Abstract
A sublethal-challenge model was established in BALB/c mice by using protection from the development of severe splenomegaly as an indicator of vaccinogenic activity for evaluation of the protective efficacies of vaccine candidates. To determine the immunodominant antigens as defined by reaction to an infection-derived antibody, mouse sera from different stages of experimental infection with various doses of Coxiella burnetii were tested by immunoblotting. Proteins with molecular masses of 14, 16, 21, 28, 32, 45 to 50, 57, and 60 kDa were recognized as immunodominant antigens. Antibody responses in whole-cell antigen (WCA)-vaccinated mice were compared with those in unvaccinated mice by immunoblotting using two-dimensional gel-separated C. burnetii antigens. The results indicated that there were significantly different antibody responses during different stages of vaccination and challenge, suggesting that several specific immunogenic antigens may play critical roles in the protection of mice against challenge. To clone these immunogenic antigens, a genomic DNA library of Nine Mile phase I was screened with convalescent-phase antisera from mice. Eighteen novel immunoreactive proteins with molecular masses ranging from ∼14 to 67 kDa were cloned and identified. Interestingly, several recombinant proteins reacted with sera from both early-stage infected and WCA-vaccinated prechallenged mice. These results suggest that these proteins may play critical roles in the development of protective immunity and that they are logical candidates for vaccine and serodiagnostic reagents.
Coxiella burnetii is an obligate intracellular bacterium that multiplies within the phagolysosomes of eukaryotic cells and causes a worldwide zoonotic disease, Q fever. Human Q fever is generally acquired via the respiratory route by inhalation of infectious aerosols produced by domestic livestock and can be acute or chronic (27, 28). Acute Q fever is a flu-like illness that is usually self-limiting and is effectively treated by antibiotics (19). In contrast, chronic Q fever is a severe, sometimes fatal disease, and patients have responded poorly to various antibiotic therapies (16, 26). Endocarditis is the most common chronic manifestation, while vascular infection, bone infection, and chronic hepatitis are also reported (28). Infection in most animals is mainly subclinical, but abortion and infertility are common manifestations in ruminants (2). Domestic animals, especially cattle, sheep and goats, are important reservoirs of the agent responsible for infection of humans (12, 20).
Since the clinical diagnosis of Q fever is difficult and the isolation of the causative agent is time-consuming and hazardous, the diagnosis of Q fever is mainly based on the results of serological tests. Several serological methods, including microagglutination (14), complement fixation (24, 25), immunofluorescence assay (IFA) (4, 23), and enzyme-linked immunosorbent assay (ELISA) (36, 47), have been developed for detecting C. burnetii antibodies. However, the antigens currently used in these tests are purified phase I and phase II organisms. Organisms for both phase I and phase II are purified from infected yolk sacs, infected tissue culture cells, and infected guinea pig spleens to provide these antigens. Since cultivation of C. burnetii is difficult and hazardous and requires special equipment, the antigens cannot be prepared in most clinical laboratories and therefore are of limited use for routine diagnosis of Q fever and for large-scale investigations. The need for standardization of diagnostic antigens is a strong rationale for the development of new serodiagnostic reagents. However, the immunodominant antigens of C. burnetii during infection are not well characterized. The ability of single or multiple selected proteins to serve as an alternative to purified whole bacteria as antigens for serological diagnostic tests is untested.
Previous studies demonstrated that formalin-inactivated phase I whole-cell vaccine was effective in protecting against the disease in humans and animals (42, 44). However, vaccination with whole-cell materials can induce severe local and occasional systemic reactions (39, 40). Efforts to overcome this problem by developing a subunit vaccine have been encouraging. Zhang et al. demonstrated that a partially purified 67-kDa antigen from C. burnetii was able to confer full protection in both guinea pigs and mice (48). Williams and coworkers demonstrated that a partially purified 29-kDa protein, P1, from C. burnetii could confer protection from a lethal challenge on mice (43). These studies suggested that subunit protein vaccines were able to provide protection against C. burnetii infection. However, since the two proteins were not cloned or well characterized, there is no protective antigen(s) from C. burnetii that has been clearly identified and characterized.
Antigen structures, including lipopolysaccharide (LPS) and membrane proteins, have been demonstrated to play a role in the development of protective immunity to infection by C. burnetii. To determine specific virulence determinants and potential vaccinogenic antigens, several studies have focused on the identification of immunogenic antigens of C. burnetii. Williams and Stewart reported that the 27.5-, 28- to 31-, and 53-kDa antigens were shared by phase I and phase II, while the 80- and 92-kDa antigens were unique to phase II (46). Recently, Ho et al. showed that eight antigens of 13, 15.7, 26.5, 28, 29.5, 55, 67, and 83 kDa were common to 13 prototype strains and 5 Japanese isolates (11). Although these studies identified several immunogenic antigens of C. burnetii, there has been no systematic study to determine the immunodominant antigens during infection and protective vaccination with C. burnetii, and only a few immunogenic proteins (Hsp60, Com1, Cbmip, and P1) have been cloned and characterized (10, 21, 37, 38). Since immunodominant antigens, as defined by reaction to an infection-derived antibody, may be diagnostic targets or subunit vaccine candidates, we have engaged in a comprehensive study to identify and clone these immunodominant antigens. We predict that this description will facilitate the development of novel, improved serological diagnostic assays and subunit vaccines based on the recombinant antigens.
In this study, a sublethal-challenge infection model that can be used to evaluate the protective efficacies of vaccine candidates was established in BALB/c mice. To identify the immunodominant antigens during infection and vaccination, mouse sera from experimental infection and vaccination with Nine Mile phase I (see below) were tested by immunoblotting with one- or two-dimensional (1- or 2-D) gel-separated phase I or phase II C. burnetii antigens. To clone these antigens, a genomic DNA library of Nine Mile phase I was constructed in the expression vector Lambda ZAP Express (Stratagene, La Jolla, Calif.) and screened with convalescent-phase mouse antiserum.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
C. burnetii (Nine Mile phase I RSA493) was grown in embryonated yolk sacs and purified as previously described (29). Escherichia coli XL1-Blue MRF′ cells were transfected with bacteriophage Lambda ZAP Express vector and grown in top agar on NZY agar plates.
Experimental infection of BALB/c mice with Nine Mile phase I.
