Abstract
Chlamydial delayed-type hypersensitivity antigens were analyzed by using the subcutaneous salpingeal autotransplant model of Macaca nemestrina infected with Chlamydia trachomatis serovar E. Heat shock protein 60 was the only antigen shown to induce delayed-type hypersensitivity among other antigens tested, including UV-inactivated organisms, recombinant major outer membrane protein, purified outer membrane proteins, and heat shock protein 10.
Chlamydia trachomatis infection continues to be one of the most important sexually transmitted diseases in the world and one of the most common sexually transmitted diseases in the United States. In the majority of cases, chlamydial infections are mild and self-limiting. However, in a subset of women, the extension of a cervical infection into the upper genital tract may induce pelvic inflammatory disease and salpingitis, which may lead to fallopian tube obstruction and infertility. The pathogenesis leading to tubal obstruction has not been defined. A genetic predisposition to pelvic inflammatory disease in women and in the macaque model has been noted (3, 9). However, clinical and animal studies have shown that the induction of a delayed-type hypersensitivity (DTH) reaction to C. trachomatis antigens leading to repeated and persistent infection of the upper reproductive tract plays an important role in tubal obstruction (10, 16). One of the chlamydial antigens which have been implicated in this immunopathogenesis is heat shock protein 60 (HSP60) (4, 13, 14, 17). However, whether other chlamydial antigens are involved in the immunopathogenesis of tubal obstruction has not been analyzed systematically. Therefore, in this study we utilized the subcutaneous salpingeal autotransplant (“pocket”) macaque model to analyze whether any chlamydial antigens other than HSP60 may induce DTH. The pocket model allows us to test multiple antigens in a single animal.
(Part of this research was presented at Chlamydia 2002, Helsinki, Finland, 20 to 23 August 2002 [A. B. Lichtenwalner, D. L. Patton, W. C. Van Voorhis, Y. T. Cosgrove Sweeney, and C.-C. Kuo, Proc. 4th Meet. Eur. Soc. Chlamydia Res., p. 197-198].)
Sexually mature female pigtailed macaques (Macaca nemestrina) were used. Animals were housed at the University of Washington National Primate Research Center. The animal use protocol for this study was approved by the Animal Care Committee at the University of Washington.
The autotransplantation of salpingeal fimbrial tissue into subcutaneous abdominal “pockets” at multiple sites has been described previously (11, 12). Two weeks after the surgery, baseline histologic data were obtained by excising transplant tissue from each animal for histologic evaluation by standard hematoxylin and eosin staining. The remaining pockets were then inoculated with 105 inclusion-forming units of C. trachomatis serovar E in 50 μl of the chlamydial transport medium SPG, a phosphate buffer containing sucrose and glutamic acid (sucrose, 75 g; KH2PO4, 0.52 g; Na2HPO4, 1.22 g; glutamic acid, 0.72 g; H2O to 1 liter [pH 7.4 to 7.6]). At 21 days postinoculation, two pockets were removed from each animal for the evaluation of baseline inflammatory reactions. One monkey was used for testing the dose response, and the remaining three monkeys were used for testing variously treated chlamydial antigens. The chlamydial antigens tested were UV-inactivated whole organisms, recombinant chlamydial HSP60 (rcHSP60), rcHSP10, major outer membrane protein (MOMP), and outer membrane complex protein (OMP). Control antigens used were recombinant glutathione S-transferase (rGST), which was used for cloning recombinant chlamydial antigens, and SPG. Fifty micrograms of antigen in 50 μl was inoculated into each pocket. Groups of two to six pockets per animal were randomly assigned to each test antigen. Pocket tissues were removed 48 h after inoculation for histologic examination. All surgical procedures were conducted while animals were under general anesthesia with ketamine and atropine.
