Abstract
Acute exacerbation is a frequent complication of chronic obstructive pulmonary disease (COPD). Recent studies suggested a role for bacteria such as Streptococcus pneumoniae in the development of acute exacerbation. For this study, we investigated the following in COPD patients: (i) the epidemiology of pneumococcal colonization and infection, (ii) the effect of pneumococcal colonization on the development of exacerbation, and (iii) the immunological response against S. pneumoniae. We cultured sputa of 269 COPD patients during a stable state and during exacerbation of COPD and characterized 115 pneumococcal isolates by use of serotyping. Moreover, we studied serum immunoglobulin G (IgG) antibody titers, antibody avidities, and functional antibody titers against the seven conjugate vaccine serotypes in these patients. Colonization with only pneumococci (monocultures) increased the risk of exacerbation, with a hazard ratio of 2.93 (95% confidence interval, 1.41 to 6.07). The most prevalent pneumococcal serotypes found were serotypes 19F, 3, 14, 9L/N/V, 23A/B, and 11. We calculated the theoretical coverage for the 7- and 11-valent pneumococcal vaccines to be 60 and 73%, respectively. All patients had detectable IgG levels against the seven conjugate vaccine serotypes. These antibody titers were significantly lower than those in vaccinated healthy adults. Finally, on average, a 2.5-fold rise in serotype-specific and functional antibodies in S. pneumoniae-positive sputum cultures was observed during exacerbation. Our data indicate that pneumococcal colonization in COPD patients is frequently caused by vaccine serotype strains. Moreover, pneumococcal colonization is a risk factor for exacerbation of COPD. Finally, our findings demonstrate that COPD patients are able to mount a significant immune response to pneumococcal infection. COPD patients may therefore benefit from pneumococcal vaccination.
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality in adults. According to the Global Burden of Disease Study, COPD is the fifth most common disease and fourth leading cause of death in the world. Both the prevalence and mortality rate are still expected to increase in the coming decades (11). The chronic course of this disease is frequently interrupted by acute exacerbation, which has a major impact on the morbidity and mortality of COPD patients (17). Acute exacerbation of COPD is characterized by an acute sustained worsening of the patient's condition from a stable state, beyond normal day-to-day variations, which occurs one to three times a year and may warrant additional treatment (3, 18). Bacterial infections are thought to contribute to the pathogenesis and clinical course of COPD (20). Several recent studies have shown a clear association between the isolation of bacterial species such as Streptococcus pneumoniae, Moraxella catarrhalis, and Haemophilus influenzae and acute exacerbation (18). Patel et al. were the first investigators who observed a significant relation between lower airway colonization in the stable state and exacerbation frequency. However, their study population was too small to investigate the correlation for individual bacterial species (12). In contrast, Sethi et al. showed a significant increase in the occurrence of exacerbation when S. pneumoniae and M. catarrhalis were isolated (18).
We hypothesize that the prevention of bacterial growth in the airways of COPD patients will significantly decrease the occurrence of exacerbation and thus improve the quality of life for COPD patients. One of the pathogens that is frequently involved in colonization and infection of COPD patients is S. pneumoniae. For this pathogen, a 23-valent polysaccharide vaccine and a 7-valent conjugate vaccine are available. The 23-valent vaccine is protective against pneumococcal invasive disease in adults and elderly patients, but not in children under the age of two or immunocompromised patients (14). In addition, the 23-valent polysaccharide vaccine has not shown a significant reduction in cases of pneumonia in elderly patients (7). Moreover, the polysaccharide vaccine induces no immunological memory, which necessitates revaccination (2). In contrast, the conjugate vaccine is effective in young children against invasive as well as mucosal diseases and elicits immunological memory, but it covers fewer (7) serotypes. To investigate the relevance of pneumococcal colonization and infection in COPD patients, we determined the effect of pneumococcal colonization on the development of exacerbation. In addition, to assess the theoretical role of pneumococcal vaccination in COPD patients, we determined the serotype distribution among pneumococcal sputum isolates found during colonization and infection. Finally, we investigated the presence of immunoglobulin G (IgG) antibodies against pneumococcal capsular polysaccharides in COPD patients and the development of capsule-specific antibodies as a result of pneumococcal infection during exacerbation.