To establish a mouse challenge model and monitor the immunodominance of C. burnetii antigens, BALB/c mice (8 weeks old) were infected intraperitoneally with various doses (100, 105, 106, 107, 108, and 109 organisms, as measured by the optical density at 600 nm) of Nine Mile phase I RSA493. Three days postinfection, high-dose (107, 108, and 109 organisms) infected mice developed severe illness: reduced movement, ruffled fur, and body weight loss. After infection with C. burnetii for 2, 3, or 5 weeks, four mice at each infection dose were sacrificed and their spleen weights were measured. Sera were collected from each infection dose group at week 2, 3, or 5 and used for identification of the immunodominant antigens during infection.
Evaluation of protective efficacy of WCA in BALB/c mouse model.
The vaccinogenic activity of whole-cell antigen (WCA) was tested in a BALB/c mouse model. Twenty-four BALB/c mice (6 weeks old) were randomly separated (eight mice per group) into normal control, unvaccinated, and vaccinated groups. The mice in the vaccinated group were immunized with formalin-inactivated Nine Mile phase I WCA in Titermax adjuvant (Sigma, St. Louis, Mo.) three times at 14-day intervals. At each immunization, the mice were subcutaneously injected with 50 μl of antigen (containing 20 μg of antigen) mixed with 50 μl of Titermax. Unvaccinated mice were immunized with phosphate-buffered saline (PBS) in adjuvant. After the third immunization, sera were collected from four mice in each group and used for identification of the immunodominant antigens during vaccination. Fourteen days after the third immunization, except for normal control animals, unvaccinated and WCA-vaccinated mice were challenged by intraperitoneal injection with 108 Nine Mile phase I cells in 0.4 ml of 0.25 M sucrose phosphate buffer per mouse. Four mice from each group were terminated, and the spleen weights were measured at both 2 and 4 weeks postchallenge.
Immunoblotting with 1- or 2-D gel-separated antigens.
To determine the immunodominant antigens during infection, mouse sera from different stages of infection with various doses of C. burnetii were tested by immunoblotting for antibodies against phase I antigens. Briefly, WCAs were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred electrophoretically to polyvinylidene difluoride (PVDF) membranes in Tris-glycine buffer (containing 0.1 M Tris base, 0.192 M glycine, and 10% methanol) at 150 mA for 90 min. The membranes were blocked for 2 h at room temperature in PBS with 0.05% Tween 20 (PBST) and 10% nonfat dry milk and then were incubated with diluted mouse anti-Nine Mile phase I serum. After being washed with PBST buffer, the membranes were incubated with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) or anti-mouse IgM (1:10,000 dilution) for 2 h at room temperature. The reactions were detected by using an ECL Western blot detection kit (Amersham Pharmacia, Piscataway, N.J.).
To determine whether the immunodominant antigens recognized by mouse sera were protein or LPS antigens, the IgG and IgM antibodies to proteinase K-treated phase I and phase II antigens were also tested by immunoblotting as described above. Briefly, 10 μg of phase I and phase II whole cells were treated as follows: the cells were (i) boiled in Laemmli sample buffer for 5 min only, (ii) digested with 10 μg of proteinase K at 55°C for 4 h and then boiled in Laemmli sample buffer for 5 min, or (iii) boiled in Laemmli sample buffer for 5 min and then digested with 10 μg of proteinase K at 55°C for 4 h, and separated by SDS-PAGE.
To identify the immunodominant antigens during vaccination and challenge that may play a critical role in protective immunity, the antibody responses of unvaccinated and WCA-vaccinated challenged mice were compared by immunoblotting them with 2-D gel-separated antigens. A 2-D gel system using tube gels (pH range, 3 to 10) in the first-dimension electrophoresis described by O'Farrell (22) with modified Laemmli sample buffer was developed and used to separate C. burnetii antigens. Briefly, 200 μg of purified Nine Mile phase I cells was dissolved in modified sample buffer (containing 2.5% SDS, 10% 2-mercaptoethanol, and 10% glycerol in 60 mM Tris-HCl, pH 6.8) and boiled for 5 min. The sample was loaded onto 2.4-mm (internal diameter) by 14-cm isoelectric-focusing tube gels of 2% ampholytes (pH 3 to 10), 4% acrylamide, 2% NP-40, and 9.1 M urea. The sample was focused at 200 V for 1 h, 350 V for 30 min, 500 V for 30 min, and 750 V for 16 h. In the second-dimension gel electrophoresis, C. burnetii antigens that were separated by pI in the first-dimension tube gel were separated on a 13.5% polyacrylamide gel at 80 V for 12 h. The transfer of 2-D gel-separated antigens to PVDF and immunoblotting were performed as described above.
Construction of a genomic DNA library of C. burnetii.
A genomic DNA library of Nine Mile phase I was constructed using Lambda ZAP Express vector that had been predigested with BamHI and treated with calf intestinal phosphatase. Genomic DNA was partially digested with Sau3AI and fractionated by agarose gel electrophoresis. DNA fragments between 2 and 9 kb were purified from the agarose gel using a GeneClean II kit (Bio 101, Vista, Calif.) and ligated with 1.0 μg of BamHI-digested Lambda ZAP Express vector. Recombinant phage DNA was packaged in vitro using Gigapack III Gold packaging extract (Stratagene). The library was titrated by infecting XL1-Blue MRF′ cells with aliquots of packaged phage and plated onto NZY agar plates.
Screening of the genomic library.
The library was screened by plaque immunoblot assay using sera from mice that had recovered from C. burnetii infection (5 weeks postinfection with 109 organisms). About 8,000 plaques were plated (400 plaques per plate) and lifted onto IPTG (isopropyl-β-d-thiogalactopyranoside)-preabsorbed nitrocellulose membranes (Stratagene). The membranes were blocked in PBST and 10% nonfat dry milk and then screened by immunoblotting as described above. In vivo excision of the recombinant pBK-CMV plasmid from Lambda ZAP Express was achieved according to the protocol of the supplier.
SDS-PAGE and immunoblot analysis of expressed proteins.
Total proteins expressed by cloned C. burnetii DNA in E. coli XLOLR were analyzed by SDS-PAGE and immunoblotting. E. coli XLOLR containing the recombinant plasmid was cultured in Luria broth at 37°C overnight. The cells were pelleted by centrifugation and resuspended in 10 mM Tris-HCl (pH 8.0). The cells were mixed with an equal volume of 2× SDS sample buffer and boiled for 10 min. The proteins were separated by electrophoresis on 13.5% polyacrylamide gels and transferred onto PVDF membranes. Immunoreactive proteins of C. burnetii were detected by immunoblotting using infection- and vaccination-derived mouse sera as described above.
DNA sequence analysis.
