Inhibition of p110α or of the downstream PI3K signaling pathway components PDK1 and Akt, as well as phosphoinositide sequestration, blocks invadopodia formation in breast cancer cells.
Abstract
Invadopodia are extracellular matrix–degrading protrusions formed by invasive cancer cells that are thought to function in cancer invasion. Although many invadopodia components have been identified, signaling pathways that link extracellular stimuli to invadopodia formation remain largely unknown. We investigate the role of phosphoinositide 3-kinase (PI3K) signaling during invadopodia formation. We find that in human breast cancer cells, both invadopodia formation and degradation of a gelatin matrix were blocked by treatment with PI3K inhibitors or sequestration of D-3 phosphoinositides. Functional analyses revealed that among the PI3K family proteins, the class I PI3K catalytic subunit p110α, a frequently mutated gene product in human cancers, was selectively involved in invadopodia formation. The expression of p110α with cancerous mutations promoted invadopodia-mediated invasive activity. Furthermore, knockdown or inhibition of PDK1 and Akt, downstream effectors of PI3K signaling, suppressed invadopodia formation induced by p110α mutants. These data suggest that PI3K signaling via p110α regulates invadopodia-mediated invasion of breast cancer cells.
Introduction
Degradation of ECM that is present in the basement membrane and tumor stroma is essential for local invasion and formation of metastatic sites by malignant cancer cells (Kessenbrock et al., 2010). Invadopodia, which were first described by Chen (1989), are ECM-degrading membrane protrusions formed on the ventral surface of invasive cancer cells and are thought to play a role in cancer cell invasion (Yamaguchi et al., 2005b; Weaver, 2006; Buccione et al., 2009; Madsen and Sahai, 2010). Invadopodia have been observed in a variety of invasive cancer cell lines, including mammary adenocarcinoma, colon carcinoma, melanoma, and glioma as well as in primary invasive tumor cells derived from glioblastoma and head and neck cancers (Clark et al., 2007; Stylli et al., 2008). In the case of breast cancer cell lines, the ability to form invadopodia is closely related to their invasive and metastatic properties in vivo (Coopman et al., 1998; Yamaguchi et al., 2005a, 2009). Additionally, invadopodia-like protrusions in breast cancer cells have been observed during intravasation by intravital imaging (Condeelis and Segall, 2003; Yamaguchi et al., 2005b). A recent study showed that invasive cancer cells use invadopodia to breach the basement membrane and penetrate into the stroma (Schoumacher et al., 2010). Moreover, Eckert et al. (2011) recently reported that Twist, an inducer of epithelial–mesenchymal transition, induces invadopodia formation to promote tumor metastasis and provided evidence of invadopodia formation in vivo in sections of invasive primary tumors.
Many components of invadopodia, such as various proteins involved in actin polymerization, cell signaling, membrane trafficking, cell–ECM adhesion, and ECM degradation, have been reported to date (Linder, 2007; Gimona et al., 2008; Caldieri and Buccione, 2010). We and other researchers previously reported that invadopodia formation is induced by stimulation with serum and growth factors (Tague et al., 2004; Yamaguchi et al., 2005a; Mandal et al., 2008; Eckert et al., 2011). However, the signaling pathways that link these extracellular stimuli to invadopodia formation remain largely unknown.
The phosphoinositide 3-kinases (PI3Ks) are a family of lipid kinases that phosphorylate phosphoinositides at the D-3 position of the inositol headgroup and, thus, produce D-3 phosphoinositides (Cantley, 2002). PI3Ks mediate the signal transduction of extracellular stimuli and regulate diverse cellular events, such as mitogenesis, survival, membrane transport, and cell migration (Engelman et al., 2006; Cain and Ridley, 2009). PI3Ks are subdivided into three general classes (I–III) in mammals on the basis of their enzyme domain structures and substrate specificities (Fruman et al., 1998). Specifically, the class I subfamily consists of four catalytic subunits, including three class IA subunits (p110α, p110β, and p110δ) and a single class IB subunit (p110γ). However, the class II PI3K group consists of three isoforms, PI3K-C2α, PI3K-C2β, and PI3K-C2γ. Finally, mammals have a single class III isoform, namely, Vps34, which is a homologue of the sole PI3K present in yeast.
Uncontrolled activation of the PI3K signaling pathway leads to several pathological phenomena, including tumorigenesis and tumor malignancy (Cantley, 2002). This is indicated by the finding that the expression and activity of several members of the PI3K signaling pathway are frequently altered in a variety of human cancers (Yuan and Cantley, 2008). For instance, the PIK3CA gene, which encodes the class IA PI3K catalytic subunit p110α, is one of the most frequently amplified and mutated genes identified in human cancers (Yuan and Cantley, 2008; Zhao and Vogt, 2008). Clinical studies involving human breast cancer patients revealed that mutations leading to the activation of PIK3CA are associated with the development of invasive and metastatic phenotypes and poor patient prognosis (Saal et al., 2005; Li et al., 2006; Maruyama et al., 2007). Moreover, a previous study has shown that introduction of the mutant PIK3CA gene into a breast cancer cell line enhanced lung metastasis in mice (Pang et al., 2009). However, the detailed mechanisms by which the PIK3CA gene product p110α contributes to cancer invasion and metastasis are yet to be determined.
It is established that 3-phosphoinositide–dependent protein kinase-1 (PDK1) is a serine/threonine kinase that mediates PI3K signaling during various cellular responses (Toker and Newton, 2000). PDK1 is recruited to cell membranes upon PI3K activation, where it phosphorylates and activates Akt, the major mediator of the PI3K signaling pathway (Stephens et al., 1998). Both PDK1 and Akt are overexpressed in human breast cancers and are thought to be critical components of the oncogenic PI3K signaling pathway (Dillon et al., 2007; Maurer et al., 2009; Sheng et al., 2009). Furthermore, previous studies have demonstrated that PDK1 and Akt are involved in the invasive and metastatic phenotypes of human cancer cells (Xie et al., 2006; Dillon et al., 2007; Liu et al., 2009; Sheng et al., 2009). However, the roles of PDK1 and Akt in invadopodia formation remain unclear. In the present study, we investigate the role of PI3K signaling during invadopodia formation in invasive human breast cancer cells.
Results
PI3K activity is required for invadopodia formation in human breast cancer cells
The formation of invadopodia in human cancer cells and podosomes, which are structures functionally similar to invadopodia, in Src-transformed fibroblasts requires the activity of PI3K (Nakahara et al., 2003; Mandal et al., 2008; Oikawa et al., 2008). In the present study, the role of PI3K in invadopodia formation was investigated in detail in the highly invasive human breast cancer cell line MDA-MB-231 (Neve et al., 2006). MDA-MB-231 cells form invadopodia in vitro and have, therefore, been widely used in studies investigating various aspects of these invasive structures (Chen et al., 1994). MDA-MB-231 cells were seeded onto fluorescent gelatin-coated coverslips in the presence or absence of each of two PI3K inhibitors, LY294002 and wortmannin, and stained for two invadopodia markers, cortactin and F-actin. Invadopodia were observed as dotlike clusters of cortactin and F-actin on the ventral membrane of cells, which corresponded with the degradation sites on the gelatin matrix (Fig. S1 A). To quantify the invadopodia-mediated degradation of the gelatin matrix for each treatment, we calculated the area of the degradation sites. Both LY294002 and wortmannin significantly inhibited the formation of invadopodia and gelatin degradation in a dose-dependent manner, with half-maximal inhibitory concentration (IC50) values of 3.3 µM and 3.6 nM for LY294002 and wortmannin, respectively (Fig. S1, B–D). Furthermore, the percentage of cells with invadopodia and the number of invadopodia per cell were also reduced in cells treated with either PI3K inhibitor (Fig. S1, E and F).
We also examined the effect of PI3K inhibition on the stability of preformed invadopodia. MDA-MB-231 cells expressing GFP-actin were seeded onto plates coated with a gelatin matrix, and cells were observed using time-lapse microscopy upon treatment with LY294002. LY294002 treatment of cells exhibiting GFP-actin–positive invadopodia resulted in the degradation of invadopodia within 1 min of treatment (Fig. S1 G and Video 1). A similar result was obtained when cells expressing Venus-cortactin were analyzed in the same manner (Video 2). Quantification of the intensity of GFP-actin signals at the invadopodia revealed that the actin core structures of invadopodia disassembled immediately after the addition of LY294002, whereas the invadopodia of cells treated with DMSO did not disassemble (Fig. S1 H). Collectively, these results indicate that PI3K activation is required for both the formation and stability of invadopodia in human breast cancer cells.
