Abstract
Cancer immunotherapy has been the focus of intense research since the late 19th century when Coley observed that bacterial components can contribute to cancer regression by eliciting an antitumor immune response. Successful activation and maturation of tumor-specific immune cells is now known to be mediated by bacterial endotoxin, which activates Toll-like receptor 4 (TLR4). TLR4 is expressed on a variety of immune as well as tumor cells, but its activation can have opposing effects. While TLR4 activation can promote antitumor immunity, it can also result in increased tumor growth and immunosuppression. Nevertheless, TLR4 engagement by endotoxin as well as by endogenous ligands represents notable contribution to the outcome of different cancer treatments, such as radiation or chemotherapy. Further research of the role and mechanisms of TLR4 activation in cancer may provide novel antitumor vaccine adjuvants as well as TLR4 inhibitors that could prevent inflammation-induced carcinogenesis.
1. Introduction
Immune system plays a crucial role not only in defense against microbial infection but also in control and surveillance of malignant neoplasms. Immune cells scan tissues with the objective to remove newlyformed malignant cells before they turn into fully formed tumors. Malignant cells developed intricate mechanisms that enable them to inhibit immune cells through secretion of specific cytokines that create an immunosuppressive environment [1]. Tumors can even directly kill tumor-infiltrating lymphocytes, which are CD95 sensitive, by expressing the CD95L (Fas ligand) [2].
Innate immunity is the first line of defense against microbial infection. Innate immune cells recognize the intruding pathogen and trigger appropriate immune response with the help of Toll-like receptors (TLRs), arguably the most important vertebrate innate immune receptors. TLRs recognize different molecules of microbial origin, called pathogen-associated molecular patterns. TLRs are located on the cell surface (TLR1, 2, 4, 5, 6) or in the endosomal compartments (TLR3, 7, 8, 9), where they safeguard the organism against infection. After recognition of their respective ligands, TLRs dimerize and trigger a cytoplasmic signaling pathway that leads to activation of several nuclear factors (e.g., NFκB, IRF) responsible for transcription of immune genes [3].
Toll-like receptor signaling in immune cells is critical for regulation of innate and adaptive immune responses, such as DC maturation and antigen presentation as well as CD8+ T-cell cytotoxicity, all of which are important factors in antitumor immunity [4]. On the other hand, TLR stimulation can also result in enhanced regulatory T-cell proliferation and suppressor function favoring tumor development [5–7]. TLR expression is not limited to immune cells, and indeed many tumor cells have been found to express TLRs, signaling through which can promote tumor growth and immune evasion [8, 9]. On the other hand, TLR signaling in tumor cells was also shown to reduce the proliferative capacity of tumor cells [10]. We will focus on reports concerning TLR4 signaling and its involvement in cancer development and progression as well as the therapeutic benefit that could come from TLR4 stimulation.
2. Toll-Like Receptor 4 in Health and Disease
TLRs are homologues of Toll, a receptor found in insects, that is involved in establishing dorsoventral polarity during embryogenesis as well as in immune response against fungal infections [11, 12]. The first discovered human Toll homologue was TLR4. It recognizes endotoxin (i.e., lipopolysaccharide), an outer membrane component of Gram-negative bacteria, that is composed of a conserved amphipathic lipid A component and of variable polysaccharides. The mechanism of TLR4 activation is quite complex and (unlike other TLRs) involves several auxiliary proteins (LBP, CD14) as well as a coreceptor (MD-2) [3] (Figure 1). It is in fact MD-2 and not TLR4 that directly recognizes and binds endotoxin [13, 14]. MD-2 is a soluble protein with a large hydrophobic pocket that represents the binding site for the acyl chains of lipid A. Lipid A is usually composed of 6 acyl chains, but only 5 of them bind into the hydrophobic pocket of MD-2. The 6th acyl chain protrudes out of the pocket and interacts with hydrophobic residues on TLR4. These interactions are crucial for MD-2/TLR4 heterodimerization and therefore prerequisite for the activation of the TLR4 signaling cascade [15, 16]. The endotoxin/MD-2/TLR4 heterodimer can, unlike other TLR signaling complexes, recruit two distinct intracellular adaptor proteins (i.e., MyD88/TIRAP and TRIF/TRAM) and can therefore activate two parallel signaling pathways and trigger the transcription of both proinflammatory cytokines as well as type I interferons [3]. Immune effects of TLR4 activation are indeed extensive; LPS alone can activate over 1000 genes [17]. It is therefore not too surprising that TLR4 activation affects not only the immune response against invading Gram-negative bacteria but is also involved in chronic inflammation, autoimmune diseases, and malignancies. TLR4 signaling in cancer is considered a double-edged sword. If TLR4 is activated on immune cells, it can enhance anti-tumor immunity. On the other hand, chronic inflammation is a major risk factor in cancer development [18].
Figure 1.
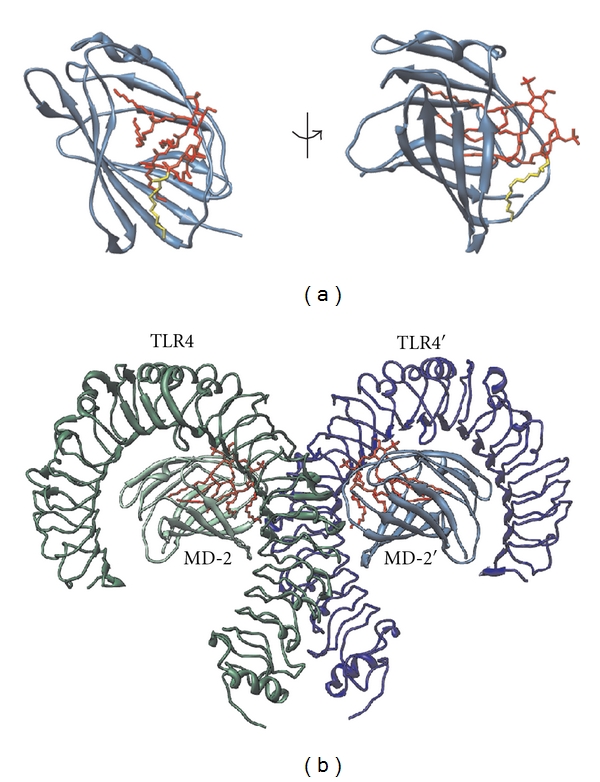
TLR4/MD-2 receptor complex recognizes and binds endotoxin. (a) MD-2 (shown in blue ribbons) is a soluble protein with a large hydrophobic pocket that directly binds bacterial endotoxin (red). One of the acyl chains of endotoxin (yellow) remains outside the hydrophobic pocket and mediates crucial interactions with TLR4 that bind the TLR4/MD-2 heterodimer together. Left: direct view of the MD-2 hydrophobic pocket. Right: side view showing the protruding endotoxin acyl chain. (b) The TLR4/MD-2/endotoxin heterodimer. Only the extracellular domains of TLR4 whose crystal structures were determined are shown [16].
3. TLR4 Expression in Cancer Cells
Progress in cancer research over the past decade has been immense, and the original fundamental characteristics of cancer (sustained proliferative signaling, evasion of growth suppressors, resistance to cell death, replicative immortality, induction of angiogenesis, invasion, and metastasis) [19] have recently been revisited and updated. Evasion of immune destruction rises as a new emerging hallmark of cancer [20]. Tumors utilize multiple mechanisms that help them turn the immune balance in their favor. They can secrete immunosuppressive cytokines (TGFβ, IL-10, etc.), express antiapoptotic molecules, or downregulate tumor antigens and MHC1 expression [1]. TLRs are expressed by a variety of tumor cell lines, both in mouse and in human (Tables 1 and 2). Many of them are not limited to a single TLR but rather utilize an assortment of different TLRs (similarly to immune cells).
Table 1.
Murine tumor cell lines that express TLR4.