Plasmid DNAs were purified by the alkaline lysis method, using a QIAprep Miniprep kit (Qiagen Inc., Valencia, Calif.), from cultures of E. coli XLOLR clones containing different C. burnetii DNA inserts. All the cloned DNAs were sequenced from one end of the insert at Gene Technologies Laboratories, Biology Department, Texas A&M University. A BLAST search against the complete genome sequence of Nine Mile phase I (The Institute for Genomic Research database) allowed identification of the cloned genes.
RESULTS
C. burnetii infection in BALB/c mice.
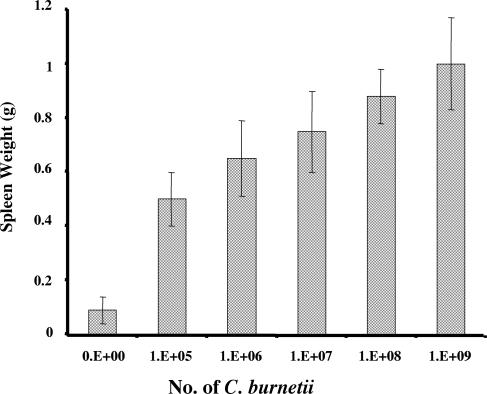
BALB/c mice were infected by the intraperitoneal route with serial dilutions of Nine Mile phase I to establish a mouse challenge model for evaluation of the protective efficacies of vaccine candidates. Three days postinfection, all mice at each infection dose developed clinical disease: reduced movement, ruffled fur, and body weight loss. Mice infected with the higher doses showed more severe illness. However, all the mice recovered from overt illness by day 10 after infection, and no deaths occurred at any dose. Low-dose (105 and 106 organisms) infected mice recovered sooner than high-dose (107, 108, and 109 organisms) infected mice, as observed by comparison of clinical signs. Splenomegaly was observed in mice infected at all doses at both 2 and 3 weeks postinfection, but at 5 weeks postinfection, only high-dose (107, 108, and 109 organisms) infected mice showed splenomegaly. Figure 1 shows the spleen weights of mice 2 weeks postinfection with different doses. Comparison of spleen weights between mice infected with different doses demonstrated that mouse spleen weight directly correlated with the infectious dose. These results suggest that BALB/c mice may provide a useful sublethal-infection model to evaluate the protective efficacies of vaccine candidates, with splenomegaly as a reporter for vaccinogenic activity.
FIG. 1.
Comparison of spleen weights of mice infected with different doses of C. burnetii. The spleen weights are for each group of mice 2 weeks postchallenge. The spleen weight of each group is the average with standard deviation for four mice.
Protective activity of WCA in BALB/c mouse model.
To test whether the BALB/c mouse model using splenomegaly as a reporter can be used to evaluate the vaccinogenic activities of vaccine candidates, the protective efficacy of WCA was tested in the model. After three immunizations, WCA-immunized mice produced a strong antibody response to a wide range of C. burnetii antigens, as detected by immunoblotting. Three days postchallenge, unvaccinated mice developed severe illness, while WCA-vaccinated mice did not show clinical disease. After euthanasia, spleen weights were measured for all mice at 2 or 4 weeks postchallenge. Severe splenomegaly was observed in unvaccinated mice, but splenomegaly was significantly reduced in WCA-vaccinated mice. Comparison of spleen weights from mice 2 and 4 weeks postchallenge indicated that there was a significant difference between unvaccinated and WCA-vaccinated mice. These results demonstrated that WCA elicited near-complete protection against challenge with virulent phase I organisms in the sublethal splenomegaly model.
Immunodominant antigens of C. burnetii recognized by mouse infection-derived sera.
To determine the immunodominant antigens during infection, mouse sera from different stages of infection with various doses of C. burnetii were tested by immunoblotting for antibodies against phase I antigens. Figure 2A shows an immunoblot of phase I antigens using a 1:500 dilution of mouse sera from different stages of infection with various doses (105, 108, or 109) of the organism. At all doses, infected mice developed a significant antibody response to C. burnetii antigens, particularly to 14-, 21-, 28-, 32-, and 60-kDa antigens. Low-dose (105 and 106 organisms) infected mice recognized 28-, 32-, and 60-kDa antigens as immunodominant antigens while high-dose (107, 108, and 109 organisms) infected mice also strongly responded to 14-, 21-, 45- to 50-, and 57-kDa antigens. High-dose infected mice developed a strong immune response to a wider range of C. burnetii antigens than low-dose infected mice. Immunoblotting also demonstrated that there were significantly different immune responses to C. burnetii antigens in early and late stages of infection. Two weeks postinfection, mice strongly responded to 28-, 32-, and 60-kDa antigens. However, 5 weeks postinfection, the mice also recognized 14-, 21-, 34-, 45- to 50-, and 57-kDa antigens as immunodominant antigens. These results indicate that 14-, 21-, 28-, 32-, 34-, 45- to 50-, 57-, and 60-kDa antigens are the immunodominant antigens during infection in mice, as measured by the ability to elicit a strong humoral response. Figure 2B and C shows immunoblots of proteinase K-digested and undigested phase I and phase II antigens with 1:500-diluted mouse sera from weeks 2 and 5 postinfection with 108 organisms, respectively. The results indicate that the 14-, 21-, 28-, 32-, and 60-kDa immunodominant antigens are shared by phase I and phase II, while the 55- and 80-kDa antigens are unique to phase II. Proteinase K digestion of phase I and phase II antigens prior to separation on SDS-PAGE eliminated the reactivity of these immunodominant antigens. Figure 2D shows IgM activity against phase I and proteinase K-digested phase I antigens in 1:25-diluted mouse sera from week 5 postinfection. Low-level IgM antibodies to 14- and 18-kDa proteinase K-treated phase I antigens were detected in mouse sera from late-stage infection but were undetectable in mouse sera from early-stage infection (data not shown). These results suggested that the immunodominant antigens predominantly recognized by mouse sera from the early and late stages of infection were protein antigens, not LPS.
FIG. 2.
Immunoblots of C. Burnetii antigens with infection-derived mouse sera. (A) Immunoblot of phase I C. burnetii antigen with sera from mice infected with various doses of C. burnetii. Lane 1, uninfected mouse sera. Lanes 2 to 4, weeks 2, 3, and 5, respectively, postinfection with 106 organisms. Lanes 5 to 7, weeks 2, 3, and 5, respectively, postinfection with 108 organisms. Lanes 8 to 10, weeks 2, 3, and 5, respectively, postinfection with 109 organisms. (B and C) Immunoblots of proteinase K-digested and undigested phase I and phase II WCAs with mouse sera from weeks 2 and 5, respectively, postinfection with 108 organisms. WC, whole cell; WCL, whole-cell lysates. (D) Immunoblot showing IgM activity against proteinase K-digested and undigested phase I antigens in 1:25-diluted mouse sera from week 5 postinfection. PK-I, proteinase K-treated phase I antigen.
