Abstract
Background:
Prawns and shrimp are a frequent cause of seafood allergy mediated by IgE antibodies. Penaeus monodon and Penaeus latisulcatus, commonly known as black tiger prawn and king prawn, respectively, are among the most frequently consumed prawns in Malaysia. The aim of this study was to identify the IgE-binding proteins of these 2 prawn species.
Methods:
Raw and boiled prawn extracts were prepared and then resolved by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). IgE-immunoblotting was then performed using sera from patients with positive skin prick tests to the raw prawn extracts.
Results:
SDS-PAGE analysis of the raw extracts of both prawn species revealed 23 protein bands; the boiled extracts yielded fewer protein bands. The bands in the range of 40 to 100 kDa were sensitive to heat and therefore were not found in the boiled extracts. Immunoblot of raw extracts of black tiger prawns and king prawns yielded 14 and 11 IgE-binding proteins, respectively, with molecular weights of between 15 and 200 kDa. Proteins at 36, 42, and 49 kDa were detected as the major allergens in both species of prawns. A protein of 75 kDa was also identified as a major allergen in black tiger prawns. Other potential allergens were also observed at various molecular masses.
Conclusion:
Proteins of 36, 42, and 49 kDa were identified as the major allergens of both species of prawns. The 36 and 42 kDa proteins are hypothesised to be tropomyosin and arginine kinase, respectively. A high molecular weight protein of 75 kDa was found to be an additional major allergen in black tiger prawns.
Keywords: allergens, allergy and clinical immunology, hypersensitivity, Penaeus, immunoblotting, tropomyosin
Introduction
Prawns are classified in the phylum Arthropoda, subphylum Crustacean, class Malacostraca, and order Decapods. The term “prawn” is commonly used to describe any large shrimp (1). According to Holthuis (2), prawns include approximately 33 genera with approximately 2500 species, of which less than 300 species are of economic interest throughout the world. More than 20 species of prawns are found in Malaysia, including the black tiger prawn, the king prawn, the white prawn, the sharp-rostrum prawn, and the yellow prawn (3). Local data has reported that more than 60 000 tonnes of marine prawns are landed annually, with the number increasing every year (3).
IgE-mediated hypersensitivity reactions have become important global health issues (4). In the United States alone, a recent survey reported that 1 in 50 Americans has a type of shellfish allergy, including allergy to prawns (5). Exposure to prawn allergens can occur through ingestion of prawn-containing meals, or by inhalation or skin contact while cooking or working (6,7). Sensitised individuals can develop urticaria, angio-oedema, asthma, or life-threatening anaphylactic reactions (8–10).
Over the last decade, various worldwide studies have identified tropomyosin as the major seafood allergen (9,11,12). Tropomyosin, a myofibrillar protein consisting of 2 subunits with molecular masses of 34–38 kDa, has been well-documented as the major cross-reactive allergen of various species of prawns and shrimp, including brown shrimp (Pen a 1), black tiger shrimp (Pen m 1), and white leg pacific shrimp (Lit v 1) (13–15). Besides tropomyosin, a 40-kDa p known as arginine kinase has also been detected as an important minor allergen in black tiger shrimp (4,10). More recently, 2 new prawn allergens, sarcoplasmic calcium-binding protein and myosin light-chain, have also been characterised (1,16).
Penaeus monodon (black tiger prawn) and Penaeus latisulcatus (king prawn), 2 important aquaculture species in Asia, are commonly consumed in Malaysia. Both of these prawns are mainly caught in the coastal waters off the Indo-West Pacific, Southeast Asia, and South China Sea (3). There have not been any local studies characterising prawn allergens. Thus, the purpose of this study was to identify the major allergens of these 2 species of prawn among our local patients with prawn allergy.
Materials and Methods
Prawn extract preparation
Fresh black tiger prawns and king prawns were obtained from a prawn aquaculture pond in Selangor, Malaysia. Raw and boiled extracts were prepared following the procedure described by Daul et al. (9), with slight modifications. Briefly, the shell and meat of each species were blended in 1 M phosphate-buffered saline (pH 7.2) and extracted overnight at 4 °C under constant mixing. The homogenates were centrifuged at 14 000 rpm at 4 °C for 15 minutes. The supernatants were then sterile-filtered, lyophilised, and stored at −20 °C until use. For preparation of the boiled extracts, the homogenates of the prawns were boiled for 5 minutes before extraction, as described above. The protein concentration of each extract was determined using the Total Protein Kit (Sigma-Aldrich, UK) and bovine serum albumin as a standard.