D-3 phosphoinositides are required for invadopodia formation
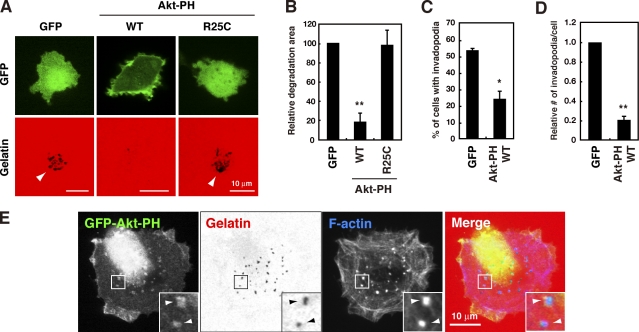
We next investigated the role of D-3 phosphoinositides synthesized by PI3Ks in invadopodia formation. The pleckstrin homology (PH) domain of Akt interacts with phosphatidylinositol 3,4,5-trisphosphate (PI(3,4,5)P3) and phosphatidylinositol 3,4-bisphosphate (PI(3,4)P2), which are two major products of PI3K, and its overexpression results in the sequestration and inhibition of the function of these phosphoinositides (Várnai et al., 2005). In the present study, the PH domain of Akt was overexpressed in MDA-MB-231 cells as a GFP fusion protein (GFP-Akt-PH). This construct, which localized to the plasma membrane, inhibited the formation of invadopodia, as measured by both the percentage of cells with invadopodia and the number of invadopodia per cell, and gelatin degradation (Fig. 1, A–D). In contrast, a mutant form of the Akt PH domain (R25C), in which an essential amino acid for phosphoinositide binding is mutated (Várnai et al., 2005), did not localize to the plasma membrane or inhibit gelatin degradation (Fig. 1, A and B). Furthermore, to examine the localization of D-3 phosphoinositides at invadopodia sites, a cell line expressing the GFP-Akt-PH construct at an extremely low level, ∼13 times less than transient expression (Fig. S2 A), was established, which allows the cells to retain invadopodia. In these cells, signals corresponding to GFP-Akt-PH were significantly concentrated at F-actin–rich invadopodia and at the gelatin degradation sites (Fig. 1 E). This accumulation of GFP signals at invadopodia was not observed when cells expressing GFP alone were examined in the same manner (Fig. S2 B). These results indicate that PI(3,4,5)P3 and/or PI(3,4)P2 produced as downstream effectors of PI3K have an essential role in invadopodia-mediated ECM degradation.
Figure 1.
D-3 phosphoinositides are necessary for invadopodia formation. (A) MDA-MB-231 cells transfected with the GFP, GFP-Akt-PH wild-type (WT), or GFP-Akt-PH R25C mutant construct were cultured on fluorescent gelatin-coated coverslips for 7 h and imaged by confocal microscopy. Arrowheads denote degradation sites on the gelatin matrix. (B–D) Degraded areas on the gelatin matrix (B), the percentage of cells with invadopodia (C), and the relative number of invadopodia per cell (D) were quantified for transfected cells as described in the Materials and methods. (E) MDA-MB-231 cells stably expressing GFP-Akt-PH WT were cultured on fluorescent gelatin-coated coverslips for 3 h, stained for F-actin, and observed by confocal microscopy. Insets are magnified images of the boxed regions. Arrowheads denote invadopodia where GFP-Akt-PH signals were accumulated. Data in B–D are represented as means + SEM of four independent determinations. *, P < 0.01; and **, P < 0.005 by Student’s t test.
The class I PI3K catalytic subunit p110α is an essential regulator of invadopodia formation
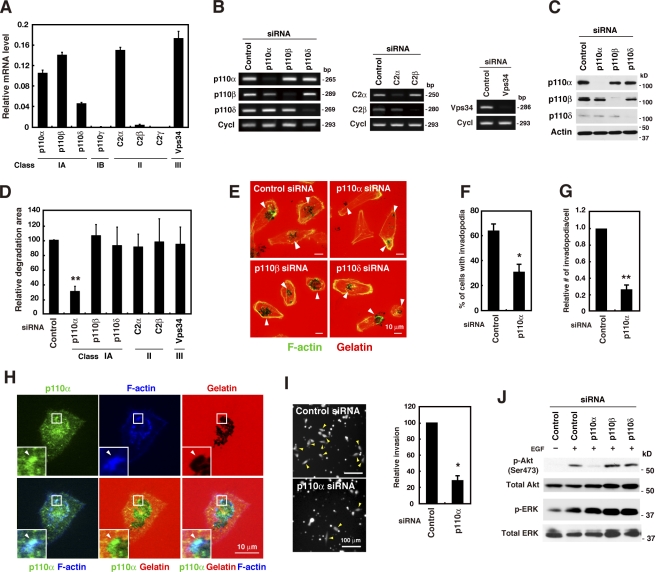
Mammalian cells contain eight PI3K enzymes, which are further classified into classes I, II, and III (Fruman et al., 1998). In the present study, the expression levels of the PI3K family of proteins were examined in MDA-MB-231 cells by real-time quantitative PCR and standard semiquantitative PCR analyses performed using different sets of primers specific for the PI3K isoforms (Fig. 2 A and Fig. S3 A). The class I subunits p110α, p110β, and p110δ, the class II subunit C2α, and the class III subunit Vps34 were abundantly expressed in these cells. Furthermore, the expression of the class II subunit C2β was weak but detectable. However, these cells did not express the class I subunit p110γ or the class II subunit C2γ.
Figure 2.
Class I PI3K catalytic subunit p110α is an essential regulator of invadopodia formation. (A) Real-time quantitative PCR analysis of the expression of PI3K isoforms in MDA-MB-231 cells. The relative mRNA levels of PI3K isoforms normalized with the mRNA levels of cyclophilin B are shown. (B and C) MDA-MB-231 cells were transfected with siRNAs targeting individual PI3K isoforms for 48 h, and the expression profiles of PI3K isoforms were determined by RT-PCR (B) and immunoblot analyses (C). Cyclophilin B (Cycl) and β-actin were used as internal controls. (D) MDA-MB-231 cells transfected with the indicated siRNAs were cultured on fluorescent gelatin-coated coverslips for 7 h, and the degraded areas on the gelatin matrix were quantified. (E) Representative images of cells transfected with siRNAs targeting p110 isoforms and stained for F-actin. Arrowheads denote the gelatin degradation sites. (F and G) The percentage of cells with invadopodia (F) and the relative number of invadopodia per cell (G) were determined in cells transfected with control or p110α siRNA. (H) MDA-MB-231 cells plated onto fluorescent gelatin-coated coverslips for 4 h were stained with anti-p110α antibody and phalloidin. Insets are magnified images of the boxed regions. Arrowheads denote accumulation of p110α signals at invadopodia. (I) MDA-MB-231 cells transfected with control or p110α siRNA were labeled with CellTracker green and analyzed for invasion through Matrigel-coated Transwell inserts for 24 h. Invaded cells were then imaged by fluorescent microscopy and counted. Arrowheads denote invaded cells. Smaller dots represent pores of the membrane of Transwell inserts. (J) MDA-MB-231 cells transfected with the indicated siRNAs were serum-starved overnight and stimulated with 8 nM EGF for 10 min. The cells were then analyzed by immunoblotting to determine the phosphorylation status of Akt (p-Akt) and ERK (p-ERK). Data are represented as means + SEM of three (A and I), eight (D), and five (F and G) independent determinations. *, P < 0.01; and **, P < 0.0002 by Student’s t test.
siRNA knockdown experiments were performed to determine the contribution of individual PI3K isoforms to invadopodia formation. MDA-MB-231 cells were transfected with siRNAs targeting each PI3K enzyme and subsequently examined for invadopodia formation and gelatin degradation. The efficiency and selectivity of the siRNAs in knocking down individual PI3K isoforms were confirmed by RT-PCR analysis (Fig. 2 B), and the knockdown of class I p110 enzymes was also confirmed by immunoblotting (Fig. 2 C). Cells with reduced p110α levels showed a significant decrease in invadopodia formation and gelatin degradation activity (Fig. 2, D–G). Similar results were obtained with three other siRNAs targeting different regions of the p110α gene (Fig. S4, A and B). However, cells transfected with siRNAs targeting other class I PI3K enzymes (i.e., p110β and p110δ) did not show decreased invadopodia formation or gelatin degradation activity (Fig. 2, D and E). Furthermore, knockdown of classes II and III PI3Ks, including C2α, C2β, and Vps34, did not affect gelatin degradation activity (Fig. 2 D). Examination of the localization of endogenous p110α by immunocytochemistry revealed the presence of strong signals corresponding to endogenous p110α at invadopodia that were enriched with F-actin and were associated with gelatin degradation sites (Fig. 2 H). To ascertain whether invadopodia formation mediated by p110α reflects the invasiveness of cancer cells, an in vitro Matrigel invasion assay was performed. MDA-MB-231 cells transfected with p110α siRNA showed markedly reduced invasion through Matrigel in comparison to cells transfected with control siRNA (Fig. 2 I). Collectively, these results indicate that among the PI3K family proteins, p110α is specifically involved in invadopodia-mediated invasion of human breast cancer cells.
The effect of p110α knockdown on invadopodia formation was assessed in other invasive breast cancer cell lines, namely BT-549 and Hs578T. BT-549 cells treated with two different p110α siRNAs showed a significant decrease in invadopodia-mediated gelatin degradation (Fig. S4, C and D). As Hs578T cells were sensitive to siRNA transfection under the present experimental conditions, a short hairpin RNA (shRNA) targeting the p110α gene was introduced into Hs578T cells by lentiviral transduction. Transduction of Hs578T cells with p110α shRNA resulted in a marked reduction of the expression of p110α and a concomitant decrease in gelatin degradation activity as compared with cells with control shRNA (Fig. S4, E–G).
The PI3K signaling pathway activation status was determined by measuring the amount of phosphorylated Akt, a major downstream effector of the PI3K signaling pathway. Knockdown of p110α suppressed Akt phosphorylation upon EGF stimulation (Fig. 2 J), whereas knockdown of p110β or p110δ had almost no effect. Thus, p110α is likely the primary mediator of growth factor–stimulated PI3K signaling in this cell type. Importantly, EGF-induced phosphorylation of ERK was not affected by p110α knockdown (Fig. 2 J). This result suggests that p110α inhibition does not affect MAPK signaling, a pathway that has been implicated in invadopodia formation in human melanoma cells (Tague et al., 2004).
