Abstract
Chiral PCB congeners are major components of PCB mixtures and undergo enantioselective biotransformation to hydroxylated (OH-)PCBs by cytochrome P450 enzymes. While it is known that biotransformation results in an enantiomeric enrichment of the parent PCB, it is currently unknown if OH-PCBs are formed enantioselectively. The present study screened seven commercial capillary gas chromatography columns containing modified β- or γ-cyclodextrins for their potential to separate the atropisomers of methylated derivatives of OH-PCB. The atropisomers of 3-, 4- and 5-methoxy derivatives were at least partially separated on one or more columns. A subsequent biotransformation study was performed with rat liver microsomes to assess if hydroxylated metabolites are formed enantioselectively from PCBs 91, 95, 132 and 149. The OH-PCBs were extracted from the microsomal incubations, derivatized with diazomethane and analyzed as the respective methoxylated (MeO-)PCB derivatives using selected columns. The 5-hydroxylated metabolites of PCBs 91, 95, 132 and 149 were the major metabolites, which is consistent with PCB’s biotransformation by cytochrome P450 2B enzymes. All 5-hydroxylated metabolites displayed a clear, congener-specific enantiomeric enrichment. Overall, this study demonstrates for the first time that chiral PCBs, such as PCB 91, 95, 132 and 149, are enantioselectively metabolized to OH-PCBs by cytochrome P450 enzymes.
Keywords: metabolites, atropisomers, enantioselective gas chromatography, cytochrome P450 enzymes
Introduction
Polychlorinated biphenyls (PCBs) remain an important class of environmental contaminants, even forty years after their production was banned in the US. PCB levels did not show a clear decline in the general US population from 1999 to 2004 (1), thus suggesting ongoing PCB exposure via the diet (2) and by inhalation of indoor air (3). Human epidemiological data consistently show a negative association between developmental exposure to environmental PCBs and cognitive function in infancy or childhood (4). Specifically, in utero and lactational exposures to multiple-ortho substituted PCB congeners correlate with decreased intelligence quotients, impaired learning and memory, attentional deficits, and lowered reading comprehension. These PCB congeners and their hydroxylated metabolites cause developmental neurotoxicity in laboratory studies by mechanisms involving altered Ca2+ signaling, interference with thyroid hormone signaling and decreased dopamine content (5). Ryanodine receptor (RyR) sensitization has been shown to be the most sensitive mechanism mediating PCB’s effect on Ca2+ signaling. A recent study demonstrates that PCB 136 enantiospecifically sensitizes RyRs, with only (-)-PCB 136 being active (6).
Although PCBs are considered to be persistent organic pollutants, many PCB congeners, especially congeners with vicinal hydrogen substituents, are biotransformed through complicated metabolic pathway to hydroxylated (OH-PCB) and methylsulfonylated metabolites (MeSO2-PCB) (7). OH-PCB can be formed by direct insertion of oxygen into an aromatic C-H bond or via an epoxide intermediate. The epoxide may rearrange to OH-PCBs, be conjugated with glutathione in a glutathione transferase catalyzed reaction or react with other cellular nucleophiles, such as proteins and DNA. Several OH-PCBs, but not analogous MeSO2-PCBs, have been shown to be neurotoxic and to sensitize RyRs (8). Many of these neurotoxic metabolites display axial chirality and exist as rotational isomers which are non-superimposable mirror images of each other (9). Such non-superimposable molecules are called enantiomers or, in the case of the rotational isomers of multiple-ortho substituted PCB congeners, atropisomers. Chiral PCB metabolites can be formed by biotransformation of both chiral or prochiral PCBs.
Analogous to the parent compounds (9), it is likely that chiral OH- and MeSO2-PCBs also undergo enantiomeric enrichment in vivo and display enantioselective toxicity. Indeed, several studies report enantiomeric enrichment of MeSO2-PCB in wild-life (10–14), human (15) and laboratory animal studies (16, 17). A study by Norström et al. showed that meta- and para-methylsulfonyl metabolites of PCB 132 are formed enantiospecifically in rats (17). In contrast, the enantiomeric enrichment of chiral OH-PCBs is poorly investigated and to date only one study demonstrated the enantiomeric enrichment of two OH-PCBs after intraperitoneal administration of racemic PCB 136 in rats (18).
It is currently unclear if the enantiomeric enrichment of chiral PCB metabolites, in particular OH-PCBs, in vivo is due to their enantioselective formation by cytochrome P450 enzymes (P450 enzymes) or other, enantioselective phase II biotransformation processes. A recent study by Warner et al. provides indirect evidence that PCB metabolism by cytochrome P450 enzymes may result in the enantioselective formation of OH-PCBs (19). While the authors demonstrated that PCB 132 atropisomers are enantioselectively metabolized by recombinant rat and human cytochrome P450 enzymes, it is unclear which metabolites were formed and if these metabolites displayed enantiomeric enrichment. In the present study we use a suite of putative metabolites of neurotoxic PCBs 91, 95, 132 and 149 to first develop gas chromatographic methods for the separation of the OH-PCB atropisomers. Subsequently, these separation methods were utilized to investigate the enantioselective formation of OH-PCB by hepatic microsomes. These studies demonstrate the enantioselective formation of 5- hydroxylated PCBs by cytochrome P450 enzymes, an observation that has implication for understanding the mechanisms of PCB neurotoxicity and, ultimately, assessing the risk of developmental neurotoxicity in PCB exposed populations.