Table 2.
Human tumor cell lines that express TLR4.
| Tumor type | Human tumor cell line | References |
|---|---|---|
| Bladder cancer | T24 | [22] |
| Breast cancer | MDA-MB-231 | [23] |
| Colon cancer | SW480, HT29, KM20 | [24, 25] |
| Laryngeal and oral cancer | PCI-1, PCI-30 | [26] |
| Melanoma | SkMEL-28, BN1, 9923M, ME5, ME16, ME17 | [27, 28] |
| Neuroblastoma | NB-1 | [29] |
| Ovarian cancer | SKOV3, AD10, A2780, CP70 | [9, 30, 31] |
Expression of TLR4 was confirmed by RT-PCR and FACS analysis on a large number of murine tumor cells, such as colon, breast, prostate, lung, and melanoma cancer cells. TLR4 signaling was shown to be unimpaired and could induce the synthesis of soluble immune mediators that could help the tumor to withstand the immune attack [8]. MC26 cells, for example, were shown to express functional TLR4 that (when activated by endotoxin) triggered activation of NF-κB, ERK, and JNK kinases as well as the synthesis of iNOS, IL-6, and IL-12p70 [8]. iNOS and IL-6 have immunosuppressive effects [32–34], but IL-12p70 is generally not considered favorable for tumor development since it activates NK cells, induces T-cell proliferation, and promotes specific allogenic CTL reactions [35]. Some papers indicate that IL-12p70 can also have suppressive effects on allogenic or tumor-specific CTL generation [36, 37], but since evidence undisputedly demonstrates anti-tumor effects for IL-12 its production by tumor cells is possibly just a side product of TLR4 activation and subsequent NF-κB activation.
Supernatants from endotoxin-stimulated tumor cells were shown to inhibit T-cell proliferation and NK-cell cytotoxicity. Furthermore, blockade of tumor TLR4 signaling with anti-TLR4 siRNA or with inhibitory TLR4 peptide treatment prolongs the survival of MC26-bearing mice [8].
Functional TLR4 signaling was also demonstrated on human tumor cells. On colon carcinoma cells TLR4 signaling, in addition to production of immunosuppressive factors, also improved tumor cell apoptosis resistance [24]. Moreover, endotoxin stimulation of human prostate epithelial cancer cells elicited production of immunosuppressive and proangiogenic factors (TGF-beta and VEGF, resp.) [38].
TLR4 is expressed not only on malignant cells but also on normal tissues and benign tumors [30, 31]. Much remains to be studied concerning the function of TLR4 on normal nonimmune tissues in correlation with cancer development. But we must not forget to examine the expression of other contributing proteins in the TLR4 signaling cascade, for example, the adapter protein MyD88 (myeloid differentiation 88) that is essential for pro-inflammatory signaling. Although TLR4 expression was shown in normal ovarian epithelium, MyD88 was not expressed, therefore rendering TLR4 signaling via the proinflammatory MyD88-dependent pathway nonfunctional [9, 30]. Similar observation was made in a variety of colorectal carcinoma cell lines where tumor cells expressed TLR4 but failed to coexpress CD14, an important auxiliary protein in the endotoxin receptor complex [39] (Table 3).
Table 3.
Human tumors expressing TLR4.
4. Chronic Inflammation Mediated by TLR4 in Cancer Development and Progression
Numerous links exist between inflammation and tumor development [18]. At the same time inflammatory cytokines are indispensable for immune cell activation and antitumor function. Therefore, there is an apparent contradiction when we consider the role of inflammation in cancer. It is plausible that part of the answer to this puzzle lies not in the inflammatory stimulation per se but in its timing, duration, and intensity.
Chronic inflammation is often associated with cancer and can be the result of different causes, such as autoimmune disease or microbial infection.
An example of microbial infection that can predispose an individual to cancer development is Helicobacter pylori infection. Infection with H. pylori is a known risk factor in gastric cancer and has been classified as a human carcinogen by the International Agency for Research on Cancer [45]. H. pylori infection is chronic and persistent, because H. pylori has the ability to evade immune system recognition. It has unusual endotoxin that exhibits very low endotoxic activity compared to the more common hexa-acylated form of endotoxin, usually found in enterobacteria (e.g., Escherichia coli) [46]. In spite of the inability to stimulate TLR4 on its own, H. pylori actively promotes inflammation by upregulating TLR4 expression via TLR2 and MEK1/2-ERK1/2 pathway giving way to TLR4 activation by endotoxin from other bacteria that pass through the gastrointestinal tract [47, 48]. TLR4 expression was indeed observed on gastric carcinoma tumor cells as well as on gastric epithelium with intestinal metaplasia and dysplasia [42].
Persistent inflammation is also a characteristic of colitis-associated neoplasms. Patients with ulcerative colitis have a five to eight times higher risk of developing colorectal cancer than the rest of the population [49, 50]. TLR4 expression is upregulated in colitis-associated cancer lesions from patients with ulcerative colitis but not in the surrounding tissue [51]. TLR4 seems to promote the development of colitis-associated colorectal tumors, and mice deficient in TLR4 are markedly protected against the development of neoplasia [52]. The reason behind this phenomenon could lie in the TLR4-Cox2-PGE2 signaling axis. Cyclooxygenase-2 (Cox-2) is aberrantly expressed in the majority of colorectal tumors and is (along with its enzymatic product prostaglandin E2) involved in the development of colorectal cancer [53]. It was recently shown that oral administration of high dosages of PGE2 can by-pass the protective effect exhibited by TLR4-deficient mice, which implicates PGE2 as an important TLR4 downstream molecule in colorectal cancer development as well as a potential target for more effective prevention of colitis-associated colorectal cancer [54].
TLR4 also has the potential to become a disease progression marker in patients with colon cancer or premalignant lesions [55] as well as a biomarker of the aggressive tumor phenotype in laryngeal carcinoma and breast cancer [41, 56]. Its high expression correlates with poor prognosis in colorectal cancer patients [57] and in murine models [58]. Furthermore, TLR4 is associated with liver metastasis; researchers showed an increase in TLR4 expression in steatotic murine livers following diet-induced obesity. In a metastatic model of colorectal cancer animals with steatotic livers had increased metastatic tumor mass within the liver compared to lean controls. Silencing of TLR4 on tumors lowered the tumor burden, indicating that tumor cell TLR4 signaling promotes metastatic growth [58]. On the contrary other studies concerning colorectal carcinoma showed correlation between reduced TLR4 expression and increased metastatic potential of the tumor [39].
TLR4 is associated with metastasis also in other types of cancer, such as melanoma, where TLR4 activation induces cell migration [28], and prostate cancer. It was shown that highly metastatic human prostate cancer cell lines, such as PC3 or DU145, express higher levels of TLR4 compared to poorly metastatic cell lines. Moreover, downregulation of TLR4 expression by siRNA can inhibit prostate cancer cell invasion in vitro and can improve survival of tumor-bearing animals [59]. Similar results were shown in human breast cancer cell line, where downregulation of TLR4 significantly reduced tumor cell proliferation [23].
Conversely, another study [60] reports a decrease in TLR4 expression in human prostate tissue samples that correlates with histopathological grade of prostate cancer. TLR4 expressed in normal and low-grade tumors could therefore be a contributing factor in chronic inflammation that promotes carcinogenesis [61], while decreased TLR4 expression in more aggressive high-grade tumors could result from loss of cell differentiation that accompanies cancer progression [60].
A similar phenomenon, though with a different underlying cause, can be seen in the case of cervical cancer, where Yu and coworkers [62] observed downregulation of TLR4 expression during progression of cervical neoplasia. They have attributed this downregulation to the immunosuppressive effect that persistent human papilloma virusinfection has on the host immune response [62]. A degree of prudence is therefore recommended when conclusions are made from the data currently available, because of major discrepancies between studies with respect to different species, cell culture, or cancer type studied.