MATERIALS AND METHODS
Study population.
Two hundred sixty-nine patients, aged 40 to 75 years, in the outpatient department of Medisch Spectrum Twente Hospital, Enschede, The Netherlands, participating in the COPE study and with a history of COPD, were recruited from May 1999 through March 2000 (19). The patients had to meet the following criteria: (i) a clinical diagnosis of stable COPD, as defined by American Thoracic Society criteria; (ii) no history of asthma; (iii) no exacerbation in the month prior to enrollment; (iv) current or former smoker; (v) age between 40 and 75 years; (vi) a baseline prebronchodilator forced expiratory volume in 1 s (FEV1) of 25 to 80% that predicted; (vii) a prebronchodilator ratio of FEV1 to inspiratory vital capacity value of 60% or less; (viii) reversibility value of FEV1 postinhalation of 80 μg of ipratropium bromide via a metered dose inhalator with Aerochamber of 12% the predicted value or less; (ix) a total lung capacity that was higher than the predicted total lung capacity minus 1.64 × the standard deviation; (x) no maintenance treatment with oral steroids or antibiotics; (xi) no medical condition with a low survival rate or serious psychiatric morbidity (e.g., cardiac insufficiency or alcoholism); (xii) absence of any other active lung disease (e.g., sarcoidosis). The use of medication such as nasal corticosteroids, theophyllines, chronic use of acetylcysteine, and all other bronchodilators was allowed (19).
None of the patients were vaccinated with pneumococcal vaccines before entering the study. Sputum samples were retrieved at 0, 4, 7, and 10 months, provided that the disease was stable, and during exacerbation. Serum was collected during acute exacerbation (acute-phase serum) and 2 weeks after exacerbation (convalescent-phase serum). The diagnosis of exacerbation was made based on the following criteria: (i) an objective exacerbation, with a >15% decrease in lung function (FEV1) and/or a 300-ml decrease in FEV1; (ii) a subjective exacerbation assessed by means of a symptom checklist and a 2-week diary; and (iii) a clinical exacerbation, as judged by the patient's own chest physician (19).
Sputum cultures.
Sputa were collected at scheduled visits to the outpatient department and in cases of exacerbation. The sputum samples were collected in sterile vials and transported to the microbiology laboratory within 4 h. Sputum samples were judged microscopically with a low-magnification lens (×100) and had to contain <10 epithelial cells and >25 leukocytes per field to be considered representative bronchial samples. A fixed volume of a 1:100 dilution of sputum was plated onto blood agar, chocolate agar, MacConkey agar, and Sabouraud agar. The identification of bacterial growth was performed by standard techniques (10). S. pneumoniae, H. influenzae, M. catarrhalis, Pseudomonas aeruginosa, Staphylococcus aureus, Aspergillus, and Candida species and other gram-negative rods were considered to be potential pathogens. Other bacterial species were classified as normal flora.
Bacterial colonization was defined as the presence of bacteria in a culture retrieved from the lower respiratory tract of a clinically stable COPD patient (5). A bacterial infection was defined by the presence of one or more potential pathogens in a COPD patient with a clinical exacerbation. A monoculture was defined as the growth of a single bacterial species, whereas the presence of multiple bacterial species was defined as a mixed culture.
Serotyping of pneumococcal isolates.
In total, 115 pneumococcal isolates were cultured and serotyped by the capsular swelling method (Quellung reaction), using commercially available antisera (Statens Serum Institute, Copenhagen, Denmark) and microscopic observation.
EIA for measuring anti-pneumococcal polysaccharide IgG concentrations.
Concentrations of IgG antibodies were measured by an enzyme immunoassay (EIA) method as described previously (9). The results are given in micrograms per milliliter, calculated from the officially assigned IgG values of the 89-SF reference serum (15), or were converted into units per milliliter by comparison with the 89-SF reference serum, which was considered to contain 100 U/ml, in cases for which no IgG values were available.