SDS-PAGE of prawn extracts
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described by Nakano et al. (17), with some modifications. The samples of the extracts were heated at 97 °C for 4 minutes, and Precision Plus Protein Standards (Bio-Rad, USA) were used as protein markers. Protein bands were resolved by SDS-PAGE using 12% separating gels with 5% stacking gels using a Mini Protean 3 Apparatus (Bio-Rad, USA) at 120 mA for 45 minutes. The resulting protein bands were stained with Coomassie Brilliant Blue R-250 (Bio-Rad, USA) and analysed using a densitometer instrument (Bio-Rad, USA).
Sera
Sera from patients with histories of prawn allergy and demonstrated positive skin prick tests to raw extracts of black tiger prawn (20 patients) and king prawn (22 patients) were used in this study. Skin prick tests were performed at the Allergy Clinic, Hospital Kuala Lumpur, by a medical officer. This study was approved by the Medical Research and Ethics Committee, Ministry of Health, Malaysia.
IgE-immunoblotting
IgE-immunoblotting experiments were performed to determine the specific IgE-binding proteins in the raw extracts of black tiger prawn and king prawn. After electrophoresis, the resolved proteins were electrophoretically transferred onto nitrocellulose 0.45 μm membranes using the Mini Trans-Blot System (Bio-Rad, USA) at 100 V for 70 minutes. The membranes were stained with Ponceau S (Sigma-Aldrich, USA) and cut into 3-mm strips. The strips were washed with Tween-20 Tris-buffered saline (Bio-Rad, USA) and blocked with blocking buffer containing 5% non-fat milk in Tris-buffered saline for 2 hours. The blocked strips were incubated overnight with each individual patient’s serum at 4 °C under constant mixing. The strips were incubated with biotinylated goat anti-human IgE (Kirkergaard and Perry Laboratories, UK) as a secondary antibody for 30 minutes, followed by incubation with streptavidin-conjugated alkaline phosphatase (Bio-Rad, USA) for 30 minutes. Detection of the antibody-bound complexes was conducted using the Alkaline Phosphatase Conjugate Substrate Kit (Bio-Rad, USA). Immunoblotting results were analysed using a densitometer analyser (Bio-Rad, USA). Each plate or set of strips contained a blank (no serum) and a negative control (normal serum).
Results
SDS-PAGE of prawn extracts
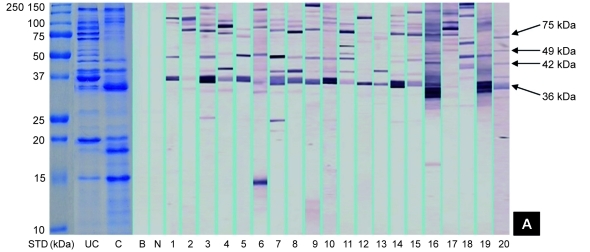
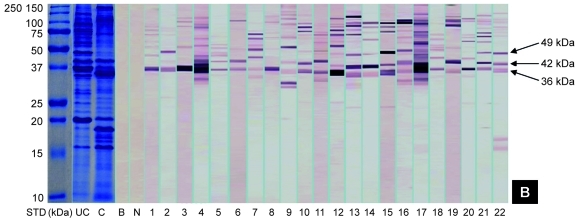
SDS-PAGE gels of raw and boiled extracts of both prawn species are shown in Figure 1. Protein profile of raw extracts of both prawns revealed approximately 23 protein bands at various molecular weights between 15 and 200 kDa, whereas the boiled extracts of black tiger prawns and king prawns contained 18 and 16 protein bands, respectively. Several protein bands between 40 and 100 kDa were not detected in the boiled extracts of both prawns. However, enhancement of the band at 18 kDa was found in both boiled extracts. A 36-kDa protein, which is likely to be tropomyosin, was detected in all extracts.
Figure 1:
Protein profiles and IgE-binding patterns of raw (A) black tiger prawns and (B) king prawns. Lane STD consists of molecular weight markers (in kDa). Lanes UC and C contain protein profiles of raw and boiled extracts, respectively. Lanes B and N contain blank and negative controls, respectively. Lanes 1 to 22 contain the immunoblotting results of different serum samples.