Immunodominant antigens of C. burnetii recognized by the mouse immune system during vaccination and challenge.
Our hypothesis is that WCA-vaccinated mice developed a significant immune response to immunodominant antigens during vaccination and challenge that is critical for protection of the mice against challenge. Since immunoblotting with 1-D gel-separated antigens failed to detect differences in antibody responses to C. burnetii antigens during vaccination and challenge, a 2-D gel system was developed and used to separate C. burnetii proteins. Figure 3A shows the silver staining of 2-D gel-separated C. burnetii proteins. More than 500 distinctive protein spots were counted in the gel by processing scanned images using Imagemaster 2D Elite software version 3.10 (Amersham Pharmacia Biotech), providing a greatly enhanced ability to distinguish specific immunogenic proteins by immunoblotting. Figure 3B shows the immunoblot of 2-D gel-separated antigens using a 1:600 dilution of sera from prechallenge WCA-vaccinated mice. Several strong immunoreactive spots were observed in the high pI range (8 to 10), while most reactive spots were of higher molecular weight in the low pI range (4 to 6). Significant immunodominant proteins, including the 14-, 21-, 28-, 32-, and 60-kDa proteins with pI ranges from 8 to 10 and 36- to 60-, 100-, 130-, and 200-kDa proteins with pI ranges from 4 to 6 were recognized. This result indicated that mice vaccinated three times with WCA elicited a significant antibody response to a wide range of C. burnetii antigens. Figure 3C shows the immunoblot of 2-D gel-separated antigens using a 1:600 dilution of sera from unvaccinated mice 2 weeks postchallenge. The sera recognized immunoreactive protein spots that are similar to those seen using sera from prechallenge vaccinated mice. However, several additional specific immunoreactive spots, including the 30-kDa region and 47- (pI 9.4), 32- (pI 6), and 25-kDa (pI 4.5 and 5) proteins were observed. Figure 3D shows the immunoblot of 2-D gel-separated antigens using a 1:600 dilution of sera from WCA-vaccinated mice 2 weeks postchallenge. Distinctly different immune responses were found in WCA-vaccinated mice prechallenge and 2 weeks postchallenge (Fig. 3B and D). Antibodies against 14-, 21-, 28-, and 32-kDa proteins in high pI ranges from 8 to 10 were dramatically reduced after challenge, but a strong antibody response was generated to 14- and 35-kDa proteins in a pI range from 5 to 6. N-terminal amino acid sequence analysis and immunoblotting of 2-D gel-separated antigens with monoclonal antibody demonstrated that the 35-kDa protein is the translation elongation factor Ts (33) (data not shown). Figure 3E shows the immunoblot of 2-D gel-separated antigens using a 1:2,000 dilution of sera from unvaccinated mice 4 weeks postchallenge. This result indicates that 4 weeks postinfection, mice generated high-titer antibodies only to 14-, 28-, 32-, and 60-kDa proteins that have been identified as immunodominant proteins during the early stage of infection. Figure 3F shows the immunoblot of 2-D gel-separated antigens using a 1:2,000 dilution of sera from WCA-vaccinated mice 4 weeks postchallenge. The result indicates that 4 weeks postchallenge, vaccinated mice developed extremely strong antibody responses to a wide range of C. burnetii antigens, especially to the proteins that were recognized by sera from WCA-vaccinated mice prechallenge.
FIG. 3.
A 2-D gel protein profile of C. burnetii and immunoblots of 2-D gel-separated C. burnetii antigens with sera from WCA-vaccinated and unvaccinated infected mice. (A) Silver stain of 2-D gel-separated C. burnetii proteins. (B to F) Immunoblots of 2-D gel-separated C. burnetii antigens with mouse sera. (B) 1:600-diluted sera from prechallenge WCA-vaccinated mice. (C) 1;600-diluted sera from 2-week-postchallenge unvaccinated control mice. (D) 1:600-diluted sera from 2-week-postchallenge WCA-vaccinated mice. (E) 1:2,000-diluted sera from 4-week-postchallenge unvaccinated control mice. (F) 1:2,000-diluted serum from 4-week-postchallenge WCA-vaccinated mouse. NMI, Nine Mile phase I.
Cloning and identifying immunodominant antigens.
In order to clone immunodominant antigens, a genomic DNA library was screened with sera from mice 5 weeks postinfection that had recovered from severe illness. One hundred and two immunoreactive clones with various signal intensities were identified by the infection-derived mouse sera from ∼8,000 plaques. Of these reactive plaques, 54 clones that consistently expressed immunoreactive antigens were purified and expressed in the excised plasmid pBK-CMV. Clones expressing immunoreactive proteins with molecular masses ranging from 14 to 67 kDa were identified by SDS-PAGE and immunoblotting (Table 1). A BLAST search of the complete genome sequence of Nine Mile phase I (The Institute for Genomic Research database) identified 2 previously cloned proteins (Com1 and Hsp60) (10, 38) and 18 novel immunoreactive proteins. Most of the cloned genes encoded potential surface-exposed (open reading frames [ORFs] 01435, 02054, 01633, 02428, 02027, 00984, 01173, and 02523) or secreted (ORFs 00701 and 01174) proteins. Interestingly, several putative lipoprotein-encoding genes (ORFs 02054, 00518, 01633, and 02027) were cloned. However, several housekeeping genes (ORFs 01019, 01632, 02703, 02705, 00264, and 02702) were also isolated, and a single type IV secretion system gene, icmG (ORF 02827), was identified. The reactivities of recombinant antigens expressed by cloned genes in E. coli XLOLR with early or late antisera (2 or 5 weeks postinfection) from infected mice were tested by immunoblotting. Early sera reacted with recombinant proteins expressed by ORFs 01019, 00518, 01633, 02428, 02027, 01632, 02703, and 02705 (Fig. 4A, lanes 3 and 6 to 13). Late sera strongly reacted to the recombinant proteins expressed by all the clones tested (Fig. 4B). Sera from prechallenge WCA-vaccinated mice recognized recombinant proteins expressed by ORFs 01019, 00518, 01633, 02428, 02027, 02703, and 02705 (data not shown) that are similar to antigens recognized by the acute-phase sera. ORFs 02428 and 02702 encoding Com1 and Hsp60 proteins, which reacted with early and late antisera, respectively, were cloned and characterized previously (10, 38).
