (iii) defined antigen vaccines such as LeIF (35) and LACK antigen (12). Broad-spectrum vaccines used genes coding for a protective antigen such as FML antigen (34). Evaluation of the protection conferred to patients by the expanded- and broad-spectrum vaccines is still in progress. The selection of parasite antigens used in such vaccination trials is still largely empirical, since information on the factors controlling Leishmania antigen presentation in vivo is lacking. Dendritic cells (DC), a lineage of professional antigen-presenting cells (APC), are known as sentinels of the immune system (36, 23, 16). Numerous studies have indicated that their capacity to activate antigen-specific naïve CD4+ or CD8+ T cells is strongly enhanced by the recognition of microbial products. During this process of maturation, major histocompatibility complex (MHC) class II-encoded and costimulatory molecules (CD40, CD80, CD86,…) are upregulated. The recent discovery of Toll-like receptors (TLRs) which are involved in the recognition of highly conserved microbial products called pathogen-associated molecular patterns has greatly contributed to our understanding of how DC detect pathogens (for reviews, see references 1, 14, and 17). TLRs display conserved cytoplasmic domains allowing them to use common signaling pathways, involving myeloid differentiation marker 88 (MyD88), rather than the interleukin-1 receptor (10).
Splenic DC maturation has been shown to occur in vivo in response to various microbial inflammatory stimuli involving lipopolysaccharide (LPS) (8), CpG oligodeoxynucleotide (CpG ODN) (15), and polyclonal T-cell activators (24, 26). In this study, we analyzed the ability of a Leishmania donovani parasite to induce splenic DC maturation in vivo. We demonstrate that intravenous (i.v.) injection of Leishmania parasites induces migration and maturation of splenic DC in vivo. This process requires live parasites, does not require parasite internalization, and is partially abolished in MyD88-deficient mice.
MATERIALS AND METHODS
Mice, reagents, and parasites.
Female BALB/c and C57BL/6 mice (6 to 8 weeks old) were purchased from Harlan Nederland (Horst, The Netherlands); RAG mice were purchased from Jackson Laboratory (Bar Harbor, Maine). MyD88−/− mice were obtained from Shizuo Akira (Osaka University) and bred in our own animal facility. The maintenance and care of mice complied with the guidelines of the Université Libre de Bruxelles Ethic Committee for the human use of laboratory animals. Mitogenic anti-CD3e (hamster immunoglobulin) (catalog no. 145-2C11; American Type Culture Collection [ATCC]) was produced and purified in our laboratory according to standard procedures. CpG-ODN 1826 was obtained from the Coley Pharmaceutical Group (Wellesley, Mass.). Leishmania promastigotes (L. braziliensis [MHOM/PE/-/LC2043], L. donovani [MHOM/ET/67/Hu3:LV9], L. major [MHOM/IR/-/173 and LRC-L137 V121], and L. mexicana [MHOM/BZ/82/BEL21]) obtained after passage in BALB/c mice were propagated in vitro as previously described (27). Stable genomic integration of green fluorescent protein (GFP) in L. major (LRC-L137 V121 strain) has been described previously (22). Parasites harvested in stationary phase after 8 to 10 days of culture growth were centrifuged (2,500 × g, 10 min, 4°C) and washed three times in RPMI medium before being used for inoculation into the animals. Heat-killed parasites were obtained following incubation at 60°C for 20 min.
In vivo treatment.
The indicated doses of anti-CD3 monoclonal antibody (MAb), CpG-ODN, or parasites in phosphate-buffered saline (PBS) were injected i.v. into the lateral tail vein of mice. Control animals were injected with PBS.
Purification of low-density spleen cells.
Spleens were digested with collagenase (CLSIII; Worthington Bio-chemical Corp., Freehold, N.J.), further dissociated in calcium-free medium, and separated into low- and high-density fractions on a Nycodenz gradient (Nycomed, Oslo, Norway), as previously described (20).
Cytofluorometric analysis.
Cells were analyzed by flow cytometry with a FACSort cytometer (Becton Dickinson, Mountain View, Calif.). The cells were preincubated with saturating doses of 2.4G2 (a rat anti-mouse Fc receptor MAb; ATCC) for 10 min before staining to prevent antibody binding to Fc receptors. They were further labeled with phycoerythrin (PE)-coupled N418 (anti-CD11c) or (as indicated) fluorescein isothiocyanate-coupled antibodies, including AF6-120.1 (anti-I-Ab), 3.23 (anti-CD40), and 16-10A1 (anti-CD80) (all from Pharmingen; San Diego, Calif.), and DEC-205 (anti-CD205), 14.4.4-S (anti-I-E k,d), and GL1 (anti-CD86) (available through the ATCC and purified and labeled in our laboratory). Cells were gated according to size and scatter to eliminate dead cells and debris from analysis.
Immunohistochemistry.
