Abstract
Various fluorodeoxyribonucleosides were evaluated for their antiviral activities against influenza virus infections in vitro and in vivo. Among the most potent inhibitors was 2'-deoxy-2'-fluorocytidine (2'-FdC). It inhibited various strains of low and highly pathogenic avian influenza H5N1 viruses, pandemic H1N1 viruses, an oseltamivir-resistant pandemic H1N1 virus, and seasonal influenza viruses (H3N2, H1N1, influenza B) in MDCK cells, with the 90% inhibitory concentrations ranging from 0.13 µM to 4.6 µM, as determined by a virus yield reduction assay. 2'-FdC was then tested for efficacy in BALB/c mice infected with a lethal dose of highly pathogenic influenza A/Vietnam/1203/2004 H5N1 virus. 2’FdC (60 mg/kg/d) administered intraperitoneally (i.p.) twice a day beginning 24 h after virus exposure significantly promoted survival (80% survival) of infected mice (p=0.0001). Equally efficacious were the treatment regimens in which mice were treated with 2'-FdC at 30 or 60 mg/kg/day (bid × 8) beginning 24 h before virus exposure. At these doses, 70–80% of the mice were protected from death due to virus infection (p=0.0005, p=0.0001; respectively). The lungs harvested from treated mice at day four of the infection displayed little surface pathology or histopathology, lung weights were lower, and the 60 mg/kg dose reduced lung virus titers, although not significantly compared to the placebo controls. All doses were well tolerated in uninfected mice. 2'-FdC could also be administered as late as 72 h post virus exposure and still significantly protect 60% mice from the lethal effects of the H5N1 virus infection (p=0.019). Other fluorodeoxyribonucleosides tested in the H5N1 mouse model, 2’-deoxy-5-fluorocytidine and 2'-deoxy-2', 2'-difluorocytidine, were very toxic at higher doses and not inhibitory at lower doses. Finally, 2'-FdC, which was active in the H5N1 mouse model, was also active in a pandemic H1N1 influenza A infection model in mice. When given at 30 mg/kg/d (bid × 5) beginning 24 h before virus exposure), 2’-FdC also significantly enhanced survival of H1N1-infected mice (50%, p=0.038) similar to the results obtained in the H5N1 infection model using a similar dosing regimen (50%, p<0.05). Given the demonstrated in vitro and in vivo inhibition of avian influenza virus replication, 2’FdC may qualify as a lead compound for the development of agents treating influenza virus infections.
Keywords: 2'-deoxy-2'-fluorocytidine, fluorodeoxyribonucleosides, H5N1 avian influenza virus, BALB/c mouse, pandemic H1N1 virus
1. Introduction
The impact of influenza infection is felt globally each year causing apparent disease in approximately 20% of the world’s population (Moscona, 2005). Human influenza causes a variety of infections ranging from acute lower respiratory disease that can culminate in acute respiratory distress syndrome (ARDS) to a mild, self-limiting febrile illness (http://www.cdc.gov/flu/avian/gen-info/facts.htm). Seasonal influenza A and pandemic H1N1 influenza A viruses have the potential to cause pandemic disease that periodically has been associated with high mortality rates. In addition, there is great concern that highly pathogenic avian influenza H5N1 viruses that infect and cause inordinate amount of lethal disease in humans could evolve to pose a pandemic risk (Kwon et al., 2011).
Usually, “avian influenza virus” refers to influenza A viruses causing severe, epidemic infections in birds, although the severe and lethal infections with these viruses can occur in humans. The risk of infection for humans from these highly pathogenic avian influenza viruses is generally low because the viruses are not readily transmissable to humans from birds or from infected to uninfected humans (Kwon et al., 2011). However, the virus can be very lethal in humans with mortality rates approaching 50% (http://www.who.int/csr/disease/avian_influenza/country/en/), thus making this a virus of extreme concern. The H5N1 viruses are endemic in Asia and have spread into Europe and America (http://www.who.int/csr/disease/avian_influenza/country/en/). Human H5N1 influenza infection was first recognized in 1997 when this virus infected 18 people in Hong Kong, causing 6 deaths (Centers for Disease Control and Prevention 1997; 1998a; 1998b; 1998c; Claas et al., 1998; Suarez et al., 1998). Almost all cases of avian influenza infection in humans have resulted from contact with infected chickens or ducks or surfaces contaminated with secretion/excretions from infected birds (http://www.cdc.gov/flu/avian/gen-info/facts.htm). Up to 16 March 2011, more than 534 human H5N1 cases have been diagnosed around the world and more than half of those infected have died as a result of the virus (http://www.who.int/csr/disease/avian_influenza/country/en/). The Centers for Disease Control and Prevention (CDC) anticipate that there will be more cases, more hospitalizations and more deaths associated with this virus because the population has little to no effective protective immunity against these virus strains (http://www.cdc.gov/flu/avian/gen-info/facts.htm).
Although vaccination is the primary strategy for the prevention of influenza infections, vaccines are ineffective against rapidly emerging mutant viral antigens (Verity et al., 2011). Vaccine production by current methods cannot be carried out with the speed required to halt the progress of a new influenza virus strain (Hayden, 2004), although recently some experimental H5N1 and H1N1 pandemic influenza virus vaccine candidates have been rapidly developed using synthetic DNA approaches, including site-directed mutagenesis of DNA encoding related virus strains, and rapid generation of virus using synthetic DNA cloned into plasmid vectors (Verity et al., 2011). In addition, vaccines that are specific for newly arising strains require several months of preparation, and although the development of a vaccine against H5N1 influenza has been under way for a number of years, none is yet approved for use in the United States (http://www.cdc.gov/flu/avian/gen-info/facts.htm). Efforts have been intensified in recent years to understand pathogenesis of influenza infections and to develop antiviral therapies to treat seasonal, pandemic as well as H5N1 influenza disease.
There are four anti-influenza drugs approved for use in the United States. The M2 ion channel inhibitors amantadine and rimantadine and the influenza virus neuraminidase (NA) inhibitors oseltamivir and zanamivir (Preziosi, 2011). Some studies have indicated that many of the avian influenza H5N1 viruses are resistant to both amantadine and rimantadine (Trampuz et al., 2004), with the viruses having an M gene containing mutations associated with this resistance (Puthavathana et al., 2005). Oseltamivir (Tamiflu) is a widely used, effective treatment for all strains of influenza. In addition, i.v.-administered peramivir has been approved by the United States Food and Drug Administration for emergency use (Hernandez et al., 2011). It is orally administered to directly target the site of infection, the respiratory tract. However, of the existing antiviral drugs, only oseltamivir and zanamivir seem to inhibit the avian H5N1 viruses, although the inhibition in some cases is modest and there is the concern of development of resistance if these two drugs were routinely used to treat H5N1 infections (Govorkova et al., 2001; Leneva et al., 2000; Naughtin et al., 2011). Therefore, there is a great need to develop easily synthesizable, alternative potent anti-influenza drugs to combat human disease caused by highly pathogenic avian influenza H5N1 viruses (HPAIV) and to address the potential need to treat newly emerging drug-resistant strains of influenza viruses (Aoki et al., 2007; Thorlund et al., 2011; Ujike et al., 2011). In this regard, a promising experimental anti-influenza drug, favipiravir (6-fluoro-3-hydroxy-2-pyrazinecarboxamide, T-705) has been shown to inhibit a variety of influenza viruses, including HPAIV (Furuta et al., 2002; Sidwell et al., 2007).
The antiviral effects of 2'-deoxy-2'-fluorocytidine (2’FdC) were first reported for the herpes virus, pseudorabies virus and equine abortion virus (Wohlrab et al., 1985). Bajramovic et al. reported that 2'-fluoro-2'-deoxycytidine exhibited potent antiviral activity against Borna disease virus (BDV) (Bajramovic et al., 2004). Stuyver et al. demonstrated that 2’FdC is a potent inhibitor of the hepatitis C virus RNA replicon in Huh-7 cells (Stuyver et al., 2004). They suggested that 2’FdC was shown to affect both a viral target and a cellular target. On the other hand, Skoog et al. first reported that 2’-azido-2’-deoxycytidine is an inhibitor of DNA replication in mammalian cells (Skoog et al., 1977). Bjursell et al. further confirmed that 2’-azido-2’-deoxycytidine blocked the initiation of polyoma virus DNA synthesis (Bjursell et al., 1977). It also has been previously reported that 2’-azido-2’-deoxycytidine inhibited HIV (Bianchi et al., 1994; Giacca et al., 1996). In addition, 5-fluoro-2’-deoxycytidine was found to inhibit thymidine kinase-deficient (TK-) mutant strains of herpes simplex virus (HSV) (De Clercq et al., 1987).
2'-Fluororibosides have also specifically been investigated as potential antiviral agents. A series of 2'-fluororibosides were evaluated for anti-influenza activity in cell culture and in a mouse pneumonia model (Tisdale, 1993; Tuttle et al., 1993). Many were found to be inhibitors of influenza A. Of the compounds tested in MDCK cells, 2'-deoxy-2'-fluoroguanosine and 2'-deoxy-2'-fluorocytosine were the most potent inhibitors of influenza virus replication with EC50 values of 18.2 and 2.6 µM, respectively. 2'-deoxy-2'-fluoroguanosine was a more potent inhibitor of influenza virus replication in chicken embryo fibroblasts and both compounds were found to be equally inhibitory for influenza virus replication in human tracheal cultures. 2'-deoxy-2'-fluoroguanosine was found to be broad-spectrum in vitro inhibitor of influenza viruses, inhibiting influenza AH1N1, H2N2, H3N2, and influenza B viruses nearly equally. 2'-Deoxy-2'- fluoroguanosine was found to selectively inhibit the influenza transcriptase complex and was less inhibitory to transcriptase complexes isolated from 2'-deoxy-2'-fluoroguanosine partially resistant influenza virus (Tisdale et al., 1995) In another study, it was shown that the differences of in vitro anti-influenza virus activity of twenty purine fluororibonucleosides was based on the amount of nucleoside triphosphates of each compound that was generated in MDCK cells and CEF cells (Tuttle et al., 1993). In vivo, 2'-deoxy-2'-fluoroguanosine (40 mg/kg) delivered orally at 0.5, 4 and 20 h after virus exposure was shown to reduce lung virus titers at 24 after treatment more effectively than amantadine or ribavirin and to protect 50% of mice from death when administered subcutaneously twice a day for 5 days beginning 4 h prior to exposure to virus. (Tisdale, 1993). In that same study, 2’FdC had in vitro activity, but did not reduce virus lung titers in the mouse model at 24–48 h post virus exposure. In ferrets, 2'-deoxy-2'-fluoroguanosine at 20 mg/kg given 1 h after virus exposure reduced virus replication in the lungs, amelioration of fever, and nasal inflammation (Jakeman et al., 1994). Thus, 2'-fluororibosides may warrant further evaluation of influenza virus inhibition in vitro and in vivo, especially against highly pathogenic avian influenza A viruses such as the H5N1 strains.
In the current study, we tested the effects of fluorocytidine analogs for inhibition of highly pathogenic avian influenza H5N1 viruses as well as other influenza viruses in vitro. Several inhibitory analogs were then tested for efficacy in BALB/c mice infected with a lethal dose of the highly pathogenic influenza A/Vietnam/1203/2004 H5N1 virus or with a pandemic H1N1 virus.
2. Materials and Methods
2.1. Viruses and cells
Influenza A/Vietnam/1203/2004 (H5N1), A/Thailand/2(Kan-1)/16/2004 (H5N1),A/Hong Kong/213/2003 (H5N1), and A/CA/04/09 (H1N1) viruses were obtained from the CDC (Atlanta, GA). Influenza A/Hong Kong/213/2003 (H5N1), A/Gull/PA/4175/83 (H5N1), and A/Duck/MN/1525/81 (H5N1) viruses were provided by Dr. Robert G. Webster, St. Jude Children’s Research Hospital (Memphis, TN). Influenza A/Hong Kong/213/2003 × Ann Arbor/6/60 strain (A/Hong Kong/213/2003H) and A/Vietnam/1203/04 × Ann Arbor/6/60 (A/Vietnam/1203/04H) hybrid viruses were also used (George Kemble, Medlmmune Vaccines, Inc., Mountain View, CA). These latter viruses are attenuated hybrid viruses expressing the A/Vietnam/1203/2004 or A/Hong Kong/213/2003 hemagglutinin (HA) and NA proteins, but with a replication core from the cold-adapted Ann Arbor H1N1 virus, which contains the PB1, PB2, PA, NP, M, and NS gene segments of the latter virus generated by the use of reverse genetics. The HA gene was genetically modified to remove the stretch of basic amino acids connecting the HA1 and HA2 domains of HA, as described by Suguitan et al. (2006). A/Influenza A/Sydney/05/97 (H3N2), A/Brisbane/10/2007 (H3N2), influenza A/CA/07/2009 (H1N1) viruses and A/Hong Kong/2369/2009 (H1N1) virus with the NA 274Y mutation for oseltamivir resistance were from CDC (Chen et al., 2009). The influenza B viruses, B/Malaysia/2506/2004 and B/Florida/4/2006, were received from CDC. All the viruses used in cell culture experiments were passaged through Madin-Darby canine kidney (MDCK) cells (American Type Culture Collection, Manassas, VA) at least once to prepare pools. The pools were then titrated in MDCK cells before use. The cells were grown in MEM containing 5% fetal bovine serum (Thermo Fisher Scientific Inc., Logan, UT) and 0.18% sodium bicarbonate with no antibiotics in a 5% CO2 incubator. Viral propagation and assays were done in MDCK cells in MEM, 0.18% sodium bicarbonate, 10 units of trypsin/ml, and 1.0 µg EDTA/ml.
