Abstract
Aims
Although left ventricular (LV) relaxation is well recognized as a predictor of mitral annulus (MA) early diastolic (E′) velocity, its significance relative to other predictors of E′ is less well understood.
Methods and results
We assessed 40 healthy volunteers, 43 patients with acutely decompensated chronic systolic heart failure (HF), and 36 patients with hypertrophic obstructive cardiomyopathy (HOCM) using echocardiography and right or left heart catheterization. Data were obtained at baseline. In addition, in healthy volunteers haemodynamics were varied by graded saline infusion and low body negative pressure, while in HF patients it was varied by vasoactive drug treatment. E- and A-wave velocity (E/A) ratio of the mitral valve inflow, systolic MA velocity integral (s′ integral) and E′ and late velocity (A′) of lateral and septal MA pulsed wave velocities were assessed by echocardiography. Time constant of isovolumic pressure decay τ0) was calculated from isovolumic relaxation time/[ln(aortic dicrotic notch pressure) – ln(LV filling pressure)]. In all three groups, s′ integral was the strongest predictor of E′ (partial r= 0.53–0.79; 0.81 for three groups combined), followed by E/A ratio (partial r= 0.10–0.78; 0.26 for all groups combined) and τ0 (partial r= −0.1 to 0.023; −0.21 for all groups combined).
Conclusion
In healthy adults, patients with systolic HF, or patients with HOCM, E′ is related to LV long-axis function and E/A ratio, a global marker of LV filling. E′ appears less sensitive to LV relaxation.
Keywords: Echocardiography, Relaxation, Tissue Doppler, Heart failure
Introduction
Early diastolic velocity (E′) of the mitral annulus used either as an isolated parameter, as the ratio between early (E) velocity of mitral inflow and E′ (E/E′ ratio),1 or in a classification algorithm,2 predicts survival in patients with heart failure (HF) or after myocardial infarction. An accepted explanation of these observations is that, in contrast to the E-wave of mitral inflow, E′ is less sensitive to preload, making it a marker of left ventricular (LV) relaxation.3–6 However, a factor that influences E′ is LV long-axis systolic function, as the length of the downward path of the mitral annulus of the diastole must be identical to its upward path during systole.7 An indirect proof of this proposition is that E′ and peak systolic annular velocity scale symmetrically with the heart size.8
In this retrospective, cross-sectional, observational study we explored the relationship between E′ and LV relaxation, systolic long-axis function and global ventricular filling pattern in three groups of subjects: patients with severe hypertrophic obstructive cardiomyopathy (HOCM), acutely decompensated patients with severe systolic HF, and healthy volunteers. Our hypotheses were that: (1) long-axis systolic function and global LV filling pattern are strongly associated with E′, and (2) that E′ is correlated with LV relaxation parameters even after accounting for systolic long-axis function and global LV filling pattern in each individual group, and in all subjects analysed jointly.
Methods
Patients
The study population consisted of 119 subjects stratified into Group 1 (healthy volunteers), Group 2 (systolic HF patients), or Group 3 (HOCM patients), as described below. The rationale of studying these specific groups is that, although the basic relationships between diastolic haemodynamic and echocardiography parameters should be independent of underlying pathology, it appears that the strength of these relationships is modulated by changes in the LV ejection fraction9 and presence of concentric LVH.10
All subjects satisfied the following inclusion criteria: (i) normal sinus rhythm, atrial sensed-ventricular paced rhythm, or atrial and ventricular paced rhythm, (ii) discernible E- and A-waves on the mitral inflow, (iii) no previous mitral valve surgery, or severe mitral regurgitation according to American College of Cardiology/American Heart Association guidelines, (iv) spontaneous ventilation, and (v) no cardiac transplantation.
Group 1 consisted of healthy volunteers who participated in a study that combined invasive and Doppler echocardiography measures to assess the effects of ageing on LV early diastolic function.11 It was composed of 15-young healthy volunteers <50 year (mean age 35 ± 9 years; 12 men) and 25 healthy seniors >65 year (mean age 69 ± 4 year; 13 men). Inclusion criteria and haemodynamic protocol have already been published.,11,12 Briefly, all subjects were screened for the presence of arterial hypertension, obstructive coronary artery disease, or structural heart disease, using 24-h blood pressure recordings and baseline and exercise ECGs and echocardiograms. Subjects were excluded if any of the following were present: mean daytime blood pressure >140/90 mmHg, ECG changes suggestive of ischaemic heart disease, left bundle branch block, atrial flutter/fibrillation, atrioventricular block greater than first degree, baseline or exercise-induced wall motion abnormalities, valvular heart disease other than mild valvular insufficiency, right ventricular or LV hypertrophy, untreated thyroid disorders, chronic lung disease, regular cigarette smoking within the previous 10 year, body mass index of ≥30, or use of any cardiovascular medications (e.g. β-blockers, etc.). The Cleveland Clinic Institutional Review Board approved this research project, and informed consent was obtained in all subjects.