The immunohistochemical techniques used in this study are described in detail elsewhere (30). Briefly, spleen samples were harvested and frozen at −80°C. Frozen sections (6 to 10 μm thick) were fixed in acetone for 10 min and transferred to PBS. The sections were treated for 30 min with blocking reagent (Boehringer) (1% in PBS) to saturate the sites of nonspecific reactions. The slides were then stained with biotinylated N418 (anti-CD11c Mab; ATCC). They were further incubated with avidin-biotin-peroxidase complex (Vectastain ABC kit; Vector Laboratories, Burlingame, Calif.) and stained with a solution of diaminobenzidine tetrahydrochloride (DAB tablets; Sigma, St. Louis, Mo.), giving brown precipitates. Digitized images were captured using a charge-coupled-device color camera (Ikegami Tsushinki, Tokyo, Japan) and analyzed using CorelDraw 7 software (Corel, Ottawa, Ontario, Canada).
RESULTS
Leishmania parasites induce migration of splenic DC from marginal zones to T-cell areas.
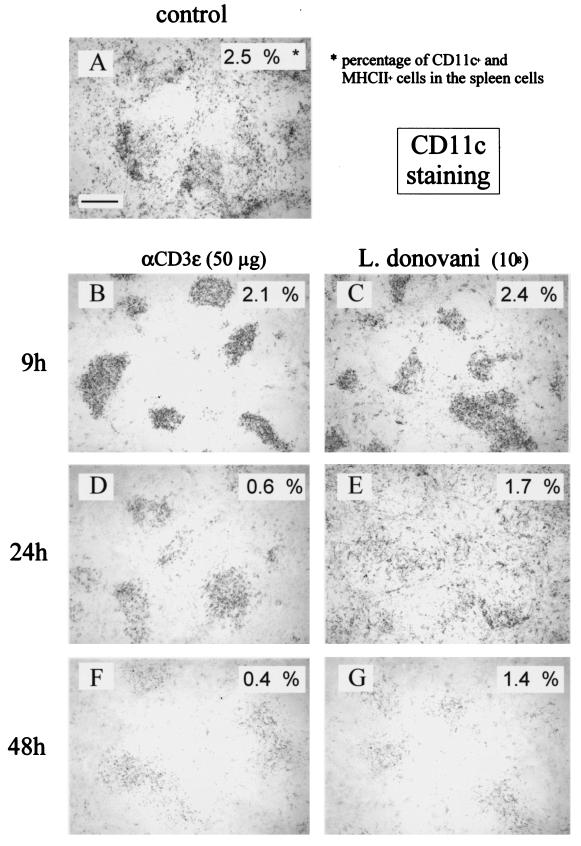
Since intrasplenic migration is the first detectable reaction of splenic DC in response to microbial or inflammatory stimuli in vivo, we studied such migration by immunohistochemistry within the 48 h following i.v. injection of mice with 108 L. donovani parasites. In nontreated animals, most DC (identified as CD11c-expressing cells) were found in splenic marginal zones and T-cell zones (Fig. 1A). Injection of mitogenic anti-CD3 antibody (as a positive control) (24) or L. donovani led to the rapid (9 h) relocalization of most CD11c+ cells to the white pulp in T-cell-rich areas around the central arteriole (Fig. 1B and C). At 24 h after anti-CD3 MAb treatment, CD11c+ cells were present in the T-cell area (Fig. 1D). While they were found in red pulp, marginal zones, and T-cell areas after L. donovani injection (Fig. 1E), CD11c+ cells were gradually (9 to 48 h) lost following both treatments, as seen by immunohistochemistry results (note the weak CD11c-specific labeling in Fig. 1F and G) and flow cytometry results (CD11c+ cells levels were shown to be 2.5% in control mice, 0.4% after the 48-h anti-CD3 treatment, and 1.4% after the 48-h Leishmania treatment). Reconstitution of splenic DC populations was observed (consistent with their rapid turnover) 3 to 4 days after treatment (18) (data not shown).
FIG. 1.
Leishmania parasites induce DC migration and loss in vivo. The results of phosphatase staining of cryosections of spleens from control (PBS-injected) and mitogenic anti-CD3 (50 μg)- or parasite (108)-injected (i.v.) C57BL/6 mice are shown. Times of injection are indicated. Sections were incubated with biotinylated antibodies against CD11c. The percentages of CD11C-positive cells present in spleen cells from mice of the same experiment were determined by cytofluorometry and are given in the upper right corner of each picture. These results are representative of three independent experiments. Bar, 150 μm.
Leishmania parasites induce DC maturation in vivo.
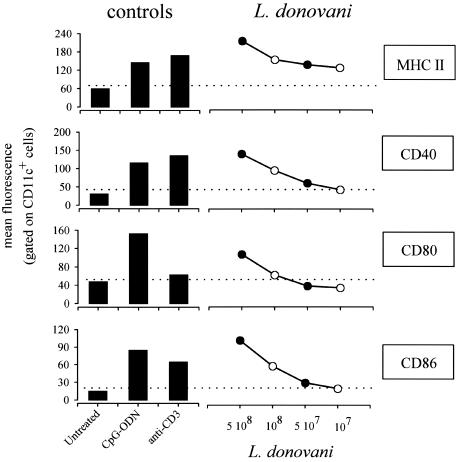
To investigate the capacity of L. donovani parasites to induce splenic DC maturation in vivo, the expression of MHC class II, CD40, CD80 (B7.1), and CD86 (B7.2) was analyzed by flow cytometry. DC-enriched low-density spleen cells were purified from L. donovani-, mitogenic anti-CD3 antibody (50 μg)-, or CpG ODN (50 μg)-treated C57BL/6 mice and examined 9 h later for their expression of markers known to be upregulated during DC maturation. As expected from previous observations, anti-CD3 (24) and CpG (15) injection led to increased expression of MHC class II, CD40, CD80, and CD86 (Fig. 2). Similarly, administration of 1 × 108 to 5 × 108 L. donovani parasites led to a dose-dependent upregulation of these markers (Fig. 2). Both CD11c-expressing DC subsets (CD11c+ B220− CD8− and CD11c+ B220− CD8+) matured (data not shown).