The test antigens were prepared as follows. (i) To obtain purified chlamydial organisms, C. trachomatis serovar E (E/UW-5/Cx) elementary bodies (EBs) were grown in HeLa 229 cells and purified by density gradient centrifugation with diatrizoate meglumin (Hypaque-76; Winthrop-Breon Laboratories, New York, N.Y.) (6). (ii) To obtain inactivated organisms, chlamydial EBs were inactivated by UV irradiation (7). (iii) To obtain chlamydial outer membrane proteins, chlamydial proteins were fractionated into Sarkosyl-insoluble and -soluble fractions according to the method of Caldwell et al. (2). The Sarkosyl-insoluble fraction (MOMP) contains chlamydial outer membrane complexes, and the Sarkosyl-soluble fraction (OMP) contains the majority of the other chlamydial EB proteins, including HSP10 and HSP60. (iv) To obtain recombinant proteins, affinity-purified rcHSP60 (8) was obtained from R. Morrison and rcHSP10 was obtained from G. Bryne (8). rGST from Schistosoma japonicum was used to express rcHSP. All proteins were tested and found to be free of endotoxins (sensitivity, 0.1 endotoxin unit).
Swab samples were obtained for culture and ligase chain reaction for the detection of chlamydial infection from the removed tissues, which were subsequently placed in 10% formalin and processed for histologic examination. Fixed tissues were embedded in paraffin, thin sectioned, and stained with hematoxylin and eosin for light microscopic examination. T lymphocytes were identified by staining with a pan-T-cell stain, and polymorphonuclear leukocytes (were identified by a myeloperoxidase stain. Cells infiltrating the submucosa were counted in five randomly chosen high-power fields (×400 magnification), and the average number of cells was calculated. The observer was blind as to the tissue origins. Data were analyzed by analysis of variance (ANOVA) followed by Tukey's multiple-comparison test. P values of 0.05 or less were considered significant.
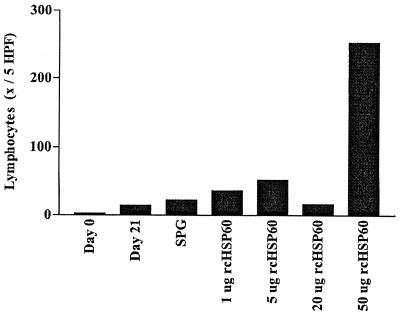
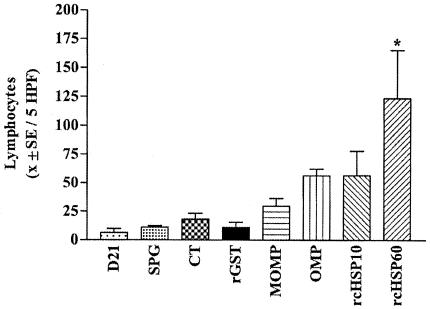
Histologic examination of uninoculated pocket tissues showed no inflammation. A mild inflammation was observed 21 days after inoculation with live chlamydial organisms and before testing for DTH reactions (11). The dose-response experiment, involving a series of rcHSP60 concentrations (0, 5, 20, and 50 μg), showed a dose-dependent lymphocytic response (Fig. 1) characteristic of DTH at 48 h (11). The maximum reaction was observed at the 50-μg concentration. Tests with various antigens showed that only rcHSP60 elicited a significant lymphocytic reaction (P < 0.05, ANOVA and Tukey's multiple-comparison test) (Fig. 2). Mild lymphocytic reactions to MOMP, OMP, and rcHSP10 were also observed. However, the differences from controls were not statistically significant (Fig. 2). No plasma cell or polymorphonuclear leukocyte cell response was observed. No inflammatory reaction was observed in the tissues tested with control antigens. The OMP fraction contains chlamydial HSP60 (cHSP60) in addition to other proteins. In this protein fraction, the concentration of cHSP60 may not be high enough to induce a significant DTH reaction. The weak response of the whole EBs, which should contain cHSP60 in the chlamydial envelope, may be due to a proportionally smaller amount of cHSP60 relative to the total amount of protein injected into the pocket.
FIG. 1.