Experimental section
Methoxylated derivatives of PCBs
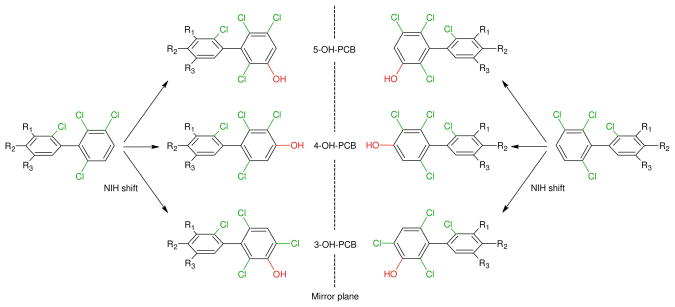
A series of racemic 4-, 5-methoxy and 4,5-di-methoxy- derivatives of PCBs 91, 95, 132 and 149 and respective NIH-shift products (Figure 1) were synthesized and characterized as described previously (18) (see Table S1 for a list of compounds). The nomenclature of the PCBs is according to the revised Ballschmiter nomenclature (20). The nomenclature of the metabolites follows the recommendation of Maervoet et al. (21) and is presented in Table S1.
Figure 1.
Formation of putative metabolites of PCB atropisomers with the OH-group in the 2,3,6-trichlorinated ring by rat liver microsomes (PCB 91: R2 = Cl; R1 = R3 = H; PCB 95: R3 = Cl; R1 = R2;= H; PCB 132: R1 = R2 = Cl; R3 = H; PCB 149: R2 = R3 = Cl; R1 = H).
Enantioselective gas chromatography
Atropisomers of methoxylated PCBs were separated using an Agilent 7890A gas chromatograph equipped with an electron capture detector (μ-ECD) using the following enantioselective columns: HP-Chiral-20B (20B) and Cyclosil-B (CB) from Agilent, Chirasil-Dex (CD) from Varian, BGB-172 (BGB) for BGB Analytic, and Chiral-Dex B-DM (BDM), Chiral-Dex B-PM (B-PM) and Chiral-Dex G-TA (GTA) columns from Supelco Analytical (see Table S3 for details). The compounds were first analyzed individually using the following temperature program: 50°C for 1 min, 10°C/min to 140°C, hold for 20 min, 1°C/min to column maximum temperature, hold for 20 min (22) to establish retention times and elution order as well as identify atropisomers of methoxylated PCBs separating on these columns for further optimization. The injector and detector were kept at 250°C. The flow was set to 1 mL/min.
Subsequently, the separation of methoxylated PCBs that partially separated or showed significant peak widening on a particular column was further optimized. Because the resolution of PCB atropisomers improves with decreasing analysis temperature (Figure S1) (18, 23), temperature programs with a long isothermal hold at progressively lower temperatures were used: 50°C for 1 min, 10°C/min to X, 10°C to 225°C, hold for 10 min, where X is in the range from 140°C to 180°C. The flow in these analyses was 3 mL/min to achieve elution in a reasonable amount of time. The length of the temperature programs varied from 220 min at 180°C to 500 min at 140°C. In some cases an analysis time of 8 h was not sufficient to elute the methoxylated metabolite. These long analysis times were considered impractical for enantioselective analysis, even if improvement of separation was observed. Atropisomers of PCBs in the microsomal incubation mixtures (see below) were analyzed on the CD column using the following temperature program: 2°C/min from 100°C to 150°C, 0.2°C/min to 185°C, 15°C to 200°C (22, 24). The conditions provided acceptable resolution (from 0.63 to 0.89) for all four studied congeners.
Microsomal incubations and extraction
Microsomal incubations consisted of 0.1 M phosphate buffer (pH 7.4), 3 mM magnesium chloride, 0.5 mM NADPH and 0.77 mg/mL of microsomal protein in a final volume of 16 mL. After 5 min of pre-incubation, 10 mM solution of PCB in DMSO was added (80 μl, 0.5% total volume) and the samples were incubated at 37±1°C in a shaking water bath for 30 min. Control samples with DMSO alone were incubated at the same time and no metabolites were detected in these blank samples. The reaction was quenched by adding 2 mL of ice-cold 0.5M NaOH and subsequent heating of the samples at 90°C for 10 min. Metabolites and remaining parent compound were extracted from the acidified reaction mixture (1 mL of 6M HCl) with 2-propanol (2 mL) and hexane-MTBE (5 mL, 1:1 v/v) (24, 25). Metabolites were separated from the parent compound by partitioning of OH-PCBs into KOH (10 mL, 0.5M) and re-extracting them with hexane-MTBE (8 mL, 9:1 v/v) from acidified solution (3 mL of 6M HCl). After exchanging the solvent to hexane, OH-PCBs were derivatized with diazomethane (26) and the MeO-PCBs were cleaned-up using sulfuric acid (27, 28) before gas chromatographic analysis.