FIG. 2.
Maturation of splenic DC following L. donovani injection. C57BL/6 mice were injected (i.v.) with a control solution (PBS) (Untreated), with CpG-ODN (50 μg), with mitogenic anti-CD3 (50 μg), or with 5 × 108 (5 108), 1 × 108, 5 × 107 (5 107), or 1 × 107 L. donovani parasites. At 9 h after treatment, mice were sacrificed and spleens were collected. Pooled low-density spleen cells from three individuals in each group were double stained with PE-labeled anti-CD11c and with biotinylated anti-CD40, anti-CD80, anti-CD86, and anti-MHC class II followed by streptavidin-APC treatment. The data represent the fluorescence of cells gated for high levels of SCD11c expression. These results are representative of four independent experiments.
Other experiments were performed by injecting 108 parasites. To further analyze the DC-maturing properties of Leishmania parasites, we analyzed the importance of administration methods and mouse strains. Comparison of i.v. versus intraperitoneal injection of mitogenic anti-CD3 and L. donovani in BALB/c and C57BL/6 mice showed equivalent upregulation of MHC class II and costimulatory markers on DC (data not shown).
Leishmania parasite-mediated DC maturation requires live parasites.
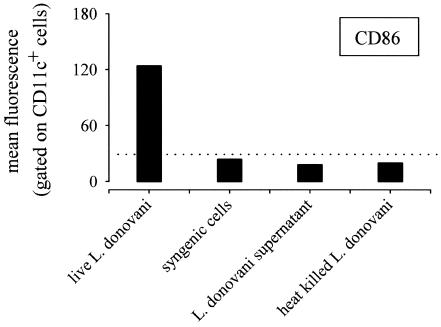
Interestingly, the requirement for live parasites appeared absolute for the promotion of DC maturation, as demonstrated by the fact that heat-killed parasites did not induce DC maturation (Fig. 3). Note that neither 108 syngeneic spleen cells nor parasite culture supernatant injected i.v. induced detectable DC maturation (Fig. 3), demonstrating that parasite-mediated DC maturation was specific.
FIG. 3.
Leishmania parasite-mediated DC maturation requires live parasites. C57BL/6 mice were injected (i.v.) with a control solution (PBS), 108 syngeneic spleen cells, L. donovani culture supernatant, 108 heat-killed L. donovani parasites, or 108 viable L. donovani parasites (as indicated in the figure). At 9 h after treatment, mice were sacrificed and spleens were collected. Pooled low-density spleen cells from three individuals in each group were double stained with PE-labeled anti-CD11c and with biotinylated anti-CD86 followed by streptavidin-APC treatment. The data represent the fluorescence of cells gated for high levels of CD11c expression. These results are representative of two independent experiments.
Various Leishmania species induce DC maturation in vivo.
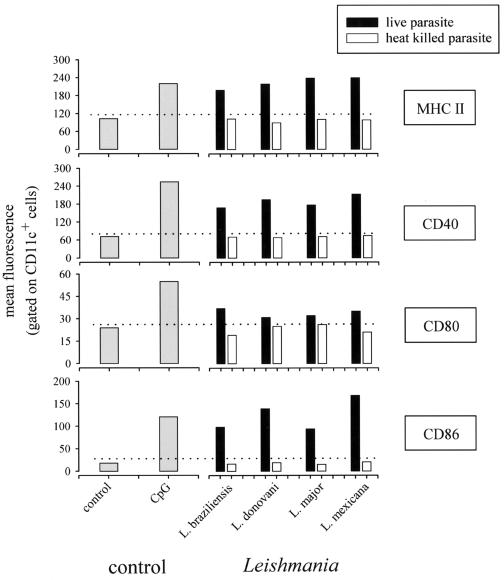
Since studies have shown conflicting results regarding the ability of Leishmania species to activate cells of the DC lineage in vitro (3, 31, 38), we compared the abilities of L. braziliensis, L. donovani, L. major, and L. mexicana to induce DC maturation in vivo (Fig. 4). We observed that all tested species induced DC maturation, as demonstrated by the upregulation of MHC class II, CD40, and CD86. As previously observed for L. donovani, live parasites are strictly required for the induction of maturation.
FIG. 4.