IgE-immunoblotting
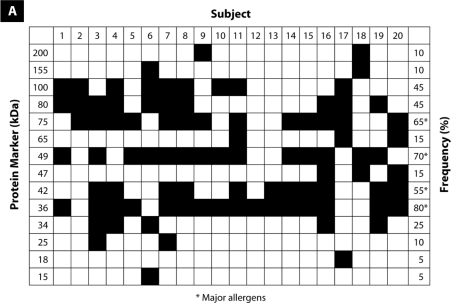
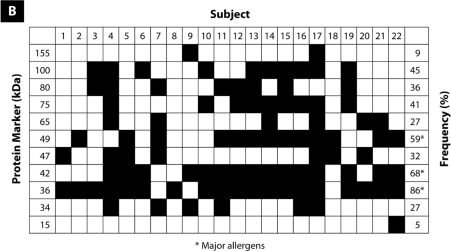
As shown in Figure 1, immunoblotting of the raw black tiger prawn extracts identified 14 IgE-binding proteins at various molecular weights between 15 and 200 kDa, whereas immunoblotting of king prawn extracts identified 11 IgE-binding proteins ranging from 15 to 155 kDa. Four major IgE-binding components at the molecular masses of 36, 42, 49, and 75 kDa were detected in black tiger prawn extracts, whereas the king prawn extracts revealed 3 major IgE-binding proteins at 36, 42, and 49 kDa. Several potential minor allergens at 80 and 100 kDa have also been observed in both species of prawns. IgE-binding profiles of all sera tested are summarised in Figure 2. No binding was observed in the negative control and blank strips.
Figure 2:
The frequency of specific IgE-binding of sera from patients with raw (A) black tiger prawn allergy and (B) king prawn allergy by immunoblotting analysis.
Discussion
Prawn allergy is a long-lasting and potentially life-threatening disorder (18). In Malaysia, large varieties of prawn and shrimp species are used for human consumption. Although seafood, especially prawns, has been reported to be one of the most frequent causes of allergic reactions among local patients (19), there have not been any reports on the characterisation of local prawn allergens.
In our SDS-PAGE analysis, the raw extracts of black tiger prawns and king prawns revealed similar protein profiles. Most protein bands were located approximately 20, 36, and 50 to 100 kDa. Other researchers have also demonstrated similar findings (9,20,21). Very few studies have focused on the thermal effects on seafood protein (21,22). In our study, SDS-PAGE analysis of the boiled prawn extracts yielded fewer protein bands than raw prawn extracts due to denaturation of several protein bands in the range of 40 to 100 kDa. The reduction and loss of bands in the boiled extracts might be due to a loss of secondary and tertiary protein structures (20). Previous studies have reported that at temperatures above 55 °C, proteins become unfolded and lose their secondary and tertiary structures while retaining their primary structure (20,23). In this study, bands at 18, 20, 36, and ∼150 kDa were found to be heat-resistant, with enhanced intensity at the 18-kDa band with applied heat. This finding is similar to other studies that reported several heat-resistant proteins bands at 20, 36, and 70 kDa (20,24). The enhancement of the 18-kDa band might be due to various basic-charged proteins that became exposed due to unfolding, which might have resulted in increased interactions with the Coomassie blue dye molecules (23).
In our study, we detected several major IgE-binding proteins at 36, 42, 49, and 75 kDa in black tiger prawn extracts, and at 36, 42, and 49 kDa in king prawn extracts. We found that 80% and 86% of patients with black tiger prawn and king prawn allergy, respectively, exhibited IgE-binding proteins at 36 kDa. Tropomyosin, a 34 to 36 kDa heat-resistant protein, has been established as a major allergen in invertebrates, including prawn, crab, cockroach, and house dust mites (9,11,12,21). It is possible that our 36-kDa protein might be similar to the previously reported tropomyosin allergen from other prawn and shrimp species. Currently, tropomyosin is the most well-characterised prawn allergen, and its IgE-binding epitopes have been identified (14,25).
