Abstract
Interleukin-1β, tumor necrosis factor alpha, prostaglandin E2 (PGE2), and Porphyromonas gingivalis-specific immunoglobulin G levels in gingival crevicular fluid were measured in primates immunized with a P. gingivalis vaccine followed by ligature-induced periodontitis. Only PGE2 levels were dramatically suppressed (P < 0.0001) in immunized animals versus controls. A significant correlation (P < 0.027) was also found between PGE2 levels and decreased bone loss scores. This study presents the first evidence of a potential mechanism involved in periodontitis vaccine-induced suppression of bone loss in a nonhuman primate model and offers insight into the role of PGE2 in periodontal destruction.
Periodontitis is a chronic inflammatory condition that can result in tooth loss due to bone and tissue destruction. This disease is associated with pathogenic gram-negative bacteria, such as Porphyromonas gingivalis (4, 9, 13). The mechanisms by which P. gingivalis promotes periodontitis are still incompletely understood, although lipopolysaccharide (LPS) has been implicated. Bacterial LPS is a potent stimulator of many inflammatory mediators, including interleukin-1 beta (IL-1β), tumor necrosis factor alpha (TNF-α), and prostaglandin E2 (PGE2) (3, 5, 14), each of which is elevated in the nonhuman primate model of ligature-induced periodontitis (7, 10-12). The specific role of these mediators has yet to be determined, but local production of inflammatory mediators in the gingiva has been hypothesized to cause osteoclast activation and the resultant bone resorption that defines periodontitis. We previously reported that immunization of Macaca fascicularis monkeys with formalin-killed P. gingivalis resulted in less bone loss than in nonimmunized control animals in the ligature-induced periodontitis model (15). The mechanism for this protection was not determined, however. Therefore, this study examined the levels of IL-1β, TNF-α, PGE2, and P. gingivalis-specific immunoglobulin G (IgG) antibodies in the gingival crevicular fluid (GCF) from immunized versus nonimmunized macaques.
Ten M. fascicularis monkeys were immunized at weeks 0, 3, 6, and 16 with formalin-killed P. gingivalis strain 5083 in SAF adjuvant (Syntex Laboratories, Palo Alto, Calif.), while 10 control monkeys were injected with adjuvant only (15). Ligatures were placed subgingivally around the second premolar and first and second molar teeth in one mandibular and one contralateral maxillary posterior sextant in each animal. GCF was collected with paper strips (ProFlow, Inc., Amityville, N.Y.) from the sulcus or pocket of two maxillary (nonligated) and two mandibular ligated posterior teeth on the same side at each visit during the study. Mediator and antibody assays were performed on GCF samples collected at weeks 20 and 36, corresponding with bone height measurement points. A series of sequential enzyme-linked immunosorbent assays (ELISAs) were performed with the GCF samples. First, human IL-1β was measured by ELISA according to the kit's instructions (Cistron, Pinebrook, N.J.). A 100-μl aliquot of sample or standard was added to an IL-1β-precoated microtiter plate, and after the initial incubation, the sample was then transferred to a TNF-α ELISA assay plate (Genzyme, Cambridge, Mass.) and finally to an anti-P.gingivalis ELISA plate (6). An additional GCF aliquot was used to quantitate PGE2 in a competitive inhibition assay conducted according to the manufacturer's instructions (Amersham, Arlington Heights, Ill.). IL-1β, TNF-α, and PGE2 assays were read at an optical density at 450 nm (OD450) on a microplate reader (Molecular Devices Corp., Sunnyvale, Calif.), while anti-P. gingivalis IgG levels were read at OD405. Marginal bone height measurements were calculated from periapical radiographs as described previously (15). A multivariate repeated measures analysis (MANOVA) was used to compare mean levels between control and immunized monkeys to assess the effect of immunization and to compare mean levels between ligated and nonligated sites to assess the effects of ligation. Data for inflammatory mediators were analyzed on a natural log scale to normalize their distribution. Since the effects of ligation and immunization were similar at both time points, the data from weeks 20 and 36 were combined to decrease variability. If a significant interaction between the immunization effect and ligation effect was present (P < 0.01), separate MANOVAs were also performed for ligated and nonligated sites to assess the effect of immunization. In the same manner, separate analyses were performed to assess the effect of ligation for control and immunized monkeys. For all other comparisons, the significance level was set at P = 0.05. Statistical analysis was performed with SAS software (Cary, N.C.).
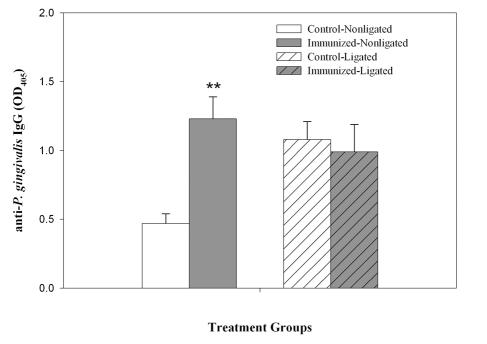
The systemic immunization of M. fascicularis with P. gingivalis resulted in an expected significant (P < 0.001) increase in anti-P.gingivalis IgG expression in GCF from nonligated sites (Fig. 1). Immunization had no apparent effect on the ligated sites, since these sites already exhibited relatively high levels of IgG in both immunized and control animals.