5. Endogenous TLR4 Ligands Responsible for TLR4 Signalization in Cancer
But what activates TLR4 signaling—is it bacterial endotoxin or perhaps other ligands? Endotoxin is ubiquitously present in air, gut, and epithelial surfaces, and perioperative exposure to it is associated with accelerated metastatic tumor growth [63]. Metastases could be the consequence of activation of the TLR4 signaling pathway that results in reduced apoptosis and increased proliferation of metastatic tumor cells. Killeen and coworkers [64] recently studied the role of endotoxin and TLR4 in invasion of extracellular matrix (ECM) and have shown that endotoxin promotes tumor cell ECM adhesion and invasion through activation of the urokinase plasminogen activator system (a serine protease that turns plasminogen into enzymically active plasmin responsible for blood clot degradation).
It is undisputed that the presence or absence of TLR4 expression on tumor (as well as nontumor) cells can influence different stages of carcinogenesis. Although many reports show clear correlation between chronic microbial infection and cancer initiation (e.g., H. pylori infection), others fail to provide evidence of the presence of endotoxin or other TLR4 ligands at cancer initiation sites. An important role is therefore attributed to different molecules of host origin that have lately arisen as potential endogenous ligands of TLR4. These proposed endogenous molecules include different components of the extracellular matrix, intracellular proteins, or modified lipids or lipoproteins (summarized in Table 4). Interestingly, many of them are proposed to activate both TLR4 and TLR2 without having any substantial structural similarity to their natural ligands (endotoxin or lipopeptides, resp.).
Table 4.
| Proposed endogenous TLR4 ligand | Reference | |
|---|---|---|
| Advanced glycation end product low-density lipoprotein | AGE-LDL | [65] |
| Angiotensin II | [66, 67] | |
| Beta defensin | [68, 69] | |
| Biglycan | [70, 71] | |
| Calprotectin | [72] | |
| Ceramide | [73] | |
| Fibrinogen | [74, 75] | |
| Fibronectin extra domain A | F-EDA | [76, 77] |
| High-mobility group box 1 | HMGB1 | [78–81] |
| Heat shock protein | HSP | [82–85] |
| Heparan sulfate | [86] | |
| Hyaluronan | [87–92] | |
| Minimally modified (oxidized) low-density lipoprotein | mmLDL | [93–95] |
| Myeloid-related protein-8/14 | MRP-8/14 | [96] |
| Oxidized Palmitoyl-arachidonoyl-phosphatidylcholine | OxPAPC | [97, 98] |
| Pancreatic adenocarcinoma upregulated factor | PAUF | [99] |
| Serum amyloid A | [100] | |
| Saturated fatty acid | SFA | [101] |
| Surfactant protein A | [102] | |
| Tenascin-C | [103] |
Because many (if not most) of the studies describing putative endogenous TLR4 ligands (Table 4) used recombinant proteins and/or commercial reagents with undetermined levels of residual endotoxin, it is reasonable to raise concerns about the purity of the putative ligands used in experiments. The most common methods used to exclude potential endotoxin contamination are the limulus amebocyte lysate (LAL) test and endotoxin neutralization with polymyxin B (PMB). Some researchers demonstrate that their proposed TLR ligands lose their activating capacity after exposure to elevated temperatures. But as described in an excellent review by Erridge [104], these methods have a major shortfall when used in studies describing novel endogenous TLR4 ligands. LAL test, for example, is unable to detect endotoxin in the presence of endotoxin-binding molecules. Furthermore, molecules that bind endotoxin can also prevent its inactivation by PMB. As for the heat sensitivity, the biological activity of endotoxin can be greatly reduced by elevated temperatures.
High-mobility group box-1 protein (HMGB1) is a putative TLR4 ligand implicated in cancer. HMGB1 is a nuclear DNA-binding protein that is actively secreted from cells following cytokine stimulation or passively released during cell death. It signals through the receptor for advanced glycation end products (RAGE) [105] and has been implicated in a variety of immune processes and pathological conditions including cancer [106, 107]. In the past few years many studies reported signalization of HMGB1 through TLR4 and declared HMGB1 an endogenous ligand of TLR4 [79, 80, 107]. HMGB1 is connected in several ways to tumor progression and metastasis [105]. On the other hand, HMGB1 released from irradiated or doxorubicin-/oxaliplatin- treated cells can improve immunogenicity of dying tumor cells and therefore help improve tumor antigen presentation [107]. A substantial number of studies show that HMGB1 however binds agonists of TLR, predominantly anionic molecules such as LPS [108], poly(IC), and CpG ODN that activate TLR4, TLR3, and TLR9; therefore, it may act as a chaperone [109, 110], similar to CD14, which stimulates activation of TLR4, TLR3, TLR7, and TLR9 by their agonists [111–113]. Additionally HMGB1 produced in mammalian cell cultures and therefore devoid of bacterial contaminants or endogenous danger signals does not activate TLR4 (unpublished observation). It should therefore be reconsidered whether these TLR4 ligands are not in fact just endotoxin-binding or endotoxin-sensitizing molecules without the intrinsic capability of binding and activating TLR4 on their own [104].
It is difficult to comprehend the multitude of the proposed TLR4 agonists that bear no structural similarity to the lipid A moiety of the LPS that is the only TLR4 agonist that has been prepared by chemical synthesis and whose molecular mechanism of activation is known [15, 16]. With respect to the plausible molecular mechanism of the direct activation of TLR4/MD-2 signaling complex oxidatively modified endogenous lipids seem to be the most likely ubiquitous endogenous agonists (Manček-Keber, manuscript in preparation).
6. Breaking the Immune Tolerance of Tumors by TLR4 Stimulation
Toll-like receptor activation is the trigger that sets the immune system into action. The application of TLR ligands in cancer therapy is therefore an attractive possibility that has been intensively studied in the past years in the context of cancer treatment or prevention (as anti-tumor vaccine adjuvants). Macrophages stimulated by endotoxin respond by secretion of chemokines and proinflammatory cytokines, including TNFα and interleukin-1β, which coordinate local and systemic inflammatory responses [17]. Dendritic cells, stimulated by endotoxin, secrete IL-12, which is important in anti-tumor immunity [114]. Furthermore, TLR4 stimulation induces DC maturation and antigen presentation, which has important effect on adaptive immune responses [4]. TLR stimulation influences antigen processing and presentation [115] by affecting the expression of costimulatory molecules on the surface of antigen-presenting cells as well as by controlling antigen uptake [116, 117] and phagosome maturation [118]. In addition to presenting antigens to lymphocytes, mature DCs are also capable of activating cancer-specific natural killer and NKT cells [119]. Inversely, TLR-stimulated NK cells facilitate in immature DC activation and maturation [120] and help intensify DC-mediated antitumor immune responses [121].
Tumors consist in large part not only of tumor but also of immune cells. It is therefore reasonable to assume that direct application of TLR ligands will affect both types of cells. TLR stimulation will possibly have even greater effect on the immune cell population, since not all tumor cells express TLR or the expression varies depending on the developmental stage of the tumor.
This is evident form an example of Bacillus Calmette-Guerin (BCG), an attenuated strain of Mycobacterium bovis that is used in the current treatment of nonmuscle invasive bladder cancer [122]. BCG promotes dendritic cell maturation, and this effect is TLR4 (as well as TLR2) dependent [123]. Furthermore, BCG can induce expression of TNF-related apoptosis-inducing ligand (TRAIL) on tumor infiltrating dendritic cells, therefore rendering them cytotoxic against tumor cells [124].