Group 2 consisted of 43 consecutive patients >18 years of age with chronic (>6 months) symptomatic systolic HF who underwent Doppler echocardiography and right heart catheterization for optimal evaluation of haemodynamic derangements at the Cleveland Clinic Heart Failure intensive care unit between September 2006 and February 2008. All patients had markedly impaired systolic LV function (defined by the LV ejection fraction 30%) and New York Heart Association class III–IV symptoms. The Cleveland Clinic Institutional Review Board approved this research project, and informed consent was obtained in all subjects.
Finally, Group 3 consisted of 36 consecutive patients, referred for consultation to our institution with suspected obstructive HOCM between October 2006 and April 2008. HOCM was defined by a hypertrophied and non-dilated left ventricle in the absence of another cardiac or systemic disease that could produce a similar magnitude of hypertrophy13–15. All patients were deemed to have significant dynamic LV outflow tract (LVOT) obstruction that was intractable to maximal medical therapy and were being considered for surgical myectomy vs. alcohol septal ablation. All patients underwent Doppler echocardiography and invasive angiography. The patients are part of a registry that is approved by Cleveland Clinic Institutional Review Board with waiver of individual informed consent.
Sample size was determined by assuming that a sample size of each of the three groups should have a single-sided power of at least 80% to detect a correlation between E′ and relaxation parameter with an absolute r-value of >0.40 or more at the two-tailed alpha level of 0.05. The r-value of 0.40 was selected as a previous study showed an r-value of −0.46 for the correlation between E′ and time constant of the isovolumic pressure decay (τ0).6
Invasive and non-invasive data acquisition
Group 1
A 6-Fr balloon-tipped fluid-filled catheter was placed under fluoroscopy through an antecubital vein into the pulmonary artery. Mean pulmonary capillary wedge pressure (PCWP) was determined at end-expiration. Arterial systolic and diastolic blood pressures were measured by cuff sphygmomanometry. After collecting initial haemodynamic and echocardiography data, lower body negative pressure was applied to decrease PCWP as previously reported.12 Briefly, the subject was placed in a Plexiglas box sealed at the level of the iliac crest. The suction was provided with the use of a vacuum pump with a variable autotransformer. Haemodynamic and echocardiography measurements were made after 5 min of LBNP at –15 and –30 mmHg. The negative pressure was then released. After repeated baseline measurements to confirm a return to haemodynamic steady state, cardiac filling was increased by rapid infusion (100 mL/min) of warm (37°C) isotonic saline. Measurements were repeated, after 10 and 20 mL/kg (elderly) or 15 and 30 mL/kg (young) had been infused, to attain similar increases in PCWP in both groups. Complete two-dimensional (2D) and Doppler echocardiography studies were performed, using ATL (Advanced Technology Laboratories; Bothell, Washington) HDI 5000CV. Apical two- and four-chamber views, PW Doppler of inflow and outflow LV tracts, and PW tissue Doppler of lateral and septal side of the mitral annulus were performed at each stage of load protocols.
Group 2
Haemodynamic and echocardiography data were simultaneously collected at baseline (within 12 h of admission) and at follow-up (after 48 h of intensive medical therapy) if the pulmonary artery catheter was still in place. Haemodynamic data, including invasively obtained radial artery blood pressure and PCWP (wedge position was verified by fluoroscopy and phasic changes in pressure waveforms), represent the average of five cycles. Arterial pressures and PCWP were assessed at end expiration. Complete 2D and Doppler echocardiography studies were performed using commercially available equipment. Apical two- and four-chamber views, pulsed-wave PW Doppler interrogation of inflow and outflow LV tracts, and PW tissue Doppler of lateral and septal side of the mitral annulus were performed.
Group 3
Invasive haemodynamics in this group were obtained from a left heart catheterization performed within a mean of 8 ± 9 days of the echocardiography, without change of oral therapy or hospitalization for HF during this period. LV end-diastolic pressures and arterial systolic and diastolic pressures were determined by the catheter placed in the mid-ventricle and ascending aorta, respectively. All patients underwent complete 2D and Doppler echocardiography study that included standard and colour apical two-, three-, four-, and five-chamber views, PW and continuous wave Doppler interrogation of inflow and outflow LV tracts, and PW tissue Doppler of lateral and septal side of the mitral annulus.
In addition, resting LVOT peak velocity was measured by continuous-wave Doppler echocardiography, and resting LVOT pressure gradient was estimated by using simplified Bernoulli equation.16 Care was taken to avoid contamination of the LVOT waveform by the mitral regurgitation jet.17 In patients with resting LVOT gradients <30 mmHg, provocative manoeuvres, including Valsalva and amyl nitrite were also used to measure a provocable LVOT gradient. Maximal LVOT gradient was defined as highest recorded gradient (resting or provocable) in a patient. Resting mitral inflow and mitral annulus Doppler data were acquired prior to applying Valsalva maneuver and/or amyl nitrate inhalation.
Data analysis
We performed following echocardiography measurements: LV volumes and ejection fraction (Simpson's biplane rule), LV mass,8 peak E and late atrial (A) wave velocities of mitral inflow and their ratio, isovolumic relaxation time, duration of E- and A-waves, and E-wave deceleration time. Additionally, we measured s′' wave integral, peak E′ and A′' velocities, and duration of E′' and A′' waves from pulsed wave tissue Doppler tracings of the septal and lateral side of the mitral annulus obtained in the four chamber apical view. For the purpose of analysis, tissue Doppler data obtained from lateral and septal side were averaged.