FIG. 1.
Effect of immunization on antibody expression. GCF samples collected from both ligated and nonligated sites from immunized and control animals were assayed for the P. gingivalis-specific antibody by ELISA. The data presented are the mean and standard error of triplicate assays. A statistically significant change from control nonligated animals is indicated by ** (P < 0.001).
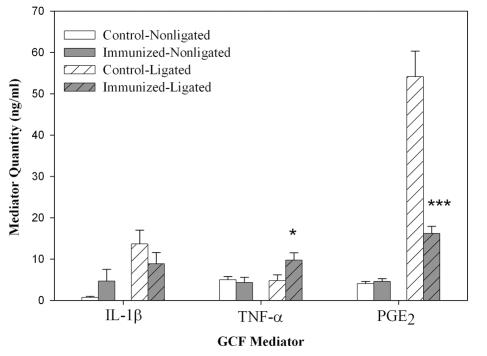
The effects of immunization on PGE2 expression were more pronounced in the GCF from ligated sites in control animals (Fig. 2). Ligation caused a 10-fold increase in PGE2 expression, which was significantly (P < 0.0001) reduced in M. fascicularis monkeys immunized with P. gingivalis. The levels of IL-1β, TNF-α, and PGE2 were low in control animals in nonligated sites, and the vaccination protocol did not appreciably decrease these already low levels. While IL-1β levels did not increase in ligated sites after immunization, the expression of TNF-α did show a small but significant increase (P < 0.05).
FIG. 2.
Effect of immunization and ligation on mediator expression. GCFs collected from immunized and control animals were assayed for the indicated inflammatory mediators. The data presented are the mean and standard error of triplicate assays. Statistically significant changes from ligated sites in control animals are indicated by * (P < 0.05) and *** (P < 0.0001).
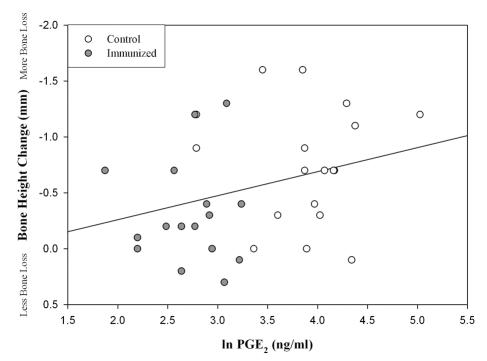
To determine the relationship between the mediator levels and bone height in our study, we plotted the mean PGE2 present in ligated sites as compared to their bone height measurements (Fig. 3). There was an apparent positive but weak correlation between PGE2 levels and bone height (r = 0.30, P = 0.027). The graph also illustrates how the majority of the immunized animals reported lower PGE2 levels and less bone loss than the control monkeys. Levels of the other mediators (IL-1β, TNF-α, and IgG) did not significantly correlate with bone height change (data not shown). A correlation trend (P < 0.07) also existed between PGE2 levels and computer-assisted densitometric image analysis bone density scores (data not shown).
FIG. 3.
Correlation of PGE2 with bone height. Shown are scatter plot and linear regression analyses of GCF levels of PGE2 compared to radiographic bone height measurements at ligated sites in control and immunized animals (P = 0.027, r = 0.30).
In a previous report, animals immunized with P. gingivalis displayed significant bone loss reduction compared to the control group (15). To investigate the possible mechanism involved in the immunization's protection against ligature-induced periodontitis, we assessed inflammatory mediator levels in the GCF of immunized and control animals at weeks 20 (first visit after ligature placement) and 36 (final visit). We chose to look at IL-1β, PGE2, and TNF-α, because their expression occurs in periodontitis along with the loss of connective tissue and bone. Although immunization minimally altered IL-1β and TNF-α levels, ligation caused a large increase in PGE2 in nonimmunized animals that was almost totally blocked by immunization. A weak but significant correlation was found between lower PGE2 levels in GCF from these sites and reduction in alveolar bone height measurements in the immunized animals (i.e., lower PGE2 corresponding to less bone loss). Therefore, other factors are also involved with PGE2 in bone loss.
While we saw slightly higher IL-1β and TNF-α levels in the immunized animals, the crevicular fluid PGE2 levels were markedly depressed, suggesting an immunization effect may have occurred prior to PGE2 production. One biological effect of PGE2 is bone demineralization (16), and therefore decreased PGE2 production would likely lead to a slower progression of alveolar bone loss and bone height. Although it is unclear why IL-1β and TNF-α levels were not reduced by immunization, decreased PGE2 production does suggest a possible mechanism for the protective effects of the vaccine. PGE2 is a major inflammatory mediator stimulated in response to a gram-negative infection (5, 8), and the importance of the levels of PGE2 in GCF may outweigh that of TNF-α and/or IL-1β. Interestingly, it is becoming clear that prostaglandins have a wider range of effects than previously thought and that localized concentrations found in periodontitis may induce other adverse health effects in the body (1, 2).