Another example of an immune activator of microbial origin that promotes dendritic cell maturation is the streptococcal agent OK-432. OK-432 is a preparation of a killed low-virulence strain of Streptococcus pyogenes that has been successfully used for over 30 years as an immunotherapeutic agent in different malignancies [125]. Its mechanism of action apparently involves TLR4 activation, since OKA-432 does not inhibit tumor growth on TLR4 knockouts as it does on wild-type mice. Moreover, patients with head and neck cancer responded to OK-432 treatment combined with fluoropyrimidine chemotherapy and radiation significantly better if they expressed TLR4 and MD-2 mRNA (compared to patients without TLR4 or MD-2 expression) [126, 127].
Stimulation of TLR4 on tumor cells can give contradicting results in terms of cancer progression versus treatment. The outcome seems to be species, tissue, and tumor type dependent. While TLR4 stimulation is on one hand associated with cancer progression (discussed above), it can also lead to anti-tumor immune response. B16 melanoma cells, for instance, that were stimulated with endotoxin in vitro exhibit reduced capability of inducing tumor growth in vivo. This response was totally independent of TLR4 expression by nontumor cells. In vitro stimulated tumor cells seem to differentially influence the phenotype of tumor infiltrating lymphocytes (TILs) so that TILs produced elevated levels of IFN-gamma and reduced levels of IL-10, thus favorably affecting the intratumoral cytokine balance [10].
7. Radio- and Chemotherapy Can Enhance Antitumor Immunity by Providing TLR Ligands
Combining immunotherapy and radiation is a new, compelling approach to cancer therapy. Though radiation is considered mostly immunosuppressive, it is noted also for its immunostimulatory effects. Patients therefore benefit from radiation therapy not only because it directly damages tumor cells but also because suppressor T-cell populations appear to be more radiosensitive than effector T lymphocytes [128]. Radiation can benefit anti-tumor immunity also by increasing expression of inflammatory cytokines by dendritic cells, therefore affecting their phenotype and function [129]. Dendritic cells are critical for anti-tumor immunity because of their ability to cross-present tumor antigens to specific CD8+ T lymphocytes. For efficient antigen cross-presentation, DCs need to receive appropriate stimulation through innate immune receptors. Since immature DCs can induce anti-tumor immunity when administered into irradiated tumors without the addition of TLR ligands [130], radiation was hypothesized to provide the necessary stimulus.
Apetoh et al. [107] recently proposed that HMGB1, which is released from irradiated tumor cells, acts as an endogenous TLR4 ligand. They demonstrated that TLR4 is essential for efficient tumor antigen cross-presentation following radio- or chemotherapy and proposed that HMGB1 binds and activates TLR4 on DCs. HMGB1 could therefore activate DCs and prevent the accelerated degradation of the phagocytosed tumor antigens within DCs promoting efficient tumor antigen processing and cross-presentation [107] (Figure 2).
Figure 2.
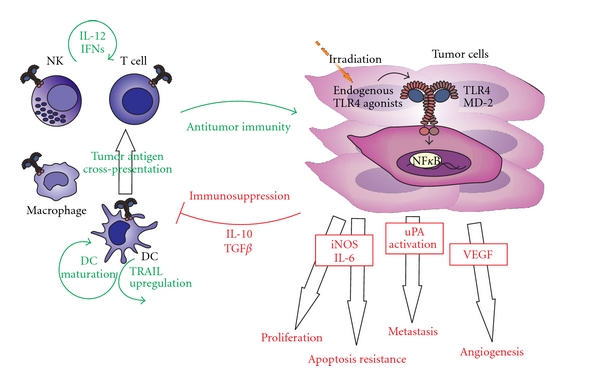
TLR4 signaling in cancer—a struggle of antitumor immunity against cancer proliferation and immune evasion. TLR4 signaling on immune cells can enhance anti-tumor immunity by different mechanisms, including IL-12 or IFNγ upregulation and promotion of DC maturation and function (left side of the figure, depicted in green). On the other hand, TLR4 signaling on tumor cells can increase their tumorigenic potential (right side of the figure, depicted in red).
The crucial role of TLR4 in immunostimulatory effects of radiation was also emphasized in a study by Paulos et al. [131], where they demonstrated elevated serum levels of endotoxin in mice following whole body irradiation. They showed that microbial endotoxin that translocated from the radiation-injured gut was responsible for enhanced anti-tumor effect of radiation. Moreover, radiation had diminished effect on tumors following removal of translocated endotoxin or in mice that were defective in the TLR4 signaling pathway [131]. These findings could be especially relevant for the treatment of gastrointestinal malignancies.
8. Cancer Vaccines Utilizing TLR4 Activation
Tumor cell lysates or purified tumor-associated antigens for vaccines have been used for therapeutic or prophylactic cancer vaccine. Although cell lysates contain endogenous danger signals that act as adjuvants, strong response against tumor-associated antigens requires additional stimulation of adaptive immune response by Toll-like receptor agonists. Agonists of TLR9 (CpG ODN), TLR3 (poly(IC), and TLR4 (endotoxin analogues) have been used to increase the innate immune response and activate antigen-presenting cells of the host. TLR4 is particularly important for development of a strong adaptive immune response by stimulation of the antibody class switching, affinity maturation, and formation of memory cells [132]. TLR4 is expressed on follicular dendritic cells that are essential for the affinity maturation in germinal centers [133, 134]. Systemic effect and toxicity of LPS preclude its application for cancer immunotherapy that started by the early attempts by William Coley. MPLA is a monophosphorylated lipid A derivative that has several orders of magnitude lower toxicity than lipid A and was reported to preferentially activate TRIF-dependent pathway [135]. MPLA has been registered as a vaccine adjuvant and used in clinical vaccines, such as Cervarix against human papilomavirus. MPLA is the only TLR4 agonist that has been clinically tested as an adjuvant for cancer vaccines. Results in clinical trials have been modest but seem to be much better if the vaccines are used in early stages of the disease, such as, for example, therapy of non-small-cell lung carcinoma (NSCLC) using MAGE-3 antigen combined with MPLA-based adjuvant AS02B rather than in late stages, when the immune system of patients is already severely compromised (reviewed in [136]). Additional alternative therapeutic approaches are based on combination of TLR4 agonist as a vaccine adjuvant with tumor-associated antigens in combination with radio- or chemotherapy or autologous dendritic cell therapy.
9. Conclusions
TLR signaling triggers immune cell activation and maturation and is indispensable for the efficient immune response against the pathogenic microorganisms as well as against malignant cells. An effective immune system is most important in the early stages of carcinogenesis when cancerous cells are few and are not limited to less immunogenic cell clones. If immunosurveillance against malignantly transformed cells is unsuccessful in the early stage, tumors quickly outgrow the immune cell cytotoxic capabilities. TLR4 expression by tumor cells can be a contributing factor that promotes tumor cell proliferation, survival, or immunosuppression.
Therapeutic interventions at the level of TLR4 stimulation is a double-edged sword since different studies demonstrate positive as well as negative effects of TLR4 stimulation on cancer development or treatment. Harnessing the beneficial effects of TLR4 stimulation while eliminating the negative ones remains the challenge for cancer researchers.
Acknowledgments
This work was supported by program and projects from the Slovenian Research Agency and by the Slovenian centre of excellence EN-FIST.