A novel mouse adapted H1N1 strain was originally obtained from Elena Govorkova (St. Jude Children’s Research Hospital, Memphis, TN). It has been designated as influenza A/CA/04/09 pandemic H1N1 virus (SJ#175190) and was adapted to mice by Natalia A. Ilyushina (St. Jude Children’s Research Hospital, Memphis, TN). The influenza A/CA/04/09 strain used in this study was first passaged in MDCK cells and then grown in embryonated chicken eggs. It was adapted to mice by 9 sequential passages through mouse lungs. The virus from the last lung passage was then plaque purified in MDCK cells and amplified in embryonated chicken eggs. The virus was amplified in MDCK cells to prepare stocks for mouse studies. Mice were exposed to 3 LD50 (103.39 PFU) of virus by the intranasal (i.n.) route.
2.2. Test compounds
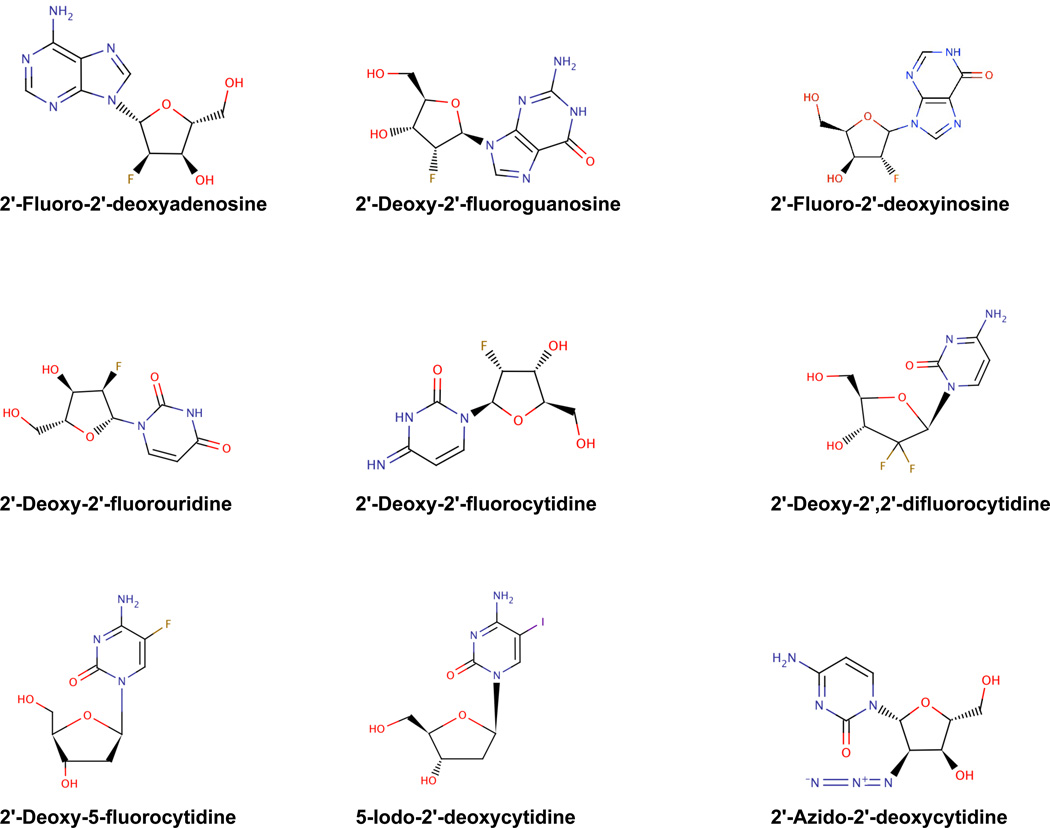
Ribavirin was provided by Z. Hong of Valeant Pharmaceuticals, Inc. (Costa Mesa, CA). Oseltamivir was purchased from a local pharmacy. 2-fluoro-2'-deoxyadenosine, 2'-deoxy-2'-fluorouridine, 2'-deoxy-2'-fluoroguanosine, 2'-azido-2'-deoxyctidine-5'-triphosphate, 2'-deoxy-5-fluorocytidine, and 2'-deoxy-2'-fluorocytosine were tested in this study. 2’FdC was obtained from ChemGenes Corporation (Wilmington, MA 01877) and from Carbosynth Ltd., (Compton, Berkshire, RG20 6NE, UK). 2'-Deoxy-2'-fluorouridine and 2'-deoxy-2'-fluoroguanosine were from Carbosynth Ltd., (Compton, Berkshire, RG20 6NE, UK). 2'-Azido-2'-deoxyctidine-5'-triphosphate was provided by TriLink BioTechnologies (San Francisco, CA). 2'-Fluoro-2'-adenosine and 2'-deoxy-5-fluorocytidine were obtained from Sigma-Aldrich (St. Louis, MO). See Figure 1 for structures. All preparations used for in vivo animal studies were suspended in 0.4% carboxymethyl cellulose (CMC) or solubilized in physiologically sterile saline (PSS), and stored at 4°C until they were used. Test compounds were dissolved in dimethyl sulfoxide (DMSO) for in vitro studies and diluted 1:100 in MEM for efficacy testing.
Figure 1.
Structures of fluoronucleoside analogs.
2.3. In vitro antiviral evaluations
Antiviral activity was determined in vitro by the following methods: inhibition of virus-induced cytopathic effect (CPE), as determined by visual (microscopic) examination of the cells; increases in the uptake of the vital dye neutral red into cells (NR assay); and virus yield reduction (VYR assay). These methods have been described previously (Smee et al., 2001). Cytopathic effect observations measure virus effects on cell morphology, which may or may not reflect physiological changes in the cell induced by virus infection. Neutral red is a vital dye, which stains functioning lysosomes and thus reflects the over all physiological status of the cell, and virus yield reduction assay measures the effects of a compound on the amount of virus released from a cell. Thus, one could expect a compound may have profound effect on one of the parameters measured, but not one of the other parameters measured that are described above. Eight one-half log10 concentrations of the test compounds were evaluated in triplicate wells containing influenza virus-MDCK cells. Standard placebo-treated virus controls, toxicity controls, and normal-medium controls were included in all assays. In vitro assays were done in MDCK cells grown in MEM supplemented with 0.18% sodium bicarbonate with 10 units of trypsin/ml, 1.0 µg EDTA/ml, and 50 µg gentamicin/ml added for the antiviral assays. All assays were incubated at 37°C. CPE inhibition data were expressed as the 50% inhibitory (viral CPE-inhibitory) concentration (IC50); the 50% cytotoxic (cell-inhibitory) concentration (CC50); and the selectivity index (SI), which was determined as CC50/IC50.
The neutral red (NR) uptake assay was performed as described (Kumaki et al., 2011). Briefly, medium was removed from each well of a plate, 0.011% neutral red (NR) was added to each well of the plate, and the plate was incubated for 2 h at 37°C in the dark. The neutral red (NR) solution was removed from the wells, the wells were rinsed and any remaining dye was extracted using Sörenson's citrate buffered ethanol. Absorbances at 540 nm/405 nm were read with a microplate reader (Opsys MR™, Dynex Technologies, Chantilly, VA). Absorbance values were expressed as percentages of untreated controls and IC50, CC50, and SI values were calculated as described above.
The titers of infectious virus produced from supernatant fluids of infected cells from antiviral CPE inhibition assays were determined as previously described (Smee et al., 2001). After scoring the CPE in each test well of a plate, each plate was frozen at −80°C and then thawed. Sample wells for one compound concentration tested were pooled and titered in MDCK cells for infectious virus by CPE assay. A total of 8 test concentrations for each compound tested were titered for virus production. The virus yield reduction IC90 value for each compound was determined by linear regression analysis and was defined as the concentration that inhibited the virus yield from cell supernatants by a factor of one-log10 (IC90) and was used to confirm the results of the CPE inhibition/NR uptake assays.
2.4. Animals
Specific pathogen-free female 14–18 g BALB/c mice were obtained from Charles River Laboratories (Wilmington, MA) for this study. They were maintained on Wayne Lab Blox and fed with standard mouse chow and tap water ad libitum. The BALB/c mice were quarantined for 24 h prior to use. The highly pathogenic avian influenza H5N1 virus animal studies were carried out in a certified bio-safety level 3+ animal facility. Animal procedures complied with the guidelines set forth by USDA and the Utah State University Institutional Animal Care and Use Committee.
2.5 Animal experimental design
Many highly pathogenic H5N1 avian influenza A viruses readily infect mice without adaptation and often cause lethal disease in these animals (Gubareva et al., 1998; Hatta et al., 2007; Lu et al., 1999). A number of parameters can be used to monitor influenza virus infections in mice with some of the more common measures including mean time to death, arterial oxygen saturation decline, lung pathology scores, lung weights, change in host weights, lung virus titers, and histopathological changes in the lung (Sidwell and Smee, 2000).
Female BALB/c mice were anesthetized with a 0.1 ml intraperitoneal (i.p.) injection of 50 mg/kg of Ketamine® and influenza A/Vietnam/1203/2004 H5N1 virus (5 PFU = 1 LD90) or A/CA/04/09 H1N1 virus (103.4 PFU = 3 LD50) was administered intranasally (i.n.) in a volume of 0.05 ml. Groups of 10 mice were administered varying concentrations of 2’FdC per os (p.o.) or i.p. twice a day for 8 days (bid × 8) beginning 24 h before virus exposure. Ribavirin (75mg/kg/d), when used a positive control drug, was also administered p.o. or i.p. 24 h before virus exposure, depending on the experiment. Other fluorocytidine compounds were only administered by the i.p. route. Fifteen mice were either treated p.o. with water or 0.4% CMC or i.p. with PSS or 0.4% CMC 24 h prior to virus exposure and then twice a day for 8 days. Mice in these two groups represented the placebo controls. Animal deaths were recorded for up to 21 days post virus exposure. Avian influenza virus-inoculated and mock-infected mice were weighed every day and clinical signs were observed. Weight loss was determined in all infected and uninfected groups of mice. Animals that lost greater than 30% of their initial body weight were humanely euthanized by CO2 asphyxiation, and the day of euthanization was designated as the day of death due to infection. For certain experiments, five mice from each group were sacrificed on day 4 after virus exposure. Each mouse lung was collected and homogenized for virus titer determination. The samples were assayed in triplicate in MDCK cells by cytopathic effect assay, and the 50% cell culture infectious dose values calculated. Parameters for determining the effects of treatment included the prevention of death through 21 days, inhibition of lung consolidation (lung score and lung weight), and lessening of lung virus titers. Toxicity controls, consisting of three mice for each compound concentration tested, were evaluated daily for weight loss and adverse events such as ruffling of fur, lethargy, paralysis, incontinence, repetitive circular motion, and aggression.
2.6. Compound toxicity determination
For the fluorocytidines tested for efficacy in mice, a dose range finding experiment was usually done to determine the maximum tolerated concentration. Five mice were used per treatment group; with weights taken prior to the start of treatment, and the animals were observed for overt signs of toxicity and death for 21 days. Adverse events for which observations were made included ruffling of fur, lethargy, paralysis, incontinence, repetitive circular motion, and aggression. Mice were also weighed every day from 24 h prior to virus infection to day 21 post virus exposure. Untreated controls were also included in each study and evaluated for healthiness as described above.