Only control animals showed a significant difference in GCF antibody levels between ligated and nonligated teeth. This is most likely due to local inoculation of P. gingivalis or other cross-reactive oral bacteria into the periodontal tissues via ligature placement in the control animals. In the immunized animals, a systemic antibody response was probably already in place, and therefore no significant increase in local antibody titers occurred from the ligature placement.
These animal model results support the association between high PGE2 levels and increased bone loss in periodontitis. They suggest that immunization may be associated with a decrease in PGE2 levels and reduced bone loss. Given that PGE2 is a major component involved in bone demineralization, further investigation into the mechanisms of blocking inflammatory bone loss by immunization is needed.
Acknowledgments
This work was supported by Public Health Service grants DE-12939, DE-00431, and DE-08555 from the National Institute of Dental and Craniofacial Research.
We thank Beth Hacker for assistance with the manuscript.
Editor: D. L. Burns
REFERENCES
- 1.Collins, J. G., H. W. Windley III, R. R. Arnold, and S. Offenbacher. 1994. Effects of a Porphyromonas gingivalis infection on inflammatory mediator response and pregnancy outcome in hamsters. Infect. Immun. 62:4356-4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deshpande, R. G., M. Khan, and C. A. Genco. 1998. Invasion strategies of the oral pathogen Porphyromonas gingivalis: implications for cardiovascular disease. Invasion Metastasis 18:57-69. [DOI] [PubMed] [Google Scholar]
- 3.Dinarello, C. A., S. Gatti, and T. Bartfai. 1999. Fever: links with an ancient receptor. Curr. Biol. 9:R147-R150. [DOI] [PubMed] [Google Scholar]
- 4.Haffajee, A. D., and S. S. Socransky. 1994. Microbial etiological agents of destructive periodontal diseases. Periodontol. 2000 5:78-111. [DOI] [PubMed] [Google Scholar]
- 5.Hersh, D., J. Weiss, and A. Zychlinsky. 1998. How bacteria initiate inflammation: aspects of the emerging story. Curr. Opin. Microbiol. 1:43-48. [DOI] [PubMed] [Google Scholar]
- 6.Houston, L. S., S. A. Lukehart, G. R. Persson, and R. C. Page. 1999. Function of anti-Porphyromonas gingivalis immunoglobulin classes in immunized Macaca fascicularis. Oral Microbiol. Immunol. 14:86-91. [DOI] [PubMed] [Google Scholar]
- 7.Hussain, M. Z., Q. P. Ghani, J. C. Zhang, B. Enriquez, C. Hayashi, and M. R. Wirthlin. 1994. Alterations of fibroblast metabolism in early ligature-induced periodontitis in the cynomolgus monkey. J. Periodontol. 65:771-775. [DOI] [PubMed] [Google Scholar]
- 8.Miyauchi, M., N. Ijuhin, H. Nikai, T. Takata, H. Ito, and I. Ogawa. 1992. Effect of exogenously applied prostaglandin E2 on alveolar bone loss—histometric analysis. J. Periodontol. 63:405-411. [DOI] [PubMed] [Google Scholar]
- 9.Moore, W. E., and L. V. Moore. 1994. The bacteria of periodontal diseases. Periodontol. 2000 5:66-77. [DOI] [PubMed] [Google Scholar]
- 10.Offenbacher, S., P. A. Heasman, and J. G. Collins. 1993. Modulation of host PGE2 secretion as a determinant of periodontal disease expression. J. Periodontol. 64:432-444. [DOI] [PubMed] [Google Scholar]
- 11.Offenbacher, S., B. M. Odle, L. D. Braswell, H. G. Johnson, C. M. Hall, H. McClure, J. L. Orkin, E. A. Strobert, and M. D. Green. 1989. Changes in cyclooxygenase metabolites in experimental periodontitis in Macaca mulatta. J. Periodontal Res. 24:63-74. [DOI] [PubMed] [Google Scholar]
- 12.Page, R. C. 1991. The role of inflammatory mediators in the pathogenesis of periodontal disease. J. Periodontal Res. 26:230-242. [DOI] [PubMed] [Google Scholar]
- 13.Page, R. C., and K. S. Kornman. 1997. The pathogenesis of human periodontitis: an introduction. Periodontol. 2000 14:9-11. [DOI] [PubMed] [Google Scholar]
- 14.Paludan, S. R. 2000. Synergistic action of pro-inflammatory agents: cellular and molecular aspects. J. Leukoc. Biol. 67:18-25. [DOI] [PubMed] [Google Scholar]
- 15.Persson, G. R., D. Engel, C. Whitney, R. Darveau, A. Weinberg, M. Brunsvold, and R. C. Page. 1994. Immunization against Porphyromonas gingivalis inhibits progression of experimental periodontitis in nonhuman primates. Infect. Immun. 62:1026-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raisz, L. G. 1999. Prostaglandins and bone: physiology and pathophysiology. Osteoarthritis Cartilage 7:419-421. [DOI] [PubMed] [Google Scholar]