TABLE 1.
Identification of predicted ORFs of cloned genes
| No. | Mass (kDa) | Early serab | TIGR ORF | Predicted pI | SignalPc | Description |
|---|---|---|---|---|---|---|
| 1 | 12 | − | ORF02638 | 10.6 | No | Hypothetical protein |
| 2 | 13 | − | ORF01453 | 10.6 | 1 | comE operon protein 1; putative |
| 3 | 16 | + | ORF01019 | 6.2 | No | Ribosomal protein L9 |
| 4 | 19 | − | ORF02054 | 5.6 | 0.995 | Peptidoglycan-associated lipoprotein |
| 5 | 22 | + | ORF00518 | 10.2 | No | Lipoprotein; putative |
| 6 | 25 | + | ORF01633 | 8.9 | 0.934 | Lipoprotein |
| 7a | 27 | + | ORF02428 | 9.1 | 1 | Com1 |
| 8 | 28 | + | ORF02027 | 9.3 | 0.974 | ABC transporter; putative lipoprotein |
| 9 | 32 | + | ORF01632 | 5.9 | No | Isoleucyl-tRNA synthetase |
| 10 | 35 | + | ORF02703 | 10.1 | No | IS111A; transposase |
| 11 | 35 | + | ORF02705 | 6.7 | No | Glycine cleavage system T protein |
| 12 | 35 | − | ORF00701 | 9.3 | 0.939 | HlyD family secretion protein |
| 13 | 36 | − | ORF00984 | 5.1 | 0.995 | Hypothetical protein |
| 14 | 37 | − | ORF01174 | 9.4 | 0.995 | HlyD family secretion protein |
| 15 | 47 | − | ORF01173 | 8.4 | 1 | Protease DO |
| 16 | 37 | − | ORF02053 | 5.4 | No | Hypothetical protein |
| 17 | 40 | − | ORF02523 | 9.2 | 1 | Hypothetical protein |
| 18 | 49 | − | ORF00264 | 5.4 | No | 2-Oxoglutarate dehydrogenase |
| 19a | 60 | + | ORF02702 | 5 | No | Chaperonin; 60-kDa subunit |
| 20 | 67 | − | ORF02827 | 5.3 | No | IcmG protein; putative |
FIG. 4.
Comparison of reactivities of recombinant C. burnetii antigens expressed by different clones in E. coli XLOLR with acute- and convalescent-phase antisera from mice experimentally infected with various doses of C. burnetii. (A) Immunoblot of recombinant proteins with sera from 2 weeks postinfection (acute phase) with 105 organisms. (B) Immunoblot of recombinant proteins with sera from 5 weeks postinfection (convalescent phase) with 109 organisms. The samples in panels A and B are the same. Lane 1, Nine Mile phase I lysates; lanes 2 to 18, lysates of E. coli XLOLR containing different recombinant plasmids encoding the following ORFs: lane 2, 01453; lane 3, 01019; lanes 4 and 5, 00518; lane 6, 01633; lanes 7 and 9, 02027; lane 8, 02428; lanes 10 and 11, 01632; lane 12, 02703; lane 13, 02705; lane 14, 00701; lane 15, 01174; lanes 16 to 18, 01173; lane 19, negative control, lysates of E. coli XLOXR containing pBK-CMV plasmid.
DISCUSSION
The mouse is the most commonly used animal model for studies of Q fever, and it has provided an initial evaluation system to monitor the immunodominance and vaccinogenicities of C. burnetii antigens (31). Mice infected with virulent C. burnetii can develop severe or fatal illness or remain relatively free of clinical signs, depending upon the strain of mouse (1, 15, 31, 45). BALB/c mice were described as intermediate in sensitivity to lethal challenge and have been used by several groups to the estimate protective efficacies of C. burnetii antigens (18, 45, 48). Previous studies have demonstrated that BALB/c mice, which do not posses a major characterized immune dysfunction, were able to generate a strong protective response to C. burnetii antigens (18, 48). Our results indicated that BALB/c mice infected with Nine Mile phase I developed dose-related clinical disease and splenomegaly. The finding that mouse spleen weight was directly correlated with the infectious dose suggests that splenomegaly may be a useful indicator to evaluate the vaccinogenic activities of C. burnetii antigens. Evaluation of vaccinogenic activities of WCA antigens in this model demonstrated that WCA generated near-complete protection against challenge from the development of clinical disease and splenomegaly. These results suggest that the sublethal BALB/c mouse model with protection from the development of clinical disease and severe splenomegaly can be a useful alternative to a lethal-challenge model to evaluate the protective efficacies of vaccine candidates.
Previously, several studies focused on the identification of immunogenic proteins of C. burnetii by immunoblotting with sera from human Q fever patients, experimentally infected mice, or hyperimmune rabbits (11, 13, 17, 41). However, there has been no report of a comprehensive and systematic analysis of the immunodominant antigens during infection by C. burnetii. To determine immunodominant antigens that were recognized by the immune system during an infection, mouse sera from different stages of experimental infection with various doses of C. burnetii were analyzed in this study. Our results indicated that BALB/c mice infected with C. burnetii developed a significant antibody response to C. burnetii antigens and that the level of response depended on the infectious dose. High-dose infected mice developed a strong immune response to a wide range of C. burnetii antigens, including the 14-, 16-, 21-, 28-, 32-, 45- to 50-, 57-, and 60-kDa proteins. Significantly different immune responses to C. burnetii antigens were also found in the early and late stages of infection. Interestingly, even low-dose infected mice generated a strong antibody response to the 21-, 28-, 32-, and 60-kDa proteins at an early stage of infection. Since antibodies appearing during the early stage of infection are particularly important for serological diagnosis of acute Q fever, the immunodominant proteins recognized by early infection-derived sera are potential serological diagnostic target antigens.