Pharmacological inhibition of p110α blocks invadopodia formation
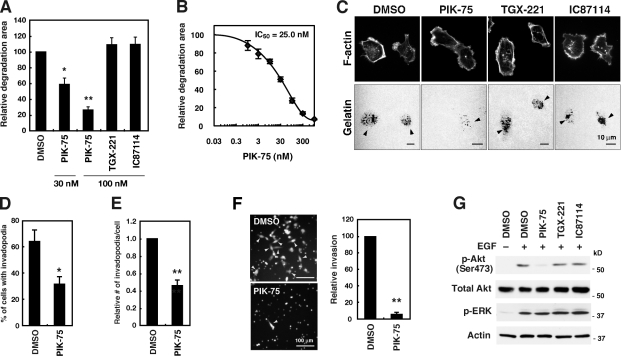
To confirm that p110α is an essential regulator of invadopodia formation, the effect of selective inhibitors of class I PI3K isoforms was investigated. Cells were cultured on fluorescent gelatin-coated coverslips in the presence of PIK-75, TGX-221, or IC87114, which are selective inhibitors of p110α, β, and δ, respectively (Knight et al., 2006; Chaussade et al., 2007). p110α inhibition by PIK-75 treatment significantly inhibited gelatin degradation in a dose-dependent manner, showing an IC50 of 25.0 nM (Fig. 3, A–C), and suppressed invadopodia formation (Fig. 3, D and E). A similar inhibition of gelatin degradation was observed when BT-549 and Hs578T breast cancer cells were treated with PIK-75 (Fig. S4, H and I). However, neither TGX-221 nor IC87114 significantly affected gelatin degradation (Fig. 3, A and C) despite their use at concentrations well above the IC50 values reported previously (Chaussade et al., 2007). PIK-75 treatment also markedly inhibited Matrigel invasion of MDA-MB-231 cells (Fig. 3 F).
Figure 3.
Effects of pharmacological inhibition of class I PI3Ks on invadopodia formation. (A) MDA-MB-231 cells were cultured on fluorescent gelatin-coated coverslips for 7 h in the presence or absence of various class I PI3K inhibitors, including PIK-75 for p110α, TGX-221 for p110β, and IC87114 for p110δ. The degraded areas on the gelatin matrix were quantified and are represented as the percentage of control DMSO-treated cells. (B) Dose–response curve of gelatin degradation obtained in the presence of increasing concentrations of PIK-75 is shown. (C) Representative images of MDA-MB-231 cells treated with various class I PI3K inhibitors are shown. Arrowheads denote the gelatin degradation sites. (D and E) The percentage of cells with invadopodia (D) and the relative number of invadopodia per cell (E) were determined in cells treated with control DMSO or 100 nM PIK-75. (F) MDA-MB-231 cells labeled with CellTracker green were analyzed for invasion through Matrigel-coated Transwell inserts in the presence or absence of 100 nM PIK-75 for 24 h. Invaded cells were then imaged by fluorescent microscopy and counted. Arrowheads denote invaded cells. (G) MDA-MB-231 cells were serum-starved overnight and treated with 300 nM of the indicated class I PI3K inhibitors for 1 h. The cells were subsequently stimulated with 8 nM EGF for 10 min and used for immunoblotting to determine the phosphorylation status of Akt (p-Akt) and ERK (p-ERK). Data are represented as means ± SEM of six (A, D, and E) and three (B and F) independent determinations. *, P < 0.01; and **, P < 0.0005 by Student’s t tests.
As expected, we found that only p110α inhibition by PIK-75 suppressed EGF-induced Akt phosphorylation (Fig. 3 G). In addition, EGF-induced phosphorylation of ERK was not affected by PIK-75 treatment (Fig. 3 G). At the concentrations used in these experiments, PIK-75 should specifically inhibit p110α activity but should not block p110β and p110δ activities based on results of previous studies (Knight et al., 2006; Chaussade et al., 2007). These results indicate that p110α plays a pivotal role in PI3K signaling and regulates the invadopodia-mediated ECM degradation activity of invasive breast cancer cells.
Activating mutations in the PIK3CA gene promote invadopodia formation
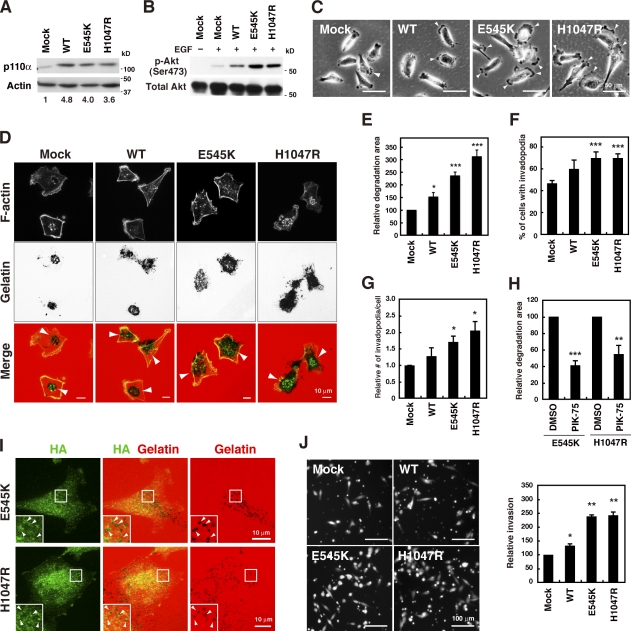
The PIK3CA gene, which encodes p110α, is one of the most frequently mutated genes in human breast cancers, and mutations in this gene are associated with invasion and metastasis (Saal et al., 2005; Maruyama et al., 2007). Most of the mutations occur at two hot spots, namely E545K in the helical domain and H1047R in the catalytic domain. These mutations constitutively activate the PI3K signaling pathway (Isakoff et al., 2005; Kang et al., 2005). Accordingly, the effect of these PIK3CA mutations on invadopodia formation was investigated in MDA-MB-231 cells, which express wild-type (WT) p110α (Hollestelle et al., 2007). MDA-MB-231 cell lines stably expressing WT, E545K, or H1047R p110α were generated. The expression levels of the ectopic proteins were ∼4–5 times higher than the expression level of the endogenous protein (Fig. 4 A). The results showed an increase in EGF-induced Akt phosphorylation in cells expressing WT p110α and a further increase in cells expressing either E545K or H1047R p110α in comparison to control mock-infected cells (Fig. 4 B). Furthermore, morphological analysis revealed that WT p110α cells tended to form more lamellipodia or membrane ruffles than control mock-infected cells (Fig. 4 C). An additional increase in the protrusive activities in E545K- and H1047R-expressing cells was observed (Fig. 4 C), which may reflect enhanced cell motility induced by these p110α mutants as described previously (Pang et al., 2009). Invadopodia formation and gelatin degradation activity were moderately increased in WT p110α cells and further enhanced in E545K- and H1047R-expressing cells (Fig. 4, D–G). The enhanced gelatin degradation activity in E545K- and H1047R-expressing cells was still sensitive to PIK-75 treatment, indicating that the enzymatic activity is crucial for invadopodia formation (Fig. 4 H). Similar to the behavior of the endogenous protein, the E545K and H1047R p110α mutants also accumulated at gelatin degradation sites (Fig. 4 I). In addition, E545K- and H1047R-expressing cells showed enhanced invasion through Matrigel compared with mock-infected cells (Fig. 4 J). These findings indicate that these activating mutations in the PIK3CA gene commonly present in human cancers promote the invadopodia-mediated invasive activity of breast cancer cells.
Figure 4.
Cancerous p110α mutations promote invadopodia formation. (A) MDA-MB-231 cells stably expressing wild-type (WT), E545K, or H1047R p110α were analyzed by immunoblotting. Numbers below represent relative expression levels of the p110α constructs. (B) Cell lines stably expressing p110α were serum-starved overnight and stimulated with 8 nM EGF for 10 min. The phosphorylation status of Akt (p-Akt) was determined by immunoblotting. (C) Phase-contrast images show the morphology of the p110α cell lines. Arrowheads denote membrane protrusions. (D) Cells stably expressing the p110α constructs were cultured on fluorescent gelatin matrices for 7 h and stained with phalloidin to visualize invadopodia. Arrowheads denote the gelatin degradation sites. (E–G) Gelatin degradation activity (E), the percentage of cells with invadopodia (F), and the number of invadopodia per cell (G) were determined in p110α cell lines. (H) Cells expressing E545K or H1047R p110α were examined for gelatin degradation in the presence or absence of 100 nM PIK-75. (I) Cells expressing E545K or H1047R p110α were cultured on fluorescent gelatin matrices for 4 h and stained with anti-HA antibody to visualize localization of E545K and H1047R p110α. Insets are magnified images of the boxed regions. Arrowheads denote colocalization of the HA signals with the gelatin degradation sites. (J) Cells labeled with CellTracker green were analyzed for invasion through Matrigel-coated Transwell inserts for 24 h. Data are represented as means + SEM of seven (E), six (F–H), and three (J) independent determinations. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 by Student’s t tests.