Lymphocyte response to rcHSP60 at 48 h. Shown are lymphocyte counts at days 0 and 21 and at 48 h following challenge inoculation with the SPG control or increasing doses of rcHSP60 (one female macaque was tested; values are averages [x] for four pockets per test antigen). The response was predominantly lymphocytic and nonplasmocytic, typical of the DTH response to Chlamydia seen in this model. No dose responses were considered to occur in either plasma cells or neutrophils (data not presented). HPF, high-power fields.
FIG. 2.
Lymphocyte response at 48 h after challenge with various chlamydial proteins. Shown are lymphocyte counts at day 21 and at 48 h following challenge inoculation with SPG, killed chlamydiae (CT) or rGST (controls), or 50 μg of chlamydial antigens (three female macaques were tested; values are averages [x] for two to four pockets per treatment). *, P ≤ 0.05 (ANOVA and Tukey's multiple-comparison test). Only for rcHSP60 was the lymphocyte response significantly different from those for the controls or for the chlamydial-protein preparations. HPF, high-power fields.
One of the limitations of this study was that the maximum systemic DTH response may not be induced by the infection of autotransplanted salpingeal tissues in the subcutaneous sites. Therefore, weak DTH reactions induced by testing with antigens other than cHSP60 may not be detected. Nevertheless, this study demonstrated that cHSP60 is the major antigen responsible for the DTH reaction in C. trachomatis salpingeal infection. Similar findings have been reported by Taylor et al. (15) for the ocular trachoma model in monkeys, for which these authors showed that formalin or UV-inactivated purified C. trachomatis EBs did not elicit DTH and neither did purified chlamydial MOMP or LPS. However, a soluble fraction of the triton extract of the organisms, known to contain cHSP60, did induce a DTH reaction.
The molecular mechanism of the DTH response to cHSP60 is of current interest. cHSP60 of C. pneumoniae has been shown to interact with Toll-like receptors, triggering innate immunity (1). Whether Toll-like receptors are involved in the immunopathogenesis of C. trachomatis genital tract infections in the pigtailed macaque has not been investigated. Such studies may further our understanding of immunopathogenesis in this model.
In conclusion, this study demonstrated that cHSP-60 does elicit DTH in the experimental salpingeal model used and supports clinical observations indicating a role for cHSP60 in the immunopathogenesis of tubal damage (4, 17). This pathogenic mechanism is similar to that involved in ocular trachoma disease in experimental monkeys (5). This hypothesis should be further tested in an animal experiment to see whether animals sensitized with cHSP60 and infected with C. trachomatis in the salpinx develop severe salpingitis and tubal damage.
Acknowledgments
This work was supported by U.S. Public Health Service grant AI22082-10A2 and by Washington National Primate Research Center grant RR-00166.
During this study, animals were housed at the University of Washington National Primate Research Center. Prior approval for the use of these animals in this protocol was obtained from the Animal Care Committee at the University of Washington.
None of the authors have commercial or other associations that might pose a conflict of interest.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Bulut, Y., E. Faure, L. Thomas, H. Karahashi, K. S. Michelsen, O. Equils, S. G. Morrison, R. P. Morrison, and M. Arditi. 2002. Chlamydial heat shock protein 60 activates macrophages and endothelial cells through Toll-like receptor 4 and MD2 in a MYD88-dependent pathway. J. Immunol. 168:1435-1440. [DOI] [PubMed] [Google Scholar]