EIA for measuring the avidity of anti-pneumococcal polysaccharide antibodies.
The relative avidities of IgG antibodies for pneumococcal capsular polysaccharides were determined by the EIA method as described by Anttila et al. (1), with some minor modifications. Briefly, microtiter plates (Maxisorp; Nunc, Roskilde, Denmark) were coated overnight with 100 μl of coating solution, containing capsule polysaccharides at a concentration of 10 μg/ml (LGC Promochem, Teddington, United Kingdom), per well. Sera were diluted 1:50 in phosphate-buffered saline (PBS) containing 5% cell wall polysaccharides (Statens Serum Institute) and incubated at 4°C overnight. Sera were diluted 1:100 in PBS-0.05% Tween 20, and threefold serial dilutions were incubated for 2 h at 37°C. After washing, 0.5 M sodium thiocyanate in PBS was added to dissociate antibody-antigen complexes. After 15 min of incubation at room temperature, the plates were washed and antibody binding was detected by the addition of alkaline-phosphatase-conjugated anti-human IgG (Sigma, St. Louis, Mo.). The color was developed by the substrate p-nitrophenyl phosphate (Sigma). The absorbance was measured at 405 nm on an EIA reader (SpectraMAX; Molecular Devices, Sunnyvale, Calif.). Results were expressed as avidity index (AI) values, assigned as the percentages of antibodies that remained bound to the antigens after thiocyanate treatment. The high-avidity antibody concentration was calculated by multiplying the antibody concentration by the AI value. The reproducibility of the assay was checked by including a control serum in each plate.
Data analysis.
The results were analyzed with the statistical software SPSS 10. The effect of pneumococcal colonization on the time to the first exacerbation episode was assessed by using multivariate Cox proportional hazard analyses. We adjusted for potential confounding variables, including age, sex, smoking status, number of exacerbation episodes in the preceding year, and FEV1% predicted, as found previously (P. van der Valk, D. Bogaert, E. Monninkhof, J. van der Palen, P. Hermans, G. Zielhuis, C. van Herwaarden, and R. Hendriks, submitted for publication). The initial concentrations of antibodies are depicted as geometric mean concentrations (GMC). Avidities are given as the means of the AI values and high-avidity antibody concentrations. Statistical comparisons between antibody levels were performed by analysis of variance and independent sample t tests. The maximum increase in antibody titer was tested for significance by using the one-sample t test. Statistical significance was set at P values of <0.05.
RESULTS
Sputum samples from 269 patients were obtained at 0, 4, 7, and 10 months in cases of stable disease. An additional sputum sample was collected at each hospital visit for an acute exacerbation of COPD. In total, 55% of the patient group developed at least one exacerbation episode during the follow-up period. In total, 918 stable state sputa and 241 exacerbation sputa were collected. Of the stable state sputa, 603 cultures were negative for potential pathogens, whereas 315 cultures were positive for at least one microorganism (34%). The exacerbation sputa showed significantly more positive cultures (49%). Mixed cultures were found in 9 and 5% of the sputum cultures during stable state and exacerbation, respectively. Monocultures were found significantly more often during exacerbation episodes (41%) than during stable states (26%). The three predominant bacterial species cultured during a stable state and exacerbation were H. influenzae (19 and 26%, respectively), S. pneumoniae (13 and 13%, respectively), and M. catarrhalis (9 and 7%, respectively).
We calculated the effect of the colonization status at the time of randomization on the time to the next exacerbation episode for 209 patients from whom sputum was available at that time. We adjusted for potential confounding variables, including age, sex, smoking status, number of exacerbations in the preceding year, and FEV1% predicted. Bacterial colonization in general did not increase the risk of a first exacerbation compared to noncolonized patients, nor did pneumococcal colonization in general (hazard ratio, 1.31; 95% confidence interval [CI], 0.743 to 2.305). However, the adjusted hazard ratio for the development of exacerbations in patients with a pneumococcal monoculture was 2.93 (95% CI, 1.41 to 6.07).