PDK1 and Akt are involved in invadopodia formation
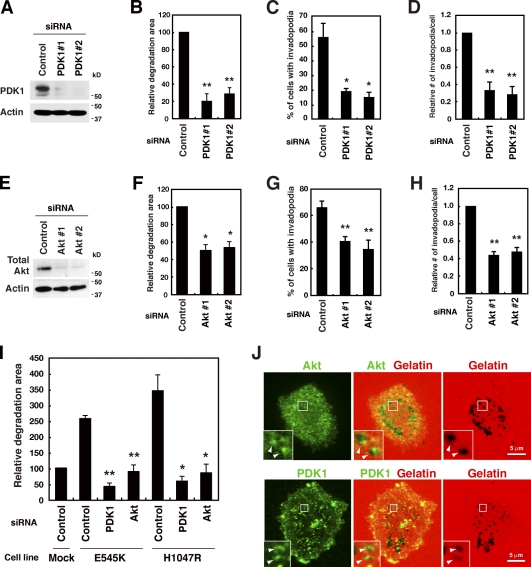
To determine the downstream target of p110α associated with invadopodia formation, the role of PDK1 was examined. PDK1 has been shown to translocate to the plasma membrane upon activation of PI3Ks, and phosphorylate downstream targets, including Akt (Toker and Newton, 2000). PDK1 expression in MDA-MB-231 cells was confirmed by immunoblotting and suppressed by two different siRNA sequences that target different regions of the PDK1 gene (Fig. 5 A). PDK1 down-regulation clearly impaired invadopodia formation in these cells and the related gelatin matrix degradation (Fig. 5, B–D).
Figure 5.
PDK1 and Akt are essential downstream effectors of p110α for invadopodia formation. (A) MDA-MB-231 cells were transfected with control or two distinct PDK1 siRNAs for 48 h and used for immunoblotting to determine the amount of PDK1. (B–D) Cells transfected with the control or PDK1 siRNA were cultured on fluorescent gelatin-coated coverslips for 7 h. Degraded areas on the gelatin matrix (B), the percentage of cells with invadopodia (C), and the number of invadopodia per cell (D) were quantified for transfected cells. (E) Cells were transfected with control or two different sets of siRNAs targeting Akt1, 2, and 3 for 48 h and used for immunoblotting analysis with the anti–pan-Akt antibody. (F–H) Degraded areas on the gelatin matrix (F), the percentage of cells with invadopodia (G), and the number of invadopodia per cell (H) were quantified for siRNA-transfected cells. (I) Cells stably expressing E545K or H1047R p110α were transfected with indicated siRNAs for 48 h and tested for invadopodia activities for 7 h. (J) MDA-MB-231 cells plated onto fluorescent gelatin-coated coverslips for 4 h were stained with the anti-Akt or anti-PDK1 antibody. Insets are magnified images of the boxed regions. Arrowheads denote the accumulation of Akt and PDK1 signals at the gelatin degradation sites. Data are represented as means + SEM of six (B, G, and H), four (C, D, and I), and three (F) independent determinations. *, P < 0.02; and **, P < 0.005 by Student’s t tests.
The role of Akt in invadopodia formation was then examined. The expression of all Akt isoforms (i.e., Akt1, Akt2, and Akt3) was detected in MDA-MB-231 cells by real-time quantitative PCR (Fig. S3 B). To avoid possible functional redundancy, all Akt isoforms were simultaneously knocked down. In cells transfected with two different sets of siRNAs, the expression of total Akt was efficiently suppressed (Fig. 5 E). Akt knockdown significantly decreased invadopodia formation and gelatin degradation (Fig. 5, F–H). Furthermore, knockdown of PDK1 or Akt markedly decreased invadopodia formation in both E545K and H1047R p110α cells (Fig. 5 I). Examination of the localization of endogenous Akt and PDK1 proteins revealed that these proteins accumulated at invadopodia-mediated gelatin degradation sites in MDA-MB-231 cells (Fig. 5 J) and BT549 cells (Fig. S4 J). These results indicate that the role of PDK1 and Akt as downstream targets of p110α is essential for invadopodia formation.
Pharmacological inhibition of PDK1 and Akt blocks invadopodia formation
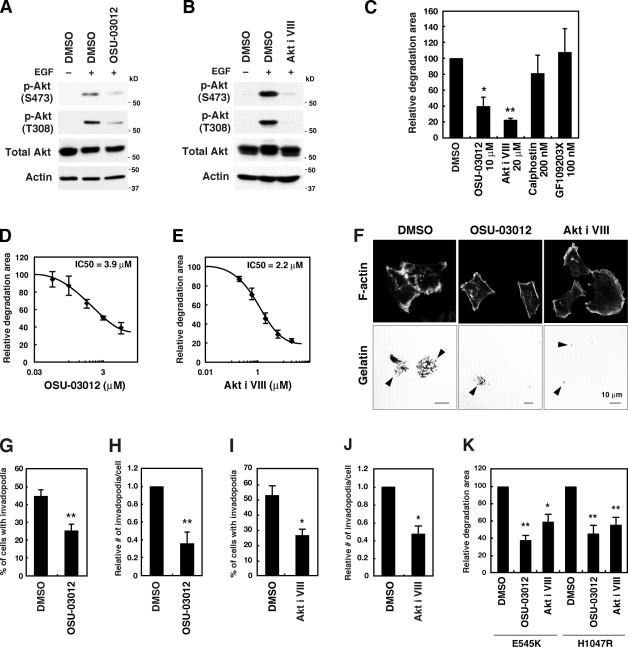
To further confirm the involvement of PDK1 and Akt, cells were treated with OSU-03012 and the Akt inhibitor VIII, which are inhibitors of PDK1 and Akt, respectively. Although its specificity may need better characterization, OSU-03012 was shown to potently inhibit PDK1 activity by competing with ATP (Zhu et al., 2004). The Akt inhibitor VIII is a PH domain–dependent specific Akt inhibitor and blocks activation of Akt (Barnett et al., 2005). Treatment of cells with these inhibitors resulted in a decrease in the levels of phosphorylated Akt (Fig. 6, A and B). These inhibitors markedly blocked gelatin degradation activity (IC50 = 3.9 µM for OSU-03012 and 2.2 µM for Akt inhibitor VIII; Fig. 6, C–F) and invadopodia formation (Fig. 6, G–J). We also examined the effect of a PKC inhibitor on invadopodia formation because PKC is another major substrate of PDK1 (Toker and Newton, 2000). When treated with the broad-range PKC inhibitors calphostin and GF109203X, MDA-MB-231 cells showed no obvious changes in gelatin degradation activity (Fig. 6 C). Moreover, OSU-03012 and the Akt inhibitor VIII significantly blocked gelatin degradation activities of cells expressing the activating mutants of p110α (Fig. 6 K).
Figure 6.
Pharmacological inhibition of PDK1 and Akt blocks invadopodia formation. (A and B) MDA-MB-231 cells were serum-starved overnight and treated with inhibitors, 10 µM OSU-03012 for PDK1 (A) or 20 µM Akt inhibitor VIII (Akt i VIII) for Akt (B) for 1 h. The cells were subsequently stimulated with 8 nM EGF for 10 min and used for immunoblotting to determine the phosphorylation status of Akt (p-Akt). (C) MDA-MB-231 cells were cultured on fluorescent gelatin-coated coverslips for 7 h in the presence of various inhibitors, including OSU-03012, Akt inhibitor VIII, and calphostin, and GF109203X for PKC. The degraded areas on the gelatin matrix were quantified. (D and E) Dose–response curves of gelatin degradation obtained in the presence of increasing concentrations of OSU-03012 (D) or Akt inhibitor VIII (E) are shown. (F) Representative images of MDA-MB-231 cells treated with 10 µM OSU-03012 and 20 µM Akt inhibitor VIII are shown. Arrowheads denote the gelatin degradation sites. (G–J) The percentage of cells with invadopodia (G and I) and the relative number of invadopodia per cell (H and J) were quantified for cells treated with 10 µM OSU-03012 (G and H) or 20 µM Akt inhibitor VIII (I and J). (K) Cells expressing E545K or H1047R p110α were examined for gelatin degradation in the presence of 10 µM OSU-03012 or 20 µM Akt inhibitor VIII. Data are represented as means ± SEM of six (C, I, and J), four (E, G, H, and K), and three (D) independent determinations. *, P < 0.02; and **, P < 0.005 by Student’s t tests.
Overexpression of Akt constructs affects invadopodia formation
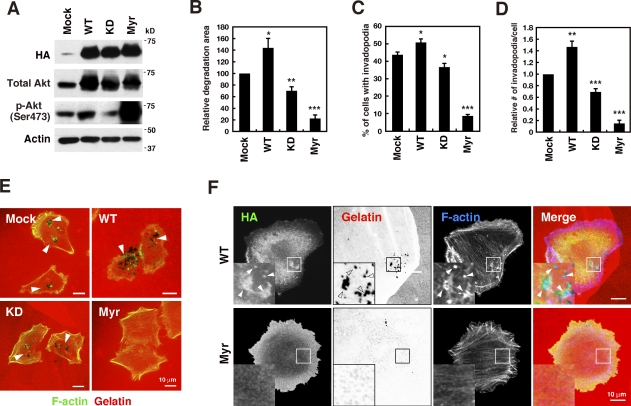
The effect of the ectopic expression of various Akt constructs was examined by generating MDA-MB-231 cell lines stably expressing WT, kinase dead (KD), or a membrane-targeted constitutively active form (myristoylated [Myr]) of Akt1. Akt phosphorylation increased in cells expressing WT Akt1 but decreased in cells expressing KD Akt1 in comparison to control mock-infected cells (Fig. 7 A). Myr Akt1 expression robustly enhanced Akt phosphorylation (Fig. 7 A). Invadopodia formation and gelatin degradation activity were increased in WT Akt1 cells but decreased in KD Akt1 cells, which is consistent with the changes in Akt phosphorylation (Fig. 7, B–E). Unexpectedly, however, cells expressing Myr Akt1 showed a marked decrease in invadopodia formation and gelatin degradation (Fig. 7, B–E). Ectopically expressed WT Akt1 accumulated at invadopodia in a similar manner to endogenous protein (Fig. 7 F). In contrast, Myr Akt1 uniformly distributed throughout the plasma membrane and showed no specific localization (Fig. 7 F). We also generated MDA-MB-231 cell lines expressing other constitutively active forms of Akt1, namely E17K and E40K, which have a higher affinity for phosphoinositides (Aoki et al., 1998; Carpten et al., 2007). Although the expression of these Akt1 mutants markedly increased Akt phosphorylation, it abrogated invadopodia-mediated gelatin degradation activity (Fig. S5, A and B). Collectively, these results confirm the role of Akt in invadopodia formation and suggest that site-specific and proper activation of Akt is necessary for efficient assembly of invadopodia.