2.7. Lung score/lung weight determinations
Lungs were scored based on surface appearance (gross pathology, hemorrhage) of lungs. Lungs were assigned a score ranging from 0 to 4, with 0 indicating that the lungs looked normal and 4 denoting that the entire surface area of the lung was inflamed and showed plum colored lung discoloration (Sidwell et al., 1995).
2.8. Lung virus titer determinations
Lung virus titers were analyzed from mice sacrificed on day 4 post virus exposure. A lobe from each mouse lung was homogenized and the tissue fragments were allowed to settle. The various dilutions of the supernatant fluids were assayed in triplicate for infectious virus in MDCK cells by CPE assay, as described previously (Sidwell et al., 2001). The titers (CCID50 values) were calculated using the Reed-Muench (1938) method.
2.9. Statistical analysis
Mice were weighed in groups prior to treatment and then every day thereafter to determine the average weight change for all animals in each treatment group. Weights were expressed as group averages for each day and evaluated for statistical significance by the two-way analysis of variance. Differences between two treatment groups were analyzed for significance by Bonferroni’s pairwise comparison tests (Jaccard et al., 1984). Survival analyses were analyzed using the Kaplan-Meier graphical method and a Logrank test (Lee and Wang, 2003). When significant differences among the treatment groups were observed, pairwise comparisons of survivor curves (placebo vs. any treatment) were analyzed by the Gehan-Breslow-Wilcoxon test (GraphPad Prism® for MAC v5), and the relative significance was adjusted to a Bonferroni-corrected significance threshold for the number of treatment comparisons done (Bland, 2000). Hazard ratios, which compare how rapidly groups of treated mice die relative to untreated control groups of mice, were determined by the Mantel-Haenszel tests as part of the survival analysis program used above (GraphPad Prism® for MAC v5). Mean day of death (deaths caused by disease) was calculated and analyzed by the Kruskal-Wallis test followed by Dunn’s posttest for evaluating the significant pairwise comparisons (Dunnett and Tamhane, 1992).Live numbers per total mice in a group differences were evaluated by contingency table analysis. Significant differences in lung weights and lung virus titers were detected by ANOVA. Subsequent pairwise comparisons were made by Newman-Keuls posttests. Significant differences in lung scores were determined by Kruskal-Wallis test followed by Dunn’s pairwise comparison posttests.
3. Results
3.1. Effects of 2’FdC on H5N1 avian influenza virus infection in vitro
We first examined the effect of 2’FdC and 2’-deoxy-5-fluorocytidine (5'-FdC) (Figure 1), two of the compounds shown to inhibit other RNA viruses, for inhibition of replication of various avian H5N1 influenza viruses in MDCK cells at 37°C. 2'-FdC most potently inhibited the Hong Kong/213/2003 (H5N1) virus with IC50 values of 0.05 µM by visual assay with an IC50 value of 0.27 µM by neutral red uptake assay (Table 1). This activity was confirmed by virus yield reduction assay; IC90 = 1.54 µM. 2'-FdC also inhibited the A/Thailand/2(Kan-1)/16/2004 in a similar fashion (NR IC50 = 0.16, NR IC50 = 0.72, VYR IC90 = 0.69). This inhibition was independent of cell cytotoxicity; the SI values for this compound ranged from 251–1954. However, the A Vietnam/1203/2004 virus was somewhat less sensitive to inhibition with IC50 values = 8.0 and 0.9 µM by visual and NR assays and an IC90 = 2.9 µM.
Table 1.
Effects of 2’-deoxyfluorocytidines on the replication of highly pathogenic avian H5N1 influenza A viruses in MDCK cells.
| Visual Assay | Neutral Red Uptake Assay | VYR Assaya | ||||||
|---|---|---|---|---|---|---|---|---|
| Virus | Compound | IC50b | CC50c | Sld | IC50 | CC50 | SI | IC90e |
| Vietnam/1203/2004 | 2’-deoxy-2’-fluorocytidine | 8.0 ± 10.4 | >100 ± 0 | >36 ± 28.0 | 0.9 ± 1.2 | 82 ± 31.2 | 196 ± 137.2 | 2.9 |
| 2'-deoxy-5-fluorocytidine | 0.19 ± 0.03 | >100 ± 0 | >535 ± 78 | 0.09 ± 0.1 | >100 ± 0 | >1100 ± 0.0 | 0.13 | |
| Thailand/2(Kan1)/16/2004 | 2’-deoxy-2’-fluorocytidine | 0.16 ± 0.04 | >100 ± 0 | >676 ± 204 | 0.72 ± 0.51 | >100 ± 0 | >251 ± 249 | 0.69 |
| 2'-deoxy-5-fluorocytidine | >100 ± 0 | >100 ± 0 | 0 ± 0 | >100 ± 0 | >100 ± 0 | 0 ± 0 | >100 | |
| Hong Kong/213/2003 | 2’-deoxy-2’-fluorocytidine | 0.05 ± 0.01 | >100 ± 0 | >1954 ± 553 | 0.27 ± 0.14 | >100 ± 0 | >447 ± 216 | 1.54 |
| 2'-deoxy-5-fluorocytidine | >100 ± 0 | >100 ± 0 | 0 ± 0 | >100 ± 0 | >100 ± 0 | 0 ± 0 | >100 | |
Values for CPE and NR assays are arithmetic means of three independent experiments. VYR assays were done one time.
Virus Yield Reduction Assay;
IC50: 50% virus inhibitory concentration;
CC50: 50% cell cytotoxic concentration of drug;
selective index: Visual and neutral red (NR) SI = CC50/IC50;
IC90: 90% virus inhibitory concentration (1log10 drop in virus titer);
5-FdC also potently and selectively inhibited the A/Vietnam/1203/2004 virus with an IC50 value of 0.19 µM by visual assay and 0.09 µM by neutral red uptake assay. SI values ranged from >535 to >1100. This inhibitory activity was confirmed by virus yield reduction assays with an IC90 calculated to be equal to 0.13 µM. However, the other two viruses from clade 1 were completely insensitive to inhibition by this compound. These observations were seen in three different experiments.
3.2. Effects of other fluororibonucleosides and 2'-azido-2'-deoxyctidine-5'-triphosphate on H5N1 avian influenza virus replication in vitro
We further examined the effects of other fluorodeoxyribonucleosides for inhibition of H5N1 influenza virus replication to see how important the nucleoside was for inhibiting virus replication (Table 2). 2'-azido–2'-deoxyctidine–5’-triphosphate (Figure 1) was also evaluated to see if the azide group contributed significantly to the antiviral effect seen previously and because 4’ azidocytidine analogs have been shown to inhibit Hepatitis C virus (Smith et al., 2009). The Hong Kong/213/2003 and Vietnam/1203/2004 viruses were modestly inhibited by 2'-deoxy-2'-fluoroguanosine (Figure 1) with IC50 values ranging from 8.5–21 µM, but this activity was only slightly selective for virus inhibition when tested up to a concentration of 100 µM. The Thailand/2/(KAN1)/16/2004 virus was not inhibited by the compound. 2-Deoxy-2'-fluoroadenosine (Figure 1) and 2'-deoxy-2'-fluorouridine (Figure 1) essentially did not inhibit any of the H5N1 viruses. 2'-azido–2'-deoxyctidine–5’-triphosphate exhibited the same strain specific inhibition that was demonstrated for 5'-FdC; it only inhibited the A/Vietnam1203/2004 virus and not the other two strains. We assume that in order to enter cells, 2’-azido-2’-deoxycytidine-5’-triphosphate will dephosphorylate and become 2’-azido-2’-deoxycytidine. The triphosphate form was the only source of this compound that we found commercially available. This inhibition of the A/Vietnam/1203/2004 strain was very potent with IC50 values averaging from 0.05 µM for the three visual assays done to 0.09 µM for the three neutral red uptake assays that were performed. The activity was very selective with SI values ranging from >2030 to >2045.
Table 2.
Effects of other cytidine analogs (µM) on the replication of highly pathogenic avian H5N1 influenza A viruses in MDCK cells.
| Visual Assay | Neutral Red Uptake Assay | ||||||
|---|---|---|---|---|---|---|---|
| Virus | Compound | IC50a | CC50c | Sld | IC50 | CC50 | SI |
| Vietnam/1203/2004 | 2’-deoxy-2’-fluoroadenosine | >100 | >100 | 0 | >100 | >100 | 0 |
| 2’-deoxy-2’-fluoroguanosine | 21 | >100 | >4.8 | 8.6 | >100 | >12 | |
| 2’-deoxy-2’-fluorouridine | >100 | >100 | 0 | >100 | >100 | 0 | |
| 2’-iodo-2’-deoxycytidine | >100 | >100 | 0 | >100 | >100 | 0 | |
| 2’-azido-2’-deoxycytidinef | 0.05 ± 0.01e | >100 ± 0 | >2045 ± 248 | 0.09 ± 0.09 | >100 ± 0 | >2030 ± 1347 | |
| Thailand/2(Kan 1)/16/2004 | 2’-deoxy-2’-fluoroadenosine | >100 | >100 | 0 | >100 | >100 | 0 |
| 2’-deoxy-2’-fluoroguanosin | >100 | >100 | 0 | 31 | >100 | >3.2 | |
| 2’-deoxy-2’-fluorouridine | >100 | >100 | 0 | 62 | >100 | >1.6 | |
| 2’-iodo-2’-deoxycytidine | >100 | >100 | 0 | >100 | >100 | 0 | |
| 2’-azido-2’-deoxycytidine | >100 ± 0e | >100 ± 0 | 0 ± 0 | >100 ± 0 | >100 ± 0 | 0 ± 0 | |
| Hong Kong/213/2003 | 2’-deoxy-2’-fluoroadenosine | >100 | >100 | 0 | >100 | >100 | 0 |
| 2’-deoxy-2’-fluoroguanosine | 8.5 | >100 | >12 | 17 | >100 | >5.9 | |
| 2’-deoxy-2’-fluorouridine | 32 | >100 | >3.1 | 32 | >100 | >3.1 | |
| 2’-iodo-2’-deoxycytidine | >100 | >100 | 0 | >100 | >100 | 0 | |
| 2’-azido-2’-deoxycytidine | >100 ± 0e | >100 ± 0 | 0 ± 0 | >100 ± 0 | >100 ± 0 | 0 ± 0 | |
Values for CPE and NR assays are arithmetic means of three independent experiments.
IC50: 50% virus inhibitory concentration;
CC50: 50% cell cytotoxic concentration of drug;
selective index (SI = CC50/IC50; virus titer);
5’-Triphosphate derivative; assay was done three times.
3.3. Effects of 2’FdC on other influenza viruses in vitro
From the data previously presented, it appears that 2’-FdC consistently inhibited all viruses tested. Thus, further experiments were done to describe the spectrum of virus inhibition by this compound. To determine the broad spectrum anti-influenza activity of 2'-FdC, the in vitro sensitivity of two hybrid strains of influenza expressing H5N1 NA from an H1N1 backbone, A/Hong Kong/213/2003H (H5N1) and Vietnam/1203/2004H (H5N1), two low pathogenic avian influenza H5N1 strains, A/Duck/MN/1525/81 and A/Gull/PA/4175/83H, two H3N2 viruses A/Sydney/05/97 and A/Brisbane/10/2007, two strains of 2009 H1N1 viruses (A/CA/07/2009 and A/Hong Kong/2369/2009 (oseltamivir resistant, H275Y mutation), and two strains of influenza B Malaysia/2506/2004 and Florida /4/2004 virus were evaluated in CPE inhibition assays, in neutral red (NR) uptake assays, and in virus yield reduction assays (Table 3). In addition, the difluoro analog of 2'-FdC approved for cancer therapy (Sai and Saito, 2011), gemcitabine, was also evaluated for virus inhibition. All viruses tested were potently sensitive (IC50 <15 µM) to inhibition by 2'-deoxy-2'-fluorocytidine. Among the viruses tested, the A/Duck/MN/1525/81(H5N1) strain was sensitive to inhibition by 2'-FdC with IC50 values equal to 0.25 and 0.21 µM by visual and NR assay, respectively (Table 3). The broad-spectrum inhibition activity of influenza viruses by 2’FdC also translated into a strong reduction in virus yield for almost all the viruses. IC90 values ranged from 0.69 µM for the Thailand/2(Kan1)/16/2004 H5N1 strain (Table 1) to 7.3 µM for the B/Malaysia/2506/2004 strain (Table 3). The IC90 values for the H3N2 and H1N1 viruses tested were in the range of 4.6–6.8 µM and for the hybrid H1N1 viruses expressing the H5N1 HA and NA proteins, 2.9–5.1 µM.