We investigated all 115 pneumococcal isolates in detail by means of serotyping. The most prevalent serotypes were serotypes 19F and 3 (13 and 10%, respectively), followed by serotypes 14, 9L/N/V, 23A/B, and 11 (9% each) (Table 1). The theoretical vaccine coverages for the 7-valent and 11-valent pneumococcal conjugate vaccines and the 23-valent polysaccharide vaccine were 34, 47, and 70%, respectively. Inclusion of the expected cross-reactive serotypes increased the theoretical coverages to 60, 73, and 88%, respectively. A separate analysis of the potential vaccine coverage of pneumococcal isolates found during a stable state and exacerbation showed no significant difference (Table 1).
TABLE 1.
Most prevalent pneumococcal serotypes observed in COPD patients
| Serotype | Total no. of isolates (%) | No. of stable state isolates (%) | No. of exacerbation isolates (%) |
|---|---|---|---|
| 14 | 10 (8.7) | 5 (5.5) | 5 (20.0) |
| 19F | 15 (13.0) | 12 (13.3) | 3 (12.0) |
| 23F | 8 (7.0) | 7 (7.8) | 1 (4.0) |
| 3 | 12 (10.4) | 11 (12.2) | 1 (4.0) |
| 6Aa | 9 (7.8) | 7 (7.7) | 2 (8.0) |
| 9 L/Na | 10 (8.7) | 5 (5.6) | 5 (20.0) |
| 11 | 9 (7.8) | 6 (6.7) | 3 (12.0) |
| 23A/Ba | 10 (8.7) | 9 (10.0) | 1 (4.0) |
| 7-valent | 39 (33.9) | 30 (33.3) | 9 (36.0) |
| 7-valentb | 69 (60.0) | 52 (57.7) | 17 (68.0) |
| 11-valent | 54 (47.0) | 44 (48.9) | 10 (40.0) |
| 11-valentb | 84 (73.0) | 66 (73.3) | 18 (72.0) |
| 23-valent | 80 (69.6) | 61 (56.8) | 19 (76.0) |
| 23-valentb | 101 (78.8) | 78 (67.8) | 23 (92.0) |
| Total | 115 (100) | 90 (100) | 25 (100) |
Cross-reactive serotype.
Including cross-reactive serotypes.
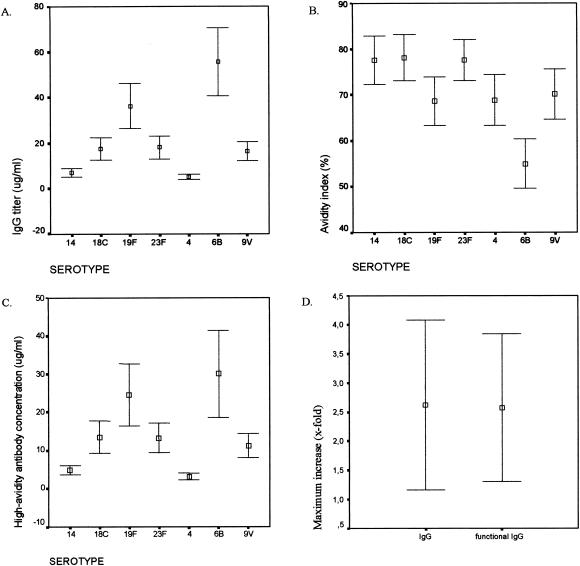
We investigated the presence of IgG antibodies and the AI of these antibodies for the serotypes included in the 7-valent conjugate vaccine (4, 6B, 9V, 14, 18C, 19F, and 23F) for serum samples obtained from 92 patients. For this purpose, we included all first serum samples obtained during the study period. Antibody levels against all seven serotypes were detectable in all patients. The GMCs of antibodies against the seven serotypes for the COPD patients as well as for 10 healthy adult volunteers before and after vaccination with the 7-valent conjugate vaccine are depicted in Table 2. The GMCs ranged from 5.04 μg/ml for serotype 4 to 55.76 for serotype 6B, with a large variability (Fig. 1A). The GMCs for healthy adults ranged from 1.7 (serotype 4) to 21.9 (serotype 6B) μg/ml before vaccination to 13.4 (serotype 9V) to 76.7 (serotype 6B) μg/ml after conjugate vaccination. The mean AIs as well as the mean high-avidity antibody concentrations with 95% CIs are also shown in Fig. 1B and C. The AIs ranged from 55% for serotype 6B to 78% for serotype 18C. The high-avidity antibody titers ranged from 3.4 μg/ml for serotype 4 to 30.7 μg/ml for serotype 6B.