Previous studies based on conventional serological methods, such as complement fixation, microagglutination, and IFA, suggested that the abilities to react with early antisera were different in phase I and phase II organisms (5-7, 35). Phase II organisms reacted with both early (<20 days postinfection) and late (>20 days postinfection) infection-derived sera, while phase I organisms were recognized predominantly by late antisera (5, 35). Experimental infection or vaccination of guinea pigs with phase I organisms generated early phase II and later phase I antibody responses, while vaccination with phase II organisms elicited only phase II antibodies (5-7, 35). Based on these observations, it was hypothesized that phase II protein antigenic determinants were present in phase I organisms but were masked by phase I LPS (2, 3, 7, 30). This model was supported in a study by Hackstadt and led to the hypothesis that the LPS of phase I, with extended carbohydrate structure, sterically blocked the access of antibodies to common phase I and phase II protein antigens of C. burnetii (9). Since there has been no clear evidence to confirm that phase I cells cannot be recognized by antibodies against common proteins, this model has not resolved whether phase I and phase II organisms share identical protein antigenic determinants. In the present study, several proteinase K-sensitive bands specific to phase II lysates reacted with phase I infection-derived sera (Fig. 2B and C). Williams et al. also identified 80- and 92-kDa antigens specific to phase II organisms (46). We speculate that it is unlikely that phase II cells express unique 80- and 55-kDa proteins, since their detection resulted from reaction with sera from mice infected with phase I organisms. Rather, it is more likely that these antigens are detected on Western blots by an unexplained difference between phase I and phase II whole cells in conformation or solubilization. Sera from phase II antigen-immunized mice compared by IFA and ELISA with phase I antigens suggested that phase I antigens reacted with phase II-specific antibodies (8). Comparison of the reactivities of formalin-inactivated phase I and phase II antigens with antisera from phase I-infected or -vaccinated mice also demonstrated that there were no significantly different reactivities in phase I and phase II antigens in ELISA (data not shown).
To determine whether the immunodominant antigens during infection with phase I organisms were predominantly protein or LPS, we also examined antibody responses to nonproteinaceous antigens, especially LPS. Proteinase K digestion of whole C. burnetii organisms suggested that the dominant antigens recognized by IgG at 2 (Fig. 2B) and 5 (Fig. 2C) weeks postinfection are proteins. Detection of IgM antibody at 5 weeks postinfection suggests that at least two molecular weight moieties were likely LPS at a 1:25 titer. These results support the notion that proteins are the predominant antigens recognized during infection in mice and support their use as serodiagnostic targets and subunit vaccines.
To identify the potential protective antigens, the antibody response to C. burnetii antigens in WCA-vaccinated mice was analyzed by immunoblotting with 2-D gel-separated C. burnetii antigens. Our results demonstrated that there are significantly different antibody responses between (i) prechallenge and 2 weeks postchallenge in vaccinated mice, (ii) 2 and 4 weeks postchallenge in vaccinated mice, (iii) vaccinated and unvaccinated mice 2 weeks postchallenge, and (iv) vaccinated and unvaccinated mice 4 weeks postchallenge. Several specific antigens were identified in WCA-vaccinated mice during vaccination and prior to challenge, suggesting that these antigens may play an important role in the protection of mice against challenge. These results suggest that immunoblotting with 2-D-separated antigens has an enhanced ability to identify the differences between stages of immune response, as well as to identify key antigens in each stage of vaccination and challenge.
Since WCA-vaccinated mice generated complete protection against challenge, the sera from these mice may have the ability to recognize protective antigens. The 14-, 21-, 28-, 32-, and 60-kDa proteins in the pI range from 8 to 10 and 36- to 60-, 100-, 130-, and 200-kDa proteins in the pI range from 4 to 6 were recognized by sera from WCA-vaccinated mice, suggesting that these proteins may play important roles in the protection of mice against challenge. Interestingly, WCA-vaccinated mice developed an antibody response to C. burnetii antigens which was similar to the response of unvaccinated mice 2 weeks postchallenge. This result suggests that the proteins that generated protective immunity in WCA-vaccinated mice may be the same proteins that were recognized by the mouse immune system during the early stage of infection. The results also identified a specific antibody response to C. burnetii antigens in WCA-vaccinated mice prechallenge and 2 and 4 weeks postchallenge. Antibodies to the 14-, 21-, 28-, and 32-kDa proteins in the high pI range from 8 to 10 dramatically decreased, but antibodies to 14- and 35-kDa proteins in the pI range from 5 to 6 increased in 2-week-postchallenge mice. Four-week-postchallenge mice developed extremely strong antibody responses against these proteins. These results suggest that WCA-vaccinated mice rapidly generated a strong secondary antibody response to the 14-, 21-, 28-, and 32-kDa proteins and that these proteins may play critical roles in the early protective immune defense. A proteomic approach that combines 2-D gel technology with C. burnetii genomics is able to identify specific proteins. Further characterization of these specific proteins may provide useful reagents for the development of a subunit vaccine and recombinant antigen based serodiagnostic assays.
Based on these results, we speculated that cloning and characterizing many of these proteins would provide valuable reagents for developing novel, specific diagnostic assays and a subunit vaccine against C. burnetii infection. In order to clone these antigens, an expression genomic DNA library was screened with convalescent-phase sera. Twenty different immunoreactive proteins with molecular masses ranging from 14 to 60 kDa were cloned and identified. A BLAST search of the complete genome sequence identified two previously cloned proteins and 18 novel immunoreactive proteins. As expected, many of the cloned proteins were predicted surface-exposed or secreted proteins. These proteins were all recognized by convalescent-phase sera, suggesting that they may play important roles in the development of protective immunity. However, identification of several housekeeping proteins by convalescent-phase sera suggested that housekeeping proteins are also exposed to the mouse immune system during infection and that they may play some role in protection against infection. Interestingly, several recombinant antigens expressed by ORFs 01019, 00518, 01633, 02428, 02027, 02703, and 02705 reacted with sera from the early stage of infection and from WCA-vaccinated prechallenge mice. These results suggest that the proteins may be useful reagents for developing subunit vaccines and novel, specific diagnostic reagents. The identification of an immunoreactive clone with sequence homology to icmG is particularly interesting. Current analysis and annotation of the complete genome sequence for the Nine Mile strain of C. burnetii indicates a complete complement of genes encoding a type IV secretion system, closely related to the Dot/Icm locus of Legionella pneumophila (32, 34). The reactivity of IcmG with infection-derived sera strongly suggests that C. burnetii expresses a type IV secretion system in vivo. Future studies will establish whether these recombinant proteins have the ability to generate a protective immune response in BALB/c mice, with the ultimate goal of identifying the key antigens in the development of protective immunity.
Acknowledgments
This study was supported by Public Health Service grants AI37744 and AI448191 from the National Institute of Allergy and Infectious Diseases.