Gas chromatographic analysis
In addition to the enantioselective analysis, samples from microsomal incubations were analyzed on Agilent 6890 gas chromatograph (GC) with a 5975 mass selective detector in both total and selective ion monitoring modes. The GC was equipped with a SLB-5ms column (Supelco, 60 m, 250 μm × 0.25 μm). The following temperature program was used: 100°C hold for 1 min, 5°C/min to 250°C, hold for 20 min, 5°C/min to 280°C, hold for 3 min. The injector temperature was 280°C and the MS temperatures were 280°C, 230°C and 150°C for transfer line, source and quadrupole, respectively. In total ion scan, a mass range of m/z 50 to 500 was recorded. In selective ion monitoring mode, ions m/z 326, 356 and 386 were used to scan for metabolites of pentachlorobiphenyls, and 360, 390 and 420 for metabolites of hexachlorobiphenyls. The same chromatographic conditions were employed for GC-ECD analyses with a SPB-1 column (Supelco, 60 m, 250 μm × 0.25 μm). The detector temperature for these analyses was 300°C and the flow rate was 1.0 mL/min.
Results and discussion
Enantioselective separation of methoxylated PCBs
A major obstacle towards studying the enantioselective metabolism of PCBs is the lack of good separation methods for methoxylated derivatives of relevant chiral OH-PCBs. Although we recently reported the separation of MeO-PCB atropisomers with the CD column, the resolution on this column was unsatisfactory (18). Therefore, a set of seven enantioselective columns was obtained from commercial sources to optimize the separation of methoxylated PCB atropisomers (Table S3). This set of columns is similar to the one used previously by Wong and Garrison (22) in a study of the separation of PCB atropisomers. Six columns contained a β-cyclodextrin-based stationary phase, a stationary phase that is frequently used in gas and liquid chromatographic separations of PCB atropisomers (22, 29) and methoxylated PCB derivatives (18, 30). These six columns differ in the modification of the β-cyclodextrin as well as the polysiloxane backbone. For example, the CD and BPM columns contain a permethylated β-cyclodextrin phase that is chemically bound to the polysiloxane backbone in the CD but not the BPM column. Additionally, a single γ-cyclodextrin column was studied (GTA). This chiral selector has also been used for the enantioselective separation of PCBs (22).
Atropisomeric separations of a few MeO-PCBs were comparatively straightforward. Specifically, the atropisomers of 5–91 (see Table S1 for the MeO-PCB nomenclature) were separated on all 7 columns used in this study (Figure S2B and S3), 5′-132 atropisomers were resolved on 6 columns (except of 20B column) and 5–95 atropisomers were separated on 5 columns (Table S1). These three compounds have a 5-methoxy group and a 2,3,6 chlorine substitution pattern in the same phenyl ring. Interestingly, Haglund reported that a 2,3,6- chlorine substitution pattern in one phenyl ring favors the separation of PCBs on β-cyclodextrin phases (29, 31). This suggests that a methoxy substituent in 5-position may have a relatively small effect on the enantioselective separation of MeO-PCBs on the columns investigated.
Similarly, all seven NIH shift products have a 2,3,4,6-substitution pattern (i.e. MeO-PCBs with a 2,4,6-chlorine substitution pattern and a 3-methoxy groups in the same phenyl ring) resolved to some extent of the different enantioselective columns, with two of the seven NIH shift products separating on four different columns (3–98 and 3–154) and all other atropisomers separating on at least one column (Table S1 and S2). The only exception was 3–50, which did not separate on any column. Similarly, chiral PCB congeners with a 2,3,4,6-substitution pattern were resolved easily on various chiral columns (22). The observation that 3–98, but not 3–50 atropisomers separated on most stationary phases is consistent with earlier observations on the CD phase (18). Analogous to the parent PCBs (29), a 2,3-dichloro substitution in the less lipophilic, non-methoxylated phenyl ring is required to achieve a separation of the MeO-PCB atropisomers of the NIH shift products on the CD column. This relationship between chemical structure and resolution appears to extend to the chiral stationary phases investigated in this study.
In contrast, the resolution of mono-methoxylated compounds with a 4-methoxy group was poorer compared to the structurally analogous 5-methoxylated PCBs. The best separations were observed for 4–91 (Rs = 0.79; BDM column), 4–95 (Rs = 0.78; BGB column) and 4–136 (Rs = 0.83; CB column), whereas 4′-132 atropisomers were not resolved on any column used in this study. Chiral PCBs with 4,5-dimethoxy groups did not resolve on any of the columns under investigation. The only exception was 4,5–95, which resolved on the BDM and CB columns. Together, these observations suggest that a 4-methoxy group, but not a 4-chlorine substituent prevents the separation of atropisomers on the chiral columns investigated. In the case of pentachlorinated PCBs with 4-methoxy groups, the more lipophilic methoxylated phenyls ring is likely to partition into the respective cavity of the chiral selector (18, 29). Therefore, one plausible explanation for the overall poor separation of PCB atropisomers with a 4-methoxy group is an unfavorable interaction of the 4-methoxy substituent with functional group in the cavity of the chiral selector. However, further studies are needed to confirm this hypothesis.