TABLE 2.
GMCs (μg/ml) of anti-pneumococcal antibodies against the seven conjugate vaccine pneumococcal serotypes
| Pneumococcal serotype | GMC (μg/ml [95% CI])
|
||
|---|---|---|---|
| COPD patients | Healthy adults | Healthy adults after conjugate vaccination | |
| 4 | 5.04 (3.93-6.15) | 1.65a (0.48-2.81) | 19.95b (5.99-33.92) |
| 6B | 55.76 (40.83-70.70) | 21.9 (7.02-36.84) | 76.70 (46.87-106.53) |
| 9V | 16.38 (12.22-20.54) | 3.39a (0.85-5.95) | 13.41 (8.51-18.31) |
| 14 | 7.06 (5.11-9.01) | 3.37 (0.28-6.47) | 30.74b (14.22-47.26) |
| 18C | 17.49 (12.59-22.40) | 9.37 (3.80-22.54) | 56.53b (27.23-85.83) |
| 19F | 36.34 (26.29-46.40) | 18.40 (4.97-31.82) | 66.76 (34.61-98.90) |
| 23F | 18.08 (13.13-23.04) | 4.08 (0.64-7.50) | 55.43b (17.65-93.21) |
Significantly lower antibody level compared to patient group.
Significantly higher antibody level compared to patient group.
FIG. 1.
Capsular antibody titers against the seven pneumococcal conjugate vaccine serotypes in 92 COPD patients at the start of the study. Error bars show 95% CIs for the GMCs for IgG antibodies (A), AIs (B), and high-avidity antibody titers (C). (D) Error bars show 95% CIs for mean maximum antibody increases and mean maximum high-avidity antibody increases for the homologous serotype during exacerbation.
Because the studied serum samples were collected randomly, i.e., during both a stable state and exacerbation, we reanalyzed our data with respect to this variable. No significant difference in antibody titers against the seven tested serotypes was observed between samples obtained during exacerbation and during a stable state (data not shown).
We investigated the development of antibodies in response to colonization with pneumococci during acute exacerbation. In total, 15 patients, who were colonized with serotypes 14 (4), 9N (4), 9V (3), 19F (3), and 11 (1), were studied during an exacerbation. We investigated the antibody levels in serum and the AIs of the antibodies against the capsular polysaccharides of the pneumococcal serotypes isolated from the patients. When available, two or three consecutive serum samples were analyzed, as initial, acute-phase, and convalescent-phase samples. Furthermore, we calculated the maximum increase in antibody titer in the consecutive sera for individual patients (Table 3). We observed a significant rise in antibodies as well as in high-avidity antibodies, with an average 2.5-fold increase (Fig. 1D). The mean avidity, which was 70%, did not change significantly.
TABLE 3.
