Abstract
Background
Squamous Cell Carcinoma of Esophagus is one of the most common malignancies in both men and women in eastern and south-eastern Africa. In Kenya, clinical observations suggest that this cancer is frequent in the Rift Valley area. However, so far, there has been no report on the molecular characteristics of esophageal squamous cell carcinoma (ESCC) in this area.
Results
We have analyzed TP53 mutations, the presence of human papilloma virus (HPV) DNA and expression of inflammation markers Cyclooxygenase 2 (Cox-2) and Nitrotyrosine (NTyR) in 28 cases (13 males and 15 females) of archived ESCC tissues collected at the Moi Teaching and Referral Hospital in Eldoret, Kenya. Eleven mutations were detected in TP53 exons 5 to 8 (39%). All ESCC samples were negative for HPV 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 70, 73 and 82. Immunohistochemical analysis of Cox-2 and NTyR showed a low proportion of positive cases (17.4% and 39.1%, respectively). No association between the above markers and suspected risk factors (alcohol or tobacco use, hot tea drinking, use of charcoal for cooking) was found.
Conclusion
Our findings suggest that mechanisms of esophageal carcinogenesis in eastern Africa might be different from other parts of the world. Low prevalence of TP53 mutation compared with other intermediate or high incidence areas of the world highlights this hypothesis. Our data did not support a possible ole of HPV in this series of cases. Further studies are needed to assess and compare the molecular patterns of ESCC from Kenya with those of high-incidence areas such as China or Central Asia.
Background
Esophageal Squamous Cell Carcinoma (ESCC) is the 6th most common cancer worldwide. About 80% of the cases occur in low and middle income countries, with large geographic variations in incidence [1]. The highest rates (over 50 per 100.000 person-years) are observed in areas of central Asia (in particular Northern Iran and central China). Intermediate incidence rates have been reported for parts of Latin America (Southern Brazil, Uruguay) and several regions in Europe (Western France, Hungary) [2]. The main risk factors in Western Countries include, combined consumption of tobacco and alcohol. In high incidence areas, a number of different factors such as hot tea consumption, low socio-economic status, low fresh fruit and vegetable intake and exposure to dietary carcinogens have been suggested to play a role [3-8]. The involvement of chronic infection by human papilloma viruses (HPV) is a controversial issue. Early results showing a high prevalence of HPV DNA in cases from high incidence areas of China [9,10] have not been confirmed in more stringent, recent studies [11,12].
ESCC has consistently been reported to be a frequent cancer in both males and females in southern and eastern Africa. In South Africa (Transkei region), molecular studies have reported a low prevalence of mutations in exons 5 to 8 of the TP53 tumor suppressor gene (17%) [13-16], well below the world average (40%) and the high prevalence observed in high incidence areas of China (70%) [17,18] or Northern Iran (90%) (Abedi-Ardekani et al, not published). So far, there is no data on TP53 mutation prevalence in ESCC from other parts of Africa.
ESCC is a common cancer in some rural areas of Kenya [19]. In the Rift Valley, ESCC is the leading malignancy in males and the third after cervical and breast cancers in females [20,21]. A recent study in the Bomet District, Western Kenya, showed that ESCC accounted for 914 (34.6%) of the 2643 cancers diagnosed between January 1999 and December 2007, with 6.3% of the patients less than 31 years [22].
We have performed a pilot study on 28 archived, paraffin embedded ESCC tissues from patients referring to the Moi Teaching and Referral Hospital (MTRH, Eldoret, Kenya). We have analysed TP53 mutations in exons 5 to 8, presence of high risk types of HPV, and Immunohistochemical (IHC) expression of two markers associated with inflammation damage (NTyR, a direct marker of protein damage by nitric oxide over expressed in ESCC linked with inflammation, [23,24] and inflammatory response (Cox-2, which is often elevated in ESCC from various high-incidence areas, [25]).
Methods
Patient selection
Patients who underwent resection for primary ESCC at MTRH, Eldoret, Kenya, between June 2003 and July 2006 were recruited into the study. Diagnosis was performed at the histopathology department, MTRH, and confirmed at the International Agency for Research on Cancer (IARC). A group of 37 properly fixed and processed ESCC samples was assembled for further molecular studies. The patients were part of a case-control study with information on risk factors obtained through questionnaires at the time of diagnosis. All patients involved in that study had signed a written consent. (Patel K. et al., unpublished, manuscript in preparation). The study was approved by Ethics Review board of MTRH and of IARC.
DNA extraction, TP53 mutation and HPV analysis
DNA was extracted from areas of tissue sections containing over 50% of cancer cells as identified by a pathologist at IARC (B.A-A) using a column-based purification method. A total of 28 samples provided enough DNA of suitable quality for molecular analyses. TP53 mutations in exons 5-8 were analyzed using the IARC reference protocol http://www-p53.iarc.fr/Download/TP53_DirectSequencing_IARC.pdf. Samples were analysed by bidirectional sequencing and the analysis was repeated with two independent PCR products. Sequencing results were compared with the reference sequence X54156 from Genbank http://p53.iarc.fr/TP53sequenceX54156.html. Sequence variations were checked with the mutation validation tool of the IARC TP53 mutation database http://www-p53.iarc.fr/MutationValidationCriteria.asp.