Various Leishmania species induce DC maturation in vivo. C57BL/6 mice were injected (i.v.) with a control solution (PBS), CpG-ODN (50 μg), 108 heat-killed parasites, or 108 viable parasites (as indicated in the figure). At 9 h after treatment, mice were sacrificed and spleens were collected. Pooled low-density spleen cells from three individuals in each group were double stained with PE-labeled anti-CD11c and with biotinylated anti-MHC class II, anti-CD40, anti-CD80, and anti-CD86 followed by streptavidin-APC treatment. The data represent the fluorescence of cells gated for high levels of CD11c expression.
Leishmania-induced maturation of DC is not due to the internalization of Leishmania parasites in DC.
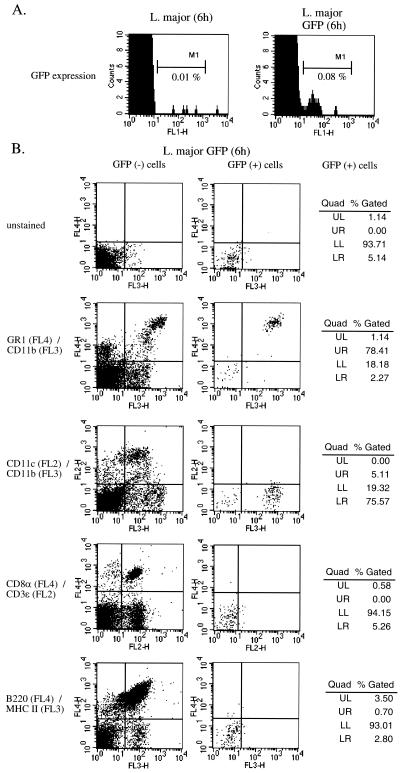
It has been shown in vitro (4) and in vivo (27, 37) that Leishmania parasites can infect DC, suggesting that parasite-induced DC maturation can result from direct interaction with DC. To further analyze the mechanism of Leishmania parasite-induced DC maturation, we used a genetically modified strain (LRC-L137 V121) of L. major expressing GFP (22). The results of a kinetic study showed that footpad lesions induced by wild-type and by GFP-expressing L. major are similar, suggesting that GFP transfection does not impair Leishmania virulence (data not shown). Furthermore, GFP+ CD11c+ cells were detected in the draining lymph nodes of infected BALB/c mice at 4 weeks following infection (data not shown). This observation demonstrates that expression of GFP does not alter the ability of L. major to infect DC in vivo. In similarity to the results seen with nonfluorescent Leishmania strains previously tested, GFP-expressing L. major induced the maturation of splenic DC (data not shown) and allowed us to identify splenic cells able to internalize Leishmania parasites in vivo. At 6 h following infection, spleen cells were recovered and GFP+ cells were detected by cytofluorometry. A small number of cells (0.08%) were found positive for GFP expression in spleen cells from GFP+ L. major-treated mice (106 cells were acquired for analysis). In contrast, spleen cells from nonfluorescent L. major-treated mice (used as an internal control) showed a background of 0.01% (Fig. 5A). The expression of cell surface markers specific for spleen cell subpopulations was analyzed with GFP− and GFP+ cells from mice injected with an L. major strain expressing a GFP marker. Data from Fig. 5B show that most (78%) GFP+ cells displayed GR1 and CD11b (Mac-1) markers. Interestingly, GFP+ cells do not express CD11c or MHC class II. Identical experiments performed 3 and 9 h after the administration of GFP+ parasites gave similar results (data not shown). These results suggest that DC maturation does not result from parasite internalization by DC.
FIG. 5.
Leishmania-induced maturation of DC is not due to internalization of Leishmania parasites by DC. (A) C57BL/6 mice (n = 3) were injected (i.v.) with 108 wild-type L. major or 108 GFP− L. major parasites. At 6 h later, mice were sacrificed and spleen cells were analyzed by cytofluorometry for GFP expression. (B) In the same experiment, spleen cells were stained with the following combinations of antibodies followed by streptavidin-peridinin chlorophyll protein (PerCP) treatment: APC-labeled anti-GR1 and PerCP-labeled anti-CD11b, PE-labeled anti-CD11c and PerCP-labeled anti-CD11b, APC-labeled anti-CD8α and PE-labeled anti-CD3ɛ, and APC-labeled anti-B220 and biotinylated anti-MHC class II. The data represent the fluorescence of cells gated for GFP-negative (left column) or GFP-positive (right column) expression. Quad, quadrant; UL, upper left; UR, upper right; LL, lower left; LR, lower right.
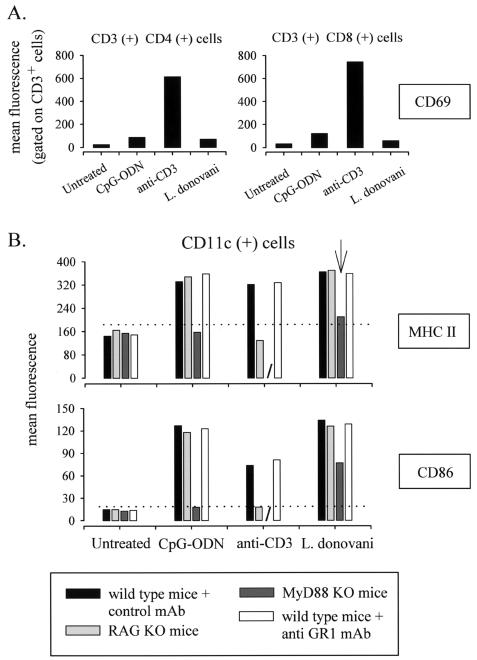
Leishmania parasite-mediated DC maturation is independent of B cells, T cells, and GR1+ cells but is partially dependent on MyD88 pathways.