The time constant of isovolumic pressure decay with a zero asymptote assumption (τ0) was estimated by the equation:18
where AoPdic is aortic dicrotic notch (i.e. end-ejection) pressure and IVRT is isovolumic relaxation time by echocardiography19. Aortic dicrotic notch pressure was by:20
where Psys is systolic, and Pdia is diastolic arterial pressure.
Owing to differences in initial protocols, aortic end-ejection pressure and LV filling pressure were calculated slightly differently in three patient groups. In Group 1, arterial pressures were obtained by cuff sphygmomanometer, while LF filling pressures were again represented by PCWP. In Group 2, arterial pressures were obtained from radial arterial pressure tracings, while LF filling pressure was assumed to be represented by PCWP. Finally, in Group 3, systolic and diastolic arterial pressures from aortic blood pressure tracings while LF filling pressure was assumed to be identical to LV end-diastolic pressure.
Statistical methods
Data are shown as mean ± SD unless otherwise noted. If data distribution deviated from normal distribution, appropriate transformations were performed prior to statistical analysis. Comparison between groups was performed by one-way analysis of variance followed by post hoc Tukey's HSD test, if appropriate.
To assess inter-observer variability of Doppler velocities and time intervals used in the study, two blinded observes measured data of 30 randomly selected patients. To assess intra-observer variability, one observer repeated measurements >1 months after initial measurements. Variability was calculated as the percent variability of the absolute difference between two measurements and expressed as mean ± SD.
To assess if relaxation (i.e. τ0) affects E′ after controlling for other parameters of LV function, we developed an E′ prediction model based solely on LV systolic long-axis function and its filling pattern. We posit three propositions: (i) systolic ascent of the mitral annulus [i.e. integral of its systolic (s′') wave] is equal to the diastolic descent (i.e. sum of the integrals of the E′' and A′' waves); (ii) the profile of the mitral inflow is similar to the profile of the diastolic mitral annular velocities; and (iii) as E- and A- waves have triangular shape, the mitral inflow profile can be represented by E- and A- wave duration and by the ratio of their velocities (E/A ratio) (see Figure 1). From these propositions, simple algebraic substitutions result in the following equation for the predicted E′ (see Supplementary data online):
where Edur and Adur are the duration of the E- and A-wave of the mitral inflow, E/A is the ratio of the mitral inflow E- and A-waves, and ∫s′ is s′ wave integral. Note that E′ predicted in this way is related to LV relaxation only through its effect on E/A ratio. To assess if relaxation has any incremental contribution as an E′ predictor, we then performed a stepwise multiple regression, using predicted E′ and τ0 as predictors of E′. The null hypothesis was that adding τ0 to predicted E′ would not significantly improve correlation with measured E′.
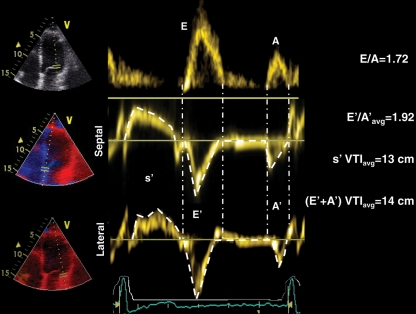
Figure 1.
An example of pulsed wave Doppler waveforms of mitral inflow (upper) and septal (middle) and lateral (lower trace) part of the mitral annulus in a patient with decompensated systolic heart failure (Group 2). Note similar profiles of mitral inflow and mitral annulus motion during diastole with similar values of E/A and average E′/A′ ratios, as well as similar duration of corresponding inflow and mitral annulus E- and A-waves. Also note similar values for average systolic and diastolic tissue Doppler integrals. Avg, average.
We also performed stepwise multiple linear regression with E′ as dependent variable, and s′ integral, E/A ratio and τ0 as predictors. To test if linear regression assumptions were satisfied, we assessed linearity of the plot of observed vs. predicted value (for linearity), the shape of residuals vs. predicted value (for homoscedascity), and tested the normality of residuals distribution. Finally, to assess the impact of multiple measures in individual patients, we applied mixed linear models approach using SPSS 11.0 (IBM Corporation, Somers, NY, USA).
All analyses were performed on whole data sets, as well as on individual subject groups. P-value <0.05 was considered statistically significant.
Results
Table 1 shows baseline clinical and echocardiography variables in our patients. As expected, LV volumes were larger in patients with systolic HF. Again expectedly, LV mass was higher in Groups 2 and 3. In total, the numbers of data-points were as follows: 188 in Group 1, 69 in Group 2, and 36 in Group 3.
Table 1.
