Abstract
Three immunizations of mice with recombinant protective antigen (rPA) by transcutaneous immunization (TCI) induced long-term neutralizing antibody titers that were superior to those obtained with aluminum-adsorbed rPA. In addition, rPA alone exhibited adjuvant activity for TCI. Forty-six weeks after completion of TCI, 100% protection was observed against lethal anthrax challenge.
Transcutaneous immunization (TCI) is a procedure that relies on application of antigen and associated adjuvant onto the outer layer of the skin and subsequent delivery to underlying Langerhans cells that serve as antigen-presenting cells (4). A variety of adjuvants have shown effectiveness in stimulating immunity by TCI, but the most widely used and most effective adjuvants have been members of the ADP-ribosylating bacterial exotoxins, such as cholera toxin or heat-labile enterotoxin (HLT) from Escherichia coli (13).
Two days prior to immunization, hair was shaved on the backs of female A/J mice (12 weeks old) purchased from the Jackson Laboratory (Bar Harbor, Maine). Recombinant protective antigen (rPA; 20 μg) (Monoclonal Antibody/Recombinant Protein Production Facility-Science Applications International Corporation, National Cancer Institute-Frederick Cancer Research and Development Center) was mixed with the indicated doses of HLT in phosphate-buffered saline (purchased from Berna Biotech, Bern, Switzerland) and applied to gauze patches placed on the backs of mice overnight. The backs were shaved 1 to 2 days prior to application of the patch. The site was hydrated with saline-soaked gauze and mildly abraded by being brushed 10 times with emery paper (GE Medical Systems, Milwaukee, Wis.) prior to patch application. Control groups consisted of HLT alone, rPA alone, or intramuscular (i.m.) immunization with 10 μg of rPA mixed with aluminum hydroxide (0.1 mg of Al3+ Rehydrogel HPA; Reheis Inc., Berkeley Heights, N.J.). Animals were bled, and sera were assayed for rPA antibodies by enzyme-linked immunosorbent assay (ELISA), as described previously (7, 9). Antiserum neutralization of Bacillus anthracis lethal toxin cytotoxicity was determined by measuring the viability of 6 × 104 J774A.1 cells in the presence of lethal toxin (100 ng of PA/ml plus 50 ng of lethal factor/ml) (10). At week 50, the mice were challenged subcutaneously with 1,000 50% lethal doses (LD50) of B. anthracis Sterne strain spores (1).
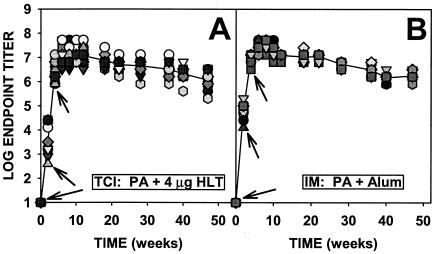
As shown in Fig. 1A, over a period of 47 weeks 100% of mice immunized with rPA by TCI with HLT as an adjuvant responded with strong antibody titers after applications of vaccine at 0, 2, and 4 weeks. All of the mice responded briskly even after a single immunization. During the 43 weeks after the last immunization the titers initially rose further and then declined gradually (less than a log) to the end of the observation period. A similar pattern, except for a more rapid rise in titer at 4 weeks, was obtained after control i.m. injection with rPA adsorbed to aluminum hydroxide (Fig. 1B).
FIG. 1.
Time course of antibody titers to rPA in mice immunized (arrows) with rPA by TCI (A) or i.m. (B). The line represents the geometric mean of the titers from the individual animals (symbols) (n = 15).
At the end of 47 weeks, ELISA antibody titers of mice that received different amounts of HLT as an adjuvant were measured (Fig. 2A). Maximal adjuvant activity of the HLT was reached even with 0.4 μg of HLT, and the antibody titer was not significantly increased with higher amounts of HLT. Figure 2A also illustrates the interesting finding that rPA by itself, not previously known as an adjuvant for inducing antibodies, had significant activity without additional adjuvant for induction of antibodies to PA by TCI. The strength of immunization with rPA alone was less than that observed after TCI with rPA combined with HLT as an additional adjuvant, and as shown in Fig. 2B, neutralizing antibody titers were much lower when HLT was not present. Neutralizing antibody titers of aluminum-adsorbed rPA were also much lower than those observed after TCI with rPA and HLT.