Table 3.
Effects of 2’-deoxy-2’-fluorocytidine and 2’-deoxy-2’, 2’-difluorocytidine on the reolication of influenza viruses in MDCK cells.
| Visul Assay | Neutral Red Uptake Assay | VYR Assaya |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Influenza Virus |
Virus Subtype | Compound (µM) | IC50b | CC50c | Sld | IC50 | CC50 | SI | IC90e |
| H1N1 | A/CA/07/2009 | 2’-deoxy-2’-fluorocytidine | 3.2 | >100 | >31 | 4.1 | >100 | >24 | 4.6 |
| 2’-deoxy-2’, 2’-difluorocytidine | 0.18 | 2.1 | 11.7 | 0.16 | 1.1 | 6.9 | 0.13 | ||
| H1N1 | A/Hong Kong/2369/2009Rf | 2’-deoxy-2’-fluorocytidine | 2.1 | >100 | >48 | 7 .8 | >100 | >12.8 | 6.3 |
| 2’-deoxy-2’, 2’-difluorocytidine | 0.05 | >100 | >200 | 0.054 | >100 | >1900 | 0.03 | ||
| H3N2 | A//Sydney/05/97 | 2’-deoxy-2’-fluorocytidine | 0.59 | >100 | >170 | 2.5 | >100 | >40 | 6.7 |
| 2’-deoxy-2’, 2’-difluorocytidine | 0.18 | 12 | 67 | 1.2 | 6.8 | 5.7 | 1.6 | ||
| H3N2 | A/Brisbane/10/2007 | 2’-deoxy-2’-fluorocytidine | 1.0 | >100 | >100 | 14 | >100 | >7.1 | 6.8 |
| 2’-deoxy-2’, 2’-difluorocytidine | 0.13 | 5.6 | 43 | 0.16 | 1.3 | 8.1 | 0.14 | ||
| H1N1 X | A/Hong Kong/213/2003H | 2’-deoxy-2’-fluorocytidine | 1.3 | >100 | >77 | 7.2 | >100 | >14 | 5.1 |
| H5N1g | 2’-deoxy-2’, 2’-difluorocytidine | <0.032 | >100 | >3100 | 0.065 | >100 | >1538 | 0.04 | |
| H1N1 X | A/Vietnam/1203/2004 H | 2’-deoxy-2’-fluorocytidine | 2.1 | >100 | >48 | 3.4 | >100 | >29 | 2.9 |
| H5N1 | 2’-deoxy-2’, 2’-difluorocytidine | <0.32 | 320 | >1000 | <0.32 | 160 | >500 | 0.32 | |
| H5N1 | A/Vietnam/1203/2004 | 2’-deoxy-2’-fluorocytidine | 1.4 | >100 | >71 | <0.032 | 26 | >81 | 1.2 |
| 2’-deoxy-2’, 2’-difluorocytidine | <0.032 | >100 | >3100 | <0.032 | >100 | >3100 | 0.03 | ||
| H5N1 | A/Gull/PA/4175/83 | 2’-deoxy-2’-fluorocytidine | 0.91 | >100 | >110 | 1.1 | >100 | >91 | 6.1 |
| 2’-deoxy-2’, 2’-difluorocytidine | 0.21 | >100 | >480 | 0.27 | >100 | 370 | 0.13 | ||
| H5N1 | A/Duck/MN/1525/81 | 2’-deoxy-2’-fluorocytidine | 0.25 | >100 | >400 | 0.21 | >100 | >480 | 5.9 |
| 2’-deoxy-2’, 2’-difluorocytidine | <0.32 | 530 | >1700 | <0.32 | 280 | >880 | 0.25 | ||
| B | Malaysia/2506/2004 | 2’-deoxy-2’-fluorocytidine | 1.9 | >100 | >53 | 4.1 | >100 | >24 | 7.3 |
| 2’-deoxy-2’, 2’-difluorocytidine | <0.032 | >100 | >3100 | 0.068 | >100 | >1500 | 0.11 | ||
| B | Florida/4/2006 | 2’-deoxy-2’-fluorocytidine | 1.0 | >100 | >100 | 1.4 | >100 | >71 | 2.1 |
| 2’-deoxy-2’, 2’-difluorocytidine | <0.32 | 440 | >1400 | <0.32 | 140 | >440 | 1.7 | ||
Values for CPE and NR assays are arithmetic means of three independent experiments. VYR assays were done one time.
Virus Yield Reduction Assay;
IC50: 50% virus inhibitory concentration;
CC50: 50% cell cytotoxic concentration of drug;
selective index: Visual and neutral red (NR) SI = CC50/IC50;
IC90: 90% virus inhibitory concentration (1log10 drop in virus titer);
R= oseltamivir resistant; A/Ann Arbor/6/60 H1N1 virus expressing hemagglutinin and neuraminidase from A/Vietnam/1203/2004 H5N1 virus.
Gemcitabine (2’-deoxy-2’, 2’-difluorocytidine, Figure 1) also potently inhibited all the virus strains tested. IC50 values ranged from <0.032 µM (A/Hong Kong/213/2003H and B/Malaysia/2506/2004) to 0.21 µM (A/Gull/PA//4175/83) by visual assay and from <0.032 µM (A/Vietnam/1203/2004) to 1.2 µM (A/Sydney/05/97) by NR assay. In some assays, some toxicity was detected with CC50 values ranging from 2.1 to 530 µM by visual assay and from 1.1 to 280 µM by neutral up take assay. Nevertheless, the inhibitory activity for most viruses was overall very selective when cytotoxicity was not a factor with SI values ranging from 5.6 to >3100. The activity discovered by visual and neutral red assays was confirmed by virus yield reduction assays with IC90 values ranging from 0.03 µM to 2.1 µM.
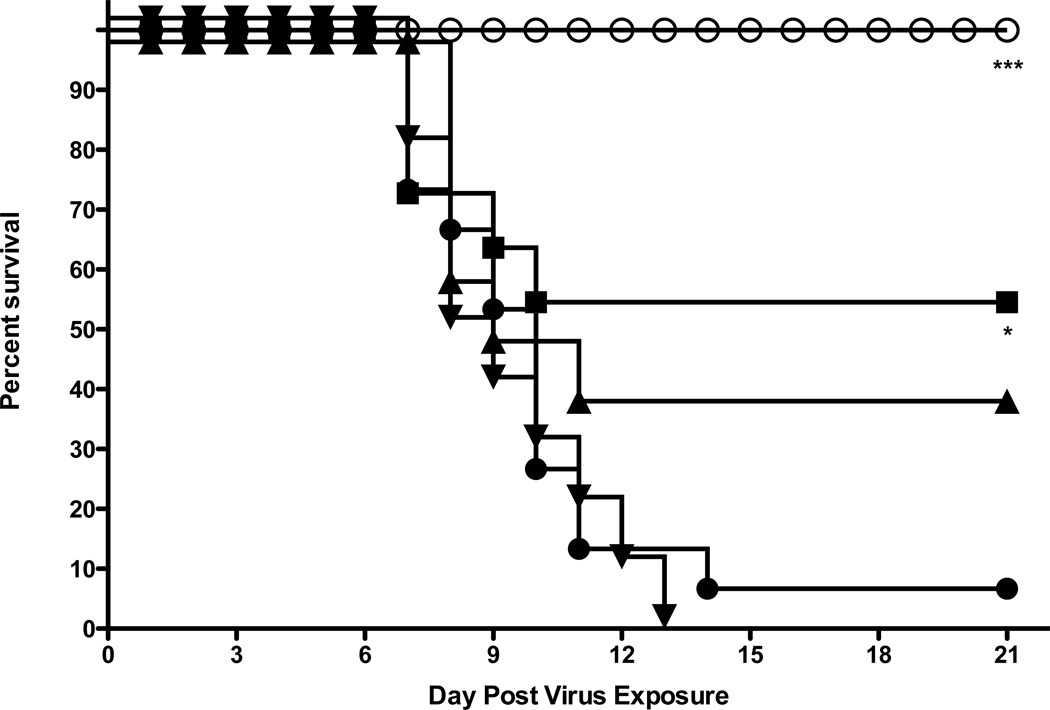
3.4. In vivo efficacy evaluation of 2’FdC against A/Vietnam/1203/2004 H5N1
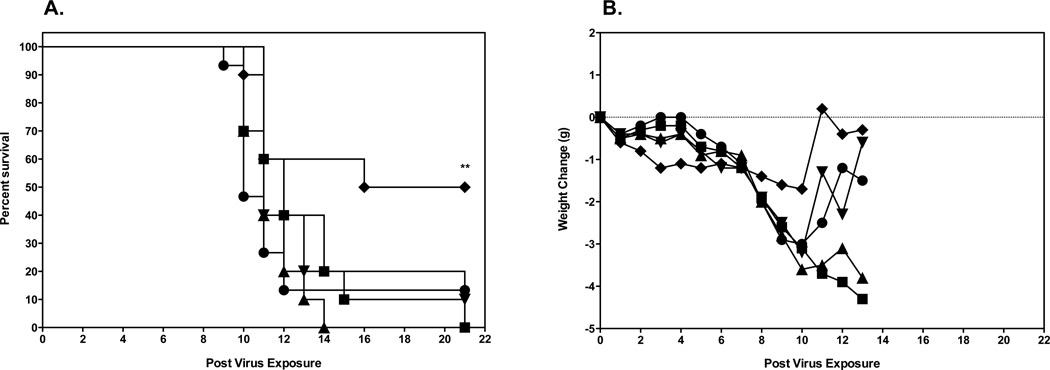
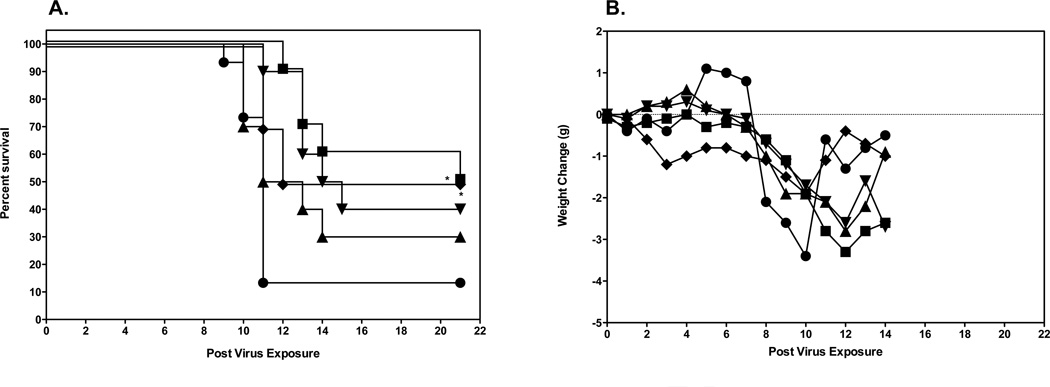
Initial evaluation of 2’FdC efficacy in a mouse model was carried out by infecting groups of 10 mice i.n. with a 100% lethal dose of highly pathogenic influenza A H5N1 virus infection. This was done by anesthetizing the mice by i.p. injection of ketamine (100/mg/kg) and instilling 50 µl of 103.5 CCID50/ml virus into the nares. The mice were then treated p.o. or i.p. with a total of 0.3, 3.0, and 30 mg/kg of 2’FdC per day twice a day for 8 days or with 75 mg/kg/day of ribavirin twice a day for 8 days beginning 24 h before virus exposure. As controls, 15 infected mice were treated p.o. with water or i.p. with PSS in parallel with 2'-deoxy-2'-fluorocytidine. Of the placebo-treated mice receiving the calculated LD90 dose 90% died as expected (Figs. 2A, p.o. administration and 2A, i.p. administration). H5N1 influenza virus-infected mice treated with any dose of 2'-FdC by the per os route of administration were not protected from mortality due to the virus infection (Fig. 2A), although ribavirin treatment delivered per os at 75 mg/kg/d did significantly protect mice from death (p<0.01). This lack of protection against death in mice treated with 2'-FdC by the per os route correlated with the data showing that the per os delivered 2'-FdC treatments did not ameliorate weight loss either (Fig. 2B). On the other hand, ribavirin-treated mice, the majority of which survived the effects of the virus infection, lost much less weight. In contrast to the data from mice treated by the p.o. route, H5N1 virus-infected mice treated with 2'-FdC at 30 mg/kg/day or ribavirin at 75 mg/kg/d by the i.p. route were partially, but significantly protected from death compared to infected mice receiving the placebo by the i.p. route (Fig. 3A, P<0.05). The protection from death was also significant for mice treated with ribavirin by the p.o. route (p<0.01). Mice receiving these treatments did lose less weight than mice treated with placebo (Fig. 3A). Surviving mice, including those receiving ribavirin or 2’FdC went on to gain weight by the end of the experiment that was nearly equal to the original body weight recorded at the initiation of the study (Fig. 3B).