Serotype-specific IgG concentrations, in initial and convalescent-phase sera and the maximum increase in serotype-specific IgG for 15 COPD patients with positive sputum cultures for S. pneumoniae during exacerbation
| Patient no. | Serotype | Gender | Age (years) | Treatment with flixotide | Serotype-specific IgG (U/ml)
|
||
|---|---|---|---|---|---|---|---|
| Initial serum | Convalescent-phase serum | Increase | |||||
| 1 | 14 | M | 66 | Yes | 97.92 | 81.25 | 0.83 |
| 2 | 14 | M | 73 | Yes | 95.24 | 428.57 | 4.50 |
| 3 | 9N | M | 47 | No | 156 | 146.43 | 0.94 |
| 4 | 19F | F | 66 | No | 1,000 | 1,300 | 1.30 |
| 5 | 19F | M | 57 | No | 75 | 100 | 1.33 |
| 6 | 9N | M | 58 | Yes | 7,000 | 9066.67 | 1.30 |
| 7 | 19F | M | 73 | No | 1,000 | 1,800 | 1.80 |
| 8 | 9V | M | 64 | No | 971.43 | 1,540 | 1.59 |
| 9 | 9V | M | 67 | Yes | 1,500 | 9,500 | 6.33 |
| 10 | 9N | M | 56 | No | 500 | 5,000 | 10.00 |
| 11 | 14 | M | 61 | Yes | 105.87 | 95.24 | 0.90 |
| 12 | 9N | M | 61 | Yes | 15733.33 | 9066.67 | 0.58 |
| 13 | 11 | M | 60 | Yes | 950 | 450 | 0.47 |
| 14 | 14 | M | 63 | Yes | 31.67 | 50 | 1.58 |
| 15 | 9V | M | 63 | Yes | 2,000 | 5,250 | 2.63 |
DISCUSSION
We investigated the effect of pneumococcal colonization on the development of exacerbation in COPD patients. Although patients colonized with bacteria in general did not show an increased risk of a first exacerbation episode compared to patients without bacterial colonization, colonization with S. pneumoniae, specifically when present as a monoculture, did increase the risk of a first exacerbation, with a hazard ratio of 2.91. Our data support the findings of Sethi et al., who also showed a significant increase in exacerbations when S. pneumoniae was isolated (18), which underscores the role of S. pneumoniae in the pathogenesis of exacerbations of COPD. Surprisingly, the presence of pneumococci in combination with other pathogens in sputum did not cause an increased risk of a next exacerbation. This might indicate that monocultures merely represent an infectious state, whereas mixed cultures are more representative of a stable state of colonization. This is supported by our data, which show that during exacerbations of COPD, monocultures of potential pathogenic microorganisms, in particular, are increased. Because this specific correlation has never been investigated, it will be worthwhile to include this variable in future analyses.
Subsequently, we calculated the theoretical coverage of the 7-valent, 11-valent, and 23-valent pneumococcal vaccines. The most prevalent serogroups among the 115 isolates investigated were serogroups 3, 9, 11, 14, 19, and 23 (10, 9, 8, 9, 14, and 16%, respectively). Because most of these serogroups are included in the conjugate vaccine, the theoretical coverage for these vaccines is 34 to 49% of all pneumococci, but is even higher (60 to 73%) when cross-reactive serotypes are considered. An analysis of just exacerbation cultures showed comparable coverage rates. This is in agreement with the data of Flamaing et al., who investigated the serotype distribution among pneumococcal isolates obtained from individuals 65 years of age and older with invasive disease in Belgium (6). The potential coverage of the 23-valent conjugate vaccine is even higher, with 70 to 88% coverage. Unfortunately, most previous studies could not find a protective effect of this vaccine against mucosal diseases.
Secondly, we investigated whether natural antibodies were already present in COPD patients but failed to protect these patients from pneumococcal colonization. GMCs for the 92 COPD patients investigated were normal to high when compared to healthy adults (M. Sluijter, unpublished observations). Moreover, as in healthy adults, GMCs for serotypes 4 and 14 were relatively low compared to the other serotypes. Finally, the quality of the antibodies, as measured by means of AIs, was sufficient. Therefore, we conclude that these patients are able to elicit an immunological response to pneumococcal challenge and thus are not immunologically deficient with respect to antibody development. The trend towards higher titers compared to healthy unvaccinated adults may represent the increased pneumococcal contacts in this patient group, although a difference in gender (50 and 98% male, respectively) and smoking history (0 and ∼100%, respectively) between the healthy adults and the patient group may also be of influence (16).