References
- 1.Igney FH, Krammer PH. Immune escape of tumors: apoptosis resistance and tumor counterattack. Journal of Leukocyte Biology. 2002;71(6):907–920. [PubMed] [Google Scholar]
- 2.Walker PR, Saas P, Dietrich PY. Tumor expression of Fas ligand (CD95L) and the consequences. Current Opinion in Immunology. 1998;10(5):564–572. doi: 10.1016/s0952-7915(98)80225-2. [DOI] [PubMed] [Google Scholar]
- 3.Kawai T, Akira S. TLR signaling. Cell Death and Differentiation. 2006;13(5):816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 4.Schreibelt G, Tel J, Sliepen KHEWJ, et al. Toll-like receptor expression and function in human dendritic cell subsets: implications for dendritic cell-based anti-cancer immunotherapy. Cancer Immunology, Immunotherapy. 2010;59(10):1573–1582. doi: 10.1007/s00262-010-0833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu H, Komai-Koma M, Xu D, Liew FY. Toll-like receptor 2 signaling modulates the functions of CD4+CD25+ regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(18):7048–7053. doi: 10.1073/pnas.0601554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutmuller RPM, den Brok MHMGM, Kramer M, et al. Toll-like receptor 2 controls expansion and function of regulatory T cells. Journal of Clinical Investigation. 2006;116(2):485–494. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kabelitz D. Expression and function of Toll-like receptors in T lymphocytes. Current Opinion in Immunology. 2007;19(1):39–45. doi: 10.1016/j.coi.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Huang B, Zhao J, Li H, et al. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Research. 2005;65(12):5009–5014. doi: 10.1158/0008-5472.CAN-05-0784. [DOI] [PubMed] [Google Scholar]
- 9.Kelly MG, Alvero AB, Chen R, et al. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Research. 2006;66(7):3859–3868. doi: 10.1158/0008-5472.CAN-05-3948. [DOI] [PubMed] [Google Scholar]
- 10.Andreani V, Gatti G, Simonella L, Rivero V, Maccioni M. Activation of Toll-like receptor 4 on tumor cells in vitro inhibits subsequent tumor growth in vivo. Cancer Research. 2007;67(21):10519–10527. doi: 10.1158/0008-5472.CAN-07-0079. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto C, Hudson KL, Anderson KV. The Toll gene of drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1988;52(2):269–279. doi: 10.1016/0092-8674(88)90516-8. [DOI] [PubMed] [Google Scholar]
- 12.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86(6):973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 13.Shimazu R, Akashi S, Ogata H, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. Journal of Experimental Medicine. 1999;189(11):1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viriyakosol S, Tobias PS, Kitchens RL, Kirkland TN. MD-2 binds to bacterial lipopolysaccharide. Journal of Biological Chemistry. 2001;276(41):38044–38051. doi: 10.1074/jbc.M105228200. [DOI] [PubMed] [Google Scholar]
- 15.Resman N, Vašl J, Oblak A, et al. Essential roles of hydrophobic residues in both MD-2 and Toll-like receptor 4 in activation by endotoxin. Journal of Biological Chemistry. 2009;284(22):15052–15060. doi: 10.1074/jbc.M901429200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458(7242):1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 17.Björkbacka H, Fitzgerald KA, Huet F, et al. The induction of macrophage gene expression by LPS predominantly utilizes Myd88-independent signaling cascades. Physiological Genomics. 2005;19:319–330. doi: 10.1152/physiolgenomics.00128.2004. [DOI] [PubMed] [Google Scholar]
- 18.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 19.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Grauer OM, Molling JW, Bennink E, et al. TLR ligands in the local treatment of established intracerebral murine gliomas. Journal of Immunology. 2008;181(10):6720–6729. doi: 10.4049/jimmunol.181.10.6720. [DOI] [PubMed] [Google Scholar]
- 22.Qian Y, Deng J, Xie H, et al. Regulation of TLR4-induced IL-6 response in bladder cancer cells by opposing actions of MAPK and PI3K signaling. Journal of Cancer Research and Clinical Oncology. 2009;135(3):379–386. doi: 10.1007/s00432-008-0478-z. [DOI] [PubMed] [Google Scholar]
- 23.Yang H, Zhou H, Feng P, et al. Reduced expression of Toll-like receptor 4 inhibits human breast cancer cells proliferation and inflammatory cytokines secretion. Journal of Experimental and Clinical Cancer Research. 2010;29(1, article 92) doi: 10.1186/1756-9966-29-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang XY, Zhu YQ, Wei B, Wang H. Expression and functional research of TLR4 in human colon carcinoma. American Journal of the Medical Sciences. 2010;339(4):319–326. doi: 10.1097/MAJ.0b013e3181cef1b7. [DOI] [PubMed] [Google Scholar]
- 25.Doan HQ, Bowen KA, Jackson LA, Evers BM. Toll-like receptor 4 activation increases Akt phosphorylation in colon cancer cells. Anticancer Research. 2009;29(7):2473–2478. [PMC free article] [PubMed] [Google Scholar]
- 26.Szczepański M, Stelmachowska M, Stryczyński Ł, et al. Assessment of expression of Toll-like receptors 2, 3 and 4 in laryngeal carcinoma. European Archives of Oto-Rhino-Laryngology. 2007;264(5):525–530. doi: 10.1007/s00405-006-0215-7. [DOI] [PubMed] [Google Scholar]
- 27.Molteni M, Marabella D, Orlandi C, Rossetti C. Melanoma cell lines are responsive in vitro to lipopolysaccharide and express TLR-4. Cancer Letters. 2006;235(1):75–83. doi: 10.1016/j.canlet.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Goto Y, Arigami T, Kitago M, et al. Activation of Toll-like receptors 2, 3, and 4 on human melanoma cells induces inflammatory factors. Molecular Cancer Therapeutics. 2008;7(11):3642–3653. doi: 10.1158/1535-7163.MCT-08-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassan F, Islam S, Tumurkhuu G, et al. Intracellular expression of Toll-like receptor 4 in neuroblastoma cells and their unresponsiveness to lipopolysaccharide. BMC Cancer. 2006;6, article 281 doi: 10.1186/1471-2407-6-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szajnik M, Szczepanski MJ, Czystowska M, et al. TLR4 signaling induced by lipopolysaccharide or paclitaxel regulates tumor survival and chemoresistance in ovarian cancer. Oncogene. 2009;28(49):4353–4363. doi: 10.1038/onc.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou M, McFarland-Mancini MM, Funk HM, Husseinzadeh N, Mounajjed T, Drew AF. Toll-like receptor expression in normal ovary and ovarian tumors. Cancer Immunology, Immunotherapy. 2009;58(9):1375–1385. doi: 10.1007/s00262-008-0650-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends in Immunology. 2003;24(6):302–306. doi: 10.1016/s1471-4906(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 33.Park SJ, Nakagawa T, Kitamura H, et al. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. Journal of Immunology. 2004;173(6):3844–3854. doi: 10.4049/jimmunol.173.6.3844. [DOI] [PubMed] [Google Scholar]
- 34.Sun R, Tian Z, Kulkarni S, Gao B. IL-6 prevents T cell-mediated hepatitis via inhibition of NKT cells in CD4+ T cell- and STAT3-dependent manners. Journal of Immunology. 2004;172(9):5648–5655. doi: 10.4049/jimmunol.172.9.5648. [DOI] [PubMed] [Google Scholar]
- 35.Xu M, Mizoguchi I, Morishima N, Chiba Y, Mizuguchi J, Yoshimoto T. Regulation of antitumor immune responses by the IL-12 family cytokines, IL-12, IL-23, and IL-27. Clinical and Developmental Immunology. 2010;2010:9 pages. doi: 10.1155/2010/832454. Article ID 832454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishioka Y, Wen H, Mitani K, et al. Differential effects of IL-12 on the generation of alloreactive CTL mediated by murine and human dendritic cells: a critical role for nitric oxide. Journal of Leukocyte Biology. 2003;73(5):621–629. doi: 10.1189/jlb.0402205. [DOI] [PubMed] [Google Scholar]
