Abstract
Intranasal immunization of mice with Rib, a cell surface protein of group B streptococcus (GBS), conjugated to or simply coadministered with the recombinant cholera toxin B subunit, induces systemic immunoglobulin G (IgG) and local IgA antibody responses and confers protection against lethal GBS infection. These findings have implications for the development of a human GBS vaccine.
Several approaches have been tried in an attempt to design an efficient vaccine against group B streptococcal (GBS) disease (7), and possible vaccine components include capsular polysaccharides and cell surface proteins of GBS (2, 8, 9, 12, 16, 17, 20, 27). Mucosal immunization is an attractive strategy, since it is noninvasive and has the potential to induce both systemic and local immune responses (3, 14, 22). Indeed, it should be advantageous to use a GBS vaccine that not only induces protective immunoglobulin G (IgG) antibodies that can be transplacentally transferred to the fetus but also induces mucosal antibodies that prevent genital colonization of the mother and thereby prevent transfer of the bacteria to the fetus.
Some human and animal studies have focused on antibodies to GBS at the female genital mucosa (8-12, 24). Although, the role of these local antibodies in the pathogenesis of GBS infection is not clear, it seems reasonable to assume that they may to some extent protect against colonization with GBS (10). It has been reported that mucosal immunization with GBS polysaccharides or inactivated GBS bacteria induces systemic and local antibody responses in mice (8, 9, 12, 24). However, there is no previous report on mucosal immunization with purified GBS proteins.
Earlier studies have shown that parenterally administered cell surface proteins of GBS elicit a systemic IgG response and confer protection against experimental GBS infection (1, 6, 17, 18, 20). This made it of interest to examine if these proteins could also be used in a mucosal GBS vaccine. In the present study, Rib, a well-characterized GBS surface protein that is expressed by many strains causing invasive neonatal infection (17, 25, 26), was combined with recombinant cholera toxin B subunit (CTB) and administered intranasally (i.n.) to mice. The systemic and local IgG and IgA responses were examined. In addition, the protective capacity of this mucosal vaccination was evaluated by lethal intraperitoneal (i.p.) challenge with GBS.
Preparation of conjugate vaccine.
The Rib protein was isolated from the high-virulence type III strain BM110 (21, 25) by several purification steps and was free of contaminating polysaccharides (17, 25). Recombinant CTB was purified from Vibrio cholerae strain 358 (19). The Rib protein was conjugated to CTB using N-succinimidyl 3-(2-pyridyldithio)propionate (SPDP; Pharmacia AB, Uppsala, Sweden) as a bifunctional coupling reagent. SPDP was added to both proteins according to the manufacturer's instructions, using pH 8.5 for Rib and pH 7.5 for CTB. A similar method has been used for coupling polysaccharides to CTB and is described in detail elsewhere (4, 5). A 10-fold molar excess was used for Rib, and a 3-fold molar excess was used for CTB. The 2-pyridyl disulfide-containing Rib was then reduced using 50 mM dithiothreitol, and the excess 2-pyridyl disulfide and dithiothreitol was removed using a Sephadex G25 prepacked PD10 column (Pharmacia AB, Uppsala, Sweden). The two derivatized proteins were mixed and allowed to react overnight at room temperature. The conjugate was purified using a Superdex 200 10/30 column connected to a BioLogic WorkStation (Bio-Rad). The fractions containing the highest-molecular-weight material were collected, pooled, and concentrated using an Amicon stirred concentration cell (Amicon). The conjugate was tested by enzyme-linked immunosorbent assay (ELISA), and both CTB and Rib were detected. The Lowry protein determination assay was used to measure the total protein content of the conjugate. However, the exact proportion of each protein could not be determined. The conjugate was stored at 4°C with 0.005% Merthiolat (Kebo AB, Stockholm, Sweden) as a preservative.
Immunizations and antibody responses.