Baseline clinical and echocardiography data
| Healthy volunteers |
||||
|---|---|---|---|---|
| Young (n= 15) | Elderly (n=25) | Systolic HF (n= 43) | HOCM (n= 36) | |
| Age (years) | 34 ± 9**,**** | 69 ± 3**,****,***** | 54 ± 12 | 57 ± 12 |
| BBB/V pace | / | / | 25 | 8 |
| Heart rate (bpm) | 63 ± 13* | 56 ± 7** | 81 ± 16 | 61 ± 17* |
| EDV (mL) | 144 ± 46** | 109 ± 38** | 241 ± 90 | 102 ± 36** |
| ESV (mL) | 57 ± 20** | 38 ± 18** | 183 ± 82 | 32 ± 15** |
| EF | 0.60 ± 0.10**,*** | 0.65 ± 0.07** | 0.26 ± 0.08 | 0.69 ± 0.08** |
| PCWP (mmHg) | 10.74 ± 1.72**,**** | 10.74 ± 1.76**,**** | 20.88 ± 7.85 | 20.65 ± 7.62 |
| τ0 (ms) | 51 ± 7**** | 67 ± 9 | 61 ± 25 | 75 ± 31* |
| E/A | 1.67 ± 0.35** | 0.91 ± 0.26** | 2.6 ± 1.34 | 1.39 ± 0.91** |
| DT (ms) | 179 ± 40*,**** | 213 ± 37** | 126 ± 36 | 245 ± 63** |
| s′ (cm/s) | 9.61 ± 1.91**,**** | 8.68 ± 1.43**,**** | 3.67 ± 1.3 | 6.73 ± 1.83** |
| E′ (cm/s) | 15.16 ± 2.98**** | 10.34 ± 1.69**,****,***** | 5.58 ± 2.03 | 6.35 ± 2.32 |
*P< 0.05 vs. systolic HF; **P< 0.001 vs. systolic HF; ***P< 0.05 vs. HOCM; ****P< 0.01 vs. HOCM; *****P< 0.01 vs. young.
BBB/V Pace, bundle branch block/ventricular pacing; DT, deceleration time of the early wave of the mitral inflow; E/A, ratio of early to late diastolic mitral inflow velocity; EF, left ventricular ejection fraction; EDV (ESV), left ventricular end-diastolic (systolic) volume; PCWP, pulmonary capillary wedge pressure; s′ (E′), mitral annulus systolic (early diastolic) wave velocity.
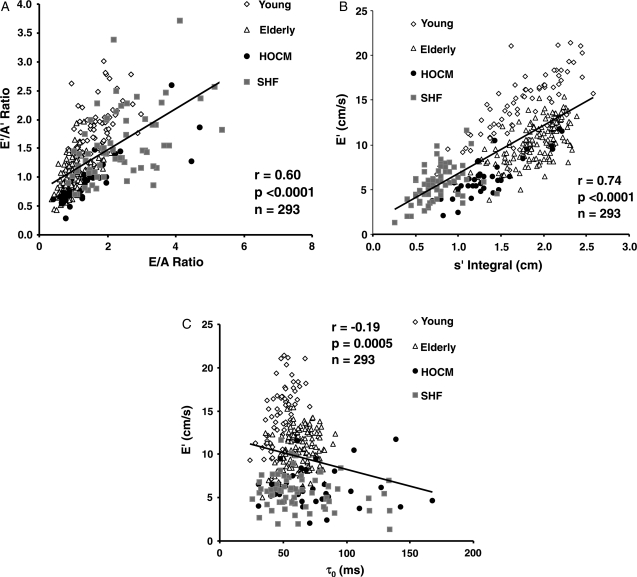
There was a moderately strong correlation between mitral inflow E/A ratio and the E′/A′ ratio of the mitral annulus, (r = 0.60. P< 0.0001) as well as between s′ integral and mitral annulus E′ (r = 0.74, P< 0.0001) (Figure 2A and B). The correlation between mitral inflow E/A ratio and the E′/A′ ratio of the mitral annulus was lowest in the SHF group (r = 0.43, P = 0.0002) and highest in the HOCM group (r= 0.83, P< 0.0001). Also, the duration of early diastolic wave of the mitral inflow and of the mitral annulus (E and E′, respectively) strongly correlated (r = 0.89, P< 0.0001), with E-wave having a duration longer by 12 ± 26 ms (P< 0.0001). Similarly, duration of atrial wave of the mitral inflow and of the mitral annulus (A and A′, respectively) correlated (r = 0.75, P< 0.0001), but now with A-wave having a duration slightly shorter by −4 ± 21 ms (P = 0.001). Correlation between mitral annulus E′ and τ0 was significant, but weak (r = −0.19, P = 0.0005; Figure 2C).
Figure 2.
(A) Correlation between the ratio of the peak early and late diastolic mitral inflow velocities (E/A ratio) and the ratio of the peak early and late diastolic velocities of the mitral annulus (E′/A′ ratio); (B) correlation between peak early diastolic velocity of the mitral annulus (E′) and the integral of the systolic velocity of the mitral annulus (s′ integral); (C) correlation between peak early diastolic velocity of the mitral annulus (E′) and the time constant of the isovolumic pressure decay (τ0). HOCM, hypetrophic obstructive cardiomyopathy; SHF, systolic heart failure; Elderly, elderly healthy volunteers; Young, young healthy volunteers.