Muraille et al. have recently reported that splenic DC maturation can be induced by T-cell-dependent (mitogenic anti-CD3, bacterial superantigens) or T-cell-independent (LPS) pathways (26). Injection (i.v.) of L. donovani parasites appeared not to induce T-cell activation, as suggested by the absence of CD69 activation markers on splenic CD3+ CD4+ or CD3+ CD8+ T cells (Fig. 6A) at 9 h after infection. Thus, it is not surprising that the absence of T and B cells in RAG−/− C57BL/6 mice did not affect the capacity of L. donovani to induce DC maturation (Fig. 6B). In contrast, mitogenic anti-CD3 (used as a control) induced CD69 expression on splenic T cells (Fig. 6A) but was unable to induce DC maturation in RAG−/− mice (Fig. 6B).
FIG. 6.
Leishmania parasite-mediated DC maturation is partially dependent of MyD88 pathways. (A) C57BL/6 mice were injected (i.v.) with a control solution (PBS), CpG (50 μg), mitogenic anti-CD3 (50 μg), or 108 viable L. donovani parasites. At 24 h after treatment, mice were sacrificed and spleens were collected. Pooled spleen cells from three individuals in each group were double stained with PE-labeled anti-CD4 or anti-CD8, as indicated in the figure, and with fluorescein isothiocyanate-labeled anti-CD69. The data represent the CD69-associated fluorescence of cells gated for CD4 or CD8 expression. These results are representative of two independent experiments. (B) Wild-type C57BL/6 mice treated with control MAb or with anti-GR1 MAb, RAG−/− C57BL/6 mice, and MyD88−/− C57BL/6 mice were injected (i.v.) with a control solution (PBS), CpG-ODN (50 μg), mitogenic anti-CD3 (50 μg), or 108 L. donovani parasites, as indicated. At 9 h after treatment, mice were sacrificed and spleens were collected. Pooled low-density spleen cells from three individuals in each group were double stained with PE-labeled anti-CD11c and with biotinylated anti-CD40, anti-CD86, and anti-MHC class II followed by streptavidin-APC treatment. The data represent the fluorescence of cells gated for a high level of CD11c expression. These results are representative of two independent experiments.
As demonstrated previously with GFP-expressing L. major, splenic GR1+ cells can internalize Leishmania parasites. To test GR1+ cells having internalized parasites for their possible contribution to the maturation of DC, we have pretreated mice 24 h before parasite inoculation with a GR1-specific MAb known to induce the depletion of GR1+ spleen cells. This treatment affected only GR1+ cells and not CD11c+ cells (data not shown). As shown in Fig. 6B, depletion of GR1+ cells did not affect the ability of L. donovani (or that of other species; data not shown) to induce the maturation of DC in vivo.
MyD88-dependent TLR pathways are involved in induction of DC maturation in response to various microbial products (10). Muraille et al. recently showed that development of a Th1 protective immune response to L. major parasites required functional MyD88-dependent transduction pathways (25). We therefore investigated the role of MyD88 protein in Leishmania-induced DC maturation. We observed a reduced ability of L. donovani to induce DC maturation in MyD88−/− C57BL/6 mice (Fig. 6B). As a control (and consistent with the results of previous studies) (13), CpG ODN-induced DC maturation is completely abolished in MyD88−/− mice (Fig. 6B). In addition, we found no difference between the results of investigations of the kinetics of migration of CD11c+ cells from wild-type and MyD88−/− C57BL/6 mice, suggesting that MyD88 deficiency impaired DC maturation but not DC migration to T-cell areas (data not shown).
DISCUSSION
It is well established that peripheral DC capture exogenous soluble antigens and migrate to draining lymph nodes, where they prime naive T cells (16). In contrast, it is unclear how immature DC detect or internalize intact pathogens such as Leishmania parasites. Furthermore, few reports have studied their subsequent migration and maturation. In vitro models have given conflicting results regarding the ability of Leishmania to activate DC-like cells (3, 31, 38). These differences might result from the use of different Leishmania species, the origin of the DC, and culture conditions. In this study, we show for the first time that systemic administration of Leishmania parasites (L. braziliensis, L. donovani, L. major, and L. mexicana) induced a full DC maturation process in vivo (i.e., DC migration to T-cell areas, upregulation of costimulatory molecules, and DC loss). Similar maturation profiles were observed for BALB/c (genetically susceptible) and C57BL/6 (genetically resistant) mice, suggesting that nonprotective responses are not due to an inability of DC to detect Leishmania parasites.