Five groups with four 10-week-old C3H/HeN female mice (Charles River, Sulzfeld, Germany) in each group were immunized i.n. with either of the following vaccines: protein Rib conjugated to CTB (Rib-CTB) (8 μg), Rib mixed with CTB (Rib+CTB) (4 μg plus 4 μg), Rib alone (Rib) (4 μg), CTB alone (CTB) (4 μg), or phosphate-buffered saline (PBS) three times 2 weeks apart. One group with four mice received 5 μg of Rib subcutaneously (s.c.) and 4 weeks later a booster of 2.5 μg s.c. The antigens were diluted in PBS to a volume of 25 μl for i.n. administration and 150 μl for s.c. administration, respectively. The mice were lightly anesthetized with methoxyflurane (Methofane) (Mallincrodt Veterinary, Mundelein, Ill.) during immunizations. Sera and genital tissue extracts were obtained from the immunized mice. Two weeks after the last vaccine dose, mice were anesthetized and bled to death from the subclavian vein. The mice were then extensively perfused with 0.1% heparin-PBS through the heart until all blood was removed. The ovaries, fallopian tubes, uterus, and vagina were taken out and then frozen at −20°C. For analyses of antibody contents, the organs were extracted by 2% (wt/vol) saponin-PBS, 1 μl/mg of organ, and allowed to thaw overnight. After centrifugation, the supernatants were stored at −20°C (5).
The titers of IgG and IgA to protein Rib in serum and in genital extracts were determined by ELISA. The wells of microtiter plates (Nunc, Roskilde, Denmark) were coated by incubation overnight at room temperature with 100 μl of a solution of Rib in PBS (0.6 μg/ml). The plates were blocked with 0.1% bovine serum albumin-PBS by incubation for 30 min at 37°C and then washed once with PBS. Samples and controls were added in threefold serial dilutions. As a reference and a positive control, a serum pool from mice immunized with Rib-CTB, Rib+CTB i.n., and Rib s.c. was used. To detect specific antibodies, the plates were washed three times with PBS containing 0.05% Tween (PBST), and horseradish peroxidase-conjugated goat anti-mouse γ chains (Jackson Immunoresearch Laboratories, Westgrove, Pa.) diluted 1:3,000 in 0.1% bovine serum albumin-PBST or goat anti-mouse IgA (Southern Biotechnology Inc., Birmingham, Ala.) diluted 1:1,000 in 0.1% bovine serum albumin-PBST was added to the wells. The plates were incubated for 90 min at room temperature. After three washes with PBST, the plates were developed using o-phenylenediamine (Sigma) and H2O2. ELISA titers are given as the reciprocal dilution giving an absorbance of 0.4 above the background level in a microplate reader (Labsystems Multiscan Plus, Helsinki, Finland) at 450 nm.
Intranasal immunization of mice with Rib-CTB or Rib+CTB elicited significant levels of serum IgG to protein Rib (Table 1). In contrast, the IgG response induced by i.n. immunization with Rib alone was approximately 100 times lower (Table 1). In agreement with previous studies (17, 18), s.c. immunization with protein Rib elicited a systemic Rib-specific IgG response (Table 1).
TABLE 1.
Titers of Rib-specific IgG in serum after immunization with different vaccine compositionsa
| Vaccine | Route of immunization | Titer of IgG, geometric mean (range)b |
|---|---|---|
| Rib-CTB | i.n. | 6,959 (1,772-27,258) |
| Rib+CTB | i.n. | 10,342 (6,288-14,988) |
| Rib | i.n. | 86 (40-261) |
| CTB | i.n. | 4 (≤3-11) |
| Rib | s.c. | 2,243 (640-4,030) |
Mice (four per group) were immunized thrice with Rib-CTB, Rib+CTB, Rib, or CTB i.n. or twice with Rib s.c.
The titers of antibody are presented as the antilog of the geometric mean and range. Titers of ≤3 were not detected. Background values from mice sham immunized with PBS are subtracted.