Figure 7.
Expression of Akt constructs affects invadopodia formation. (A) MDA-MB-231 cells stably expressing HA-tagged wild-type (WT), kinase-dead (KD), or myristoylated constitutively active (Myr) Akt1 were analyzed by immunoblotting. (B–D) Cells stably expressing the Akt constructs were cultured on fluorescent gelatin-coated coverslips for 7 h and stained for F-actin. Degraded areas on the gelatin matrix (B), the percentage of cells with invadopodia (C), and the number of invadopodia per cell (D) were quantified. Data are represented as means +SEM of six (B) and four to eight (C and D) independent determinations. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 by Student’s t tests. (E) Representative images of cells expressing the Akt constructs. Arrowheads denote the gelatin degradation sites. (F) Cells expressing WT or Myr Akt1 were cultured on fluorescent gelatin matrices for 3 h and stained with anti-HA antibody and phalloidin. Insets are magnified images of the boxed regions. Arrowheads denote localization of the HA signals at invadopodia.
Discussion
In the present study, the PI3K inhibitors LY294002 and wortmannin were shown to effectively inhibit invadopodia formation in MDA-MB-231 human breast cancer cells. This result is consistent with the previous studies describing that the formation of invadopodia in human cancer cells and podosomes in Src transformed fibroblasts requires the activity of PI3K (Nakahara et al., 2003; Mandal et al., 2008; Oikawa et al., 2008).
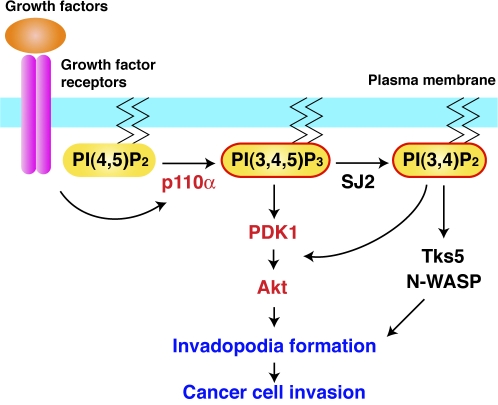
Overexpression of the Akt-PH domain, which sequesters the PI3K products PI(3,4,5)P3 and PI(3,4)P2, effectively blocked invadopodia formation. Although the predominant product of PI3K is PI(3,4,5)P3, several evidence raise the possibility that PI(3,4)P2 also plays a significant and redundant role in invadopodia formation in parallel with PI(3,4,5)P3 (Fig. 8). Chuang et al. (2004) reported that siRNA knockdown of synaptojanin-2, which generates PI(3,4)P2 via dephosphorylation of PI(3,4,5)P3, blocks invadopodia formation in glioma cells. Moreover, Oikawa et al. (2008) reported that PI(3,4)P2 regulates podosome formation by recruiting Tks5 and N-WASP, which are essential components of podosomes. Therefore, although further studies are required to precisely define the individual roles of PI(3,4,5)P3 and PI(3,4)P2, our results indicate that these D3-phosphoinositides produced by PI3K activity play an essential role in invadopodia biogenesis.
Figure 8.
A model of the function of PI3K signaling in invadopodia formation and cell invasion. p110α that is activated downstream of growth factor receptors produces the signaling lipid PI(3,4,5)P3 to regulate invadopodia formation and cancer cell invasion. PI(3,4)P2 that is generated via dephosphorylation of PI(3,4,5)P3 by synaptojanin-2 (SJ2) may regulate invadopodia formation through the Tks5/N-WASP axis in parallel with PI(3,4,5)P3. PDK1 and Akt are activated by both PI(3,4,5)P3 and PI(3,4)P2 and act as mediators of the PI3K signaling pathway for invadopodia formation.
We and other researchers have previously reported that invadopodia formation is initiated with the assembly of actin core structures followed by the accumulation of matrix metalloproteinases for ECM degradation (Yamaguchi et al., 2005a; Artym et al., 2006; Oser et al., 2009). The finding that treatment of cells with PI3K inhibitors blocked the formation of F-actin and cortactin structures of invadopodia suggests that PI3K signaling is involved in the first step of invadopodia formation. In support of this hypothesis, PI3K inhibitors disassembled the F-actin structures of invadopodia, as shown by time-lapse analysis, and that PI3K products were enriched with F-actin at the invadopodia, as detected with the GFP-Akt PH construct. Consistent with these observations, Mandal et al. (2008) recently reported that PI3K is required for the formation of F-actin cores of invadopodia induced by TGF-β stimulation.
An important finding of the present study was that among the PI3K isoforms, the class I PI3K catalytic subunit p110α is specifically involved in invadopodia formation. We showed that pharmacological inhibition of p110α blocked invadopodia-mediated ECM degradation and invasion in human breast cancer cell lines. Several inhibitors that target PI3Ks are currently being tested in clinical trials for the treatment of human cancers (Engelman, 2009). However, these broad-spectrum PI3K inhibitors can cause significant side effects caused by the multiple roles of the PI3K signaling pathway in basic cellular functions. Therefore, current research is extensively focused both on understanding the isoform-specific functions of PI3Ks and on developing isoform-specific inhibitors of the PI3K family proteins (Zhao and Vogt, 2008; Engelman, 2009; Jia et al., 2009).
Recent studies have delineated distinct functions of class I PI3K isoforms (Engelman, 2009; Jia et al., 2009). The p110α subunit was shown to predominantly mediate PI3K signaling activity in receptor tyrosine kinase signal transduction, whereas p110β responds to G protein–coupled receptors (Zhao et al., 2006; Guillermet-Guibert et al., 2008). In addition, it has been reported that immune system function is largely dependent on p110δ and p110γ (Rommel et al., 2007). Moreover, unlike PIK3CA, which encodes p110α, cancer-specific mutations have not been reported for genes encoding other class I PI3Ks (Jia et al., 2009). Based on these findings and the specific role of p110α in invadopodia formation, we hypothesize that p110α is a promising therapeutic target for the treatment of cancer invasion and metastasis with minimal side effects.
The PIK3CA mutations found in human cancers primarily occur at two hot spots: E545K in the helical domain and H1047R in the catalytic domain (Samuels and Ericson, 2006; Zhao and Vogt, 2008). These mutations are known to promote the catalytic activity of p110α, thereby leading to constitutive activation of the PI3K signaling pathway (Kang et al., 2005). We determined that the E545K and H1047R mutations in p110α enhanced invadopodia-mediated ECM degradation and invasion. This finding provides mechanistic insight into the role of p110α mutations in cancer invasion.
Although we clearly showed that basal p110α activity is required for invadopodia formation, mutations of p110α are not sufficient to trigger invadopodia formation. In fact, several breast cancer cell lines that contain p110α mutations, such as MCF-7 and T47D (Hollestelle et al., 2007), are unable to form invadopodia as reported previously (Coopman et al., 1998; Yamaguchi et al., 2009). Therefore, it is likely that activation of other factors and/or signaling pathways trigger invadopodia formation, and the concurrent activation of p110α by mutations may act as a positive modulator in this process. This concept is supported by the fact that activating p110α mutations are preferentially observed in invasive tumors (Saal et al., 2005; Maruyama et al., 2007) and often associated with other alterations, such as ERBB2 overexpression and K-ras mutations (Oda et al., 2008).
In the present study, we demonstrated, for the first time, that PDK1 and Akt are involved in invadopodia formation. Importantly, knockdown and pharmacological inhibition of Akt or PDK1 abolished the enhanced invadopodia formation induced by E545K and H1047R p110α. Previous studies have shown that PDK1 and Akt are overexpressed and/or mutated in various human cancers and have implicated these proteins in cancer invasion and metastasis (Xie et al., 2006; Pinner and Sahai, 2008; Liu et al., 2009; Sheng et al., 2009). Therefore, our findings may provide a further rationale for targeting PDK1 and Akt in addition to p110α in the development of antiinvasion and antimetastasis strategies.
Additional evidence that Akt is required for invadopodia formation was provided by the overexpression of WT and KD forms of Akt. Unexpectedly, however, overexpression of constitutively active forms of Akt markedly blocked invadopodia formation. Because we observed that Akt localized to invadopodia, site-specific and controlled activation of Akt by p110α and PDK1 may be required for proper invadopodia formation and cancer invasion. In agreement with this idea, the constitutively active form of Akt was shown to inhibit the invasion of breast cancer cells both in vitro and in vivo (Hutchinson et al., 2004; Liu et al., 2006). Further studies are necessary to elucidate the exact mechanisms underlying the regulation of invadopodia formation by the p110α–PDK1–Akt pathway.
In conclusion, our results strongly suggest that the PI3K signaling pathway mediated by p110α is a critical regulator of invadopodia-mediated invasion of human breast cancer cells. These findings identified a new cellular function of the well-known oncogene product p110α and provided new insights into the molecular mechanisms of invadopodia formation and cancer cell invasion.
Materials and methods
Cell culture
Human breast cancer cell lines MDA-MB-231, BT-549, and Hs578T were obtained from the American Type Culture Collection. MDA-MB-231 cells were maintained in a 1:1 mixture of high glucose-DME and RPMI 1640 supplemented with 10% FBS, 10 U/ml penicillin, and 10 µg/ml streptomycin. BT-549 and Hs578T cells were maintained in RPMI 1640 and DME, respectively, supplemented as described previously in this paragraph.