Formation of hydroxylated metabolites
After optimizing the enantioselective separations, a series of microsomal metabolism experiments were performed with racemic PCB 91, 95, 132 and 149 to determine the metabolite profile and, ultimately, assess if the atropisomers of the major OH-PCBs are formed enantioselectively. Highly ortho substituted PCBs with 2,3,6-trichloro substituted phenyl rings are readily metabolized in reactions catalyzed by cytochrome P450 enzymes to OH-PCBs with the OH-group in meta or para position (Figure 1). In addition, an NIH shift can result in the formation of a 2,4,6-trichloro-3-hydroxy-substitution pattern. The cytochrome P450 isoforms responsible for the metabolism of these PCB congeners are cytochrome P450 2B enzymes, such as 2B1 and 2B4 (19, 32, 33). Since rat cytochrome P450 2B enzymes are induced by phenobarbital in rat livers, the present study used liver microsomes obtained from phenobarbital-treated rats. The cytochrome P450 2B activity, measured as BROD activity, was 13100 pmol/min/mg protein, which represents almost a 100-fold increase compared to corn-oil treated animals (data not shown).
PCBs 91, 95, 132 and 149, were incubated at concentrations of 50 μM for 30 min with liver microsomes. These PCB congeners are highly neurotoxic (8) and have a 2,3,6-trichloro substitution pattern in one ring. Based on work by Schnellmann et al. with the structurally similar PCB 136 (34), a concentration of 50 μM was used to maximize the formation of metabolites and, thus, allow GC-MS detection of trace metabolites. The metabolites were extracted, separated from the excess of the parent PCB, derivatized with diazomethane and analyzed as MeO-PCB derivatives by GC-MS in both total ion and selective ion monitoring modes as well as with GC-ECD to identify the metabolites (Figures S4-S7). For all four congeners, the major metabolite formed had the OH-group in the 5-position of the 2,3,6-trichloro substituted phenyl ring. The amounts of the metabolites formed in the microsomal incubations displayed the rank order 5–91 > 5′-132 > 5–95 > 5–149 (Tables 1 and S4).
Table 1.
Comparison of the yields and enantiomeric fractions of hydroxylated metabolites of PCB 91, PCB 95, PCB 132 and PCB149 determined using different columns.$
| PCB metabolite | Yield& | BDM | CB | CD | BGB* |
|---|---|---|---|---|---|
| PCB 91 | - | - | 0.43 | - | |
| 5–91 | 20 % | 0.54a | 0.54b | 0.54a | 0.46 |
| PCB 95 | - | - | 0.64 | - | |
| 5–95 | 8.8 % | 0.36a | 0.32a | 0.33a | nr |
| X-95 | nr | 0.33 | 0.33 | 0.68 | |
| PCB 132 | - | - | 0.39 | - | |
| 5′-132 | 16 % | 0.30a | 0.31a,# | 0.31 | 0.71a,# |
| PCB 149 | - | - | 0.46 | - | |
| 5–149 | 2.6 % | 0.66 | 0.65 | 0.65 | nr |
The enantiomeric fraction of the parerent PCB is shown for comparison;
expressed as percent of the PCB used in the respective incubation (0.8 μmol);
the elution order of the atropisomers was reversed on the BGB column;
analysis conducted at 170°C;
racemic standard co-elutes with another peak;
EF of racemic standard 0.55.
Abbreviations of columns: BGB - BGB-172; BDM - Chiral-Dex B-DM, BPM - Chiral-Dex B-PM; CB - Cyclosil-B; CD - Chirasil-Dex (CD); GTA - Chiral-Dex G-TA. See Table S3 for additional information regarding the columns.
In addition to 5–91, three minor metabolites were formed in the incubation with PCB 91. We were able to identify these metabolites as 4–91, 3–100 (NIH shift product), and 4,5–91. PCB 95 formed 5–95 and a second major mono-hydroxylated pentachlorobiphenyl X-95. The retention time of X-95 did not correspond to any putative PCB 95 metabolites with the hydroxyl group in the 2,3,6-trichloro substituted ring system, which suggests that the hydroxy group of X-95 is present on the second, less chlorinated ring. This metabolite is most likely 3′-95, a metabolite that was reported in rats in vivo (35). In addition to 5′-132, three minor metabolites were detected in incubations with PCB 132. We were able to unambiguously identify these metabolites as 4′-132, 3′-140 (NIH shift product) and 4′,5′-132 using authentic standards. Only a single metabolite, 5–149, was found in incubations of PCB 149.
Under the experimental conditions employed in this study no appreciable amounts of NIH-shift products were detected, with only trace amounts of 3–100 and 3′-140 being detected in incubations with PCB 91 and PCB 132, respectively. In contrast, two in vivo studies reported the formation of NIH-shift metabolites of PCB 95 and 136 in rodent animal models (18, 35). With exception of PCB 95, no metabolites with a hydroxyl group in the second, lower chlorinated ring were observed. This is consistent with the presence of a 4-chlorine substituent in these congeners, which essentially prevents metabolic attack in this ring system. Finally, only dihydroxylated PCB 91 and PCB 132 metabolites were detected in the microsomal incubations. Since both 4- and 5–136 are readily metabolized to 4,5–136 by recombinant enzymes (33) and human liver microsomes (34), the fact that only small quantities of dihydroxylated metabolites were formed for PCBs 91 and 132 is likely due to the relatively shorter incubation time (30 min) used in the present studies.