Rib-specific IgA antibodies were found in serum and in genital tissue extracts from mice i.n. immunized with Rib-CTB. Low levels of IgA were also detected in serum from mice that received Rib+CTB (Table 2). In contrast, Rib given i.n. without CTB did not result in any measurable IgA response. The method used in this study for measuring local antibodies in mucosal tissue has previously been evaluated regarding the proportion of serum antibodies from blood retained in the tissue extracts. The proportion of serum IgA antibodies in genital tissue extracts in that control experiment was approximately 2% (13). We compared the titers of IgA in the genital tissue extracts after i.n. immunization with Rib-CTB with the corresponding titers of IgA in serum, and we found that most of the IgA antibodies in the genital tissue appeared to be locally produced, since the titer of IgA exceeded 2% of the titer in serum (Table 2). Low levels of Rib-specific IgG were detected in the genital tissue extracts from mice i.n. immunized with Rib-CTB or Rib+CTB or s.c. immunized with Rib alone (data not shown); however, most of these IgG antibodies were considered to be transuded from serum rather than locally produced, according to the observations made in the previous control experiment referred to above (13).
TABLE 2.
Titers of Rib-specific IgA in serum and genital tissue extract after immunization with different vaccine compositionsa
| Vaccine tissue | Route of immunization | Titer of IgA, geometric mean (range) inb:
|
|
|---|---|---|---|
| Serum | Genital tissue | ||
| Rib-CTB | i.n. | 53 (36-87)c | 8 (≤3-20) |
| Rib+CTB | i.n. | 8 (4-18) | 4 (≤3-8) |
| Rib | i.n. | ≤3 | ≤3 |
| CTB | i.n. | ≤3 | ≤3 |
| Rib | s.c. | 4 (≤3-5)c | 4 (≤3-9) |
Mice (four per group) were immunized thrice with Rib-CTB, Rib+CTB, Rib or CTB i.n. or twice with Rib s.c. The genital tissue was extracted with 2% saponin-PBS.
The titers of antibody are presented as the antilog of the geometric mean and range. Titers of ≤3 were not detected.
Only three sera were available for analysis of IgA.
Protection against lethal GBS infection.
Groups of 15 mice were vaccinated with Rib-CTB i.n., Rib+CTB i.n., Rib s.c., or PBS i.n., as described above. Two weeks after the booster mice were challenged i.p. with a ∼90% lethal dose of log-phase bacteria (BM110) diluted in 0.5 ml of Todd-Hewitt broth. Deaths were recorded daily for 7 days (17). Vaccine efficacy (or protective efficacy) was calculated by the following formula: (mortality in nonvaccinated − mortality in vaccinated)/(mortality in nonvaccinated). Statistical significance was estimated by Fisher's exact test and confidence intervals (CI). The confidence level was set at 0.95. A P value of less than 0.05 was considered statistically significant.
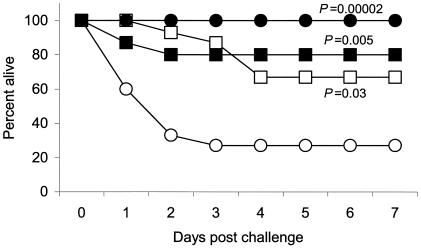
Immunization with CTB−Rib or CTB+Rib provided protection against lethal infection with the GBS type III strain BM110 expressing the Rib protein (Fig. 1). Ten of fifteen mice vaccinated with Rib-CTB i.n. and 12 of 15 mice vaccinated with Rib+CTB survived a challenge with a lethal i.p. dose of GBS. The protective efficacy for Rib-CTB was 55% (CI, 37 to 73; P = 0.03) and 73% (CI, 57 to 89; P = 0.005) for Rib+CTB. In agreement with previous reports (17, 18), s.c. vaccination with the Rib protein protected against lethal GBS infection. We observed, however, that even though the amount of the Rib protein used for s.c. immunization was reduced and was given without adjuvant, it still induced sufficient immunity to confer complete protection against lethal GBS infection (P = 0.00002) (Fig. 1).