- 37.Koblish HK, Hunter CA, Wysocka M, Trinchieri G, Lee WMF. Immune suppression by recombinant interleukin (rIL)-12 involves interferon γ induction of nitric oxide synthase 2 (iNOS) activity: inhibitors of NO generation reveal the extent of rIL-12 vaccine adjuvant effect. Journal of Experimental Medicine. 1998;188(9):1603–1610. doi: 10.1084/jem.188.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pei Z, Lin D, Song X, Li H, Yao H. TLR4 signaling promotes the expression of VEGF and TGFβ1 in human prostate epithelial PC3 cells induced by lipopolysaccharide. Cellular Immunology. 2008;254(1):20–27. doi: 10.1016/j.cellimm.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Simiantonaki N, Kurzik-Dumke U, Karyofylli G, Jayasinghe C, Michel-Schmidt R, Kirkpatrick CJ. Reduced expression of TLR4 is associated with the metastatic status of human colorectal cancer. International Journal of Molecular Medicine. 2007;20(1):21–29. [PubMed] [Google Scholar]
- 40.Kanczkowski W, Tymoszuk P, Ehrhart-Bornstein M, Wirth MP, Zacharowski K, Bornstein SR. Abrogation of TLR4 and CD14 expression and signaling in human adrenocortical tumors. Journal of Clinical Endocrinology and Metabolism. 2010;95(12):E421–E429. doi: 10.1210/jc.2010-1100. [DOI] [PubMed] [Google Scholar]
- 41.González-Reyes S, Marín L, González L, et al. Study of TLR3, TLR4 and TLR9 in breast carcinomas and their association with metastasis. BMC Cancer. 2010;10, article 665 doi: 10.1186/1471-2407-10-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmaußer B, Andrulis M, Endrich S, Müller-Hermelink H-K, Eck M. Toll-like receptors TLR4, TLR5 and TLR9 on gastric carcinoma cells: an implication for interaction with Helicobacter pylori. International Journal of Medical Microbiology. 2005;295(3):179–185. doi: 10.1016/j.ijmm.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 43.Zhang YB, He FL, Fang M, et al. Increased expression of Toll-like receptors 4 and 9 in human lung cancer. Molecular Biology Reports. 2009;36(6):1475–1481. doi: 10.1007/s11033-008-9338-9. [DOI] [PubMed] [Google Scholar]
- 44.González-Reyes S, Fernández JM, González LO, et al. Study of TLR3, TLR4, and TLR9 in prostate carcinomas and their association with biochemical recurrence. Cancer Immunology, Immunotherapy. 2011;60(2):217–226. doi: 10.1007/s00262-010-0931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pandey R, Misra V, Misra SP, Dwivedi M, Kumar A, Tiwari BK. Helicobacter pylori and gastric cancer. Asian Pacific Journal of Cancer Prevention. 2010;11(3):583–588. [PubMed] [Google Scholar]
- 46.Ogawa T, Asai Y, Sakai Y, et al. Endotoxic and immunobiological activities of a chemically synthesized lipid A of Helicobacter pylori strain 206-1. FEMS Immunology and Medical Microbiology. 2003;36(1-2):1–7. doi: 10.1016/S0928-8244(03)00093-2. [DOI] [PubMed] [Google Scholar]
- 47.Yokota SI, Okabayashi T, Rehli M, Fujii N, Amano KI. Helicobacter pylori lipopolysaccharides upregulate Toll-like receptor 4 expression and proliferation of gastric epithelial cells via the MEK1/2-ERK1/2 mitogen-activated protein kinase pathway. Infection and Immunity. 2010;78(1):468–476. doi: 10.1128/IAI.00903-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su B, Ceponis PJM, Lebel S, Huynh H, Sherman PM. Helicobacter pylori activates Toll-like receptor 4 expression in gastrointestinal epithelial cells. Infection and Immunity. 2003;71(6):3496–3502. doi: 10.1128/IAI.71.6.3496-3502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gyde SN, Prior P, Allan RN, et al. Colorectal cancer in ulcerative colitis: a cohort study of primary referrals from three centres. Gut. 1988;29(2):206–217. doi: 10.1136/gut.29.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48(4):526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fukata M, Hernandez Y, Conduah D, et al. Innate immune signaling by Toll-like receptor-4 (TLR4) shapes the inflammatory microenvironment in colitis-associated tumors. Inflammatory Bowel Diseases. 2009;15(7):997–1006. doi: 10.1002/ibd.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fukata M, Chen A, Vamadevan AS, et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133(6):1869–e2. doi: 10.1053/j.gastro.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greenhough A, Smartt HJM, Moore AE, et al. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30(3):377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 54.Hernandez Y, Sotolongo J, Breglio K, et al. The role of prostaglandin E2 (PGE 2) in Toll-like receptor 4 (TLR4)-mediated colitis-associated neoplasia. BMC Gastroenterology. 2010;10, article 82 doi: 10.1186/1471-230X-10-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cammarota R, Bertolini V, Pennesi G, et al. The tumor microenvironment of colorectal cancer: stromal TLR-4 expression as a potential prognostic marker. Journal of Translational Medicine. 2010;8, article 112 doi: 10.1186/1479-5876-8-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Starska K, Forma E, Lewy-Trenda I, et al. The expression of SOCS1 and TLR4-NFκB pathway molecules in neoplastic cells as potential biomarker for the aggressive tumor phenotype in laryngeal carcinoma. Folia Histochemica et Cytobiologica. 2009;47(3):401–410. doi: 10.2478/v10042-009-0075-2. [DOI] [PubMed] [Google Scholar]
- 57.Wang EL, Qian ZR, Nakasono M, et al. High expression of Toll-like receptor 4/myeloid differentiation factor 88 signals correlates with poor prognosis in colorectal cancer. British Journal of Cancer. 2010;102(5):908–915. doi: 10.1038/sj.bjc.6605558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Earl TM, Nicoud IB, Pierce JM, et al. Silencing of TLR4 decreases liver tumor burden in a murine model of colorectal metastasis and hepatic steatosis. Annals of Surgical Oncology. 2009;16(4):1043–1050. doi: 10.1245/s10434-009-0325-8. [DOI] [PubMed] [Google Scholar]
- 59.Hua D, Liu MY, Cheng ZD, et al. Small interfering RNA-directed targeting of Toll-like receptor 4 inhibits human prostate cancer cell invasion, survival, and tumorigenicity. Molecular Immunology. 2009;46(15):2876–2884. doi: 10.1016/j.molimm.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 60.Gatti G, Quintar AA, Andreani V, et al. Expression of Toll-like receptor 4 in the prostate gland and its association with the severity of prostate cancer. Prostate. 2009;69(13):1387–1397. doi: 10.1002/pros.20984. [DOI] [PubMed] [Google Scholar]
- 61.Kundu SD, Lee C, Billips BK, et al. The Toll-like receptor pathway: a novel mechanism of infection-induced carcinogenesis of prostate epithelial cells. Prostate. 2008;68(2):223–229. doi: 10.1002/pros.20710. [DOI] [PubMed] [Google Scholar]
- 62.Yu L, Wang L, Li M, Zhong J, Wang Z, Chen S. Expression of Toll-like receptor 4 is down-regulated during progression of cervical neoplasia. Cancer Immunology, Immunotherapy. 2010;59(7):1021–1028. doi: 10.1007/s00262-010-0825-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pidgeon GP, Harmey JH, Kay E, Da Costa M, Redmond HP, Bouchier-Hayes DJ. The role of endotoxin/lipopolysaccharide in surgically induced tumour growth in a murine model of metastatic disease. British Journal of Cancer. 1999;81(8):1311–1317. doi: 10.1038/sj.bjc.6694369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Killeen SD, Wang JH, Andrews EJ, Redmond HP. Bacterial endotoxin enhances colorectal cancer cell adhesion and invasion through TLR-4 and NF-B-dependent activation of the urokinase plasminogen activator system. British Journal of Cancer. 2009;100(10):1589–1602. doi: 10.1038/sj.bjc.6604942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hodgkinson CP, Laxton RC, Patel K, Ye S. Advanced glycation end-product of low density lipoprotein activates the Toll-like 4 receptor pathway implications for diabetic atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(12):2275–2281. doi: 10.1161/ATVBAHA.108.175992. [DOI] [PubMed] [Google Scholar]
- 66.Ji YY, Liu JT, Liu N, Wang ZD, Liu CH. PPARα activator fenofibrate modulates angiotensin II-induced inflammatory responses in vascular smooth muscle cells via the TLR4-dependent signaling pathway. Biochemical Pharmacology. 2009;78(9):1186–1197. doi: 10.1016/j.bcp.2009.06.095. [DOI] [PubMed] [Google Scholar]