Antibodies, reagents, and constructs
Alexa dyes, fluorescently labeled phalloidin, and secondary antibodies were purchased from Invitrogen. LY294002, wortmannin, anti-p110α, anti-p110β, anti-ERK, and anti-Akt antibodies were purchased from Cell Signaling Technology. The anti-p110δ antibody, calphostin, and Akt inhibitor VIII were purchased from EMD. Recombinant human EGF was purchased from Millipore. The anti-HA antibody was purchased from Covance. PIK-75 and IC87114 were purchased from Symansis. TGX-221 was purchased from Cayman Chemical. OSU-03012 was purchased from Echelon Biosciences. GF109203X was purchased from Enzo Life Sciences. The anti–β-actin antibody, gelatin, and other chemicals were purchased from Sigma-Aldrich. For GFP-Akt-PH domain constructs, the cDNA that encoded the mouse Akt-PH domain (1–111 aa) was subcloned into the pEGFP-C1 vector (Takara Bio Inc.). pBabe-puro constructs for HA-tagged WT, E545K, and H1047R forms of p110α were provided by J. Zhao (Harvard Medical School, Boston, MA; Zhao et al., 2005) through Addgene. pLNCX constructs for HA-tagged WT, KD, and constitutively active Myr forms of Akt were provided by W. Sellers (Harvard Medical School, Boston, MA; Ramaswamy et al., 1999) through Addgene. The mutagenesis basal kit (PrimeSTAR; Takara Bio Inc.) and site-directed mutagenesis kit (QuickChange Lightning; Agilent Technologies) were used to generate the Akt-PH domain R25C mutant and Akt1 E17K and E40K mutants.
Plasmid transfection, retroviral infection, lentiviral infection, and generation of stable cell lines
MDA-MB-231 cells were transfected with the indicated plasmids using Lipofectamine 2000 (Invitrogen) or Lipofectamine LTX (Invitrogen) according to the manufacturer’s instructions. To generate stable cell lines, transfected cells were selected with G418 at 1 mg/ml, and resistant clones were isolated. For retroviral infection, cDNAs were inserted into the pMXs-IP or pLNCX vector, and recombinant retroviruses were produced with the Platinum-A packaging cell line as previously described (Kitamura et al., 2003). In brief, Platinum-A cells were transfected with the retroviral constructs using Lipofectamine 2000, and the medium was changed at 1 d after transfection. Culture medium containing recombinant retroviruses was collected at 2 d after transfection and filtered through a 0.45-µm filter. Cells were immediately infected with the recombinant retroviruses in the presence of 5 µg/ml polybrene for 1 d and then selected with 1 µg/ml puromycin or 1 mg/ml G418. Control and p110α shRNA lentiviral particles were purchased from Santa Cruz Biotechnology, Inc. Lentiviral infection was performed according to the manufacturer’s instructions, and infected cells were selected with 1 µg/ml puromycin.
Immunofluorescence analysis
Cells were fixed in 4% paraformaldehyde for 15 min and permeabilized with 0.1% Triton X-100 for 5 min. To detect the localization of GFP-Akt PH construct and PDK1, cells were fixed and permeabilized in 4% paraformaldehyde, 0.1% glutaraldehyde, and 0.075 mg/ml saponin for 1 h at 37°C. The cells were blocked in 1% BSA and 1% goat serum for 30 min. The cells were incubated with primary antibodies for 1 h and then with fluorophore-conjugated secondary antibodies and phalloidin (Invitrogen) for 30 min. Samples were observed with a confocal microscope (IX81-ZDC-DSU; Olympus) equipped with a cooled charge-coupled device camera (ORCA-ER; Hamamatsu Photonics), and the imaging system was driven by MetaMorph software (Universal Imaging). All images were acquired using 60× (PLAPON60×O; NA 1.42) or 100× (UPLSAPO100×O; NA 1.4) oil objectives. Images were analyzed and processed with various software packages, including MetaMorph, ImageJ (version 1.41o; National Institutes of Health), and Photoshop (CS4; Adobe).
Time-lapse microscopic analysis
In brief, time-lapse series of cells were taken at 37°C using the aforementioned microscope (IX81-ZDC-DSU) equipped with a humidified CO2 chamber. Digital images were converted in ImageJ 1.41o, and the fluorescence intensity of GFP-actin at the invadopodia was calculated.
RNAi
All RNAi experiments were performed using Stealth RNAi molecules (Invitrogen). The Stealth RNAi molecules used in this study are shown in Table S1. Cells were transfected with 30 nM siRNA using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer’s instructions. The cells were cultured for 48–72 h and used for invadopodia formation assay and other assays.
RT-PCR
Total RNA was isolated with an RNeasy Plus Mini kit (QIAGEN). Template cDNAs were synthesized with SuperScript III (Invitrogen). Quantitative RT-PCR was performed with a quantitative PCR mix (THUNDERBIRD; TOYOBO) in a real-time PCR detection system (CFX96; Bio-Rad Laboratories). For standard PCR amplification, DNA polymerase (KOD-plus; TOYOBO) and PCR beads (puReTaq Ready-To-Go; GE Healthcare) were used. The sequences of primer pairs used in this study are shown in Table S2.
Immunoblotting
Cells were washed with ice-cold PBS twice before direct extraction in SDS-PAGE sample buffer or lysis in a buffer containing 25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS, and a protease inhibitor cocktail (Roche). The samples were resolved by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and blocked with 5% nonfat dried milk. The membranes were incubated first with primary antibodies for 1 h and then with peroxidase-conjugated secondary antibodies for 30 min. The antibodies were diluted in immunoreaction enhancer solution (Can Get Signal; TOYOBO). Immunoreactive bands were detected using an ECL-plus kit (GE Healthcare).
Invadopodia assay
Fluorescent matrix-coated dishes were prepared as previously described (Bowden et al., 2001). Gelatin was labeled with TRITC in a buffer containing 40 mM NaCl and 50 mM Na2B4O7, pH 9.3, and unbound dyes were removed by extensive dialysis against PBS at 37°C. 12-mm circular coverslips were coated with 100 µl of 25-mg/ml fluorescent gelatin and 20-mg/ml sucrose in PBS and then cross-linked with 0.5% glutaraldehyde on ice for 15 min followed by 30 min at room temperature. After extensive washing with PBS, the coverslips were treated with 5 mg/ml sodium borohydrate for 5 min to quench autofluorescence of residual glutaraldehyde. The coverslips were then sterilized with 70% ethanol for 15 min. MDA-MB-231 cells were cultured on the gelatin-coated coverslips for 3–7 h. To quantitate the gelatin degradation activity of invadopodia, we calculated the degradation area observed in images with the ImageJ 1.41o software and normalized the measurements to the total number of cells in each image. 10 randomly selected fields, usually containing 30–50 cells in total, were imaged with a 60× objective and analyzed for each experiment. The values of control cells were set to 100%, and the relative values of other cells were then calculated accordingly. The relative number of invadopodia and the percentage of cells with invadopodia were also calculated from the microscopy images.
EGF stimulation
Cells were serum-starved overnight in medium containing 0.35% BSA and stimulated with 8 nM EGF for 10 min at 37°C. The cells were subsequently washed twice with ice-cold PBS and lysed with a lysis buffer containing 50 mM Tris-HCl, pH 7.5, 1% NP-40, 2 mM EDTA, 100 mM NaCl, 1 mM sodium orthovanadate, and a protease inhibitor cocktail. The lysates were separated from cell debris by centrifugation and used for immunoblotting.
Invasion assay
Matrigel invasion assay was performed with a tumor invasion system (BioCoat: BD) composed of 24-multiwell inserts plates (8-µm pore size; Falcon FluoroBlok; BD) coated with Matrigel matrix (BD). The insert wells were rehydrated with 500 µl PBS for 2 h at 37°C. Cells were labeled with green 5-chloromethylfluorescein diacetate (CellTracker; Invitrogen) and resuspended at 1 × 105/ml in serum-free medium. 500 µl of the labeled cell suspension was added to the upper chambers, and 750 µl of growth medium containing 10% FBS was added to the lower chambers as a chemoattractant. After 24 h of incubation at 37°C, cells that invaded onto the lower surface of the filters were directly imaged with a confocal microscope (IX81-ZDC-DSU) using a 10× objective (UPLFLN 10×2PH; NA 0.3). Invaded cells were counted in five randomly selected fields per filter, the mean number of control cells was set to 100%, and the relative values of other cells were then calculated in each experiment.
Statistical analysis
Data are representative of at least three independent experiments. Statistical analysis was performed using Student’s t tests. All p-values shown are versus control cells.
Online supplemental material
Fig. S1 shows the effects of PI3K inhibitors LY294002 and Wortmannin on invadopodia formation. Fig. S2 shows the expression levels of GFP-Akt-PH and the localization of GFP in MDA-MB-231 cells. Fig. S3 shows RT-PCR analysis of the expression of PI3K and Akt isoforms in MDA-MB-231 cells. Fig. S4 shows the effects of p110α knockdown and PIK-75 treatment on invadopodia formation and localization of PDK1 and Akt at invadopodia in human breast cancer cell lines. Fig. S5 shows the effects of the expression of E17K and E40K Akt1 constructs on invadopodia formation. Table S1 and Table S2 show siRNA and primer sequences, respectively, used in this study. Video 1 and Video 2 show disassembly of invadopodia by LY294002 treatment in MDA-MB-231 cells expressing GFP-actin and Venus-cortactin, respectively. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201009126/DC1.