In most studies, 1 × 107 to 2 × 107 L. Donovani parasites are used to induce the systemic disease in mice (28, 11). Our experiments demonstrated DC maturation at high doses (1 × 108 to 5 × 108) of parasites. To detect DC maturation in vivo by flow cytofluorometry analyses, it is necessary that most DC mature. We hypothesized that doses of parasites (102 to 103 parasites) at physiological levels (2) only induce asynchronous maturation of a minor fraction of DC which would therefore be undetectable. Moreover, during systemic administration of parasites, it is likely that only a fraction of parasites can interact with splenic DC. Such a dose effect is seen in many in vivo models of DC maturation. For example, only doses of LPS (8), staphylococcal superantigens (25), or bacterial DNA (CpG ODN) (15) administered at high and nonphysiological levels (10 to 100 μg) can induce detectable in vivo maturation of splenic DC.
Leishmania parasites induce DC maturation in RAG−/− mice, demonstrating that this phenomenon is independent of the presence of T and B cells. It has been shown that Leishmania parasites can infect DC (37, 27), suggesting that parasite-induced DC maturation can result from DC infection. In addition, macrophages can also be infected with Leishmania parasites (32) and produce inflammatory factors, such as tumor necrosis factor alpha (33), that are susceptible to promoting DC maturation. To determine whether parasite internalization by DC or other splenic cells is an obligatory step for the induction of DC maturation, we used a genetically modified strain of Leishmania expressing GFP protein. Our results demonstrate that DC are not infected at the time when they mature and that deletion of GR1+ CD11b+ CD11c− cells which are infected by Leishmania parasites does not affect DC maturation in vivo. These results are in accordance with those of a study on splenic DC maturation induced by Salmonella enterica serovar Typhimurium infection in vivo (39). Using a GFP-expressing bacterial strain, these authors have demonstrated that in vitro and in vivo activated DC are mostly uninfected. In a recent study (27), Muraille et al. also investigated how Leishmania parasite infection affects DC fate in vivo. When mice were chronically infected, we observed in situ that a fraction of infected DC (identified as CD11c+ cells) in draining lymph nodes from the primary lesion have reduced levels of MHC class II expression and no detectable expression of CD86 costimulatory molecules. Taken together, these data suggest that in vivo infection of DC by parasites is not required for the induction of DC maturation. Furthermore, maturation can be inhibited even during infection.
MyD88 is an adaptor protein that has been shown to play a key role in the TLR/IL-1R family transduction pathways (1, 14, 17). Recently, Muraille et al. observed that the development of a Th1 protective immune response to L. major parasites requires functional MyD88-dependent transduction pathways (25). The partial inhibition of parasite-induced DC maturation in MyD88-deficient mice argues in favor of an important role for MyD88-adaptator protein in the detection of Leishmania parasites by DC. Recent studies have demonstrated in vitro that TLR-2 binds lipophosphoglycan purified from L. major (9) and glycosylphosphatidylinositol anchors purified from Trypanosoma cruzi (7), demonstrating the presence of TLR ligands on Trypanosomatidae parasites. Globally, these observations suggest that recognition of Leishmania antigens by TLRs might be a prerequisite for DC maturation.
In conclusion, our work demonstrates that maturation of DC in vivo can be induced by L. donovani as well as by other species of Leishmania. The pathways of activation appear to be partially dependent of MyD88-adaptor protein but independent of parasite internalization and of interactions with T cells. Our observations also suggest that since MyD88 is used during Leishmania-mediated DC maturation, TLRs could provide a new and original system for the identification of a novel set of Leishmania antigens of interest for the further development of vaccines.
Acknowledgments
We thank Domminique Le Ray (Institut of Tropical Medecine, Antwerp, Belgium) for giving us the strains of Leishmania parasites. We are indebted to Alain Wathelet for his diligent technical assistance.
This work was supported by grants from the Belgian Ministry of Scientific Policy (Action de Recherche Concertée) and the Fonds National de la Recherche Scientifique (FNRS; Crédit aux chercheurs, Brussels, Belgium) and by the Université Libre de Bruxelles (ULB; Brussels, Belgium). E.M. is supported by the Fonds pour la Recherche Médicale (FRM; Bourse d'Acceuil de Chercheur Etranger, Paris, France), and C.D.T. is supported by the ULB.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Barton, G. M., and R. Medzhitov. 2003. Toll-like receptor signaling pathways. Science 300:1524-1525. [DOI] [PubMed] [Google Scholar]
- 2.Belkaid, Y., S. Mendez, R. Lira, N. Kadambi, G. Milon, and D. Sacks. 2000. A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J. Immunol. 165:969-977. [DOI] [PubMed] [Google Scholar]
- 3.Bennett, C. L., A. Misslitz, L. Colledge, T. Aebischer, and C. C. Blackburn. 2001. Silent infection of bone marrow-derived dendritic cells by Leishmania mexicana amastigotes. Eur. J. Immunol. 31:876-883. [DOI] [PubMed] [Google Scholar]
- 4.Blank, C., H. Fuchs, K. Rappersberger, M. Rollinghoff, and H. Moll. 1993. Parasitis of epidermal Langerhans cells in experimental cutaneous leishmaniasis with Leishmania major. J. Infect. Dis. 167:418-425. [DOI] [PubMed] [Google Scholar]
- 5.Bradley, D. J. 1987. Genetics of susceptibility and resistance in the vertebrate host, p. 551-581. In W. Peters and R. Killick-Kendrick (ed.), The leishmaniases in biology and medicine. Academic Press, Ltd., London, United Kingdom.