- 67.Ji Y, Liu J, Wang Z, Liu N. Angiotensin II induces inflammatory response partly via Toll-like receptor 4-dependent signaling pathway in vascular smooth muscle cells. Cellular Physiology and Biochemistry. 2009;23(4-6):265–276. doi: 10.1159/000218173. [DOI] [PubMed] [Google Scholar]
- 68.Biragyn A, Ruffini PA, Leifer CA, et al. Toll-like receptor 4-dependent activation of dendritic cells by β-defensin 2. Science. 2002;298(5595):1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 69.Biragyn A, Coscia M, Nagashima K, Sanford M, Young HA, Olkhanud P. Murine β-defensin 2 promotes TLR-4/MyD88-mediated and NF-κB-dependent atypical death of APCs via activation of TNFR2. Journal of Leukocyte Biology. 2008;83(4):998–1008. doi: 10.1189/jlb.1007700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schaefer L, Babelova A, Kiss E, et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. Journal of Clinical Investigation. 2005;115(8):2223–2233. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Babelova A, Moreth K, Tsalastra-Greul W, et al. Biglycan, a danger signal that activates the NLRP3 inflammasome via Toll-like and P2X receptors. Journal of Biological Chemistry. 2009;284(36):24035–24048. doi: 10.1074/jbc.M109.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ehrchen JM, Sunderkötter C, Foell D, Vogl T, Roth J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. Journal of Leukocyte Biology. 2009;86(3):557–566. doi: 10.1189/jlb.1008647. [DOI] [PubMed] [Google Scholar]
- 73.Fischer H, Ellström P, Ekström K, Gustafsson L, Gustafsson M, Svanborg C. Ceramide as a TLR4 agonist; a putative signalling intermediate between sphingolipid receptors for microbial ligands and TLR4. Cellular Microbiology. 2007;9(5):1239–1251. doi: 10.1111/j.1462-5822.2006.00867.x. [DOI] [PubMed] [Google Scholar]
- 74.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through Toll-like receptor 4. Journal of Immunology. 2001;167(5):2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- 75.Kuhns DB, Priel DAL, Gallin JI. Induction of human monocyte interleukin (IL)-8 by fibrinogen through the Toll-like receptor pathway. Inflammation. 2007;30(5):178–188. doi: 10.1007/s10753-007-9035-1. [DOI] [PubMed] [Google Scholar]
- 76.Okamura Y, Watari M, Jerud ES, et al. The extra domain A of fibronectin activates Toll-like receptor 4. Journal of Biological Chemistry. 2001;276(13):10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 77.Gondokaryono SP, Ushio H, Niyonsaba F, et al. The extra domain A of fibronectin stimulates murine mast cells via Toll-like receptor 4. Journal of Leukocyte Biology. 2007;82(3):657–665. doi: 10.1189/jlb.1206730. [DOI] [PubMed] [Google Scholar]
- 78.Park JS, Svetkauskaite D, He Q, et al. Involvement of Toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. Journal of Biological Chemistry. 2004;279(9):7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 79.Jong SP, Gamboni-Robertson F, He Q, et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. American Journal of Physiology. 2006;290(3):C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 80.Yu M, Wang H, Ding A, et al. HMGB1 signals through Toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26(2):174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 81.Mittal D, Saccheri F, Vénéreau E, Pusterla T, Bianchi ME, Rescigno M. TLR4-mediated skin carcinogenesis is dependent on immune and radioresistant cells. The EMBO Journal. 2010;29(13):2242–2252. doi: 10.1038/emboj.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ohashi K, Burkart V, Flohé S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the Toll-like receptor-4 complex. Journal of Immunology. 2000;164(2):558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 83.Asea A, Rehli M, Kabingu E, et al. Novel signal transduction pathway utilized by extracellular HSP70. Role of Toll-like receptor (TLR) 2 and TLR4. Journal of Biological Chemistry. 2002;277(17):15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 84.Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the toll/interleukin-1 receptor signal pathway. Journal of Biological Chemistry. 2002;277(17):15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- 85.de Graaf R, Kloppenburg G, Kitslaar PJHM, Bruggeman CA, Stassen F. Human heat shock protein 60 stimulates vascular smooth muscle cell proliferation through Toll-like receptors 2 and 4. Microbes and Infection. 2006;8(7):1859–1865. doi: 10.1016/j.micinf.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 86.Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. Journal of Immunology. 2002;168(10):5233–5239. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- 87.Termeer C, Benedix F, Sleeman J, et al. Oligosaccharides of hyaluronan activate dendritic cells via Toll-like receptor 4. Journal of Experimental Medicine. 2002;195(1):99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. Journal of Biological Chemistry. 2004;279(17):17079–17084. doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- 89.Jiang D, Liang J, Fan J, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nature Medicine. 2005;11(11):1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 90.Taylor KR, Yamasaki K, Radek KA, et al. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. Journal of Biological Chemistry. 2007;282(25):18265–18275. doi: 10.1074/jbc.M606352200. [DOI] [PubMed] [Google Scholar]
- 91.Gariboldi S, Palazzo M, Zanobbio L, et al. Low molecular weight hyaluronic acid increases the self-defense of skin epithelium by induction of β-defensin 2 via TLR2 and TLR4. Journal of Immunology. 2008;181(3):2103–2110. doi: 10.4049/jimmunol.181.3.2103. [DOI] [PubMed] [Google Scholar]
- 92.Shimada M, Yanai Y, Okazaki T, et al. Hyaluronan fragments generated by sperm-secreted hyaluronidase stimulate cytokine/chemokine production via the TLR 2 and TLR4 pathway in cumulus cells of ovulated COCs, which may enhance fertilization. Development. 2008;135(11):2001–2011. doi: 10.1242/dev.020461. [DOI] [PubMed] [Google Scholar]
- 93.Miller YI, Viriyakosol S, Binder CJ, Feramisco JR, Kirkland TN, Witztum JL. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. Journal of Biological Chemistry. 2003;278(3):1561–1568. doi: 10.1074/jbc.M209634200. [DOI] [PubMed] [Google Scholar]
- 94.Miller YI, Viriyakosol S, Worrall DS, Boullier A, Butler S, Witztum JL. Toll-like receptor 4-dependent and -independent cytokine secretion induced by minimally oxidized low-density lipoprotein in macrophages. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(6):1213–1219. doi: 10.1161/01.ATV.0000159891.73193.31. [DOI] [PubMed] [Google Scholar]
- 95.Bae YS, Lee JH, Choi SH, et al. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: Toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circulation Research. 2009;104(2):210–218. doi: 10.1161/CIRCRESAHA.108.181040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vogl T, Tenbrock K, Ludwig S, et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nature Medicine. 2007;13(9):1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 97.Walton KA, Hsieh X, Gharavi N, et al. Receptors involved in the oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine-mediated synthesis of interleukin-8: a role for Toll-like receptor 4 and a glycosylphosphatidylinositol-anchored protein. Journal of Biological Chemistry. 2003;278(32):29661–29666. doi: 10.1074/jbc.M300738200. [DOI] [PubMed] [Google Scholar]
- 98.Imai Y, Kuba K, Neely GG, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133(2):235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Park HD, Lee Y, Oh YK, et al. Pancreatic adenocarcinoma upregulated factor promotes metastasis by regulating TLR/CXCR4 activation. Oncogene. 2011;30:201–211. doi: 10.1038/onc.2010.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sandri S, Rodriguez D, Gomes E, Monteiro HP, Russo M, Campa A. Is serum amyloid A an endogenous TLR4 agonist? Journal of Leukocyte Biology. 2008;83(5):1174–1180. doi: 10.1189/jlb.0407203. [DOI] [PubMed] [Google Scholar]