Acknowledgments
We are grateful to Drs. J. Zhao and W. Sellers for kindly providing plasmids. We thank Yumiko Konko and Keiko Takayama for their technical assistance.
This work was supported by Grants-in-Aid for Scientific Research (B), for Young Scientists (B), and for Cancer Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by a Grant-in-Aid from the Ministry of Health, Labor and Welfare of Japan for the third term Comprehensive 10-Year Strategy for Cancer Control. This work was also supported in part by the Mochida Memorial Foundation for Medical and Pharmaceutical Research, the Ono Medical Research Foundation, and the Takeda Science Foundation.
Footnotes
Abbreviations used in this paper:
- KD
- kinase dead
- Myr
- myristoylated
- PDK1
- 3-phosphoinositide-dependent protein kinase-1
- PH
- pleckstrin homology
- PI(3,4)P2
- phosphatidylinositol 3,4-bisphosphate
- PI(3,4,5)P3
- phosphatidylinositol 3,4,5-trisphosphate
- PI3K
- phosphoinositide 3-kinase
- shRNA
- short hairpin RNA
- WT
- wild type
References
- Aoki M., Batista O., Bellacosa A., Tsichlis P., Vogt P.K. 1998. The akt kinase: molecular determinants of oncogenicity. Proc. Natl. Acad. Sci. USA. 95:14950–14955 10.1073/pnas.95.25.14950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artym V.V., Zhang Y., Seillier-Moiseiwitsch F., Yamada K.M., Mueller S.C. 2006. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 66:3034–3043 10.1158/0008-5472.CAN-05-2177 [DOI] [PubMed] [Google Scholar]
- Barnett S.F., Defeo-Jones D., Fu S., Hancock P.J., Haskell K.M., Jones R.E., Kahana J.A., Kral A.M., Leander K., Lee L.L., et al. 2005. Identification and characterization of pleckstrin-homology-domain-dependent and isoenzyme-specific Akt inhibitors. Biochem. J. 385:399–408 10.1042/BJ20041140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden E.T., Coopman P.J., Mueller S.C. 2001. Invadopodia: unique methods for measurement of extracellular matrix degradation in vitro. Methods Cell Biol. 63:613–627 10.1016/S0091-679X(01)63033-4 [DOI] [PubMed] [Google Scholar]
- Buccione R., Caldieri G., Ayala I. 2009. Invadopodia: specialized tumor cell structures for the focal degradation of the extracellular matrix. Cancer Metastasis Rev. 28:137–149 10.1007/s10555-008-9176-1 [DOI] [PubMed] [Google Scholar]
- Cain R.J., Ridley A.J. 2009. Phosphoinositide 3-kinases in cell migration. Biol. Cell. 101:13–29 10.1042/BC20080079 [DOI] [PubMed] [Google Scholar]
- Caldieri G., Buccione R. 2010. Aiming for invadopodia: organizing polarized delivery at sites of invasion. Trends Cell Biol. 20:64–70 10.1016/j.tcb.2009.10.006 [DOI] [PubMed] [Google Scholar]
- Cantley L.C. 2002. The phosphoinositide 3-kinase pathway. Science. 296:1655–1657 10.1126/science.296.5573.1655 [DOI] [PubMed] [Google Scholar]
- Carpten J.D., Faber A.L., Horn C., Donoho G.P., Briggs S.L., Robbins C.M., Hostetter G., Boguslawski S., Moses T.Y., Savage S., et al. 2007. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 448:439–444 10.1038/nature05933 [DOI] [PubMed] [Google Scholar]
- Chaussade C., Rewcastle G.W., Kendall J.D., Denny W.A., Cho K., Grønning L.M., Chong M.L., Anagnostou S.H., Jackson S.P., Daniele N., Shepherd P.R. 2007. Evidence for functional redundancy of class IA PI3K isoforms in insulin signalling. Biochem. J. 404:449–458 10.1042/BJ20070003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.T. 1989. Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. J. Exp. Zool. 251:167–185 10.1002/jez.1402510206 [DOI] [PubMed] [Google Scholar]
- Chen W.T., Lee C.C., Goldstein L., Bernier S., Liu C.H., Lin C.Y., Yeh Y., Monsky W.L., Kelly T., Dai M., et al. 1994. Membrane proteases as potential diagnostic and therapeutic targets for breast malignancy. Breast Cancer Res. Treat. 31:217–226 10.1007/BF00666155 [DOI] [PubMed] [Google Scholar]
- Chuang Y.Y., Tran N.L., Rusk N., Nakada M., Berens M.E., Symons M. 2004. Role of synaptojanin 2 in glioma cell migration and invasion. Cancer Res. 64:8271–8275 10.1158/0008-5472.CAN-04-2097 [DOI] [PubMed] [Google Scholar]
- Clark E.S., Whigham A.S., Yarbrough W.G., Weaver A.M. 2007. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 67:4227–4235 10.1158/0008-5472.CAN-06-3928 [DOI] [PubMed] [Google Scholar]
- Condeelis J., Segall J.E. 2003. Intravital imaging of cell movement in tumours. Nat. Rev. Cancer. 3:921–930 10.1038/nrc1231 [DOI] [PubMed] [Google Scholar]
- Coopman P.J., Do M.T., Thompson E.W., Mueller S.C. 1998. Phagocytosis of cross-linked gelatin matrix by human breast carcinoma cells correlates with their invasive capacity. Clin. Cancer Res. 4:507–515 [PubMed] [Google Scholar]
- Dillon R.L., White D.E., Muller W.J. 2007. The phosphatidyl inositol 3-kinase signaling network: implications for human breast cancer. Oncogene. 26:1338–1345 10.1038/sj.onc.1210202 [DOI] [PubMed] [Google Scholar]
- Eckert M.A., Lwin T.M., Chang A.T., Kim J., Danis E., Ohno-Machado L., Yang J. 2011. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell. 19:372–386 10.1016/j.ccr.2011.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman J.A. 2009. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat. Rev. Cancer. 9:550–562 10.1038/nrc2664 [DOI] [PubMed] [Google Scholar]
- Engelman J.A., Luo J., Cantley L.C. 2006. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 7:606–619 10.1038/nrg1879 [DOI] [PubMed] [Google Scholar]
- Fruman D.A., Meyers R.E., Cantley L.C. 1998. Phosphoinositide kinases. Annu. Rev. Biochem. 67:481–507 10.1146/annurev.biochem.67.1.481 [DOI] [PubMed] [Google Scholar]
- Gimona M., Buccione R., Courtneidge S.A., Linder S. 2008. Assembly and biological role of podosomes and invadopodia. Curr. Opin. Cell Biol. 20:235–241 10.1016/j.ceb.2008.01.005 [DOI] [PubMed] [Google Scholar]
- Guillermet-Guibert J., Bjorklof K., Salpekar A., Gonella C., Ramadani F., Bilancio A., Meek S., Smith A.J., Okkenhaug K., Vanhaesebroeck B. 2008. The p110beta isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110gamma. Proc. Natl. Acad. Sci. USA. 105:8292–8297 10.1073/pnas.0707761105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollestelle A., Elstrodt F., Nagel J.H., Kallemeijn W.W., Schutte M. 2007. Phosphatidylinositol-3-OH kinase or RAS pathway mutations in human breast cancer cell lines. Mol. Cancer Res. 5:195–201 10.1158/1541-7786.MCR-06-0263 [DOI] [PubMed] [Google Scholar]
- Hutchinson J.N., Jin J., Cardiff R.D., Woodgett J.R., Muller W.J. 2004. Activation of Akt-1 (PKB-alpha) can accelerate ErbB-2-mediated mammary tumorigenesis but suppresses tumor invasion. Cancer Res. 64:3171–3178 10.1158/0008-5472.CAN-03-3465 [DOI] [PubMed] [Google Scholar]
- Isakoff S.J., Engelman J.A., Irie H.Y., Luo J., Brachmann S.M., Pearline R.V., Cantley L.C., Brugge J.S. 2005. Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer Res. 65:10992–11000 10.1158/0008-5472.CAN-05-2612 [DOI] [PubMed] [Google Scholar]
- Jia S., Roberts T.M., Zhao J.J. 2009. Should individual PI3 kinase isoforms be targeted in cancer? Curr. Opin. Cell Biol. 21:199–208 10.1016/j.ceb.2008.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S., Bader A.G., Vogt P.K. 2005. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc. Natl. Acad. Sci. USA. 102:802–807 10.1073/pnas.0408864102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessenbrock K., Plaks V., Werb Z. 2010. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 141:52–67 10.1016/j.cell.2010.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T., Koshino Y., Shibata F., Oki T., Nakajima H., Nosaka T., Kumagai H. 2003. Retrovirus-mediated gene transfer and expression cloning: powerful tools in functional genomics. Exp. Hematol. 31:1007–1014 [PubMed] [Google Scholar]
- Knight Z.A., Gonzalez B., Feldman M.E., Zunder E.R., Goldenberg D.D., Williams O., Loewith R., Stokoe D., Balla A., Toth B., et al. 2006. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 125:733–747 10.1016/j.cell.2006.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.Y., Rong M., Grieu F., Iacopetta B. 2006. PIK3CA mutations in breast cancer are associated with poor outcome. Breast Cancer Res. Treat. 96:91–95 10.1007/s10549-005-9048-0 [DOI] [PubMed] [Google Scholar]
- Linder S. 2007. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 17:107–117 10.1016/j.tcb.2007.01.002 [DOI] [PubMed] [Google Scholar]