- 6.Castes, M., J. Backwell, D. Trujillo, S. Formica, M. Cabrera, G. Zorrilla Arcady Rodas, P. L. Castellanos, and J. Convit. 1994. Immune response in healthy volunteers vaccinated with killed leishmanial promastigotes plus BCG. I. Skin-test reactivity, T-cell proliferation and interferon-γ production. Vaccine 12:1041-1051. [DOI] [PubMed] [Google Scholar]
- 7.Coelho, P. S., A. Klein, A. Talvani, S. F. Coutinho, O. Takeuchi, S. Akira, J. S. Silva, H. Canizzaro, R. T. Gazzinelli, and M. M. Teixeira. 2002. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins isolated from Trypanosoma cruzi trypomastigotes induce in vivo leukocyte recruitment dependent on MCP-1 production by IFN-γ-primed-macrophages. J. Leukoc. Biol. 71:837-844. [PubMed] [Google Scholar]
- 8.De Smedt, T., B. Pajak, E. Muraille, L. Lespagnard, E. Heinen, P. De Baetselier, J. Urbain, O. Leo, and M. Moser. 1996. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J. Exp. Med. 184:1413-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Veer, M. J., J. M. Curtis, T. M. Baldwin, J. A. DiDonato, A. Sexton, M. J. McConville, E. Handman, and L. Schofield. 2003. MyD88 is essential for clearance of Leishmania major: possible role for lipophosphoglycan and Toll-like receptor 2 signaling. Eur. J. Immunol. 33:2822-2831. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald, K. A., and L. A. O'Neill. 2000. The role of the interleukin-1/Toll-like receptor superfamily in inflammation and host defence. Microbes Infect. 2:933-943. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh, M., C. Pal, M. Ray, S. Maitra, L. Mandal, and S. Bandyopadhyay. 2003. Dendritic cell-based immunotherapy combined with antimony-based chemotherapy cures established murine visceral leishmaniasis. J. Immunol. 170:5625-5629. [DOI] [PubMed] [Google Scholar]
- 12.Gurunathan, S., D. L. Sacks, D. R. Brown, S. L. Reiner, H. Charest, N. Glaichenhaus, and R. A. Seder. 1997. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania donovani. J. Exp. Med. 186:1137-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 14.Imler, J. L., and J. A. Hoffmann. 2001. Toll receptors in innate immunity. Trends Cell Biol. 11:304-311. [DOI] [PubMed] [Google Scholar]
- 15.Jakob, T., P. S. Walker, A. M. Krieg, M. C. Udey, and J. C. Vogel. 1998. Activation of cutaneous dendritic cells by CpG-containing oligodeoxynucleotides: a role for dendritic cells in the augmentation of Th1 responses by immunostimulatory DNA. J. Immunol. 161:3042-3049. [PubMed] [Google Scholar]
- 16.Jenkins, M. K., A. Khoruts, E. Ingulli, D. L. Mueller, S. J. McSorley, R. L. Reinhardt, A. Itano, and K. A. Pape. 2001. In vivo activation of antigen-specific CD4 T cells. Annu. Rev. Immunol. 19:23-45. [DOI] [PubMed] [Google Scholar]
- 17.Kaisho, T., and S. Akira. 2002. Toll-like receptors as adjuvant receptors. Biochim. Biophys. Acta 1589:1-13. [DOI] [PubMed] [Google Scholar]
- 18.Kamath, A. T., S. Henri, F. Battye, D. F. Tough, and K. Shortman. 2002. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood 100:1734-1741. [PubMed] [Google Scholar]
- 19.Khalil, E. A., A. M. El Hassan, E. E Zijlstra, M. M. Mukhtar, H. W. Ghalib, B. Musa, M. E. Ibrahim, A. A Kamil, M. Elsheikh, A. Babiker, and F. Modabber. 2000. Autoclaved Leishmania major vaccine for prevention of visceral leishmaniasis: a randomised, double blind, BCG-controlled trial in Sudan. Lancet 356:1565-1569. [DOI] [PubMed] [Google Scholar]
- 20.Maldonado-Lopez, R., C. Maliszewski, J. Urbain, and M. Moser. 2001. Cytokines regulate the capacity of CD8α+ and CD8α− dendritic cells to prime Th1/Th2 cells in vivo. J. Immunol. 167:4345-4350. [DOI] [PubMed] [Google Scholar]
- 21.McMahon-Pratt, D., D. Rodriguez, J.-R. Rodriguez, Y. Zhang, K. Manson, C. Bergman, L. Rivas, J. F. Rodriguez, K. L. Lohman, N. H. Ruddle, and M. Esteban. 1993. Recombinant vaccinia viruses expressing GP-46/M-2 protect against Leishmania infection. Infect. Immun. 61:3351-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Misslitz, A., J. C. Mottram, P. Overath, and T. Aebischer. 2000. Targeted integration into a rRNA locus results in uniform and high level expression of transgenes in Leishmania amastigotes. Mol. Biochem. Parasitol. 107:251-261. [DOI] [PubMed] [Google Scholar]
- 23.Moser, M., and K. M. Murphy. 2000. Dendritic cell regulation of TH1-TH2 development. Nat. Immunol. 1:199-205. [DOI] [PubMed] [Google Scholar]