FIG. 2.
Serum IgG ELISA titers to rPA (A) and lethal toxin-neutralizing antibody titers (B) at week 47. Comparisons were made with different HLT doses used as adjuvants for TCI and i.m. immunization results obtained after i.m. immunization with aluminum-adsorbed rPA (alum). Values for IgG ELISA titers to rPA are the geometric means ± geometric mean errors of 14 or 15 samples/group. Lethal toxin-neutralizing antiserum titers (50% effective doses) were analyzed with pooled antisera.
As shown in Table 1, at every level of HLT employed, TCI resulted in 100% protection 46 weeks after the last immunization following lethal challenge at week 50 with B. anthracis spores. The protection by TCI was identical to the protection observed after i.m. injection with aluminum-adsorbed rPA, which served as a positive control. The negative-control group that received no immunization confirmed that the challenge employed was 100% lethal. It is noteworthy that immunization with rPA alone, where rPA was used as an antigen without additional adjuvant, also resulted in 100% protection. From these data it is evident that, under the conditions employed, TCI resulted in protective immunity that was at least equivalent to that obtained after i.m. immunization with aluminum-adsorbed antigen. Furthermore, if neutralizing antibody titers were important for protection, as previously suggested (12), TCI would be expected to be a stronger immunization strategy. These data are consistent with the previous observation that anti-PA immunoglobulin G (IgG) is a significant in vitro correlate of survival after lethal challenge with inhalational anthrax (10).
TABLE 1.
Survival of mice challenged by subcutaneous injection with 1,000 LD50 of B. anthracis Sterne strain spores
| Group no. | Immunization method | Antigen dose (μg) | Adjuvant (μg) | % Survival on day 21 (no. of survivors/no. challenged) |
|---|---|---|---|---|
| 1 | TCI | 20 | HLT (20) | 100 (15/15) |
| 2 | TCI | 20 | HLT (4) | 100 (15/15) |
| 3 | TCI | 20 | HLT (2) | 100 (14/14) |
| 4 | TCI | 20 | HLT (0.4) | 100 (15/15) |
| 5 | TCI | 20 | None | 100 (15/15) |
| 6 | TCI | 0 | HLT (20) | 0 (0/15) |
| 7 | i.m. | 10 | Aluminum | 100 (15/15) |
| Nonimmunized control | None | None | None | 0 (0/15) |
The present anthrax vaccines that are licensed in the United States and United Kingdom have been subjected to criticisms for numerous real or perceived suboptimal features and for frequency of adverse events (8). The vaccines are incompletely characterized and are also difficult to characterize; they are locally reactogenic like other aluminum-containing vaccines, and the dose schedule is long (2, 8). Aluminum adjuvants have the limitations of being associated with occasional severe local reactions such as erythema, IgE induction, contact hypersensitivity, and granulomatous inflammation, and they are not biodegradable and remain at the site of injection for up to a year (6). Subcutaneous nodules that can last for weeks are often found after subcutaneous injection of aluminum-containing vaccines, including the U.S. licensed anthrax vaccine (6, 11). Although aluminum adjuvants are usually viewed as relatively safe, large-scale vaccination might gain better acceptance if a less reactogenic potent adjuvant were used along with an improved immunization strategy.
The TCI vaccine strategy proposed in this study utilizes a recombinant protein (rPA), lacks aluminum adjuvant, is administered without injection, uses a potent adjuvant (HLT) that has a good safety record when administered with TCI in humans (3, 5), induces specific antibody titers that are at least equivalent to those observed after i.m. injection of aluminum-adsorbed rPA, and causes long-lived (at least 47 weeks) protective immunity against lethal (1,000-LD50) anthrax challenge.
Acknowledgments
We acknowledge the excellent technical assistance of Elaine Morrison for her work with the laboratory animals.
Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, National Research Council publication, 1996 edition.