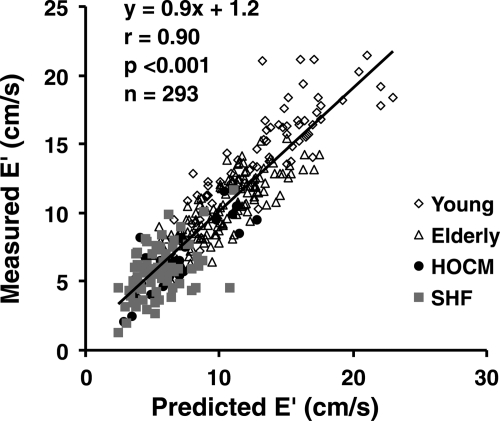
There was a strong correlation between the measured and predicted E′ (r = 0.90, P< 0.0001; Figure 3). This correlation was marginally improved by adding τ0 as a predictor (multivariate r= 0.90; partial r for τ0−0.13, P= 0.03; Table 2). When we repeated this analysis after separating the subjects into groups, τ0 was a weak independent predictor of E′ in normal subjects (partial r= −0.23, P= 0.001) but not in Group 2 (systolic HF patients), or Group 3 (HOCM patients) (Table 2).
Figure 3.
Correlation between measured peak early diastolic velocity of the mitral annulus (E′) and E′ predicted from the systolic displacement of the mitral annulus and the profile of the mitral inflow (predicted E′).
Table 2.
Estimating early diastolic velocity of the mitral annulus (E′) from predicted E′ and time constant of the isovolumic pressure decay (τ0) by multiple linear regression
| r, full model (95% ci) | Predicted E′ partial r (95% CI) | τ0 |
|||
|---|---|---|---|---|---|
| P-value | Partial r (95% CI) | P-value | |||
| All subjects | 0.90 (0.88–0.92) | 0.90 (0.88–0.92) | <0.0001 | −0.13 (–0.24 to −0.02) | 0.03 |
| HOCM | 0.83 (0.69–0.91) | 0.83 (0.69–0.91) | <0.0001 | 0.01 (−0.32 to 0.34) | 0.99 |
| SHF | 0.54 (0.35–0.69) | 0.54 (0.35–0.69) | <0.0001 | −0.064 (−0.30 to 0.18) | 0.6 |
| Healthy volunteers | 0.86 (0.82–0.89) | 0.86 (0.82–0.89) | <0.0001 | −0.23 (−0.36 to –0.09) | 0.001 |
CI, confidence interval; HOCM, hypertrophic cardiomyopathy; SHF, systolic heart failure.
Empirical approach to assessment of E′ predictors by multiple linear regression showed that the best overall predictor of E′ was s′ integral (P< 0.0001), followed by E/A ratio (P< 0.0001), with τ0 adding significantly but weakly to the model (partial r= −0.21, P= 0.0002). A mixed model that accounted for repeated measurements of the same subjects confirmed that the strongest E′ predictor was s′ integral (P< 0.0001), followed by E/A ratio (P< 0.0001), and τ0 (P= 0.0014). We then repeated this multiple linear regression approach in individual groups of subjects. In contrast to findings described in a previous paragraph, τ0 showed only a trend towards being a predictor of E′ in normal subjects (partial r= −0.10, P= 0.16). Again, τ0 was not a predictor of E′ in Group 2 (systolic HF patients) or Group 3 (HOCM patients) (Table 3).
Table 3.
Estimating early diastolic velocity of the mitral annulus from the integral of the systolic velocity of the mitral annulus (s′ integral), ratio of the early and late velocities of the mitral inflow (E/A ratio) and time constant of the isovolumic pressure decay (τ0) by multiple linear regression
| s′ integral | E/A ratio | τ0 | |||||
|---|---|---|---|---|---|---|---|
| r, full model (95% CI) | Partial r (95% CI) | P-value | partial r (95% CI) | P-value | partial r (95% CI) | P-value | |
| All subjects | 0.82 (0.77–0.85) | 0.78 (0.73–0.84) | <0.0001 | 0.28 (0.12–0.39) | <0.0001 | −0.21 (−0.34 to −0.07) | 0.0003 |
| HOCM | 0.79 (0.62–0.89) | 0.79 (0.62–0.89) | <0.0001 | 0.24 (−0.1 to 0.53) | 0.16 | 0.023 (−0.31 to 0.35) | 0.9 |
| SHF | 0.53 (0.33–0.68) | 0.53 (0.33–0.68) | <0.0001 | 0.10 (−0.14 to 0.33) | 0.4 | −0.073 (−0.31 to 0.17) | 0.55 |
| Healthy volunteers | 0.83 (0.78–0.87) | 0.60 (0.50–0.68) | <0.0001 | 0.78 (0.72 to 0.83) | <0.0001 | −0.1 (−0.24 to 0.04) | 0.16 |
CI, confidence interval; HOCM, hypertrophic cardiomyopathy; SHF, systolic heart failure.
Inter- and intra-observer variability
The inter-observer variability for mitral valve E and A, and mitral annulus E′ and A′ velocities was 5 ± 6, 6 ± 7, 6 ± 8, and 7 ± 7%, respectively. The inter-observer variability for s′ wave integral, mitral E and A duration, and IVRT was 4 ± 5, 7 ± 5, 8 ± 6, and 10 ± 9%, respectively. Intra-observer variabilities were slightly lower (mitral valve E and A, and mitral annulus E′ and A′ velocities was 4 ± 6, 6 ± 6, 6 ± 7, and 7 ± 7%, respectively; s′ wave integral, mitral E and A duration, and IVRT was 3 ± 5, 6 ± 5, 8 ± 7, and 9 ± 9%, respectively).