FIG. 1.
Protection against lethal GBS infection by i.n. immunization with the Rib protein chemically conjugated to CTB (Rib-CTB) (□) or simply coadministered with CTB (Rib+CTB) (▪) and s.c. immunization with Rib alone (•). Sham-immunized mice received PBS i.n. (○). The vaccinated mice, 15 in each group, were challenged i.p. with a ∼90% lethal dose of GBS strain BM110. Deaths were recorded daily for 7 days. Fisher's exact test was used to calculate P values.
Conclusions.
Intranasal immunization of mice with the Rib protein and CTB seems to induce systemic and local antibody responses and to confer protective immunity against GBS infection. The titers of IgG induced by immunization with Rib+CTB and Rib-CTB i.n. were higher than the titers induced by Rib s.c. (Table 1). Yet, the protective efficacy for Rib given s.c. was higher than for Rib+CTB and Rib-CTB given i.n. (Fig. 1). However, s.c. injection and mucosal application represent presentation of the vaccine antigen (Rib) to the immune system in two different ways. In addition, the mucosal route included an adjuvant (CTB). These differences may have resulted in vaccine-induced antibodies with different avidities for the Rib protein. It seems possible that the conjugation process may be further optimized, resulting in a more immunogenic preparation.
We observed a wide range of antibody responses in some mouse groups (Tables 1 and 2). This variable antibody response was concordant with the individual response patterns for antibodies in both serum and genital tissue. However, since the number of mice in each group was small, it is difficult to draw general conclusions about the levels of antibody response to the different vaccines. The local antibody levels in the female genital tract vary with the stage of the estrous cycle, and it has been shown that pretreatment of mice with progesterone before mucosal immunizations increases the number of antibody-secreting cells in the genital tract not only in response to local vaginal immunization but also in response to i.n. immunization (13). Hence, the individual variation and the level of the antibody response might have been different in the present experiment had the mice been pretreated with hormones.
A relevant question is how the antibody response would be in humans. Although only vaccination studies with humans will give the full answer to this question, some encouraging observations have been published. In one study rhesus monkeys (Macacca mulatta) were immunized i.n. with a streptococcal protein antigen (AgI/II) either chemically conjugated to or mixed with CTB, which resulted in both systemic and local IgG and IgA responses to the vaccine antigens (23). Since these nonhuman primates are phylogenetically close to humans, it seems possible that humans would respond similarly to i.n. immunization with vaccines composed of bacterial protein and CTB. Further, i.n. immunization with CTB in humans has stimulated strong systemic as well as respiratory and vaginal mucosal antibody responses (3, 14, 22). Moreover, recent studies of naturally acquired antibodies to GBS cell surface proteins in different human populations have indicated that antibodies to GBS cell surface proteins are prevalent (10, 15), indicating that these protein antigens indeed are immunogenic also in humans.
The majority of clinically important GBS strains express either of the two proteins Rib or α (17, 25). Previously, we have shown that it is possible to include these two GBS cell surface proteins in a vaccine and elicit protective immunity in mice without any sign of immunogenic competition between the vaccine antigens (18). We conclude that i.n. immunization with a vaccine containing cell surface proteins from GBS strains that are prevalent among pregnant women may be an alternative strategy in the ongoing efforts to design an efficient vaccine against neonatal GBS disease.
Acknowledgments
This work was supported by grants from the Swedish Research Council (Medicine), the Meningitis Research Foundation (United Kingdom), Sida-SAREC's programme for research on AIDS and related sexually transmitted infections, the “Förenade Liv” Mutual Group Life Insurance Company, the Swedish Society for Medical Research, the Royal Physiographic Society in Lund, and the trusts of Jerring, Kock, and Österlund.