Figure 2.
Effects of 2’FdC administered per os on survival and weight change of female BALB/c mice infected with a lethal dose of avian influenza A/Vietnam/1203/2004 H5N1 virus.
A. Survival of infected BALB/c mice treated with PSS (●), 2’FdC at 30 (■), 3.0 (▲), 0.3 (▼) mg/kg/day, or ribavirin at 75 mg/kg/day (♦) twice a day for 8 days (bid × 8). ** P<0.01, ribavirin versus PSS. A. Survival of infected BALB/c mice treated with PSS (●), 2’FdC at 30 (■), 3.0 (▲), 0.3 (▼) mg/kg/day, or ribavirin at 75 mg/kg/day (♦) (bid × 8), beginning −24 h relative to virus exposure. ** P<0.01, ribavirin versus water.
B. Weight change through day 13 in Infected BALB/c mice treated with PSS (●), 2’FdC at 30 (■), 3.0 (▲), 0.3 (▼) mg/kg/day, or ribavirin at 75 mg/kg/day (♦) (bid × 8), beginning −24 h relative to virus exposure.
Figure 3.
Effects of 2’FdC administered i.p. on survival and weight change of female BALB/c mice infected with a lethal dose of avian influenza A/Vietnam/1203/2004 H5N1 virus.
A. Survival of infected BALB/c mice treated with PSS (●), 2’FdC at 30 (■), 3.0 (▲), 0.3 (▼) mg/kg/day, or ribavirin at 75 mg/kg/day (♦) (bid × 8), beginning −24 h relative to virus exposure. * P<0.05, 2’-FdC (30 mg/kg) or ribavirin versus PSS.
B. Weight change through day 14 in Infected BALB/c mice treated with PSS (●), 2’FdC at 30 (■), 3.0 (▲), 0.3 (▼) mg/kg/day, or ribavirin at 75 mg/kg/day (bid × 8), beginning −24 h relative to virus exposure.
3.5. In vivo efficacy of higher dose 2’FdC against influenza A/Vietnam/1203/2004 H5N1 virus
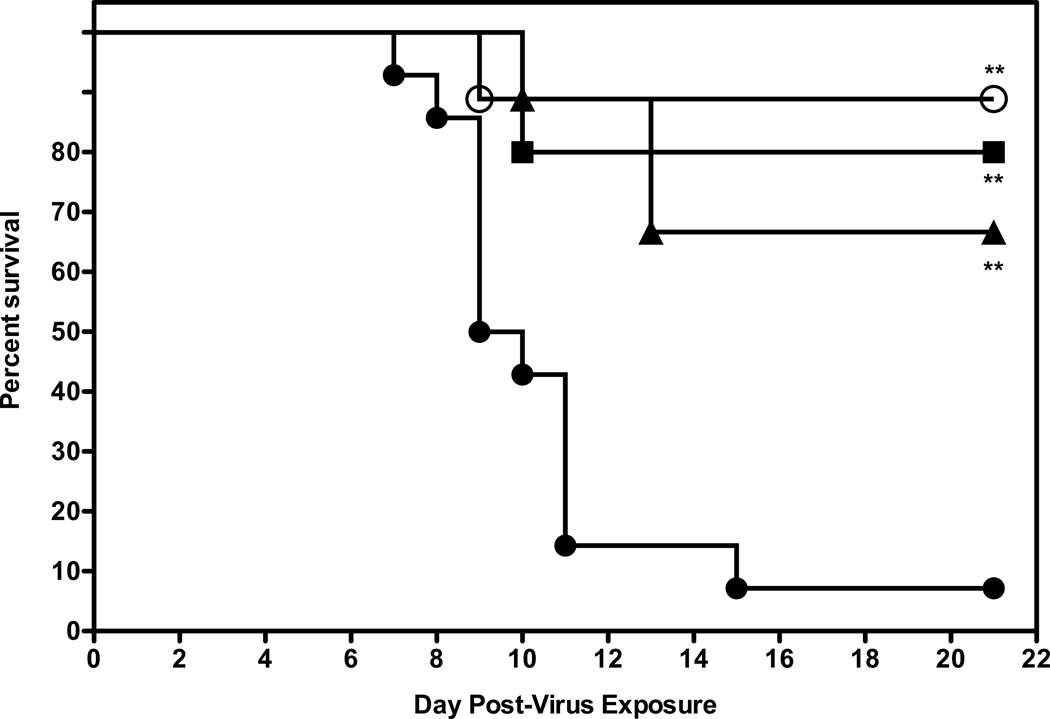
In a follow-up study, mice were administered a higher dose of 2’-FdC (60 mg/kg), using the same i.p. treatment regimen described previously. As shown before, the 30 mg/kg dose significantly protected mice against death, with 70% of the treated mice surviving in this experiment (Fig. 4), which was similarly efficacious as compared to the 60mg/kg dose, suggesting that the maximal protective effect was achieved at the 30mg/kg dose.
Figure 4.
Evaluation of higher dose of 2’FdC for efficacy against a highly pathogenic influenza H5N1 virus infection in BALB/c mice. Vehicle placebo, 2’-deoxy-2’-fluorocytidine, or ribavirin were administered intraperitoneally. Experimental groups, n = 10; placebo group, n = 15. 0.4% CMC (●), 2'-FdC at 60 (■). 30 (▲) or ribavirin at 75 mg/kg/day (○) (bid × 8), beginning −24 h relative to virus exposure. ** P= 0.0014, 2'-FdC (60 mg/kg); ** P=0.0016, 2'-FdC (30 mg/kg −24), ** P=0.0013 (ribavirin, 75 mg/kg/d).
80% of the mice survived the infection when treated with 60 mg/kg/d of 2’-FdC. At this higher dose little toxicity was observed with the exception of a tendency of a little more weight loss (data not shown). Virus lung titers at day 4 post infection were somewhat lower in mice receiving the 60 mg/kg/d treatment compared to the mice receiving placebo or the 30 mg/kg/ 2’-FdC (Table 4), although this difference was not statistically significant. Ribavirin treatment resulted in a significant reduction of almost 1000-fold in virus titer (p<0.01) compared to the lung virus titers from placebo-treated mice. The gross pathology was very mild at day 4 in all mice, yet there was some lessening of lung weights (presumably a reflection of less lung edema) in mice treated with 2'-FdC at 60 mg/kg/d and a significant reduction in lung weights for mice treated with 30 mg/kg/d 2'-FdC or ribavirin at 75 mg/kg/d (p<0.05 and p<0.01, respectively). Microscopic inspection of lung tissue (day 4 after virus exposure) after histological staining revealed little or no pathogenesis, as would be expected early in this infection even though peak virus lung titers were attained at this time of the infection (Sidwell et al., 2005). In a subsequent experiment, it was found that there were no additional benefits of treating with 80 mg/kg/d of 2’-FdC; on the contrary that dose was detrimental to weight recovery by the mice and only 40% of mice survived the infection (data not shown). Thus, the 60 mg/kg/d dose of 2'-FdC given bid × 8 was the most effective dosing regimen in protecting mice against death due to virus infection and appeared to be the maximum tolerated effective dose.
Table 4.
Effects of 2'-FdC Treatment of BALB/c Mice Infected with Influenza/A/Vietnam/1203/2004 H5N1 Virus on Various Lung Parameters at Day 4 Post Virus Exposure
| Treatment | Lung Score ± SDa |
Lung WeightSD ± SDa |
Virus Lung Titers ± SD (Log10/g)a |
|---|---|---|---|
| 0.4% CMC | 0.4 ± 0.22 | 0.17 ± 0.011 | 4.10 ± 1.052 |
| 2'-FDC (60 mg/kg/d) | 0.4 ± 0.25 | 0.14 ± 0.014 | 3.60 ± 1.303 |
| 2'-FDC (30 mg/kg/d) | 0.2 ± 0.27 | 0.13 ± 0.012* | 4.01 ± 0.713 |
| Ribavirin (75 mg/kg/d) | 0.6 ± 0.13 | 0.12 ± 0.014** | 1.42 ± 0.000** |
Values represent the arithmetic the standard deviation.
P<0.05,
P<0.01 vs. 0.4% CMC treatment group by ANOVA and Newman-Keuls pairwise comparison test.
3.6. Effects of frequency of dosing with 2’FdC on survival of BALB/c mice infected with influenza A/Vietnam/1203/2004 H5N1 virus
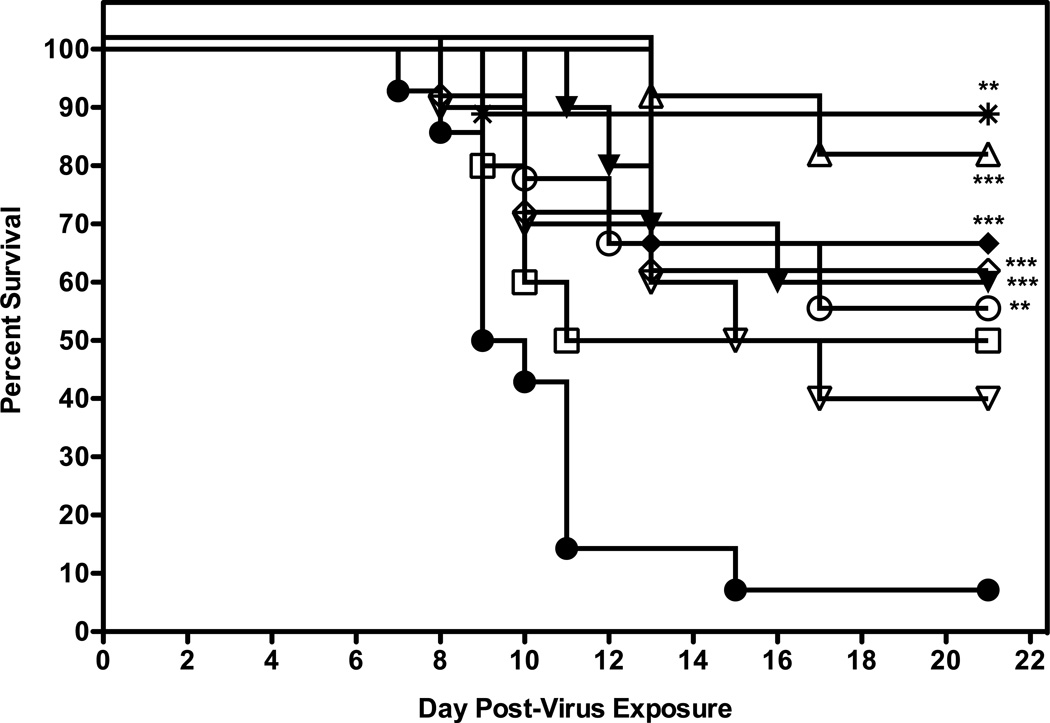
The next experiment was to determine if efficacy could be achieved with less frequent or more frequent dosing with 2'-FdC. Groups of 10 mice were administered 2'-FdC i.p. at 60 or 30 mg/kg/day i.p. for 8 days (bid, tid or qd × 8) beginning at 24 h before virus exposure. In addition, some mice were treated with 60 mg/kg/d of 2'-FdC i.p. bid × 8 beginning at various times post virus exposure. For this experiment, 90% of the placebo-treated mice receiving the calculated LD90 dose died as expected (Fig. 5). A number of the dosing regimens provided significant protection from death as compared to vehicle, although there were no significant differences between the treatment regimens. 2’FdC at 60 mg/kg/day given twice a day for 8 days, beginning 24 h after virus exposure was the regimen which had the greatest number of mice surviving the infection (p=0.0001, Fig. 5; Table 5, live per/total ratio, p<0.05). It appeared that this treatment not only promoted total survival, but also indicated that the mice in this treatment group were 10-fold less likely to die than mice treated with placebo (Table 5, hazard ratio = 10.6). In addition, treating infected mice with 2’-FdC at 72 h post virus exposure significantly protected 60% of mice from the lethal effects of virus challenge (p= 0.0191) and a hazard ratio of 4.04. Treatment with drug administered 48 h after infection prolonged the day of death to the point that day 16 after virus exposure (Table 5, mean and median days of death) was the day on which the largest number of mice died (see median day of death) but did not significantly protect mice against death (p >0.05). Other treatment regimens evaluated were designed to examine the effects of varying the frequency of treatment beginning treatment at −24 h and then bid × 8 days, but providing the mice with the same total doses per day that were previously shown to provide protection. For example, the treatment regimen which involved administering 2’FdC at 60 mg/kg/day once a day for 8 days was significantly efficacious for protecting mice from the lethality of the virus infection (p=0.0048). In contrast, 30mg/kg given once a day did not afford mice significant protection against death compared to mice in the vehicle control group, despite reaching 50% protection (p>0.05). However, mice receiving the dosing regimens involving administration of drug three times a day were significantly protected against death regardless of the dose of 2'-FdC used (60 mg/kg, p=0.0005; 30 mg/kg, p= 0.0005). These latter treatment regimens were as effective as treating with 2'FdC at 60 mg/kg/day given twice a day for 8 days.