Overall the metabolite profiles observed for all four congeners are in agreement with previous in vitro and in vivo studies. Two unidentified mono-hydroxylated metabolites were reported by Warner et al. in an in vitro experiment where PCB 95 was metabolized by rat cytochrome P450 2B1 enzyme (19). Also, a single mono-hydroxylated metabolite was reported for PCB 91 in the same study. A comparative in vivo study by Sundstrom et al. (35) reported 4′-95 as the major metabolite in quail, whereas 5–95 was the major metabolite in the rat and mouse, with 3–103 (NIH shift product) and 4′-95 being only minor metabolites. In the rat, mouse and guinea pig, 5′-132 was also the major metabolite of PCB 132. 4′- and 4′,5′-132 were also formed, but in different ratios depending on the species (36). There are currently no studies reporting the formation of hydroxylated metabolites of PCB 149.
Enantioselective analysis of microsomal incubations of chiral PCBs
Four chiral columns were used to determine the enantiomeric enrichment of the major OH-PCBs formed in the microsomal incubations discussed above (Tables 1 and S4; Figures S8-S11). All enantioselective analyses were performed isothermally at 160 °C unless otherwise stated (Table 1). The BDM column provided baseline separation of all major, 5-substituted metabolites of the PCB congeners investigated (Figure S2A). The EF values obtained with this column, as well as the CB and CD columns were comparable and revealed a congener-dependent enantiomeric enrichment for all OH-PCBs. The results from the BGB column showed good agreement with BDM, CB and CD columns; however, the elution order of the atropisomers was reversed. Since the absolute configurations of neither the parent compounds nor the corresponding OH-PCBs shown in Figure 1 has been established, it is currently not possible to determine which of the atropisomers shown in Figure 1 is formed selectively and if there is a congener-specific difference in how R and S atropisomers are metabolized.
The parent PCBs showed a congener specific enantiomeric enrichment (Table 1). In the case of PCBs 91, 132 and 149 the second eluting atropisomers were enriched, whereas the first eluting atropisomer was enriched for PCB 95. In the case of PCB 132 and 149 this corresponds to an enrichment of (+)-PCB 132 and (+)-PCB 149 (31, 37). The most pronounced enantiomeric enrichment was observed for PCB 95 (EF=0.64) and PCB 132 (EF=0.39). These observations are in agreement with a previous study by Warner demonstrating an enantiomeric enrichment of PCBs due to metabolism by recombinant rat and human cytochrome P450 enzymes (19) as well as several in vivo studies reporting an enantiomeric enrichment of these congeners in rodents and humans. For example, in a study by Kania-Korwel et al. (38), the EFs of PCB 95 (EF = 0.63) and PCB 149 (EF = 0.45) in the liver of rats treated with Aroclor 1254 were comparable to the results obtained in this study.
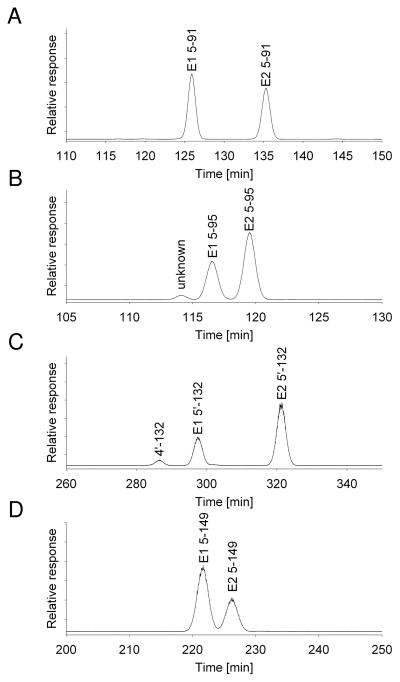
The most intriguing observation of the present study is that the major OH-PCB metabolites formed in the microsomal incubations displayed a clear, congener-specific enantiomeric enrichment. There was very little enrichment of the first eluting atropisomer observed for 5–91, with an EF of 0.54. In the case of 5–149, the first eluting congener was also enriched (EF = 0.65). Both 5–95 and the unknown mono-methoxylated PCB 95 (X-95) showed an enrichment of the second eluting atropisomer, with comparable EFs of approximately 0.33 for both metabolites. An enrichment of the second eluting atropisomer was also observed for 5′-132 (EF = 0.31). Due to the comparatively low levels of the minor metabolites and co-elution problems, it was not possible to determine the EFs of the minor metabolites; however, it is likely that these metabolites also display some enantiomeric enrichment. Overall, this study demonstrates for the first time that chiral PCBs, such as PCB 91, 95, 132 and 149, are enantioselectively metabolized to OH-PCBs by cytochrome P450 enzymes.