Uncertainty analysis
The largest potential source of error in our study is the accuracy of our τ0 measurements. We have shown that our measurement of τ0 correlates strongly with τ0 measured using both Weiss and shifting asymptote method.18,11 However, to further estimate the maximal potential correlation between τ0 and E′ after adjusting for s′ and E/A ratio, we first subtracted the variance attributable to E′ measurement from the residual sum of squares of multiple linear regression of E′ predictors. Assuming that τ0 explained the entire residual sum of squares after this subtraction, its partial correlation coefficient was only −0.47, a value still not high enough for E′ to be a clinically useful surrogate for τ0.
Discussion
In this paper, we analyse the way LV long-axis function, filling pattern, and relaxation relate to E′, a marker of diastolic function. Partial correlations we obtained by multiple regression indicate that the dominant factors associated with E′, in three groups tested either separately or jointly, were the LV long-axis function followed by LV filling pattern, with LV relaxation having a smaller impact. These findings are further strengthened by showing that adding LV relaxation to E′ calculated by a mathematical equation that incorporates LV long-axis function and filling pattern only minimally improves accuracy of E′ prediction.
E′ as a predictor of relaxation
Several studies assessed E′ as an estimate of invasively quantified LV relaxation.5,21−24 Nagueh et al studied 10 dogs that were subjected to various haemodynamic p τ0erturbations to show a strong relationship between E′ and τ with an r of −0.83. In a similar study, Firstenberg et al.21 showed that, while E′ and τ correlated with an r of −0.70, E′ and E-wave velocities changed by a similar percentage in the setting of an isolated change in preload, indicating that E′ is preload dependent. In contrast, clinical studies that assessed the same issue came up with lower correlations. Ommen et al. showed in a study of 100 subjects a correlation of r of −0.46.6 Similarly, in study of 12 control patients and 43 subjects with HF and normal ejection fraction, Kasner et al.22 obtained an r of −0.33, while Mizuno et al.23 in a study of 117 patients showed a correlation of −0.40 (using a standard zero-asymptote method for τ measurement). Our study in 119 subjects shows a correlation between E′ and τ that, when compared with these studies, was even lower, although with confidence intervals approaching the values reported before (r= −0.19, 95% CI: −0.01 to −0.36). The reason for lower correlation values obtained in this study may be multifactorial. While previously validated,11,18 our method of calculating τ should be viewed as an estimation. Additionally, some studies suggest that relaxation more strongly predicts E′ in the setting of decreased systolic function,6 and as most of our subject had normal ejection fractions, our findings may be biased. However, in our study τ0 was not a strong predictor of relaxation even in patients with systolic HF.
Relationship between left ventricular long-axis systolic displacement and early diastolic myocardial velocity
Conservation of mass necessitates that systolic and diastolic displacements are identical, which makes systolic and diastolic velocities interdependent. Indeed, Mogelvang et al.25 showed a correlation between s′ and E′ velocity of r = 0.52 even after adjustment for age, sex, body mass index, and heart rate. Furthermore, s′ and E′ velocity decrease in parallel in the systolic and diastolic HF.7 We extend these findings by showing that E′ velocity strongly depends on the s′ integral, a measure of systolic displacement. We furthermore show that our predicted E′, which is very much determined by systolic displacement, efficiently tracks true E′ values.
Left ventricular filling pattern and the local profiles of myocardial diastolic velocities
LV filling pattern in sinus rhythm has two components: E-wave of early, and A-wave of late filling. The determinants of LV filling are well-known, with the interaction of relaxation and ventricular stiffness determining early, and atrial contractility and load determining late filling. LV filling pattern, by default, is the product of local ventricular behaviour. Given that myocardial structure throughout ventricle carries some amount of homogeneity one can surmise that this homogeneity of structure translates to some homogeneity of function (i.e. local relaxation and stiffness parameters), resulting in regional myocardial velocities having at least some between-region similarity. Indeed, we do show that E/A ratios of mitral inflow and long-axis velocities, while not identical, are similar.
Clinical implications
The echocardiographic detection of LV diastolic dysfunction has been made more difficult by the confusing terminology, including the question of what exactly constitutes diastolic dysfunction. Elements of diastolic function, such as active relaxation and chamber compliance that are traditionally measured using invasive pressure and volume data, do not have direct echocardiographic counterparts. Rather, echocardiographic indices reflect combined influences of relaxation, compliance, and interventricular dependence. As a result, diagnosis of elevated filling pressures by echocardiography is not straightforward. Our findings reinforce the idea that, instead of relying on a single parameter for the assessment of LV diastolic function (or filling pressures), several parameters should be combined.9 Of note, while decision-making algorithms were proposed by leading international echocardiography associations,9 there is still a paucity of data how well they perform in the clinical setting, especially when discordant individual parameters occur. Additionally, E′ measurement may have prognostic implications even beyond diastolic function assessment.1,26 These issues have to addressed by future studies.