Healthy adults vaccinated once with the 7-valent conjugate vaccine displayed higher GMCs than the patient group, although they were only significant for serotypes 4, 14, 18C, and 23F due to the relatively large CIs. Because a similar response from our patient group can be expected upon vaccination, we suggest that vaccination of our patient group may increase antibody levels and hence may elicit a higher level of protection against pneumococcal disease. This is in agreement with the results of Jonsson et al., who have shown that vaccination of COPD patients with a serotype 6B conjugate vaccine or a 23-valent polysaccharide vaccine elicits antibody levels comparable to those in vaccinated healthy adults (8). One might question, however, whether the present levels of antibodies prevent both colonization and infection with pneumococci in our patients. It has been hypothesized that the prevention of mucosal disease requires higher levels of anti-pneumococcal antibodies than the prevention of invasive disease. Previous work has shown that higher antibody levels are necessary to protect children against acute otitis media than against invasive diseases (13). Alternatively, one might suggest that anatomical damage to the lungs and the thick mucous layers prevents the immune system from recognizing or attacking colonizing bacteria. This is supported by the data of Davis et al., who have shown that vaccination of COPD patients with a 14-valent pneumococcal polysaccharide vaccine induces significant antibody responses but not protection against pneumonia and death (4). In contrast to our findings, most pneumococci observed during infection in that study were nonvaccine serotypes, which may explain the observed vaccine failure. We determined whether the presence of pneumococci during exacerbation elicits a natural immune response to the homologous serotype. Overall, a 2.5-fold increase in IgG antibody levels and high-avidity antibody levels was found. Although a small patient group was studied and a small number of consecutive sera per patient were available, these data suggest that the immune systems of two-thirds of the COPD patients were capable of mounting an antibody response towards pneumococci, whereas one-third of the patients did not show an increase in antibodies upon the presence of pneumococci. Obviously, one could argue whether these antibodies protect COPD patients against pneumococcal reinfection. Follow-up studies are necessary in which the protective potentials of these antibodies are investigated in order to speculate about the effect of pneumococcal conjugate vaccination in COPD patients.
In conclusion, our data indicate that pneumococcal colonization in COPD patients is frequently caused by vaccine serotype strains. Moreover, pneumococcal colonization is a risk factor for exacerbations in these patients. Finally, our findings show that the majority of COPD patients mount an anticapsular immune response during pneumococcal colonization and infection. Clinical studies are needed to investigate the protective potentials of conjugate vaccination for COPD patients.
Acknowledgments
This study was financially supported by the Sophia Foundation for Medical Research, Rotterdam, The Netherlands (grant 268), and the Dutch Science Foundation (grant SGO-Inf. 005).
Editor: J. N. Weiser
REFERENCES
- 1.Anttila, M., J. Eskola, H. Ahman, and H. Kayhty. 1998. Avidity of IgG for Streptococcus pneumoniae type 6B and 23F polysaccharides in infants primed with pneumococcal conjugates and boosted with polysaccharide or conjugate vaccines. J. Infect. Dis. 177:1614-1621. [DOI] [PubMed] [Google Scholar]
- 2.Artz, A. S., W. B. Ershler, and D. L. Longo. 2003. Pneumococcal vaccination and revaccination of older adults. Clin. Microbiol. Rev. 16:308-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burge, S., and J. A. Wedzicha. 2003. COPD exacerbations: definitions and classifications. Eur. Respir. J. 41(Suppl.):46S-53S. [DOI] [PubMed] [Google Scholar]
- 4.Davis, A. L., C. P. Aranda, G. Schiffman, and L. C. Christianson. 1987. Pneumococcal infection and immunologic response to pneumococcal vaccine in chronic obstructive pulmonary disease. A pilot study. Chest 92:204-212. [DOI] [PubMed] [Google Scholar]
- 5.Ewig, S., R. Rodriguez-Roisin, and A. Torres. 2002. Indications for and choice of antibiotics in COPD, p. 201-220. In T. Similowski, W. Whitelaw, and J. Derenne (ed.), Clinical management of chronic obstructive pulmonary disease. Marcel Dekker, New York, N.Y.