This work was performed under a Cooperative Research and Development Agreement between Walter Reed Army Institute of Research, Silver Spring, Md., and Iomai Corporation, Gaithersburg, Md. Funding for the study was provided by the Biological Defense Research Program, U.S. Army Medical Research and Materiel Command, Fort Detrick, Md., and Iomai Corporation.
The information contained herein reflects the views of the authors and should not be construed to represent those of the Department of the Army or the Department of Defense.
Editor: A. D. O'Brien
REFERENCES
- 1.Fellows, P. F., M. K. Linscott, B. E. Ivins, M. L. M. Pitt, C. A. Rossi, P. H. Gibbs, and A. M. Friedlander. 2001. Efficacy of a human anthrax vaccine in guinea pigs, rabbits, and rhesus macaques against challenge by Bacillus anthracis isolates of diverse geographical origin. Vaccine 19:3241-3247. [DOI] [PubMed] [Google Scholar]
- 2.Friedlander, A. M., S. L. Welkos, and B. E. Ivins. 2002. Anthrax vaccines. Curr. Top. Microbiol. Immunol. 271:33-60. [DOI] [PubMed] [Google Scholar]
- 3.Glenn, G. M., D. N. Taylor, X. Li, S. Frankel, A. Montemarano, and C. R. Alving. 2000. Transcutaneous immunization: a human vaccine delivery strategy using a patch. Nat. Med. 6:1403-1406. [DOI] [PubMed] [Google Scholar]
- 4.Glenn, G. M., R. T. Kenney, L. R. Ellingsworth, S. A. Frech, S. A. Hammond, and J. P. Zoeteweij. 2003. Transcutaneous immunization and immunostimulant strategies: capitalizing on the immunocompetence of the skin. Expert Rev. Vaccines 2:253-267. [DOI] [PubMed] [Google Scholar]
- 5.Güereña-Burgueño, F., E. R. Hall, D. N. Taylor, F. J. Cassels, D. A. Scott, M. K. Wolf, Z. J. Roberts, G. V. Nesterova, C. R. Alving, and G. M. Glenn. 2002. Safety and immunogenicity of a prototype enterotoxigenic Escherichia coli vaccine administered transcutaneously. Infect. Immun. 70:1874-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta, R. K. 1998. Aluminum compounds as vaccine adjuvants. Adv. Drug Delivery Rev. 32:155-172. [DOI] [PubMed] [Google Scholar]
- 7.Iacono-Connors, L. C., S. L. Welkos, B. E. Ivins, and J. M. Dalrymple. 1991. Protection against anthrax with recombinant virus-expressed protective antigen in experimental animals. Infect. Immun. 59:1961-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joellenbeck, L. M., L. L. Zwanziger, J. S. Durch, and B. L. Strom (ed.). 2002. The anthrax vaccine. Is it safe? Does it work? National Academy Press, Washington, D.C. [PubMed]
- 9.Matyas, G. R., and C. R. Alving. 1996. Protective prophylactic immunity against intranasal ricin challenge induced by liposomal ricin A subunit. Vaccine Res. 5:163-172. [Google Scholar]
- 10.Pitt, M. L. M., S. F. Little, B. E. Ivins, P. Fellows, J. Barth, J. Hewetson, P. Gibbs, M. Dertzbaugh, and A. M. Friedlander. 2001. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine 19:4768-4773. [DOI] [PubMed] [Google Scholar]
- 11.Pittman, P. R. 2002. Aluminum-containing vaccine associated adverse events: role of route of administration and gender. Vaccine 20:S48-S50. [DOI] [PubMed] [Google Scholar]
- 12.Reuveny, W., M. D. White, Y. Y. Adar, Y. Kafri, Z. Altboum, Y. Gozes, D. Kobiler, A. Shafferman, and B. Velan. 2001. Search for correlates of protective immunity conferred by anthrax vaccine. Infect. Immun. 69:2888-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scharton-Kersten, T., J. Yu, R. Vassell, D. O'Hagan, C. R. Alving, and G. M. Glenn. 2000. Transcutaneous immunization on the skin with bacterial ADP-ribosylating exotoxins, subunits and unrelated adjuvants. Infect. Immun. 68:5306-5313. [DOI] [PMC free article] [PubMed] [Google Scholar]