Editor: D. L. Burns
REFERENCES
- 1.Atzpodien, E., W. Baumgartner, A. Artelt, and D. Thiele. 1994. Valvular endocarditis occurs as a part of a disseminated Coxiella burnetii infection in immunocompromised Balb/c (H-2d) mice infected with the Nine Mile isolate of C. burnetii. J. Infect. Dis. 170:223-226. [DOI] [PubMed] [Google Scholar]
- 2.Baca, O. G., and D. Paretsky. 1983. Q fever and Coxiella burnetii: a model for host-parasite interactions. Microbiol. Rev. 47:127-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brezina, R., and J. Urvolgyi. 1962. Study of the antigenic structure of Coxiella burnetii. I. Extraction of phase I antigenic component by means of trichloroacetic acid. Acta Virol. 6:84-88. [Google Scholar]
- 4.Dupuis, G., O. Peter, M. Peacock, W. Burgdorfer, and E. Haller. 1985. Immunoglobulin responses in acute Q fever. J. Clin. Microbiol. 22:484-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiset, P. 1957. Phase variation of Rickettsia (Coxiella) burnetii: study of the antibody response in guinea pigs and rabbits. Can. J. Microbiol. 3:435-445. [DOI] [PubMed] [Google Scholar]
- 6.Fiset, P., and R. A. Ormsbee. 1968. The antibody response to antigens of Coxiella burnetii. Zentbl. Bakteriol. Parasitenkd. Infektionskr. Hyg. Abt. 1 Orig. 206:321-329. [PubMed] [Google Scholar]
- 7.Fiset, P., R. A. Ormsbee, R. Silberman, M. Peacock, and S. H. Spielman. 1969. A microagglutination technique for detection and measurement of rickettsial antibodies. Acta Virol. 13:60-66. [PubMed] [Google Scholar]
- 8.Gajdosova, E., E. Kovacova, R. Toman, L. Skultety, M. Lukacova, and J. Kazar. 1995. Immunogenicity of Coxiella burnetii whole cells and their outer membrane components. Acta Virol. 38:339-344. [PubMed] [Google Scholar]
- 9.Hackstadt, T. 1988. Steric hindrance of antibody binding to surface proteins of Coxiella burnetti by phase I lipopolysaccharide. Infect. Immun. 56:802-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hendrix, L. R., L. P. Mallavia, and J. E. Samuel. 1993. Cloning and sequencing of Coxiella burnetii outer membrane protein gene com1. Infect. Immun. 61:470-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho, T., A. Hotta, G. Q. Zhang, S. V. Nguyen, M. Ogawa, T. Yamaguchi, H. Fukushi, and K. Hirai. 1998. Antigenic characteristics of polypeptides of Coxiella burnetii isolates. Microbiol. Immunol. 42:81-85. [DOI] [PubMed] [Google Scholar]
- 12.Ho, T., K. K. Htwe, N. Yamasaki, G. Q. Zhang, M. Ogawa, T. Yamaguchi, H. Fukushi, and K. Hirai. 1995. Isolation of Coxiella burnetii from dairy cattle and ticks, and some characteristics of the isolates in Japan. Microbiol. Immunol. 39:663-671. [DOI] [PubMed] [Google Scholar]
- 13.Jerrells, T. R., D. J. Hinrichs, and L. P. Mallavia. 1974. Cell envelope analysis of Coxiella burnetii phase I and phase II. Can. J. Microbiol. 20:1465-1470. [Google Scholar]
- 14.Kazar, J., R. Brezina, S. Schramek, A. Palanova, and B. Tvrda. 1981. Suitability of the microagglutination test for detection of post-infection and post-vaccination Q fever antibodies in human sera. Acta Virol. 25:235-240. [PubMed] [Google Scholar]
- 15.Kazar, J., and S. Schramek. 1984. Sensitization of mice to rickettsial toxin by Coxiella burnetii. Acta Virol. 28:309-316. [PubMed] [Google Scholar]
- 16.Kimbrough, R. C., R. A. Ormsbee, M. Peacock, R. Rogers, R. W. Bennetts, J. Raff, A. Krause, and C. Gardner. 1979. Q fever endocarditis in the United States. Ann. Intern. Med. 91:400-402. [DOI] [PubMed] [Google Scholar]
- 17.Kovacova, E., M. Vavrekova, M. Lukacova, A. B. Daiter, N. K. Tokarevich, N. A. Karceva, E. N. Gorbachev, J. Urvolgyi, E. Kocianova, J. Rehacek, et al. 1994. Immunochemical and antigenic characterization of Coxiella burnetii strains isolated in Europe and Mongolia. Eur. J. Epidemiol. 10:9-15. [DOI] [PubMed] [Google Scholar]
- 18.Lukacova, M., E. Gajdosova, L. Skultety, E. Kovacova, and J. Kazar. 1994. Characterization and protective effect of a 29 kDa protein isolated from Coxiella burnetii by detergent Empigen BB. Eur. J. Epidemiol. 10:227-230. [DOI] [PubMed] [Google Scholar]
- 19.Marrie, T. J. 1990. Acute Q fever, p. 125-160. In T. J. Marrie (ed.), Q fever, vol. 1. The disease. CRC Press, Boca Raton, Fla.
- 20.Marrie, T. J. 1990. Epidemiology of Q fever, p. 49-70. In T. J. Marrie (ed.), Q fever, vol. I. The disease. CRC Press, Boca Raton, Fla.
- 21.Mo, Y. Y., N. P. Cianciotto, and L. P. Mallavia. 1995. Molecular cloning of a Coxiella burnetii gene encoding a macrophage infectivity potentiator (Mip) analogue. Microbiology 141:2861-2871. [DOI] [PubMed] [Google Scholar]
- 22.O'Farrell, P. 1975. High resolution two dimensional electrophoresis of proteins. J. Biol. Chem. 250:4007-4021. [PMC free article] [PubMed] [Google Scholar]
- 23.Peacock, M. G., R. N. Philip, J. C. Williams, and R. S. Faulkner. 1983. Serological evaluation of Q fever in humans: enhanced phase I titers of immunoglobulins G and A are diagnostic for Q fever endocarditis. Infect. Immun. 41:1089-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peter, O., G. Dupuis, W. Burgdorfer, and M. Peacock. 1985. Evaluation of the complement fixation and indirect immunofluorescence tests in the early diagnosis of primary Q fever. Eur. J. Clin. Microbiol. 4:394-396. [DOI] [PubMed] [Google Scholar]
- 25.Peter, O., G. Dupuis, M. G. Peacock, and W. Burgdorfer. 1987. Comparison of enzyme-linked immunosorbent assay and complement fixation and indirect fluorescent-antibody tests for detection of Coxiella burnetii antibody. J. Clin. Microbiol. 25:1063-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raoult, D. 1993. Treatment of Q fever. Antimicrob. Agents Chemother. 37:1733-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raoult, D., and T. Marrie. 1995. Q Fever. Clin. Infect. Dis. 20:489-496. [DOI] [PubMed] [Google Scholar]
- 28.Raoult, D., A. Raza, and T. J. Marrie. 1990. Q fever endocarditis and other forms of chronic Q fever, p. 179-200. In T. J. Marrie (ed.), Q fever, vol. 1. The disease. CRC Press, Boca Raton, Fla.