We thank Margaretha Stålhammar-Carlemalm for her help and for purified protein Rib, and we thank Gun Wallerström for laboratory assistance.
Editor: D. L. Burns
REFERENCES
- 1.Areschoug, T., M. Stålhammar-Carlemalm, C. Larsson, and G. Lindahl. 1999. Group B streptococcal surface proteins as targets for protective antibodies: identification of two novel proteins in strains of serotype V. Infect. Immun. 67:6350-6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker, C. J., M. A. Rench, M. S. Edwards, R. J. Carpenter, B. M. Hays, and D. L. Kasper. 1988. Immunization of pregnant women with a polysaccharide vaccine of group B streptococcus. N. Engl. J. Med. 319:1180-1185. [DOI] [PubMed] [Google Scholar]
- 3.Bergquist, C., E. L. Johansson, T. Lagergård, J. Holmgren, and A. Rudin. 1997. Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infect. Immun. 65:2676-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergquist, C., T. Lagergård, and J. Holmgren. 1997. Anticarrier immunity suppresses the antibody response to polysaccharide antigens after intranasal immunization with the polysaccharide-protein conjugate. Infect Immun. 65:1579-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergquist, C., T. Lagergård, M. Lindblad, and J. Holmgren. 1995. Local and systemic antibody responses to dextran-cholera toxin B subunit conjugates. Infect. Immun. 63:2021-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brodeur, B. R., M. Boyer, I. Charlebois, J. Hamel, F. Couture, C. R. Rioux, and D. Martin. 2000. Identification of group B streptococcal Sip protein, which elicits cross-protective immunity. Infect. Immun. 68:5610-5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards, M. S., and C. J. Baker. 2001. Group B streptococcal infections, p. 1091-1156. In J. S. Remington and J. O. Klein (ed.), Infectious diseases of the fetus and newborn infant. W. B. Saunders Company, Philadelphia, Pa.
- 8.Hordnes, K., A. Digranes, I. L. Haugen, D. E. Helland, M. Ulstein, R. Jonsson, and B. Haneberg. 1995. Systemic and mucosal antibody responses to group B streptococci following immunization of the colonic-rectal mucosa. J. Reprod. Immunol. 28:247-262. [DOI] [PubMed] [Google Scholar]
- 9.Hordnes, K., T. Tynning, T. A. Brown, B. Haneberg, and R. Jonsson. 1997. Nasal immunization with group B streptococci can induce high levels of specific IgA antibodies in cervicovaginal secretions of mice. Vaccine 15:1244-1251. [DOI] [PubMed] [Google Scholar]
- 10.Hordnes, K., T. Tynning, A. I. Kvam, L. Bevanger, T. A. Brown, R. Jonsson, and B. Haneberg. 1998. Cervical secretions in pregnant women colonized rectally with group B streptococci have high levels of antibodies to serotype III polysaccharide capsular antigen and protein R. Scand. J. Immunol. 47:179-188. [DOI] [PubMed] [Google Scholar]
- 11.Hordnes, K., T. Tynning, A. I. Kvam, R. Jonsson, and B. Haneberg. 1996. Colonization in the rectum and uterine cervix with group B streptococci may induce specific antibody responses in cervical secretions of pregnant women. Infect. Immun. 64:1643-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter, S. K., M. E. Andracki, and A. M. Krieg. 2001. Biodegradable microspheres containing group B streptococcus vaccine: immune response in mice. Am. J. Obstet. Gynecol. 185:1174-1179. [DOI] [PubMed] [Google Scholar]
- 13.Johansson, E. L., C. Rask, M. Fredriksson, K. Eriksson, C. Czerkinsky, and J. Holmgren. 1998. Antibodies and antibody-secreting cells in the female genital tract after vaginal or intranasal immunization with cholera toxin B subunit or conjugates. Infect. Immun. 66:514-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansson, E. L., L. Wassen, J. Holmgren, M. Jertborn, and A. Rudin. 2001. Nasal and vaginal vaccinations have differential effects on antibody responses in vaginal and cervical secretions in humans. Infect. Immun. 69:7481-7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lachenauer, C. S., C. J. Baker, M. J. Baron, D. L. Kasper, C. Gravekamp, and L. C. Madoff. 2002. Quantitative determination of immunoglobulin G specific for group B streptococcal beta C protein in human maternal serum. J. Infect. Dis. 185:368-374. [DOI] [PubMed] [Google Scholar]