Limitations
This was a retrospective study with slightly different protocols in each of the studied groups. However, sample size of each group was large enough to adequately detect a relevant correlation between E′ and relaxation. Inclusion of patients with mitral regurgitation, paced ventricular rhythms, and severe LV hypertrophy may reduce the correlation between E′ and LV relaxation. Also, while it appears that E′ is less sensitive to LV relaxation than to systolic long-axis function and E/A ratio in patients we studied, it is uncertain if that can be generalized to all cardiovascular states, such as patients with mild to moderate systolic dysfunction, hypertensive heart disease, restrictive cardiomyopathies, or diabetic heart disease.
E′-τ0 correlation performed after controlling for E/A values may underestimate intrinsic relationship between E′ and τ0. Also, our method of τ0 estimation is less sophisticated and may be more prone to errors than non-linear regression methods that are traditionally used in rigorous haemodynamic assessment and was measured slightly differently in three patient groups. Our method is further compromised by lack of simultaneity in echo and invasive measurements in HOCM patients, which may introduce additional measurement error. Because of this, our ability to detect weak E′ correlations with tau after accounting for systolic long-axis function and global LV filling pattern (hypothesis 2). However, weak correlations are less relevant, it has been shown that our τ0 estimation method correlates satisfactorily with a standard zero—and shifting—asymptote methods,11,18 and has been previously used in clinical research.11,27
Another technical limitation is a known presence of measurement error that is inherent in measurement of echocardiography variables, especially in the setting of decreased mitral annulus velocities.
Conclusion
In this paper, we show that E′ is related LV long-axis displacement during systole and that the diastolic pattern of long-axis motion largely follows the mitral inflow pattern. E′ is comparatively less sensitive to changes in LV relaxation in healthy adults, patients with systolic HF, or patients with HOCM.
Supplementary data
Supplementary data are available at European Journal of Echocardiography online.
Conflict of interest: none declared.
Funding
This research was supported by Grant No. 2005191 from the United States-Israel Binational Science Foundation (BSF); by the National Space Biomedical Research Institute through NASA Grant NCC-9–58 (Houston, TX); and by Grant No. AG17479–02 from the National Institutes of Health (Bethesda, MD, USA).
Supplementary Material
References
- 1.Troughton RW, Prior DL, Frampton CM, Nash PJ, Pereira JJ, Martin M, Fogarty A, Morehead AJ, Starling RC, Young JB, Thomas JD, Lauer MS, Klein AL. Usefulness of tissue doppler and color M-mode indexes of left ventricular diastolic function in predicting outcomes in systolic left ventricular heart failure (from the ADEPT study) Am J Cardiol. 2005;96:257–62. doi: 10.1016/j.amjcard.2005.03.055. [DOI] [PubMed] [Google Scholar]
- 2.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 3.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–33. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 4.Nagueh SF, Mikati I, Kopelen HA, Middleton KJ, Quinones MA, Zoghbi WA. Doppler estimation of left ventricular filling pressure in sinus tachycardia. A new application of tissue doppler imaging. Circulation. 1998;98:1644–50. doi: 10.1161/01.cir.98.16.1644. [DOI] [PubMed] [Google Scholar]
- 5.Nagueh SF, Sun H, Kopelen HA, Middleton KJ, Khoury DS. Hemodynamic determinants of the mitral annulus diastolic velocities by tissue Doppler. J Am Coll Cardiol. 2001;37:278–85. doi: 10.1016/s0735-1097(00)01056-1. [DOI] [PubMed] [Google Scholar]
- 6.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–94. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 7.Yip G, Wang M, Zhang Y, Fung JW, Ho PY, Sanderson JE. Left ventricular long axis function in diastolic heart failure is reduced in both diastole and systole: time for a redefinition? Heart. 2002;87:121–5. doi: 10.1136/heart.87.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Popovic ZB, Sun JP, Yamada H, Drinko J, Mauer K, Greenberg NL, Cheng Y, Moravec CS, Penn MS, Mazgalev TN, Thomas JD. Differences in left ventricular long-axis function from mice to humans follow allometric scaling to ventricular size. J Physiol. 2005;568:255–65. doi: 10.1113/jphysiol.2005.090779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–93. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 10.Geske JB, Sorajja P, Nishimura RA, Ommen SR. Evaluation of left ventricular filling pressures by Doppler echocardiography in patients with hypertrophic cardiomyopathy: correlation with direct left atrial pressure measurement at cardiac catheterization. Circulation. 2007;116:2702–8. doi: 10.1161/CIRCULATIONAHA.107.698985. [DOI] [PubMed] [Google Scholar]