Besides tropomyosin, arginine kinase, with a molecular weight of 40 kDa, has also been reported as a crustacean allergen (10). Previous studies have identified a heat-sensitive prawn allergen from Penaeus monodon, Pem m 2, as arginine kinase (4,10). In this study, we hypothesise that our 42-kDa protein, which is present in 55% and 68% of our subjects allergic to black tiger prawn and king prawn, respectively, could be arginine kinase. Arginine kinase is a phosphagen kinase that plays a key role in energy metabolism in invertebrates (10). It has also been identified as a pan-allergen in other invertebrates, such as the moth, cockroach, and lobster (1).
Additional major allergens were also detected in our immunoblotting experiment. A protein at 49 kDa was identified as a major allergen in both prawn species, whereas a protein at 75 kDa was found to be a major allergen in only black tiger prawns. Although there have not been reports on the characterisation of these 2 allergens, other immunoblotting studies have identified these proteins as minor allergens (14,19,26). However, the detailed identification of these proteins has not yet been performed.
Conclusion
Our study identified proteins at the molecular masses of 36, 42, and 49 kDa as the major allergens of black tiger prawns and king prawns. Black tiger prawns contained an additional major allergen at 75 kDa. However, to confirm the results, further identification and characterisation of these major allergens should be done using immunological analyses of larger populations.
Acknowledgments
We wish to thank the Director General of Health for his permission to publish this paper. This work was supported by a grant from the Ministry of Health (research ID: JPP-IMR 07-035, ethical approval: NMRR-08-763-1918).
Footnotes
Authors’ Contributions
Conception and design, analysis and interpretation of the data, critical revision of the article: SS, RM, ZHMY
Obtaining of funding: ZHMY
Drafting of the article: SS
Final approval of the article: JM, SM
Administrative, technical, or logistic support: ZHMY
References
- 1.Ayuso R, Grishina G, Bardina L, Carrillo T, Blanco C, Ibanez MD, et al. Myosin light chain is a novel shrimp allergen, Lit v 3. J Allergy Clin Immunol. 2008;122(4):795–802. doi: 10.1016/j.jaci.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 2.Holthuis LB. FAO species catalogue. Vol. 1. Shrimps and prawns of the world. An annotated catalogue of species of interest to fisheries. FAO Fisheries Synopsis 125 (Vol. 1) New York (NY): Unipub; 1980. [Google Scholar]
- 3.Landings and marine fish at west coast of Peninsular Malaysia. Putrajaya (MY): Department of Fisheries Malaysia; 2006. [cited 2008 Jul 2] Available from: http://www.dof.gov.my. [Google Scholar]
- 4.Yu CJ, Lin YF, Chiang BL, Chow LP. Proteomics and immunological analysis of a novel shrimp allergen, Pen m 2. J Immunol. 2003;170(1):445–453. doi: 10.4049/jimmunol.170.1.445. [DOI] [PubMed] [Google Scholar]
- 5.Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of seafood allergy in the United States determined by a random telephone survey. J Allergy Clin Immunol. 2004;114(1):159–166. doi: 10.1016/j.jaci.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 6.Seitz CS, Brocker EB, Trautmann A. Occupational allergy due to seafood delivery: Case report. J Occup Med Toxicol. 2008;3:11. doi: 10.1186/1745-6673-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehrer SB, Ayuso R, Reese G. Current understanding of food allergens. Ann N Y Acad Sci. 2002;964:69–85. doi: 10.1111/j.1749-6632.2002.tb04133.x. [DOI] [PubMed] [Google Scholar]