- 24.Muraille, E., F. Andris, B. Pajak, K. M. Wissing, T. De Smedt, F. Desalle, M. Goldman, M. L. Alegre, J. Urbain, M. Moser, and O. Leo. 1999. Downregulation of antigen-presenting cell functions after administration of mitogenic anti-CD3 monoclonal antibodies in mice. Blood 94:4347-4357. [PubMed] [Google Scholar]
- 25.Muraille, E., C. De Trez, M. Brait, P. De Baetselier, O. Leo, and Y. Carlier. 2003. Genetically resistant mice lacking MyD88-adapter protein display a high susceptibility to Leishmania major infection associated with a polarized Th2 response. J. Immunol. 170:4237-4241. [DOI] [PubMed] [Google Scholar]
- 26.Muraille, E., C. De Trez, B. Pajak, M. Brait, J. Urbain, and O. Leo. 2002. T cell-dependent maturation of dendritic cells in response to bacterial superantigens. J. Immunol. 168:4352-4360. [DOI] [PubMed] [Google Scholar]
- 27.Muraille, E., C. De Trez, B. Pajak, F. A. Torrentera, P. De Baetselier, O. Leo, and Y. Carlier. 2003. Amastigote load and cell surface phenotype of infected cells from lesions and lymph nodes of susceptible and resistant mice infected with Leishmania major. Infect. Immun. 71:2704-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy, M. L., U. Wille, E. N. Villega, C. A. Hunter, and J. P. Farrell. 2001. IL-10 mediates susceptibility to Leishmania donovani infection. Eur. J. Immunol. 31:2848-2856. [DOI] [PubMed] [Google Scholar]
- 29.Nascimento, E., W. Mayrink, C. A. da Costa, M. S. Michalick, M. N. Melo, G. C. Barros, M. Dias, C. M. F. Antunes, M. S. Lima, D. C. Taboada, and T. Y. Liu. 1990. Vaccination of humans against cutaneous leishmaniasis: cellular and humoral immune responses. Infect. Immun. 58:2198-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pajak, B., T. De Smedt, V. Moulin, C. De Trez, R. Maldonado-Lopez, G. Vansanten, E. Briend, J. Urbain, O. Leo, and M. Moser. 2000. Immunohistowax processing, a new fixation and embedding method for light microscopy, which preserves antigen immunoreactivity and morphological structures: visualization of dendritic cells in peripheral organs. J. Clin. Pathol. 53:518-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi, H., V. Popov, and L. Soong. 2001. Leishmania amazonensis-dendritic cell interactions in vitro and the priming of parasite-specific CD4+ T cells in vivo. J. Immunol. 167:4534-4542. [DOI] [PubMed] [Google Scholar]
- 32.Reiner, S. L., and R. M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151-177. [DOI] [PubMed] [Google Scholar]
- 33.Ritter, U., A. Meissner, J. Ott, and H. Korner. 2003. Analysis of the maturation process of dendritic cells deficient for TNF and lymphotoxin-alpha reveals an essential role for TNF. J. Leukoc. Biol. 74:216-222. [DOI] [PubMed] [Google Scholar]
- 34.Santos, W. R., E. Paraguai de Souza, M. Palatnik, and C. B. Palatnik de Sousa. 1999. Vaccination of Swiss Albino mice against experimental visceral leishmaniasis with the FML antigen of Leishmania donovani. Vaccine 17:2554-2561. [DOI] [PubMed] [Google Scholar]
- 35.Skeiky, Y. A. W., J. A. Guderian, D. R. Benson, O. Bacelar, E. M. Carvalho, M. Kubin Badaro, G. Trinchieri, and S. G. Reed. 1995. A recombinant Leishmania antigen that stimulates human peripheral blood mononuclear cells to express a Th1-type cytokine profile and to produce interleukin 12. J. Exp. Med. 181:1527-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinman, R., M. Pack, and K. Inaba. 1997. Dendritic cells in the T-cell areas of lymphoid organs. Immunol. Rev. 156:25-37. [DOI] [PubMed] [Google Scholar]
- 37.Stenger, S., N. Donhauser, H. Thuring, M. Rollinghoff, and C. Bogdan. 1996. Reactivation of latent leishmaniasis by inhibition of inducible nitric oxide synthase. J. Exp. Med. 183:1501-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von Stebut, E., Y. Belkaid, T. Jakob, D. L. Sacks, and M. C. Udey. 1998. Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin-derived dendritic cells: implications for the initiation of anti-Leishmania immunity. J. Exp. Med. 188:1547-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yrlid, U., M. Svensson, A. Håkansson, B. J. Chambers, H.-G. Ljunggren, and M. J. Wick. 2001. In vivo activation of dendritic cells and T cells during Salmonella enterica serovar Typhimurium infection. Infect. Immun. 69:5726-5735. [DOI] [PMC free article] [PubMed] [Google Scholar]