- 11.Popovic ZB, Prasad A, Garcia MJ, Arbab-Zadeh A, Borowski A, Dijk E, Greenberg NL, Levine BD, Thomas JD. Relationship among diastolic intraventricular pressure gradients, relaxation, and preload: impact of age and fitness. Am J Physiol Heart Circ Physiol. 2006;290:H1454–9. doi: 10.1152/ajpheart.00902.2005. [DOI] [PubMed] [Google Scholar]
- 12.Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110:1799–805. doi: 10.1161/01.CIR.0000142863.71285.74. [DOI] [PubMed] [Google Scholar]
- 13.Martin RP, Rakowski H, French J, Popp RL. Idiopathic hypertrophic subaortic stenosis viewed by wide-angle, phased-array echocardiography. Circulation. 1979;59:1206–17. doi: 10.1161/01.cir.59.6.1206. [DOI] [PubMed] [Google Scholar]
- 14.Henry WL, Clark CE, Griffith JM, Epstein SE. Mechanism of left ventricular outlfow obstruction in patients with obstructive asymmetric septal hypertrophy (idiopathic hypertrophic subaortic stenosis) Am J Cardiol. 1975;35:337–45. doi: 10.1016/0002-9149(75)90025-9. [DOI] [PubMed] [Google Scholar]
- 15.Pollick C, Rakowski H, Wigle ED. Muscular subaortic stenosis: the quantitative relationship between systolic anterior motion and the pressure gradient. Circulation. 1984;69:43–9. doi: 10.1161/01.cir.69.1.43. [DOI] [PubMed] [Google Scholar]
- 16.Nakatani S, Marwick TH, Lever HM, Thomas JD. Resting echocardiographic features of latent left ventricular outflow obstruction in hypertrophic cardiomyopathy. Am J Cardiol. 1996;78:662–7. doi: 10.1016/s0002-9149(96)00386-6. [DOI] [PubMed] [Google Scholar]
- 17.Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, Cecchi F, Maron BJ. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348:295–303. doi: 10.1056/NEJMoa021332. [DOI] [PubMed] [Google Scholar]
- 18.Scalia GM, Greenberg NL, McCarthy PM, Thomas JD, Vandervoort PM. Noninvasive assessment of the ventricular relaxation time constant (tau) in humans by Doppler echocardiography. Circulation. 1997;95:151–5. doi: 10.1161/01.cir.95.1.151. [DOI] [PubMed] [Google Scholar]
- 19.Thomas JD, Flachskampf FA, Chen C, Guererro JL, Picard MH, Levine RA, Weyman AE. Isovolumic relaxation time varies predictably with its time constant and aortic and left atrial pressures: implications for the noninvasive evaluation of ventricular relaxation. Am Heart J. 1992;124:1305–13. doi: 10.1016/0002-8703(92)90416-s. [DOI] [PubMed] [Google Scholar]
- 20.Hebert JL, Lecarpentier Y, Zamani K, Coirault C, Daccache G, Chemla D. Relation between aortic dicrotic notch pressure and mean aortic pressure in adults. Am J Cardiol. 1995;76:301–6. doi: 10.1016/s0002-9149(99)80086-1. [DOI] [PubMed] [Google Scholar]
- 21.Firstenberg MS, Greenberg NL, Main ML, Drinko JK, Odabashian JA, Thomas JD, Garcia MJ. Determinants of diastolic myocardial tissue Doppler velocities: influences of relaxation and preload. J Appl Physiol. 2001;90:299–307. doi: 10.1152/jappl.2001.90.1.299. [DOI] [PubMed] [Google Scholar]
- 22.Kasner M, Westermann D, Steendijk P, Gaub R, Wilkenshoff U, Weitmann K, Hoffmann W, Poller W, Schultheiss HP, Pauschinger M, Tschope C. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation. 2007;116:637–47. doi: 10.1161/CIRCULATIONAHA.106.661983. [DOI] [PubMed] [Google Scholar]
- 23.Mizuno J, Arita H, Hanaoka K, Kusakari Y, Kurihara S. Novel assessment of intracellular calcium transient decay in cardiac muscle by curve-fitting with half-logistic function. Masui. 2008;57:408–19. [PubMed] [Google Scholar]
- 24.Slama M, Ahn J, Peltier M, Maizel J, Chemla D, Varagic J, Susic D, Tribouilloy C, Frohlich ED. Validation of echocardiographic and Doppler indexes of left ventricular relaxation in adult hypertensive and normotensive rats. Am J Physiol Heart Circ Physiol. 2005;289:H1131–6. doi: 10.1152/ajpheart.00345.2004. [DOI] [PubMed] [Google Scholar]
- 25.Mogelvang R, Sogaard P, Pedersen SA, Olsen NT, Marott JL, Schnohr P, Goetze JP, Jensen JS. Cardiac dysfunction assessed by echocardiographic tissue Doppler imaging is an independent predictor of mortality in the general population. Circulation. 2009;119:2679–85. doi: 10.1161/CIRCULATIONAHA.108.793471. [DOI] [PubMed] [Google Scholar]
- 26.Liang HY, Cauduro SA, Pellikka PA, Bailey KR, Grossardt BR, Yang EH, Rihal C, Seward JB, Miller FA, Abraham TP. Comparison of usefulness of echocardiographic Doppler variables to left ventricular end-diastolic pressure in predicting future heart failure events. Am J Cardiol. 2006;97:866–71. doi: 10.1016/j.amjcard.2005.09.136. [DOI] [PubMed] [Google Scholar]
- 27.Rivas-Gotz C, Khoury DS, Manolios M, Rao L, Kopelen HA, Nagueh SF. Time interval between onset of mitral inflow and onset of early diastolic velocity by tissue Doppler: a novel index of left ventricular relaxation: experimental studies and clinical application. J Am Coll Cardiol. 2003;42:1463–70. doi: 10.1016/s0735-1097(03)01034-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.