- Liu H., Radisky D.C., Nelson C.M., Zhang H., Fata J.E., Roth R.A., Bissell M.J. 2006. Mechanism of Akt1 inhibition of breast cancer cell invasion reveals a protumorigenic role for TSC2. Proc. Natl. Acad. Sci. USA. 103:4134–4139 10.1073/pnas.0511342103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wang J., Wu M., Wan W., Sun R., Yang D., Sun X., Ma D., Ying G., Zhang N. 2009. Down-regulation of 3-phosphoinositide-dependent protein kinase-1 levels inhibits migration and experimental metastasis of human breast cancer cells. Mol. Cancer Res. 7:944–954 10.1158/1541-7786.MCR-08-0368 [DOI] [PubMed] [Google Scholar]
- Madsen C.D., Sahai E. 2010. Cancer dissemination—lessons from leukocytes. Dev. Cell. 19:13–26 10.1016/j.devcel.2010.06.013 [DOI] [PubMed] [Google Scholar]
- Mandal S., Johnson K.R., Wheelock M.J. 2008. TGF-beta induces formation of F-actin cores and matrix degradation in human breast cancer cells via distinct signaling pathways. Exp. Cell Res. 314:3478–3493 10.1016/j.yexcr.2008.09.013 [DOI] [PubMed] [Google Scholar]
- Maruyama N., Miyoshi Y., Taguchi T., Tamaki Y., Monden M., Noguchi S. 2007. Clinicopathologic analysis of breast cancers with PIK3CA mutations in Japanese women. Clin. Cancer Res. 13:408–414 10.1158/1078-0432.CCR-06-0267 [DOI] [PubMed] [Google Scholar]
- Maurer M., Su T., Saal L.H., Koujak S., Hopkins B.D., Barkley C.R., Wu J., Nandula S., Dutta B., Xie Y., et al. 2009. 3-Phosphoinositide-dependent kinase 1 potentiates upstream lesions on the phosphatidylinositol 3-kinase pathway in breast carcinoma. Cancer Res. 69:6299–6306 10.1158/0008-5472.CAN-09-0820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara H., Otani T., Sasaki T., Miura Y., Takai Y., Kogo M. 2003. Involvement of Cdc42 and Rac small G proteins in invadopodia formation of RPMI7951 cells. Genes Cells. 8:1019–1027 10.1111/j.1365-2443.2003.00695.x [DOI] [PubMed] [Google Scholar]
- Neve R.M., Chin K., Fridlyand J., Yeh J., Baehner F.L., Fevr T., Clark L., Bayani N., Coppe J.P., Tong F., et al. 2006. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 10:515–527 10.1016/j.ccr.2006.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda K., Okada J., Timmerman L., Rodriguez-Viciana P., Stokoe D., Shoji K., Taketani Y., Kuramoto H., Knight Z.A., Shokat K.M., McCormick F. 2008. PIK3CA cooperates with other phosphatidylinositol 3′-kinase pathway mutations to effect oncogenic transformation. Cancer Res. 68:8127–8136 10.1158/0008-5472.CAN-08-0755 [DOI] [PubMed] [Google Scholar]
- Oikawa T., Itoh T., Takenawa T. 2008. Sequential signals toward podosome formation in NIH-src cells. J. Cell Biol. 182:157–169 10.1083/jcb.200801042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oser M., Yamaguchi H., Mader C.C., Bravo-Cordero J.J., Arias M., Chen X., Desmarais V., van Rheenen J., Koleske A.J., Condeelis J. 2009. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J. Cell Biol. 186:571–587 10.1083/jcb.200812176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang H., Flinn R., Patsialou A., Wyckoff J., Roussos E.T., Wu H., Pozzuto M., Goswami S., Condeelis J.S., Bresnick A.R., et al. 2009. Differential enhancement of breast cancer cell motility and metastasis by helical and kinase domain mutations of class IA phosphoinositide 3-kinase. Cancer Res. 69:8868–8876 10.1158/0008-5472.CAN-09-1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinner S., Sahai E. 2008. PDK1 regulates cancer cell motility by antagonising inhibition of ROCK1 by RhoE. Nat. Cell Biol. 10:127–137 10.1038/ncb1675 [DOI] [PubMed] [Google Scholar]
- Ramaswamy S., Nakamura N., Vazquez F., Batt D.B., Perera S., Roberts T.M., Sellers W.R. 1999. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc. Natl. Acad. Sci. USA. 96:2110–2115 10.1073/pnas.96.5.2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommel C., Camps M., Ji H. 2007. PI3K delta and PI3K gamma: partners in crime in inflammation in rheumatoid arthritis and beyond? Nat. Rev. Immunol. 7:191–201 10.1038/nri2036 [DOI] [PubMed] [Google Scholar]
- Saal L.H., Holm K., Maurer M., Memeo L., Su T., Wang X., Yu J.S., Malmström P.O., Mansukhani M., Enoksson J., et al. 2005. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 65:2554–2559 10.1158/0008-5472-CAN-04-3913 [DOI] [PubMed] [Google Scholar]
- Samuels Y., Ericson K. 2006. Oncogenic PI3K and its role in cancer. Curr. Opin. Oncol. 18:77–82 10.1097/01.cco.0000198021.99347.b9 [DOI] [PubMed] [Google Scholar]
- Schoumacher M., Goldman R.D., Louvard D., Vignjevic D.M. 2010. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J. Cell Biol. 189:541–556 10.1083/jcb.200909113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng S., Qiao M., Pardee A.B. 2009. Metastasis and AKT activation. J. Cell. Physiol. 218:451–454 10.1002/jcp.21616 [DOI] [PubMed] [Google Scholar]
- Stephens L., Anderson K., Stokoe D., Erdjument-Bromage H., Painter G.F., Holmes A.B., Gaffney P.R., Reese C.B., McCormick F., Tempst P., et al. 1998. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 279:710–714 10.1126/science.279.5351.710 [DOI] [PubMed] [Google Scholar]
- Stylli S.S., Kaye A.H., Lock P. 2008. Invadopodia: at the cutting edge of tumour invasion. J. Clin. Neurosci. 15:725–737 10.1016/j.jocn.2008.03.003 [DOI] [PubMed] [Google Scholar]
- Tague S.E., Muralidharan V., D’Souza-Schorey C. 2004. ADP-ribosylation factor 6 regulates tumor cell invasion through the activation of the MEK/ERK signaling pathway. Proc. Natl. Acad. Sci. USA. 101:9671–9676 10.1073/pnas.0403531101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker A., Newton A.C. 2000. Cellular signaling: pivoting around PDK-1. Cell. 103:185–188 10.1016/S0092-8674(00)00110-0 [DOI] [PubMed] [Google Scholar]
- Várnai P., Bondeva T., Tamás P., Tóth B., Buday L., Hunyady L., Balla T. 2005. Selective cellular effects of overexpressed pleckstrin-homology domains that recognize PtdIns(3,4,5)P3 suggest their interaction with protein binding partners. J. Cell Sci. 118:4879–4888 10.1242/jcs.02606 [DOI] [PubMed] [Google Scholar]
- Weaver A.M. 2006. Invadopodia: specialized cell structures for cancer invasion. Clin. Exp. Metastasis. 23:97–105 10.1007/s10585-006-9014-1 [DOI] [PubMed] [Google Scholar]
- Xie Z., Yuan H., Yin Y., Zeng X., Bai R., Glazer R.I. 2006. 3-phosphoinositide-dependent protein kinase-1 (PDK1) promotes invasion and activation of matrix metalloproteinases. BMC Cancer. 6:77 10.1186/1471-2407-6-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H., Lorenz M., Kempiak S., Sarmiento C., Coniglio S., Symons M., Segall J., Eddy R., Miki H., Takenawa T., Condeelis J. 2005a. Molecular mechanisms of invadopodium formation: the role of the N-WASP–Arp2/3 complex pathway and cofilin. J. Cell Biol. 168:441–452 10.1083/jcb.200407076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H., Wyckoff J., Condeelis J. 2005b. Cell migration in tumors. Curr. Opin. Cell Biol. 17:559–564 10.1016/j.ceb.2005.08.002 [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Takeo Y., Yoshida S., Kouchi Z., Nakamura Y., Fukami K. 2009. Lipid rafts and caveolin-1 are required for invadopodia formation and extracellular matrix degradation by human breast cancer cells. Cancer Res. 69:8594–8602 10.1158/0008-5472.CAN-09-2305 [DOI] [PubMed] [Google Scholar]
- Yuan T.L., Cantley L.C. 2008. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 27:5497–5510 10.1038/onc.2008.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J.J., Liu Z., Wang L., Shin E., Loda M.F., Roberts T.M. 2005. The oncogenic properties of mutant p110alpha and p110beta phosphatidylinositol 3-kinases in human mammary epithelial cells. Proc. Natl. Acad. Sci. USA. 102:18443–18448 10.1073/pnas.0508988102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J.J., Cheng H., Jia S., Wang L., Gjoerup O.V., Mikami A., Roberts T.M. 2006. The p110alpha isoform of PI3K is essential for proper growth factor signaling and oncogenic transformation. Proc. Natl. Acad. Sci. USA. 103:16296–16300 10.1073/pnas.0607899103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Vogt P.K. 2008. Class I PI3K in oncogenic cellular transformation. Oncogene. 27:5486–5496 10.1038/onc.2008.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Huang J.W., Tseng P.H., Yang Y.T., Fowble J., Shiau C.W., Shaw Y.J., Kulp S.K., Chen C.S. 2004. From the cyclooxygenase-2 inhibitor celecoxib to a novel class of 3-phosphoinositide-dependent protein kinase-1 inhibitors. Cancer Res. 64:4309–4318 10.1158/0008-5472.CAN-03-4063 [DOI] [PubMed] [Google Scholar]