- 2.Caldwell, H. D., J. Kromhout, and J. Schachter. 1981. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect. Immun. 31:1161-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen, C. R., S. S. Sinei, E. A. Bukusi, J. J. Bwayo, K. K. Holmes, and R. C. Brunham. 2000. Human leukocyte antigen class II DQ alleles associated with Chlamydia trachomatis tubal infertility. Obstet. Gynecol. 95:72-77. [DOI] [PubMed] [Google Scholar]
- 4.Eckert, L. O., S. E. Hawes, P. Wolner-Hanssen, D. M. Money, R. W. Peeling, R. C. Brunham, C. E. Stevens, D. A. Eschenbach, and W. E. Stamm. 1997. Prevalence and correlates of antibody to chlamydial heat shock protein in women attending sexually transmitted disease clinics and women with confirmed pelvic inflammatory disease. J. Infect. Dis. 175:1453-1458. [DOI] [PubMed] [Google Scholar]
- 5.Grayston, J. T., S.-P. Wang, S.-K. Yeh, and C.-C. Kuo. 1985. Importance of reinfection in the pathogenesis of trachoma. Rev. Infect. Dis. 7:717-725. [DOI] [PubMed] [Google Scholar]
- 6.Kuo, C.-C., and J. T. Grayston. 1976. Interaction of Chlamydia trachomatis organisms and HeLa 229 cells. Infect. Immun. 13:1103-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuo, C.-C. 1978. Immediate cytotoxicity of Chlamydia trachomatis for mouse peritoneal macrophages. Infect. Immun. 20:613-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaVerda, D., L. N. Albanese, P. E. Ruther, S. G. Morrison, R. P. Morrison, K. A. Ault, and G. I. Byrne. 2000. Seroreactivity to Chlamydia trachomatis Hsp10 correlates with severity of human genital tract disease. Infect. Immun. 68:303-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lichtenwalner, A. B., D. L. Patton, Y. T. C. Sweeney, L. K. Guar, and W. E. Stamm. 1997. Evidence of genetic susceptibility to Chlamydia trachomatis-induced pelvic inflammatory disease in the pig-tailed macaque. Infect. Immun. 65:2250-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patton, D. L., C.-C. Kuo, S.-P. Wang, and S. A. Halbert. 1987. Distal tubal obstruction induced by repeated Chlamydia trachomatis salpingitis infection in pig-tailed macaques. J. Infect. Dis. 155:1292-1299. [DOI] [PubMed] [Google Scholar]
- 11.Patton, D. L., Y. T. Sweeney, and C.-C. Kuo. 1994. Demonstration of delayed hypersensitivity in Chlamydia trachomatis salpingitis in moneys: a pathogenic mechanism of tubal damage. J. Infect. Dis. 169:680-683. [DOI] [PubMed] [Google Scholar]
- 12.Patton, D. L., P. Wolner-Hanssen, and K. K. Holmes. 1990. The effect of Chlamydia trachomatis on the female reproductive tract of the Macaca nemestrina after a single tubal challenge following repeated cervical inoculation. Obstet. Gynecol. 76:643-650. [PubMed] [Google Scholar]
- 13.Peeling, R. W., J. Kimani, E. M. Plummer, I. Maclean, M. Cheang, J. Bwayo, and R. C. Brunham. 1997. Antibody to chlamydial hsp60 predicts an increased risk for chlamydial pelvic inflammatory disease. J Infect. Dis. 175:1153-1158. [DOI] [PubMed] [Google Scholar]
- 14.Peeling, R. W., D. L. Patton, Y. T. Cosgrove Sweeney, M. S. Cheang, A. B. Lichtenwalner, R. C. Brunham, and W. E. Stamm. 1999. Antibody response to the chlamydial heat-shock protein 60 in an experimental model of chronic pelvic inflammatory disease in monkeys (Macaca nemestrina). J. Infect. Dis. 180:774-779. [DOI] [PubMed] [Google Scholar]
- 15.Taylor, H. R., S. L. Johnson, J. Schachter, H. D. Caldwell, and R. A. Prendergast. 1987. Pathogenesis of trachoma: the stimulus for inflammation. J. Immunol. 138:3023-3027. [PubMed] [Google Scholar]
- 16.Van Voorhis, W. C., L. K. Barrett, Y. T. Cosgrove Sweeney, C.-C. Kuo, and D. L. Patton. 1997. Repeated Chlamydia trachomatis infection of Macaca nemestrina fallopian tubes produces a Th1-like cytokine response associated with fibrosis and scarring. J. Infect. Dis. 65:2175-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witkin, S. S., J. Jeremias, M. Toth, and W. J. Ledger. 1993. Cell-mediated immune response to the recombinant 57-kDa heat-shock protein of Chlamydia trachomatis in women with salpingitis. J. Infect. Dis. 167:1379-1383. [DOI] [PubMed] [Google Scholar]