Considering the high cytochrome P450 2B activity in the microsomal preparation used in the incubations, it is likely that the enantioselective formation of 5-hydroxylated PCB metabolites is due to cytochrome P450 2B enzymes, which is consistent with PCB metabolism studies using recombinant enzymes. The enantioselective formation of OH-PCBs therefore in part explains the enantiomeric enrichment of the respective parent compounds in in vitro and in vivo studies (9). Further studies are needed to better understand the role of cytochrome P450 2B enzymes in the enantiomeric enrichment of both PCBs and OH-PCBs, and the toxicity of pure OH-PCB atropisomers. The later question is of particular interest from an environmental health perspective because of the enantiomeric enrichment of OH-PCBs reported in this study and the recently documented enantiospecific effect of OH-PCBs on Ryanodine receptor sensitization (8).
Supplementary Material
Figure 2.
Enantiomeric enrichment of 5-hydroxylated metabolites of PCB 91 (A), PCB 95 (B), PCB 132 (C) and PCB 149 (D) in incubations with rat liver microsomes. Samples were analyzed on the DBM column at 160°C.
Acknowledgments
The authors would like to thank Drs. Stelvio Bandiera and Eugene Hrycay (University of British Columbia) for the characterization of the microsomes, Drs. Yang Song, Sandhya M. Vyas and Sudhir N. Joshi (University of Iowa) for the synthesis of the methoxylated PCB standards, and Ananya Pramanik (University of Iowa) for help with the microsomal metabolism studies. The OH- and MeO-PCB 136 metabolites were a generous gift from E.A. Mash and S.C. Waller of the Synthetic Chemistry Facility Core of the Southwest Environmental Health Sciences Center, funded by NIH grant ES06694. The project described was supported by grants ES05605, ES013661 and ES017425 from the National Institute of Environmental Health Sciences.
Footnotes
Brief
Hydroxylated PCB metabolites are enantioselectively formed by liver microsomes from male rats.
Supporting Information Available
Description of microsomes preparation and cytochrome P450 enzyme activities; structures, nomenclature and resolution of all studied methoxylated PCBs on all columns investigated, both in temperature programmed and isothermal analysis; description of enantioselective columns; enantiomeric fractions of methoxylated PCBs in microsomal incubations; dependence of resolution on temperature in isothermal analysis for 5–91 and 5′-132; resolution of all methoxylated PCBs on BDM column and programmed temperature; resolution of 5–91 on all columns and programmed temperature; GC-MS and GC-ECD analysis of OH-PCBs formed in microsomal incubations of PCB 91, PCB 95, PCB 132 and PCB 149; comparison of the enantiomeric enrichment of 5-methoxylated derivatives of PCB 91, PCB 95, PCB 132 and PCB 149 isolated from microsomal incubations to the racemic standard. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Fourth National Report on Human Exposure to Environmental Chemicals. Center for Disease Control and Prevention; Atlanta, GA: 2009. www.cdc.gov/exposurereport/pdf/FourthReport_ExecutiveSummary.pdf. [Google Scholar]
- 2.Schecter A, Colacino J, Haffner D, Patel K, Opel M, Papke O, Birnbaum L. Perfluorinated Compounds, Polychlorinated Biphenyl, and Organochlorine Pesticide Contamination in Composite Food Samples from Dallas, Texas. Environ Health Perspect. 2010;118(6):796–802. doi: 10.1289/ehp.0901347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrad S, Ibarra C, Robson M, Melymuk L, Zhang X, Diamond M, Douwes J. Polychlorinated biphenyls in domestic dust from Canada, New Zealand, United Kingdom and United States: Implications for human exposure. Chemosphere. 2009;76(2):232–238. doi: 10.1016/j.chemosphere.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 4.Schantz SL, Widholm JJ, Rice DC. Effects of PCB exposure on neuropsychological function in children. Environ Health Perspect. 2003;111(3):357–376. doi: 10.1289/ehp.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mariussen E, Fonnum F. Neurochemical Targets and Behavioral Effects of Organohalogen Compounds: An Update. Crit Rev Toxicol. 2006;36(3):253–289. doi: 10.1080/10408440500534164. [DOI] [PubMed] [Google Scholar]