- 8.Bush RK. Approach to patient with symptoms of food allergy. Am J Med. 2008;121(5):376–378. doi: 10.1016/j.amjmed.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 9.Daul CB, Slattery M, Reese G, Lehrer SB. Identification of major brown shrimp (Penaeus aztecus) allergen as the muscle protein tropomyosin. Int Arch Allergy Immunol. 1994;105(1):49–55. doi: 10.1159/000236802. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Orozco KD, Aispuro-Hernandez E, Yepiz-Plascencia G, Calderon-de-la-Barca AM, Sotelo-Mundo RR. Molecular characterization of arginine kinase, an allergen from the shrimp Litopenaeus vannamei. Int Arch Allergy Immunol. 2007;144(1):23–28. doi: 10.1159/000102610. [DOI] [PubMed] [Google Scholar]
- 11.Lehrer SB, Ayuso R, Reese G. Seafood allergy and allergens: A review. Mar Biotechnol. 2003;5(4):339–348. doi: 10.1007/s10126-002-0082-1. [DOI] [PubMed] [Google Scholar]
- 12.Liang Y, Cao M, Su W, Zhang L, Huang Y, Liu G. Identification and characterisation of the major allergen of Chinese mitten crab (Eriocheir sinensis) Food Chem. 2008;111(4):998–1003. [Google Scholar]
- 13.Ayuso R, Reese G, Leong-Kee S, Plante M, Lehrer SB. Molecular basis of arthropod cross-reactivity: IgE-binding cross-reactive epitopes of shrimp, house dust mite and cockroach tropomyosins. Int Arch Allergy Immunol. 2002;129(1):38–48. doi: 10.1159/000065172. [DOI] [PubMed] [Google Scholar]
- 14.Reese G, Ayuso R, Lehrer SB. Tropomyosin: An invertebrate pan-allergen. Int Arch Allergy Immunol. 1999;119(4):247–258. doi: 10.1159/000024201. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Matsuo H, Morita E. Cross-reactivity among shrimp, crab and scallops in a patient with a seafood allergy. J Dermatol. 2006;33(3):174–177. doi: 10.1111/j.1346-8138.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- 16.Shiomi K, Sato Y, Hamamoto S, Mita H, Shimakura K. Sarcoplasmic calcium-binding protein: Identification as a new allergen of the black tiger shrimp Penaeus monodon. Int Arch Allergy Immunol. 2008;146(2):91–98. doi: 10.1159/000113512. [DOI] [PubMed] [Google Scholar]
- 17.Nakano S, Yoshinuma T, Yamada T. Reactivity of shrimp allergy-related IgE antibodies to krill tropomyosin. Int Arch Allergy Immunol. 2008;145(3):175–181. doi: 10.1159/000109286. [DOI] [PubMed] [Google Scholar]
- 18.Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001–2006. J Allergy Clin Immunol. 2007;119(4):1016–1024. doi: 10.1016/j.jaci.2006.12.622. [DOI] [PubMed] [Google Scholar]
- 19.Shahnaz M, Gendeh BS, Nasuruddin A. Skin test reactivity to inhalant and food allergens in patients with allergic rhinitis. Int Med Res J. 2001;5(2):69–73. [Google Scholar]
- 20.Samson KT, Chen FH, Miura K, Odajima Y, Iikura Y, Naval Rivas M, et al. IgE binding to raw and boiled shrimp proteins in atopic and nonatopic patients with adverse reaction to shrimp. Int Arch Allergy Immunol. 2004;133(3):225–232. doi: 10.1159/000076828. [DOI] [PubMed] [Google Scholar]
- 21.Shimakura K, Tonomura Y, Hamada Y, Nagashima Y, Shiomi K. Allergenicity of crustacean extractives and its reduction by protease digestion. Food Chem. 2005;91(2):247–253. [Google Scholar]
- 22.Paschke A, Besler M. Stability of bovine allergens during food processing. Ann Allergy Asthma Immunol. 2002;89(6 Suppl 1):16–20. doi: 10.1016/s1081-1206(10)62117-5. [DOI] [PubMed] [Google Scholar]
- 23.Davis PJ, William SC. Protein modification by thermal processing. Allergy. 1998;53(46 Suppl):102–105. doi: 10.1111/j.1398-9995.1998.tb04975.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhenxing L, Hong L, Limin C, Jamil K. Impact of irradiation and thermal processing on the immunoreactivity of shrimp (Penaeus vannamei) proteins. J SciFood Agr. 2007;87(6):951–956. [Google Scholar]
- 25.Chiou YH, You CY, Wang LY, Huang SP. Detection of cross-reactivity for atopic immunoglobulin E against multiple allergens. Clin Diagn Lab Immunol. 2003;10(2):229–232. doi: 10.1128/CDLI.10.2.229-232.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leung PS, Chow WK, Duffey S, Kwan HS, Gershwin ME, Chu KH. IgE reactivity against a cross-reactive allergen in crustacean and mollusca: Evidence for tropomyosin as the common allergen. J Allergy Clin Immunol. 1996;98(5 Pt 1):954–961. doi: 10.1016/s0091-6749(96)80012-1. [DOI] [PubMed] [Google Scholar]