Figure 5.
Evaluation of different dosing regimens of 2’FdC in 0.4% CMC for efficacy against a highly pathogenic influenza H5N1 virus infection in BALB/c mice. Vehicle placebo, 2’-deoxy-2’-fluorocytidine, or ribavirin were administered intraperitoneally. Experimental groups, n = 10; placebo group, n = 15. 0.4% CMC (●), 60 (▲) and 30 (♦) mg/kg/day three times a day (tid); 60 (○) and 30 (□) mg/kg/day, once a day (qd) 24 h prior to challenge with virus; 60 mg/kg/day twice a day 24 h post virus exposure (Δ), 48 h post virus exposure (▽), 72 h (◇) post virus exposure, or ribavirin at 75 mg/kg/day (✴). All were administered a total of 8 days, regardless of time of addition.
*** P=0.0005, 2'-FdC (60 mg/kg, tid, −24 h); *** P=0.0005, 2'-FdC (30 mg/kg, tid, −24 h); ** P=0.0048, 2'-FdC (60 mg/kg, qd, −24 h); *** P= 0.0001, 2'-FdC (60 mg/kg, bid, +24 h); ** P= 0.0019, 2'-FdC (60 mg/kg, bid, +72 h); **P=0.0013, ribavirin (75 mg/kg, bid,−24 h) all versus 0.4% CMC.
Table 5.
Effects of 2'-FdC Treatment of BALB/c Mice Infected with Influenza/A/Vietnam/1203/2004 H5N1 Virus on Various Death Parameters
| Treatment | Live/Total | Median Day of Death |
Mean Day of Death ± SD |
Hazard Ratio (95% CI)a |
|---|---|---|---|---|
| 0.4% CMC | 1/14 | 9.5 | 10.5 ± 2.0 | − |
| 2'-FDC (60 mg/kg/d, tid × 8 beg. −24 h) | 6/10 | 10 | 13.0 ± 2.2 | 6.81 (2.27–20.4) |
| 2'-FDC (30 mg/kg/d, tid × 8 beg. −24 h) | 6/10 | Undefined | 13.0 ± 0.0 | 7.64 (2.47–23.7) |
| 2'-FDC (60 mg/kg/d, qd × 8 beg. −24 h) | 5/10 | Undefined | 12.3 ± 3.3 | 4.73 (1.62–13.8) |
| 2'-FDC (30 mg/kg/d, qd × 8 beg. −24 h) | 5/10 | Undefined | 9.80 ± 0.8 | 3.13 (1.08–9.12) |
| 2'-FDC (60 mg/kg/d, bid × 8 beg. +24 h) | 8/10* | Undefined | 15.0 ± 2.8 | 10.6 (3.34–33.8) |
| 2'-FDC (60 mg/kg/d, bid × 8 beg. +48 h) | 4/10 | 16 | 12.2 ± 3.4 | 3.26 (1.17–9.01) |
| 2'-FDC (60 mg/kg/d bid × 8 beg. +72 h) | 6/10 | Undefined | 10.3 ± 2.1 | 4.04 (1.40–11.6) |
| Ribavirin (75 mg/kg/d, bid × 8 beg. −24 h) | 8/9 | Undefined | 9.00 ± 0.0 | 8.87 (2.72–29.0) |
Hazards ratios are relative to the 0.2% CMC control.
P<0.05 vs. 0.4% CMC treatment group by Chi-square analysis and Fisher’s exact test for pairwise comparisons.
3.7. In vivo efficacy of 2’-deoxy-5-fluorocytidine and 2'-deoxy-2',2'-difluorocytidine on the survival mice infected with influenza A/Vietnam/1203/2004 H5N1 virus
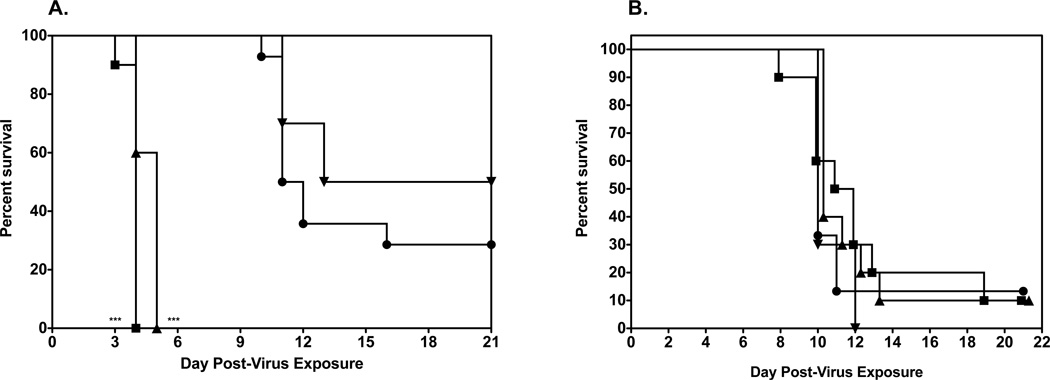
In the in vitro screens of various fluorocytidines, it was found that by moving the fluoro group to the 5 position of the nucleoside base, that the A/Vietnam/1203/2004 virus was even more potently inhibited by 2’-deoxy-5-fluorocytidine (5-FdC) than by 2'-deoxy-2'-fluorocytidine, although the other HPAIV strains tested were not sensitive to inhibition by 5-FdC (Table 1). In addition, adding another fluoro group to the 2' position of the sugar to get 2'-deoxy-2', 2'-difluorocytidine (gemcitabine) also greatly enhanced the potency against the influenza viruses (Table 3), although in some assays gemcitabine was cytotoxic. Thus, evaluation of 5-FdC and gemcitabine was carried out by infecting groups of mice i.n. with a 100% lethal dose of highly pathogenic influenza A H5N1 virus infection. Treatment for each compound was by the i.p. route, since this route of administration was found to be the most efficacious way of delivering 2'-FdC. For 5-FdC, the mice were treated i.p. with 2'-deoxy-5-fluorocytidine at 60, 30, and 3.0 mg/kg/d in 0.4% CMC twice a day for 8 days beginning 24 h before virus exposure. As controls, the infected mice were treated i.p. with 0.4% CMC in parallel with 2'-deoxy-5-fluorocytidine. H5N1 influenza virus-infected mice receiving 5-FdC by the i.p. route were not shielded from death compared to infected mice receiving 0.4% CMC (Fig. 6A). Mice treated with the 60 and 30 mg/kg doses died in greater numbers than did untreated mice (p<0.0001). In fact, in untreated mice there was considerable toxicity in the form of severe weight loss at the 30 and 60 mg/kg/d doses and some deaths (data not shown). The 3.0 mg/kg dose, although well- tolerated, was not very effective in promoting survival of infected mice suggesting this structure enhanced toxicity and while lowering efficacy compared to 2'-FdC.
Figure 6.
Evaluation of other 2’-deoxy-fluorocytidine analogs on survival of female BALB/c mice infected with a lethal dose of avian influenza A/Vietnam/1203/2004 H5N1 virus.
A. Survival of infected BALB/c mice treated i.p. with 0.4% CMC (●), gemcitabine at 0.75 mg/kg (■), 0.25 mg/kg (▲), 0.01 mg/kg (▼) (bid × 8), beginning −24 h prior to virus exposure. *** P<0.001, 5'-FdC (60, 30 mg/kg) versus 0.4% CMC.
B. Survival of infected BALB/c mice treated i.p. with 0.4% CMC (●), 2’-deoxy-5-fluorocytidine at 60 (■), 30 (▲), 3 (▼) mg/kg/day (bid × 8), beginning −24 h prior to virus exposure.
Using a similar treatment regimen, a maximum tolerated dose study was done with gemcitabine in on the suspicion that doses similar to those doses used for 2'-FdC would be toxic as was shown for 5-FdC. Indeed, the data from that study suggested that only a dose below 3 mg/kg/d could be used without the mice quickly succumbing to death (data not shown). Thus, for the efficacy study mice were administered gemcitabine at 0.75, 0.25, and 0.01 mg/kg/d in 0.4% CMC, bid × 8 beginning 24 h before virus exposure. Although these doses were well tolerated in uninfected mice (data not shown), they were not significantly effective in protecting mice against the lethal challenge dose of A/Vietnam/1203/2004 virus (Fig. 6B).
These data suggest that altering the substitutions on sugar, be either moving the fluoro group to the 5 position of he nucleoside base or adding another fluoro to the 2' position of the sugar, results in a less efficacious and more toxic compound when administered to mice.
3.8. Effects of 2’FdC on survival of BALB/c mice infected with influenza A/CA/04/2009 pandemic H1N1 virus
Finally, 2'-FdC was evaluated against a pandemic H1N1 influenza a virus from 2009 to determine if 2’-FdC was as also a broad-spectrum inhibitor of influenza viruses in vivo as it was in vitro. Using the same dosing regimen as described for the initial evaluation of 2'-FdC for efficacy against H5N1 A/Vietnam/1203/2004 virus, 2'-FdC was administered to mice 24 h before virus exposure at 30, 3, and 0.3 mg/kg/d (bid × 8) (Fig. 7). The 30 mg/kg dose significantly protected 50% of the mice from the lethal effects of the H1N1 virus infection (p=0.038) as did this dose with mice infected with the H5N1 virus in the initial efficacy study. In addition, mice in the 30 mg dosage group were almost 3 times less likely to die from infection than the mice in placebo treatment group (Table 6, see hazards ratio). Thus, the data suggest that 2'-FdC is a broad spectrum, relative non-toxic inhibitor of influenza viruses in vitro and in vivo.
Figure 7.
Effects of 2’FdC on the survival of female BALB/c mice infected with a lethal dose of influenza A/CA/04/09 pandemic H1N1 virus. 0.4% CMC (●), 2’FdC at 30 (■), 3.0 (▲), 0.3 (▼) mg/kg/day, or ribavirin at 75 mg/kg/day (○), (bid × 5) beginning 24 h prior to virus exposure. * P= 0.038, 2'-FdC (30 mg/kg) versus 0.4% CMC; *** P<0.001 ribavirin versus 0.4% CMC
6.
Effects of 2’-FdC On Various Death Parameters for BALB/C Mice Infected with Pandemic Influenza A/CA/04/09 H1N1 Virus
| Treatment | Live/Total | Median Day of Death |
Mean Day of Death ± SD |
Hazard Ratio (95% CI)a |
|---|---|---|---|---|
| PSS Dunn’s posttest | 1/14 | 10.0 | 8.9 ± 1.6 | − |
| 2'-FdC (30 mg/kg/d) | 5/10 | Undefined | 7.8 ± 1.3 | 2.95 (1.06–8.17) |
| 2'-FdC (3 mg/kg/d) | 4/10 | 10.0 | 8.9 ± 1.2 | 2.17 (0.81–5.82) |
| 2'-FdC (0.3 mg/kg/d) | 0/10 | 6.0 | 9.3 ± 2.1 | 0.76 (0.29–1.99) |
| Ribavirin (75 mg/kg/d) | 10/10** | Undefined | >21*** | 47.7 (14.1–162) |
Hazards ratios are relative to the PSS control.
P<0.001 versus PSS control by Kruskal-Wallis test and Dunn’s posttest for pairwise comparisons.
P = 0.0095 versus PSS control by Chi-square analysis and Fisher’s exact test for pairwise comparisons.