- 6.Pessah IN, Lehmler HJ, Robertson LW, Perez CF, Cabrales E, Bose DD, Feng W. Enantiomeric specificity of (−)-2,2′,3,3′,6,6′-hexachlorobiphenyl toward ryanodine receptor types 1 and 2. Chem Res Toxicol. 2009;22(1):201–207. doi: 10.1021/tx800328u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James MO. Polychlorinated biphenyls: Metabolism and metabolites. In: Larry W, Robertson LGH, editors. PCBs: Recent Advances in Environmental Toxicology and Health Effects. The University Press of Kentucky; Lexington, KY: 2001. pp. 35–46. [Google Scholar]
- 8.Pessah IN, Hansen LG, Albertson TE, Garner CE, Ta TA, Do Z, Kim KH, Wong PW. Structure-activity relationship for noncoplanar polychlorinated biphenyl congeners toward the ryanodine receptor-Ca2+ channel complex type 1 (RyR1) Chem Res Toxicol. 2006;19(1):92–101. doi: 10.1021/tx050196m. [DOI] [PubMed] [Google Scholar]
- 9.Lehmler HJ, Harrad SJ, Hühnerfuss H, Kania-Korwel I, Lee CM, Lu Z, Wong CS. Chiral polychlorinated biphenyl transport, metabolism and distribution: A review. Environ Sci Technol. 2009;44(8):2757–2766. doi: 10.1021/es902208u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jorundsdottir H, Norström K, Olsson M, Pham-Tuan H, Hühnerfuss H, Bignert A, Bergman A. Temporal trends of bis(4-chlorophenyl) sulfone, methylsulfonyl-DDe and -PCBs in Baltic guillemot (Uria aalge) egg 1971–2001- A comparison to 4,4′-DDE and PCB trends. Environ Poll. 2006;141:226–237. doi: 10.1016/j.envpol.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 11.Larsson C, Norström K, Athanansidais I, Bignert A, König WA, Bergman A. Enantiomeric specificity of methylsulfonyl-PCBs and distribution of bis(4-chlorophenyl) sulfone, PCB and DDE methyl sulfones in grey seal tissues. Environ Sci Technol. 2004;38:4950–4955. doi: 10.1021/es049361v. [DOI] [PubMed] [Google Scholar]
- 12.Wiberg K, Letcher R, Sandau CD, Duffe J, Norstrom R, Haglund P, Bidleman TF. Enantioselective gas chromatography/mass spectrometry of methylsulfonyl PCBs with application of arctic marine mammals. Anal Chem. 1998;70:3845–3852. doi: 10.1021/ac980064g. [DOI] [PubMed] [Google Scholar]
- 13.Chu S, Covaci A, Haraguchi K, Voorspoels S, van de Vijver K, Das K, Bouquegneau JM, de Coen W, Blust R, Schepens P. Levels and enantiomeric signatures of methyl sulfonyl PCB and DDE metabolites in livers of harbor porpoises (Phocoena phocoena) from the Southern North Sea. Environ Sci Technol. 2003;37:4573–4578. doi: 10.1021/es034241t. [DOI] [PubMed] [Google Scholar]
- 14.Karasek L, Hajslova J, Rosmus J, Hühnerfuss H. Methylsulfonyl PCB and DDE metabolites and their enentioselective gas chromatographuc separation in human adipose tissues, seal blubber and pelican muscle. Chemosphere. 2007;67:S22–227. doi: 10.1016/j.chemosphere.2006.05.081. [DOI] [PubMed] [Google Scholar]
- 15.Ellerichmann T, Bergman A, Franke S, Hühnerfuss H, Jakobsson E, König WA, Larsson C. Gas chromatographic enantiomer separations of chiral PCB methyl sulfons and identification of selctively retained enantiomers in human liver. Fres Environ Bull. 1998;7:244–257. [Google Scholar]
- 16.Larsson C, Ellerichmann T, Hühnerfuss H, Bergman A. Chiral PCB methyl sulfones in rat tissues after exposure to technical PCBs. Environ Sci Technol. 2002;36:2833–2838. doi: 10.1021/es025512n. [DOI] [PubMed] [Google Scholar]
- 17.Norström K, Eriksson J, Haglund J, Silvari V, Bergman A. Enantioselective formation of methyl sulfone metabolites of 2,2′,3,3′,4,6′-hexachlorobiphenyl in rat. Environ Sci Technol. 2006;40(24):7649–7655. doi: 10.1021/es061584t. [DOI] [PubMed] [Google Scholar]
- 18.Kania-Korwel I, Vyas S, Song Y, Lehmler HJ. Gas chromatographic separation of methoxylated polychlorinated biphenyl atropisomer. J Chromatogr A. 2008;1207:146–154. doi: 10.1016/j.chroma.2008.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warner NA, Martin JW, Wong CS. Chiral polychlorinated biphenyls are biotransformed enantioselectively by mammalian cytochrome P-450 isozymes to form hydroxylated metabolites. Environ Sci Technol. 2009;43:114–121. doi: 10.1021/es802237u. [DOI] [PubMed] [Google Scholar]
- 20.Ballschmiter K, Bacher R, Mennel A, Fischer R, Riehle U, Swarev M. The determination of chlorinated biphenyls, chlorinated dibenzodioxins and chlorinated dibenzofurans by GC-MS. J High Resol Chromatogr. 1992;15:260–270. [Google Scholar]
- 21.Maervoet J, Covaci A, Schepens P, Sandau CD, Letcher R. A reassessment of the nomenclature of polychlorinated biphenyl (PCB) metabolites. Environ Health Perspect. 2004;112(3):291–294. doi: 10.1289/ehp.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong CS, Garrison AW. Enantiomer separation of polychlorinated biphenyl atropisomers and polychlorinated biphenyl retention behavior on modified cyclodextrin capillary gas chromatography columns. J Chromatogr A. 2000;866(2):213–220. doi: 10.1016/s0021-9673(99)01104-8. [DOI] [PubMed] [Google Scholar]