- 101.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. Journal of Clinical Investigation. 2006;116(11):3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guillot L, Balloy V, McCormack FX, Golenbock DT, Chignard M, Si-Tahar M. Cutting edge: the immunostimulatory activity of the lung surfactant protein-A involves Toll-like receptor 4. Journal of Immunology. 2002;168(12):5989–5992. doi: 10.4049/jimmunol.168.12.5989. [DOI] [PubMed] [Google Scholar]
- 103.Midwood K, Sacre S, Piccinini AM, et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nature Medicine. 2009;15(7):774–780. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- 104.Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? Journal of Leukocyte Biology. 2010;87(6):989–999. doi: 10.1189/jlb.1209775. [DOI] [PubMed] [Google Scholar]
- 105.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annual Review of Immunology. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 106.Ellerman JE, Brown CK, de Vera M, et al. Masquerader: high mobility group box-1 and cancer. Clinical Cancer Research. 2007;13(10):2836–2848. doi: 10.1158/1078-0432.CCR-06-1953. [DOI] [PubMed] [Google Scholar]
- 107.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nature Medicine. 2007;13(9):1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 108.Youn JH, Oh YJ, Kim ES, Choi JE, Shin JS. High mobility group box 1 protein binding to lipopolysaccharide facilitates transfer of lipopolysaccharide to CD14 and enhances lipopolysaccharide-mediated TNF-α production in human monocytes. Journal of Immunology. 2008;180(7):5067–5074. doi: 10.4049/jimmunol.180.7.5067. [DOI] [PubMed] [Google Scholar]
- 109.Tsan M-F. Heat shock proteins and high mobility group box 1 protein lack cytokine function. Journal of Leukocyte Biology. 2011;89(6):247–852. doi: 10.1189/jlb.0810471. [DOI] [PubMed] [Google Scholar]
- 110.Hreggvidsdottir HS, Östberg T, Wähämaa H, et al. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. Journal of Leukocyte Biology. 2009;86(3):655–662. doi: 10.1189/jlb.0908548. [DOI] [PubMed] [Google Scholar]
- 111.Lee HK, Dunzendorfer S, Soldau K, Tobias PS. Double-stranded RNA-mediated TLR3 activation is enhanced by CD14. Immunity. 2006;24(2):153–163. doi: 10.1016/j.immuni.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 112.Baumann CL, Aspalter IM, Sharif O, et al. CD14 is a coreceptor of Toll-like receptors 7 and 9. Journal of Experimental Medicine. 2010;207(12):2689–2701. doi: 10.1084/jem.20101111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 114.Kadowaki N, Ho S, Antonenko S, et al. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens. Journal of Experimental Medicine. 2001;194(6):863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440(7085):808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 116.West MA, Wallin RPA, Matthews SP, et al. Enhanced dendritic cell antigen capture via Toll-like receptor-induced actin remodeling. Science. 2004;305(5687):1153–1157. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- 117.Garrett WS, Chen LM, Kroschewski R, et al. Developmental control of endocytosis in dendritic cells by Cdc42. Cell. 2000;102(3):325–334. doi: 10.1016/s0092-8674(00)00038-6. [DOI] [PubMed] [Google Scholar]
- 118.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from Toll-like receptors. Science. 2004;304(5673):1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 119.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 120.Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA. Functional interactions between dendritic cells and NK cells during viral infection. Nature Immunology. 2003;4(2):175–181. doi: 10.1038/ni880. [DOI] [PubMed] [Google Scholar]
- 121.Pham TNN, Hong CY, Min JJ, et al. Enhancement of antitumor effect using dendritic cells activated with natural killer cells in the presence of Toll-like receptor agonist. Experimental and Molecular Medicine. 2010;42(6):407–419. doi: 10.3858/emm.2010.42.6.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sylvester RJ. Bacillus Calmette-Guérin treatment of non-muscle invasive bladder cancer. International Journal of Urology. 2011;18(2):113–120. doi: 10.1111/j.1442-2042.2010.02678.x. [DOI] [PubMed] [Google Scholar]
- 123.Tsuji S, Matsumoto M, Takeuchi O, et al. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium boris bacillus Calmette-Guérin: involvement of Toll-like receptors. Infection and Immunity. 2000;68(12):6883–6890. doi: 10.1128/iai.68.12.6883-6890.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Roux S, Apetoh L, Chalmin F, et al. CD4+CD25+ Tregs control the TRAIL-dependent cytotoxicity of tumor-infiltrating DCs in rodent models of colon cancer. Journal of Clinical Investigation. 2008;118(11):3751–3761. doi: 10.1172/JCI35890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ryoma Y, Moriya Y, Okamoto M, Kanaya I, Saito M, Sato M. Biological effect of OK-432 (Picibanil) and possible application to dendritic cell therapy. Anticancer Research. 2004;24(5C):3295–3301. [PubMed] [Google Scholar]
- 126.Okamoto M, Oshikawa T, Tano T, et al. Involvement of Toll-like receptor 4 signaling in interferon-γ production and antitumor effect by streptococcal agent OK-432. Journal of the National Cancer Institute. 2003;95(4):316–326. doi: 10.1093/jnci/95.4.316. [DOI] [PubMed] [Google Scholar]
- 127.Okamoto M, Oshikawa T, Tano T, et al. Mechanism of anticancer host response induced by OK-432, a streptococcal preparation, mediated by phagocytosis and Toll-like receptor 4 signaling. Journal of Immunotherapy. 2006;29(1):78–86. doi: 10.1097/01.cji.0000192106.32206.30. [DOI] [PubMed] [Google Scholar]
- 128.North RJ. Radiation-induced, immunologically mediated regression of an established tumor as an example of successful therapeutic immunomanipulation. Preferential elimination of suppressor T cells allows sustained production of effector T cells. Journal of Experimental Medicine. 1986;164(5):1652–1666. doi: 10.1084/jem.164.5.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shigematsu A, Adachi Y, Koike-Kiriyama N, et al. Effects of low-dose irradiation on enhancement of immunity by dendritic cells. Journal of Radiation Research. 2007;48(1):51–55. doi: 10.1269/jrr.06048. [DOI] [PubMed] [Google Scholar]
- 130.Kim KW, Kim SH, Shin JG, et al. Direct injection of immature dendritic cells into irradiated tumor induces efficient antitumor immunity. International Journal of Cancer. 2004;109(5):685–690. doi: 10.1002/ijc.20036. [DOI] [PubMed] [Google Scholar]
- 131.Paulos CM, Wrzesinski C, Kaiser A, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. Journal of Clinical Investigation. 2007;117(8):2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kasturi SP, Skountzou I, Albrecht RA, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470(7335):543–550. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Garin A, Meyer-Hermann M, Contie M, et al. Toll-like receptor 4 signaling by follicular dendritic cells is pivotal for germinal center onset and affinity maturation. Immunity. 2010;33(1):84–95. doi: 10.1016/j.immuni.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 134.El Shikh MEM, El Sayed RM, Wu Y, Szakal AK, Tew JG. TLR4 on follicular dendritic cells: an activation pathway that promotes accessory activity. Journal of Immunology. 2007;179(7):4444–4450. doi: 10.4049/jimmunol.179.7.4444. [DOI] [PubMed] [Google Scholar]
- 135.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316(5831):1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 136.Cluff CW. Monophosphoryl lipid A (MPL) as an adjuvant for anti-cancer vaccines: clinical results. Advances in Experimental Medicine and Biology. 2009;667:111–123. doi: 10.1007/978-1-4419-1603-7_10. [DOI] [PubMed] [Google Scholar]