- 16.Lagergård, T., J. Shiloach, J. B. Robbins, and R. Schneerson. 1990. Synthesis and immunological properties of conjugates composed of group B streptococcus type III capsular polysaccharide covalently bound to tetanus toxoid. Infect. Immun. 58:687-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsson, C., M. Stålhammar-Carlemalm, and G. Lindahl. 1996. Experimental vaccination against group B streptococcus, an encapsulated bacterium, with highly purified preparations of cell surface proteins Rib and α. Infect. Immun. 64:3518-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsson, C., M. Stålhammar-Carlemalm, and G. Lindahl. 1999. Protection against experimental infection with group B streptococcus by immunization with a bivalent protein vaccine. Vaccine 17:454-458. [DOI] [PubMed] [Google Scholar]
- 19.Lebens, M., S. Johansson, J. Osek, M. Lindblad, and J. Holmgren. 1993. Large-scale production of Vibrio cholerae toxin B subunit for use in oral vaccines. Bio/Technology (New York) 11:1574-1578. [DOI] [PubMed] [Google Scholar]
- 20.Madoff, L. C., J. L. Michel, E. W. Gong, A. K. Rodewald, and D. L. Kasper. 1992. Protection of neonatal mice from group B streptococcal infection by maternal immunization with beta C protein. Infect. Immun. 60:4989-4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musser, J. M., S. J. Mattingly, R. Quentin, A. Goudeau, and R. K. Selander. 1989. Identification of a high-virulence clone of type III Streptococcus agalactiae (group B streptococcus) causing invasive neonatal disease. Proc. Natl. Acad. Sci. USA 86:4731-4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudin, A., E. L. Johansson, C. Bergquist, and J. Holmgren. 1998. Differential kinetics and distribution of antibodies in serum and nasal and vaginal secretions after nasal and oral vaccination of humans. Infect. Immun. 66:3390-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell, M. W., Z. Moldoveanu, P. L. White, G. J. Sibert, J. Mestecky, and S. M. Michalek. 1996. Salivary, nasal, genital, and systemic antibody responses in monkeys immunized intranasally with a bacterial protein antigen and the cholera toxin B subunit. Infect. Immun. 64:1272-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen, X., T. Lagergård, Y. Yang, M. Lindblad, M. Fredriksson, and J. Holmgren. 2000. Systemic and mucosal immune responses in mice after mucosal immunization with group B streptococcus type III capsular polysaccharide-cholera toxin B subunit conjugate vaccine. Infect. Immun. 68:5749-5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stålhammar-Carlemalm, M., L. Stenberg, and G. Lindahl. 1993. Protein Rib: a novel group B streptococcal cell surface protein that confers protective immunity and is expressed by most strains causing invasive infections. J. Exp. Med. 177:1593-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wästfelt, M., M. Stålhammar-Carlemalm, A. M. Delisse, T. Cabezon, and G. Lindahl. 1996. Identification of a family of streptococcal surface proteins with extremely repetitive structure. J. Biol. Chem. 271:18892-18897. [DOI] [PubMed] [Google Scholar]
- 27.Wessels, M. R., L. C. Paoletti, D. L. Kasper, J. L. DiFabio, F. Michon, K. Holme, and H. J. Jennings. 1990. Immunogenicity in animals of a polysaccharide-protein conjugate vaccine against type III group B streptococcus. J. Clin. Investig. 86:1428-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]