- 23.Vetter W. Enantioselctive fate of chiral chlorinated hydrocarbons and their metabolites in environmental samples. Food Rev Int. 2001;17(2):113–182. [Google Scholar]
- 24.Milanowski B, Lulek J, Lehmler H-J, Kania-Korwel I. Assesment of disposition of chirl polychlorinated biphenyls in female mdr 1a/b knockout versus wild-type mice using multivariate analyses. Environ Int. 2010;36(8):884–892. doi: 10.1016/j.envint.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kania-Korwel I, El-Komy MHME, Veng-Pedersen P, Lehmler HJ. Clearance of polychlorinated biphenyl atropisomers is enantioselective in female C57Bl/6 mice. Environ Sci Technol. 2010;44(8):2828–2835. doi: 10.1021/es901781p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kania-Korwel I, Zhao H, Norstrom K, Li X, Hornbuckle KC, Lehmler HJ. Simultaneous extraction and clean-up of polychlorinated biphenyls and their metabolites from small tissue samples using pressurized liquid extraction. J Chromatogr A. 2008;1214:37–46. doi: 10.1016/j.chroma.2008.10.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kania-Korwel I, Hornbuckle KC, Peck A, Ludewig G, Robertson LW, Sulkowski WW, Espandiari P, Gairola CG, Lehmler HJ. Congener specific tissue distribution of Aroclor 1254 and a highly chlorinated environmental PCB mixture in rats. Environ Sci Technol. 2005;39:3513–3520. doi: 10.1021/es047987f. [DOI] [PubMed] [Google Scholar]
- 28.Kania-Korwel I, Hornbuckle KC, Robertson LW, Lehmler H-J. Influence of dietary fat on the enantioselective disposition of 2,2′,3,3′,6,6′-hexachlorobiphenyl (PCB 136) in female mice. Food Chem Toxicol. 2008;46(2):637–644. doi: 10.1016/j.fct.2007.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haglund P. Enantioselective separation of polychlorinated biphenyl atropisomers using chiral high performance liquid chromatography. J Chromatogr. 1996;724:219–228. [Google Scholar]
- 30.Pham-Tuan H, Larsson C, Hoffmann F, Bergman A, Fröba M, Hühnerfuss H. Enantioselective semipreparative HPLC separation of PCB metabolites and their absolute structure elucidation using electronic and vibrational circular dichroism. Chirality. 2005;17:266–280. doi: 10.1002/chir.20158. [DOI] [PubMed] [Google Scholar]
- 31.Haglund P, Wiberg K. Determination of the gas chromatographic elution sequences of the (+) and (−) enantiomers of stable enantiomeric PCBs on Chirasil-Dex. J High Resol Chromatogr. 1996;19:373–376. [Google Scholar]
- 32.Duignan D, Sipes I, Leonard T, Halpert J. Purification and characterization of the dog hepatic cytochrome P-450 isozyme responsible for the metabolism of 2, 2′,4, 4′, 5, 5′-hexachlorobiphenyl. Arch Biochem Biophys. 1987;255:290–303. doi: 10.1016/0003-9861(87)90396-1. [DOI] [PubMed] [Google Scholar]
- 33.Waller SC, He YA, Harlow GR, He YQ, Mash EA, Halpert JR. 2,2′,3,3′,6,6′-hexachlorobiphenyl hydroxylation by active site mutants of cytochrome P450 2B1 and 2B11. Chem Res Toxicol. 1999;12(8):690–699. doi: 10.1021/tx990030j. [DOI] [PubMed] [Google Scholar]
- 34.Schnellmann R, Putnam C, Sipes I. Metabolism of 2,2′,3,3′,6,6′-hexachlorobiphenyl and 2,2′,4,4′,5,5′-hexachlorobiphenyl by human hepatic microsomes. Biochem Pharmacol. 1983;32(21):3233–3239. doi: 10.1016/0006-2952(83)90209-5. [DOI] [PubMed] [Google Scholar]
- 35.Sundström G, Jansson B. The metabolism of 2,2′,3,5′,6-pentachlorobiphenyl in rats, mice and quails. Chemosphere. 1975;4(6):361–370. [Google Scholar]
- 36.Haraguchi K, Kato Y, Koga N, Degawa M. Species differences in the tissue distribution of catechol and methylsulphonyl metabolites of 2,4,5,2′,5′-penta- and 2,3,4,2′,3′,6′-hexachlorobiphenyls in rats, mice, hamsters and guinea pigs. Xenobiotica. 2005;35(1):85–96. doi: 10.1080/00498250400026456. [DOI] [PubMed] [Google Scholar]
- 37.Harju MT, Haglund P. Determination of the rotational energy barriers of atropisomeric polychlorinated biphenyls. Fres J Anal Chem. 1999;364:219–223. [Google Scholar]
- 38.Kania-Korwel I, Garrison AW, Avants JK, Hornbuckle KC, Robertson LW, Sulkowski WW, Lehmler HJ. Distribution of chiral PCBs in selected tissues in the laboratory rat. Environ Sci Technol. 2006;40:3704–3710. doi: 10.1021/es0602086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.