- 6.Flamaing, J., J. Verhaegen, and W. E. Peetermans. 2002. Streptococcus pneumoniae bacteraemia in Belgium: differential characteristics in children and the elderly population and implications for vaccine use. J. Antimicrob. Chemother. 50:43-50. [DOI] [PubMed] [Google Scholar]
- 7.Jackson, L. A., K. M. Neuzil, O. Yu, P. Benson, W. E. Barlow, A. L. Adams, C. A. Hanson, L. D. Mahoney, D. K. Shay, and W. W. Thompson. 2003. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N. Engl. J. Med. 348:1747-1755. [DOI] [PubMed] [Google Scholar]
- 8.Jonsson, S., G. Vidarsson, H. Valdimarsson, G. Schiffman, R. Schneerson, and I. Jonsdottir. 2002. Vaccination of COPD patients with a pneumococcus type 6B tetanus toxoid conjugate vaccine. Eur. Respir. J. 20:813-818. [DOI] [PubMed] [Google Scholar]
- 9.Kayhty, H., H. Ahman, P. R. Ronnberg, R. Tillikainen, and J. Eskola. 1995. Pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine is immunogenic in infants and children. J. Infect. Dis. 172:1273-1278. [DOI] [PubMed] [Google Scholar]
- 10.Lenette, E., A. Balows, W. Hauser, Jr., and H. Shadomy (ed.). 1985. Manual of clinical microbiology. American Society for Microbiology, Washington, D.C.
- 11.Murray, C. J., and A. D. Lopez. 1997. Alternative projections of mortality and disability by cause. 1990-2020 Global Burden of Disease Study. Lancet 349:1498-1504. [DOI] [PubMed] [Google Scholar]
- 12.Patel, I. S., T. A. Seemungal, M. Wilks, S. J. Lloyd-Owen, G. C. Donaldson, and J. A. Wedzicha. 2002. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax 57:759-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pelton, S. I., R. Dagan, B. M. Gaines, K. P. Klugman, D. Laufer, K. O'Brien, and H. J. Schmitt. 2003. Pneumococcal conjugate vaccines: proceedings from an interactive symposium at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy. Vaccine 21:1562-1571. [DOI] [PubMed] [Google Scholar]
- 14.Poland, G. A. 1999. The burden of pneumococcal disease: the role of conjugate vaccines. Vaccine 17:1674-1679. [DOI] [PubMed] [Google Scholar]
- 15.Quataert, S. A., C. S. Kirch, L. J. Wiedl, D. C. Phipps, S. Strohmeyer, C. O. Cimino, J. Skuse, and D. V. Madore. 1995. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89-S. Clin. Diagn. Lab. Immunol. 2:590-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sankilampi, U., R. Isoaho, A. Bloigu, S. L. Kivela, and M. Leinonen. 1997. Effect of age, sex and smoking habits on pneumococcal antibodies in an elderly population. Int. J. Epidemiol. 26:420-427. [DOI] [PubMed] [Google Scholar]
- 17.Seemungal, T. A., G. C. Donaldson, A. Bhowmik, D. J. Jeffries, and J. A. Wedzicha. 2000. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 161:1608-1613. [DOI] [PubMed] [Google Scholar]
- 18.Sethi, S., N. Evans, B. J. Grant, and T. F. Murphy. 2002. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N. Engl. J. Med. 347:465-471. [DOI] [PubMed] [Google Scholar]
- 19.van der Valk, P., E. Monninkhof, J. van der Palen, G. Zielhuis, and C. van Herwaarden. 2002. Effect of discontinuation of inhaled corticosteroids in patients with chronic obstructive pulmonary disease: the COPE study. Am. J. Respir. Crit. Care Med. 166:1358-1363. [DOI] [PubMed] [Google Scholar]
- 20.Wedzicha, J. A. 2002. Exacerbations: etiology and pathophysiologic mechanisms. Chest 121:136S-141S. [DOI] [PMC free article] [PubMed]