4. Discussion
In this report, we have demonstrated that 2’FdC was inhibitory to a broad spectrum of influenza A and influenza B viruses, including three strains of highly pathogenic avian influenza H5N1 virus in MDCK cells, with the 90% inhibitory concentrations ranging from 0.69 µM to 7.3 µM, as determined by a virus yield reduction assay (Tables 1 and 3). We found that selected substitutions on the sugar moiety of the cytidine nucleoside, specifically the addition of a second fluoro group at the 2' position or moving the fluoro group to the 5 position of the nucleoside enhanced the potency of inhibition of influenza viruses in vitro, although adding the second fluoro group to the 2' position might have resulted in increased cytotoxicity in some in vitro assays that were done. Overall, gemcitabine appeared to be a slightly more potent inhibitor of influenza A virus replication as assessed by virus yield reduction assay (IC90 values = 0.03–1.7 µM) than 2'-FdC (IC90 values = 1.2–7.3 µM), although there was cytotoxicity detected in some assays when using gemcitabine. Other fluorodeoxyribonucleosides were either not inhibitory to the influenza viruses or marginally active (guanosine fluorodeoxynucleoside). In a much earlier report, 2'-deoxy-2'-fluoroguanosine and 2'-deoxy-2'-fluorocytosine were shown to be the most potent inhibitors of influenza virus replication with EC50 values of 18.2 and 2.6 µM, respectively. In that study 2'-deoxy-2'-fluoroguanosine was a more potent inhibitor of influenza virus replication in chicken embryo fibroblasts, but 2'-deoxy-2'-fluorocytodine was totally inactive (Tisdale, 1993). Both compounds were found to be equally inhibitory of influenza virus replication in human tracheal cultures (Tisdale, 1993; Jakeman et al., 1994). Based on the efficacy data from CEF cells, the investigators reporting the earlier study chose to pursue the 2'-deoxy-2'-fluoroguanosine ribonucleoside for animal testing. Our data support some of these previous findings. We found that 2'-deoxy-2'-fluoroguanosine and 2’FdC inhibited influenza A highly pathogenic H5N1 avian influenza species in CPE reduction assays (4–5 day duration) in MDCK cells (IC 50 = 8.5–21 µM, 0.05–8 µM, respectively) as potently as the two compounds inhibited the H1N1 virus in the previous study in which plaque reduction assays (2 day duration)) in MDCK cells (IC50 = 18uM, 2.6 µM, respectively) were used, with 2'FdC being the more potent of the two compounds in MDCK cells in that study. In both our study and the study done by Tisdale et al. (1993), both compounds were relatively non-toxic. In the current study as well as in the study by Tisdale et al. (1993), 2'-deoxy-2'-fluoroadenosine was not active. In the previous study, the higher IC50 value for 2'-deoxy-2'-fluoroguanosine (2'-FdG) in MDCK cells was shown to be due the rate limiting step of triphosphate formation in MDCK cells. Interestingly, other guanosine nucleoside-like analogs such as favipiravir and ribavirin that inhibit influenza viruses and perhaps the virus transcriptase complex seem likely to be phosphorylated to the triphosphate form quite well in MDCK cells (Furuta et al., 2005).
It has been suggested that 2'-fluorodeoxyribonucleosides may very well act as ribonucleosides, since it has been confirmed that both 2′-deoxy-2′-fluororibonucleosides and- ribonucleosides adopt a 3′-endo conformation (Blandin et al., 1974). Because 2’-FdC may assume a 3′-endo conformation and thus act as a ribonucleoside, 2’-FdC may actually act as a substrate for the influenza RNA polymerase complex. 2'-FdG-triphosphate has already been demonstrated to be a substrate and inhibitor of influenza polymerase (Tisdale et al., 1995). 2'-FdC may also inhibit other RNA polymerases. Shi et al. reported that 2’-FdC may also act as a substrate for the HCV RNA polymerase and that the 2' fluoro group is the group that is actually recognized by the HCV polymerase (Shi et al., 2005). Thus, it is conceivable that 2'-FdC might be acting as a general viral RNA polymerase inhibitor. Alternatively, 2'FdC may act as an immunomodulator in vivo, since it has been shown to be cytostatic in cells (Stuyver et al., 2004). In addition, other cytidine analogs such as the closely related gemcitabine (2',2'-difluorodeoxycytidine) have been shown to inhibit ribonucleotide reductase (Shao et al., 2006) and cytidine synthetase (Heinemann et al., 1995), the inhibition of which may also be mechanisms for inhibiting influenza virus infections. The lack of protection and toxicity at high doses of 2'FdC may be due to inhibition of host DNA polymerases (Richardson et al., 2000).
We further examined the efficacy of 2’FdC in a BALB/c mouse model infected with a lethal dose of highly pathogenic influenza A/Vietnam/1203/2004 H5N1 virus. The dosing regimen of 60 mg/kg administered intraperitoneally 24 h after virus exposure twice a day for eight days resulted in an 80% survival rate and some reduction in virus lung titers and probably in edema of the lungs associated with the virus pathogenesis. Using 2’-FdC at 30 mg/kg/d was nearly as effective as the higher dose of 2'FdC, but neither dose reduced virus lung titers comparable to those virus titers from lungs of mice treated with ribavirin. This could suggest that immunomodulation, as discussed above, may have contributed to the efficacy of 2'-FdC in facilitating the survival of influenza-infected mice. Other regimens of almost equal efficacy in significantly inhibiting mortality of mice due to virus infection included dosing 24 h prior to virus exposure (bid × 8). Parceling the 60 mg/kg/d dose out as three equal doses over a period of 12 hours (tid × 8) was also very efficacious. Even a single dose at 60 mg/kg given over a period of 8 days significantly protected mice against death due to virus infection compared to placebo-treated mice. The inherent variability in this experiment is apparent from the treatment group starting 72h post infection providing numerically better protection against death compared to the number of deaths in the treatment group receiving compound starting at 48h post infection. In summary, the data suggests that 30 and 60mg/kg dosing regimens provide similar levels of protection within the variability of the experiment with the exception of the 30 mg/kg dose administered once a day. Dosing starting before infection does not provide any significant benefit as compared to dosing starting after infection. It is also interesting to note that dosing at 60 mg/kg tid versus QD administration of the same dose provided similar levels of protection.
Finally, similar efficacy against influenza A virus H1N1-infected mice treated with 2'FdC at 30 mg/kg/d twice a day for 5 days was achieved as was attained for mice infected with H5N1 highly pathogenic avian influenza A virus in mice using the same dose and treatment regimen. However, this treatment of influenza A virus H1N1-infected mice with 2'FdC at 30 mg/kg/d twice a day for 5 days was not as effective as the treatment of influenza A H1N1-infected with ribavirin at 75 mg/kg/d using the same treatment regimen. Similar efficacy may have been achieved if mice had been treated with the 60 mg/kg/d dose of 2'FdC, which is closer to a molar equivalent of ribavirin used at 75 mg/kg/d.
These studies support our hypothesis that cytidine nucleoside analogs, in particular, cytidine deoxyribonucleosides might represent good platforms from which to develop potent inhibitors of RNA viruses, including influenza viruses. Thus, our study might provide the basis for the development of additional potent and clinically useful inhibitors for use as anti-influenza agents, including highly pathogenic avian influenza A H5N1 viruses.
Highlights.
2'-deoxy-2'-fluorocytidine potently inhibited H5N1 influenza viruses.
2'-FdC protected H5N1 influenza-virus infected BALB/c mice from death.
2'-FdC was administered 72 h post virus exposure and still significantly protect mice from death.
2-FdC also significantly enhanced survival in mice infected with a pandemic H1N1 virus.
Acknowledgments
We thank Miles K. Wandersee, Aaron J. Smith, Kevin W. Bailey, Zachary G. Vest, Nathan M. Nelson, Bentley J. Anderson, Samuel M. Orwin, Michael A. Morrey, for kindly providing technical assistance; Heather L. Greenstone (NIAID, NIH) for scientific discussion. The contents of this article do not necessarily reflect the position or policy of the government and no official endorsement should be inferred. The animal experiments were conducted in accordance with the approval of the Institutional Animal Care and Use Committee of Utah State University in the AAALAC-accredited Laboratory Animal Research Center. Work was conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
This project was supported in part with Federal funds from the Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract N01-AI-30048 and a subcontract from Southern Research Institute, N01-A1-30063, from the Virology Branch, National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
References
- Aoki F, Boivin G, Roberts N. Infuluenza virus susceptibility and resistance to oseltamivir. Antiviral Ther. 2007;12:603–616. [PubMed] [Google Scholar]
- Bajramovic JJ, Volmer R, Syan S, Pochet S, Gonzalez-Dunia D. 2'-Fluoro-2'-deoxycytidine inhibits Borna disease virus replication and spread. Antimicrob. Agents Chemother. 2004;48:1422–1425. doi: 10.1128/AAC.48.4.1422-1425.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi V, Borella S, Calderazzo F, Ferraro P, Chieco Bianchi L, Reichard P. Inhibition of ribonucleotide reductase by 2'-substituted deoxycytidine analogs: possible application in AIDS treatment. Proc. Natl. Acad. Sci. USA. 1994;91:8403–8407. doi: 10.1073/pnas.91.18.8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjursell G, Skoog L, Thelander L, Söderman G. 2'-Deoxy-2'-azidocytidine inhibits the initiation of polyoma DNA synthesis. Proc. Natl. Acad. Sci. USA. 1977;74:5310–5313. doi: 10.1073/pnas.74.12.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland M. An introduction to medical statistics. 3rd ed. New York: Oxford University Press, Oxford; 2000. [Google Scholar]
- Blandin M, Tran-Dinh-Son S, Catlin JC, Guschlbauer W. Nucleoside conformations. 16. Nuclear magnetic resonance and circular dichroism studies on pyrimidine-2'-fluoro-2'- deoxyribonucleo Biochim. Biophys Acta. 1974;361:249–256. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Isolation of avian influenza A(H5N1) viruses from humans–Hong Kong, May-December 1997. MMWR. 1997;46:1204–1207. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Isolation of avian influenza A(H5N1) viruses from humans–Hong Kong, May-December 1997. JAMA. 1998a;279:263–264. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Update: isolation of avian influenza A(H5N1) viruses from humans–Hong Kong, 1997–1998. JAMA. 1998b;279:347–348. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Update: isolation of avian influenza A(H5N1) viruses from humans–Hong Kong, 1997–1998. MMWR. 1998c;46:1245–1247. [PubMed] [Google Scholar]
- Chen H, Cheung CL, Tai H, Zhao P, Chan JF, Cheng VC, Chan KH, Yuen KY. Oseltamivir-resistant influenza A pandemic (H1N1) 2009 virus, Hong Kong. Emerg. Infect. Dis. 2009;15:1970–1972. doi: 10.3201/eid1512.091057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claas EC, Osterhaus AD, van Beek R, De Jong JC, Rimmelzwaan GF, Senne DA, Krauss S, Shortridge KF, Webster RG. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351:472–477. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- De Clercq E, Beres J, Bentrude WG. Potent activity of 5-fluoro-2'-deoxyuridine and related compounds against thymidine kinase-deficient (TK-) herpes simplex virus: targeted at thymidylate synthase. Mol. Pharmacol. 1987;32:286–292. [PubMed] [Google Scholar]