- 29.Samuel, J. E., M. E. Frazier, and L. P. Mallavia. 1985. Correlation of plasmid type and disease caused by Coxiella burnetii. Infect. Immun. 49:775-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schramek, S., R. Brezina, and J. Kazar. 1978. Influence of mild acid hydrolysis on the antigenic properties of phase I Coxiella burnetii. Acta Virol. 22:302-308. [PubMed] [Google Scholar]
- 31.Scott, G. H., J. C. Williams, and E. H. Stephenson. 1987. Animal models in Q fever: pathological responses of inbred mice to phase I Coxiella burnetii. J. Gen. Microbiol. 133:691-700. [DOI] [PubMed] [Google Scholar]
- 32.Segal, G., J. J. Russo, and H. A. Shuman. 1999. Relationships between a new type IV secretion system and the icm/dot virulence system of Legionella pneumophila. Mol. Microbiol. 34:799-809. [DOI] [PubMed] [Google Scholar]
- 33.Seshadri, R., L. R. Hendrix, and J. E. Samuel. 1999. Differential expression of translational elements by lifecycle variants of Coxiella burnetii. Infect. Immun. 67:6026-6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seshadri, R., I. T. Paulsen, J. A. Eisen, T. D. Read, K. E. Nelson, W. C. Nelson, N. L. Ward, H. Tettelin, T. M. Davidsen, M. J. Beanan, R. T. Deboy, S. C. Daugherty, L. M. Brinkac, R. Madupu, R. J. Dodson, H. M. Khouri, K. H. Lee, H. A. Carty, D. Scanlan, R. A. Heinzen, H. A. Thompson, J. E. Samuel, C. M. Fraser, and J. F. Heidelberg. 2003. Complete genome sequence of the Q-fever pathogen Coxiella burnetii. Proc. Natl. Acad. Sci. USA 100:5455-5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoker, M. B. P., and P. Fiset. 1956. Phase variation of the Nine Mile and other strains of Rickettsia burnetii. Can. J. Microbiol. 2:310-321. [DOI] [PubMed] [Google Scholar]
- 36.Uhaa, I. J., D. B. Fishbein, J. G. Olson, C. C. Rives, D. M. Waag, and J. C. Williams. 1994. Evaluation of specificity of indirect enzyme-linked immunosorbent assay for diagnosis of human Q fever. J. Clin. Microbiol. 32:1560-1565. (Erratum, 32:2343.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varghees, S., K. Kiss, G. Frans, O. Braha, and J. E. Samuel. 2002. Cloning and porin activity of the major outer membrane protein, P1, from Coxiella burnetii. Infect. Immun. 70:6741-6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vodkin, M. H., and J. C. Williams. 1988. A heat shock operon in Coxiella burnetii produces a major antigen homologous to a protein in both mycobacteria and Escherichia coli. J. Bacteriol. 170:1227-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waag, D. M., M. Kende, T. A. Damrow, O. L. Wood, and J. C. Williams. 1990. Injection of inactivated phase I Coxiella burnetii increases non-specific resistance to infection and stimulates lymphokine production in mice. Ann. N. Y. Acad. Sci. 590:203-214. [DOI] [PubMed] [Google Scholar]
- 40.Waag, D. M., and J. C. Williams. 1988. Immune modulation by Coxiella burnetii: characterization of a phase I immunosuppressive complex differentially expressed among strains. Immunopharmacol. Immunotoxicol. 10:231-260. [DOI] [PubMed] [Google Scholar]
- 41.Willems, H., D. Thiele, M. Glas-Adollah-Baik Kashi, and H. Krauss. 1992. Immunoblot technique for Q fever (technical note). Eur. J. Epid. 8:103-107. [DOI] [PubMed] [Google Scholar]
- 42.Williams, J. C., and J. L. Cantrell. 1982. Biological and immunological properties of Coxiella burnetii vaccines in C57BL/10ScN endotoxin-nonresponder mice. Infect. Immun. 35:1091-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams, J. C., T. A. Hoover, D. M. Waag, N. Banerjee-Bhatnagar, C. R. Bolt, and G. H. Scott. 1990. Antigenic structure of Coxiella burnetii: a comparison of lipopolysaccharide and protein antigens as vaccines against Q fever. Ann. N. Y. Acad. Sci. 590:370-380. [DOI] [PubMed] [Google Scholar]
- 44.Williams, J. C., M. G. Peacock, D. M. Waag, G. Kent, M. J. England, G. Nelson, and E. H. Stephanson. 1992. Vaccines against coxiellosis and Q fever. Development of a chloroform residue subunit of phase I Coxiella burnetii for the immunization of animals. Ann. N. Y. Acad. Sci. 653:88-111. [DOI] [PubMed] [Google Scholar]
- 45.Williams, J. C., V. Sanchez, G. H. Scott, E. H. Stephenson, and P. H. Gibbs. 1985. Variation in responsiveness of BALB/c sublines and congenic mice to phase I Coxiella burnetii infection and vaccination. Curr. Top. Microbiol. Immunol. 122:189-199. [DOI] [PubMed] [Google Scholar]
- 46.Williams, J. C., and S. Stewart. 1984. Identification of immunogenic proteins of Coxiella burnetii phase variants, p. 257-262. In L. Leive and D. Schlessinger (ed.), Microbiology—1984. American Society for Microbiology, Washington, D.C.
- 47.Williams, J. C., L. A. Thomas, and M. G. Peacock. 1986. Identification of phase-specific antigenic fractions of Coxiella burnetii by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 24:929-934. (Erratum, 25:588, 1987.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang, Y. X., N. Zhi, S. R. Yu, Q. R. Li, G. C. Yu, and X. Zhang. 1994. Protective immunity induced by 67 K outer membrane protein of phase I Coxiella burnetii in mice and guinea pigs. Acta Virol. 38:327-332. [PubMed] [Google Scholar]