- Dunnett CW, Tamhane AC. Comparisons between a new drug and active and placebo controls in an efficacy clinical trial. Stat. Med. 1992;11:1057–1063. doi: 10.1002/sim.4780110807. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Takahashi K, Fukuda Y, Kuno M, Kamiyama T, Kozaki K, Nomura N, Egawa H, Minami S, Watanabe Y, Narita H, Shiraki K. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob. Agents Chemother. 2002;46:977–981. doi: 10.1128/AAC.46.4.977-981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Takahashi K, Kuno-Maekawa M, Sangawa H, Uehara S, Kozaki K, Nomura N, Egawa H, Shiraki K. Mechanism of action of T-705 against influenza virus. Antimicrob Agents Chemother. 2005;49:981–986. doi: 10.1128/AAC.49.3.981-986.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacca M, Borella S, Calderazzo F, Bianchi LC, D’Agaro P, Rampazzo C, Bianchi V, Reichard P. Synergistic antiviral action of ribonucleotide reductase inhibitors and 3’-azido-3’-deoxythymidine on HIV type 1 infection in vitro. AIDS Res. Hum. Retroviruses. 1996;12:677–682. doi: 10.1089/aid.1996.12.677. [DOI] [PubMed] [Google Scholar]
- Govorkova EA, Leneva IA, Goloubeva OG, Bush K, Webster RG. Comparison of efficacies of RWJ-270201, zanamivir, and oseltamivir against H5N1, H9N2, and other avian influenza viruses. Antimicrob. Agents Chemother. 2001;45:2723–2732. doi: 10.1128/AAC.45.10.2723-2732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubareva LV, McCullers JA, Bethell RC, Webster RG. Characterization of Influenza A/Hong Kong/156/97 (H5N1) Virus in a Mouse Model and Protective Effect of Zanamivir on H5N1 Infection in Mice. J. Infect. Dis. 1998;178:1592–1596. doi: 10.1086/314515. [DOI] [PubMed] [Google Scholar]
- Hatta M, Hatta Y, Kim JH, Watanabe S, Shinya K, Nguyen T, Lien PS, Le QM, Kawaoka Y. Growth of H5N1 influenza A viruses in the upper respiratory tracts of mice. PLoS Pathog. 2007;3:1374–1379. doi: 10.1371/journal.ppat.0030133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden FG. Pandemic influenza: is an antiviral response realistic? Ped. Infect. Dis J. 2004;23:p262–p269. doi: 10.1097/01.inf.0000144680.39895.ce. [DOI] [PubMed] [Google Scholar]
- Heinemann V, Schulz L, Issels RD, Plunkett W. Gemcitabine: a modulator of intracellular nucleotide and deoxynucleotide metabolism. Semin. Oncol. 1995;22:11–18. [PubMed] [Google Scholar]
- Hernandez JE, Adiga R, Armstrong R, Bazan J, Bonilla H, Bradley J, Dretler R, Ison MG, Mangino JE, Maroushek S, Shetty AK, Wald A, Ziebold C, Elder J. Hollister AS, Sheridan W. and on behalf of the eIND Peramivir Investigators 2011. Clinical experience in adults and children treated with intravenous peramivir for 2009 influenza A (H1N1) under an Emergency IND Program in the United States. Clin. Infect. Dis. 2011;52:695–706. doi: 10.1093/cid/cir001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaccard J, Becker MA, Wood G. Pairwise multiple comparison procedures: A review. Psycholog. Bull. 1984;96:589–596. [Google Scholar]
- Jakeman KJ, Tisdale M, Russell S, Leone A, Sweet C. Efficacy of 2'-deoxy-2'-fluororibosides against influenza A and B viruses in ferrets. Antimicrob. Agents Chemother. 1994;38:1864–1867. doi: 10.1128/aac.38.8.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaki Y, Wandersee MK, Smith AJ, Zhou Y, Simmons G, Nelson NM, Bailey KW, Vest ZG, Li JK, Chan PK, Smee DF, Barnard DL. Inhibition of severe acute respiratory syndrome coronavirus replication in a lethal SARS-CoV BALB/c mouse model by stinging nettle lectin, Urtica dioica agglutinin. Antiviral Res. 2011 doi: 10.1016/j.antiviral.2011.02.003. 90–22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HI, Song MS, Pascua PNQ, Baek YH, Lee JH, Hong S-P, Rho J-B, Kim J-K, Poo H, Kim C-J, Choi YK. Genetic characterization and pathogenicity assessment of highly pathogenic H5N1 avian influenza viruses isolated from migratory wild birds in 2011 South Korea. Virus Res. 2011;160:305–315. doi: 10.1016/j.virusres.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Lee ET, Wang JW. Statistical methods for survival data analysis. 3rd ed. Hoboken, N.J.: Wiley-Interscience; 2003. [Google Scholar]
- Leneva IA, Roberts N, Govorkova EA, Goloubeva OG, Webster RG. The neuraminidase inhibitor GS4104 (oseltamivir phosphate) is efficacious against A/Hong Kong/156/97 (H5N1) and A/Hong Kong/1074/99 (H9N2) influenza viruses. Antiviral Res. 2000;48:101–115. doi: 10.1016/s0166-3542(00)00123-6. [DOI] [PubMed] [Google Scholar]
- Lu X, Tumpey TM, Morken T, Zaki SR, Cox NJ, Katz JM. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J Virol. 1999;73:5903–5911. doi: 10.1128/jvi.73.7.5903-5911.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A. J Med. Vol. 353. New England: 2005. Neuraminidase inhibitors for influenza; pp. 1363–1373. [DOI] [PubMed] [Google Scholar]
- Naughtin M, Dyason JC, Mardy S, Sorn S, von Itzstein M, Buchy P. Neuraminidase inhibitor sensitivity and receptor-binding specificity of Cambodian clade 1 highly pathogenic H5N1 influenza virus. Antimicrob. Agents Chemother. 2011;55:2004–2010. doi: 10.1128/AAC.01773-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preziosi P. Influenza pharmacotherapy: present situation, strategies and hopes. Expert Opin. Pharmacother. 2011;12:1523–1549. doi: 10.1517/14656566.2011.566557. [DOI] [PubMed] [Google Scholar]
- Puthavathana P, Auewarakul P, Charoenying PC, Sangsiriwut K, Pooruk P, Boonnak K, Khanyok R, Thawachsupa P, Kijphati R, Sawanpanyalert P. Molecular characterization of the complete genome of human influenza H5N1 virus isolates from Thailand. J. Gen. Virol. 2005;86:423–433. doi: 10.1099/vir.0.80368-0. [DOI] [PubMed] [Google Scholar]
- Reed LJ, Muench N. A simple method of estimating fifty per cent endpoints. Am. J Hyg. 1938;27:p493–p497. [Google Scholar]
- Richardson FC, Kuchta RD, Mazurkiewicz A, Richardson KA. Polymerization of 2'-fluoro- and 2'-O-methyl-dNTPs by human DNA polymerase [alpha], polymerase [gamma], and primase. Biochem Pharmacol. 2000;59:1045–1052. doi: 10.1016/s0006-2952(99)00414-1. [DOI] [PubMed] [Google Scholar]
- Sai K, Saito Y. Ethnic differences in the metabolism, toxicology and efficacy of three anticancer drugs. Expert Opin. Drug Metab. Toxicol. 2011;7:967–988. doi: 10.1517/17425255.2011.585969. [DOI] [PubMed] [Google Scholar]
- Shao J, Zhou B, Chu B, Yen Y. Ribonucleotide reductase inhibitors and future drug design. Curr. Cancer Drug Targets. 2006;6:409–431. doi: 10.2174/156800906777723949. [DOI] [PubMed] [Google Scholar]
- Shi J, Du J, Ma T, Pankiewicz KW, Patterson SE, Tharnish PM, McBrayer TR, Stuyver LJ, Otto MJ, Chu CK, Schinazi RF, Watanabe KA. Synthesis and anti-viral activity of a series of d- and l-2'-deoxy-2'-fluororibonucleosides in the subgenomic HCV replicon system. Bioorg. Med. Chem. 2005;13:1641–1652. doi: 10.1016/j.bmc.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Sidwell RW, Bailey KW, Wong MH, Barnard DL, Smee DF. In vitro and in vivo influenze virus-inhibitory effects of viramidine. Antiviral Res. 2005;68:10–17. doi: 10.1016/j.antiviral.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Sidwell RW, Bailey KW, Wong MH, Huffman JH. In vitro and in vivo sensitivity of a non-mouse-adapted influenza A (Beijing) virus infection to amantadine and ribavirin. Chemother. 1995;41:455–461. doi: 10.1159/000239382. [DOI] [PubMed] [Google Scholar]
- Sidwell RW, Barnard DL, Day CW, Smee DF, Bailey KW, Wong MH, Morrey JD, Furuta Y. Efficacy of orally administered T-705 on lethal avian influenza A (H5N1) virus infections in mice. Antimicrob. Agents. Chemother. 2007;51:845–851. doi: 10.1128/AAC.01051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidwell Rw, Smee DF. In vitro and in vivo assay systems for study for stusy of influenze virus inhibitors. Antiviral Res. 2000;48:1–16. doi: 10.1016/s0166-3542(00)00125-x. [DOI] [PubMed] [Google Scholar]
- Sidwell RW, Smee DF, Huffman JH, Barnard DL, Bailey KW, Morrey JD, Babu YS. In vivo influenza virus-inhibitory effects of the cyclopentane neuraminidase inhibitor RJW-270201. Antimicrob. Agents Chemother. 2001;45:749–757. doi: 10.1128/AAC.45.3.749-757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog L, Bjursell G, Thelander L, Hagerstrom T, Hobbs J, Eckstein F. 2-Deoxy-2-azidocytidine, a new inhibitor of DNA replication in mammalian cells. Eur. J. Biochem. 1977;72:371–378. doi: 10.1111/j.1432-1033.1977.tb11261.x. [DOI] [PubMed] [Google Scholar]
- Smee DF, Huffman JH, Morrison AC, Barnard DL, Sidwell RW. Cyclopentane neuraminidase inhibitors with potent in vitro anti-influenza virus activities. Antimicrob. Agents Chemother. 2001;45:743–748. doi: 10.1128/AAC.45.3.743-748.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DB, Kalayanov G, Sund C, Winqvist A, Maltseva T, Leveque VJP, Rajyaguru S, Pogam SL, Najera I, Benkestock K, Zhou X-X, Kaiser AC, Maag H, Cammack N, Martin JA, Swallow S, Johansson NG, Klumpp K, Smith M. The design, synthesis, and antiviral activity of monofluoro and difluoro analogues of 4’-azidocytidine against hepatitis C virus replication: the discovery o 4’-azido-2'-deoxy-2'-fluorocytidine and 4’-azido-2'-dideoxy-2',2'-difluorocytidine. J. Med. Chem. 2009;52:2971–2978. doi: 10.1021/jm801595c. [DOI] [PubMed] [Google Scholar]
- Stuyver LJ, McBrayer TR, Whitaker T, Tharnish PM, Ramesh M, Lostia S, Cartee L, Shi J, Hobbs A, Schinazi RF, Watanabe KA, Otto MJ. Inhibition of the subgenomic hepatitis C virus replicon in huh-7 cells by 2'-deoxy-2'-fluorocytidine. Antimicrob Agents Chemother. 2004;48:651–654. doi: 10.1128/AAC.48.2.651-654.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez DL, Perdue ML, Cox N, Rowe T, Bender C, Huang J, Swayne DE. Comparisons of highly virulent H5N1 influenza A viruses isolated from humans and chickens from Hong Kong. J. Virol. 1998;72:6678–6688. doi: 10.1128/jvi.72.8.6678-6688.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suguitan AL, Jr, McAuliffe J, Mills KL, Jin H, Duke G, Lu B, Luke CJ, Murphy B, Swayne DE, Kemble G, Subbarao K. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med. 2006;3:e360. doi: 10.1371/journal.pmed.0030360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorlund K, Awad T, Bovivin G, Thabane L. Systematic review of influenza resistance to the neuraminidase inhibitors. BMC Infect. Dis. 2011;11:134–146. doi: 10.1186/1471-2334-11-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale M, Appleyard G, Tuttle JV, Nelson DJ, Nusinoff-Lehrman S, Al Nakib W, Stables JN, Purifoy DJM, Powell KL, Darby G. Antiviral Chem Chemother. 1993;4:281–287. [Google Scholar]
- Tisdale M, Ellis M, Klumpp K, Court S, Ford M. Inhibition of influenza virus transcription by 2'-deoxy-2'- fluoroguanosine. Antimicrob. Agents Chemother. 1995;39:2454–2458. doi: 10.1128/aac.39.11.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trampuz A, Prabhu RM, Smith TF, Baddour LM. Avian influenza: a new pandemic threat? Mayo. Clin. Proc. 2004;79:523–530. doi: 10.4065/79.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle JV, Tisdale M, Krenitsky TA. Purine 2'-deoxy-2'-fluororibosides as antiinfluenza virus agents. J. Med. Chem. 1993;36:119–125. doi: 10.1021/jm00053a015. [DOI] [PubMed] [Google Scholar]
- Ujike M, Ejima M, Anraku A, Shimabukuro K, Obguchi M, Kishida N, Hong X, Takashita E, Fujisaki S, Yamashita K, Horikawa H, Kato Y, Oguchi A, Fujita N, Tashiro M, Odagiri T. Monitoring and characterization of oseltamivir-resistant pandemic (H1N1) 2009 virus, Japan, 2009–2010. Emerging. Infect. Dis. 2011;17:470–479. doi: 10.3201/eid1703.101188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verity EE, Camuglia S, Agius CT, Ong C, Shaw R, Barr I, Middleton D, Rockman S. Rapid generation of pandemic influenza virus vaccine candidate strains using synthetic DNA. Influenza Other Respi Viruses. 2011 doi: 10.1111/j.1750-2659.2011.00273.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlrab F, Jamieson AT, Hay J, Mengel R, Guschlbauer W. The effect of 2'-fluoro-2'-deoxycytidine on herpes virus growth. Biochim Biophys Acta. 1985;824:233–242. doi: 10.1016/0167-4781(85)90053-3. [DOI] [PubMed] [Google Scholar]