HPV typing was performed as previously described [26]. Briefly, detection and typing of 19 mucosal high-risk HPV types (types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 70, 73, and 82) was performed by a combination of multiplex PCR and HPV type-specific primers for amplification of viral DNA (E7). PCR products were typed using arrayed primer extension (APEX). Amplification of Beta-globin was used as positive reaction control.
Immunohistochemistry
Among 28 cases, IHC study was possible on 23. Deparaffinised sections (5 μM) were rehydrated and incubated with primary antibodies for either one hour at room temperature (DO7, mouse-anti p53 monoclonal antibody 1:500, Dako, Glostrup, Denmark), or overnight at 4°C (COX-2, polygoat, 1/2000, Santa Cruz Biotechnology) and NTyR (polyrabbit 1/2000, Upstate Biotechnology). Fixed antibodies were detected with secondary biotinylated anti-rabbit IgG (1/200, Vectasin Elite-ABC kit, Vector Laboratories Inc.) followed by streptavidin-peroxidase and diaminobenzidine-based detection according to standard protocols (Vector Laboratories, Inc.) A score combining intensity of staining (0 to 3) and proportion of stained cancer cells (0-10%: 0; 11-30%: 1; 31-50%: 2; over 50%: 3) was used. The combined scores ranging from 0-9 were scored into 3 final groups: 0-3 (no to weak expression), 4-6 (moderate expression), and 7-9 (strong expression).
Results
The characteristics of the 28 patients analyzed for both TP53 mutations and HPV DNA are summarized in Table 1. This group comprised an approximately equal proportion of males and females, in agreement with the known gender distribution of ESCC in the population of the Rift Valley [21]. Age at diagnosis ranged from 23 to 72 years (average of 56 years) with no difference between genders. All cases were invasive carcinoma.
Table 1.
Patient's characteristics and suspected risk factors
| Patient's characteristics, n = 28 | Number | |
|---|---|---|
| Gender | Males | 13 |
| Females | 15 | |
| Age | Average | 56.03 ± 12.30 |
| Range | 23-72 | |
| Tobacco use | Smoking (ever; male/female) | 6/0 |
| Snuff (ever; male/female) | 0/7 | |
| Any (ever/never) | 13/15 | |
| Alcohol use | (ever; male/female) | 9/1 |
| Hot tea drinking* | (yes; male/female) | 6/11 |
| Consumption of Mursik | (yes; male/female) | 8/10 |
| Oral hygiene+ | (poor; male/female) | 8/8 |
| Charcoal cooking | (yes; male/female) | 1/12 |
*: "hot" was considered as immediate drinking after pouring boiling tea
+: Estimated on the basis of tooth loss
Among possible risk factors, tobacco was predominantly used as smoked product in males and as snuff in females. Alcohol drinking showed a strong bias towards men (9 males for 1 female). Exposure to charcoal fumes was almost restricted to women (11 of 12 exposed patients were women). Other factors, such as hot tea drinking, consumption of mursik (a local fermented milk product laced with charcoal) and poor oral hygiene (as documented by tooth loss) were equally distributed between genders.
TP53 mutations (Table 2) were detected in 11 of 28 patients (39.3%). None of these mutations occurred at common TP53 mutation hotspots as reported in the IARC TP53 database. Of the mutations, 6 were missense, 3 nonsense and 2 were deletions inducing frameshift and supposed to lead to premature termination of translation. Only one mutation was a transversion (A:T to T:A at codon 255, p.I255F). Among transition mutations, 3 were at CpG sites, all leading to nonsense substitutions.
Table 2.
Characteristics of mutations in Esophageal Squamous Cell Carcinomas from Kenya
| Exon | Gene position | cDNA position | Codon and amino-acid change | Mutation | Mutation type |
|---|---|---|---|---|---|
| 5 | g.12524A > G | c.536A > G | p.H179R His-Arg | Missense | A:T > G:C |
| 6 | g.12706C > T | c.637C > T | p.R213X Arg-Stop | Nonsense | G:C > A:T at CpG |
| 6 | g.12706C > T | c.637C > T | p.R213X Arg-Stop | Nonsense | G:C > A:T at CpG |
| 7 | g.13386C > T | c.749C > T | p.P250L Pro-Leu | Missense | G:C > A:T |
| 7 | g.13400A > T | c.763A > T | p.I255F Ile-Phe | Missense | A:T > T:A |
| 8 | g.13776G > A | c.796G > A | p.G266R Gly-Arg | Missense | G:C > A:T |
| 8 | g.13813C > T | c.833C > T | p.P278L Pro-Leu | Missense | G:C > A:T |
| 8 | g.13816G > A | c.836G > A | p.G279E Gly-Glu | Missense | G:C > A:T |
| 8 | g.13896C > T | c.916C > T | p.R306X Arg-Stop | Nonsense | G:C > A:T at CpG |
| 8 | g.13811_13815del5 | c.831_835del5 | p.? | FS* | - |
| 6 | g.12729_12732del4 | c.660_663del4 | p.? | FS | - |
* FS: Frame Shift
Typing of 19 mucosal high-risk HPV types in the same DNA extracts as those used for TP53 mutation detection gave negative results (data not shown).
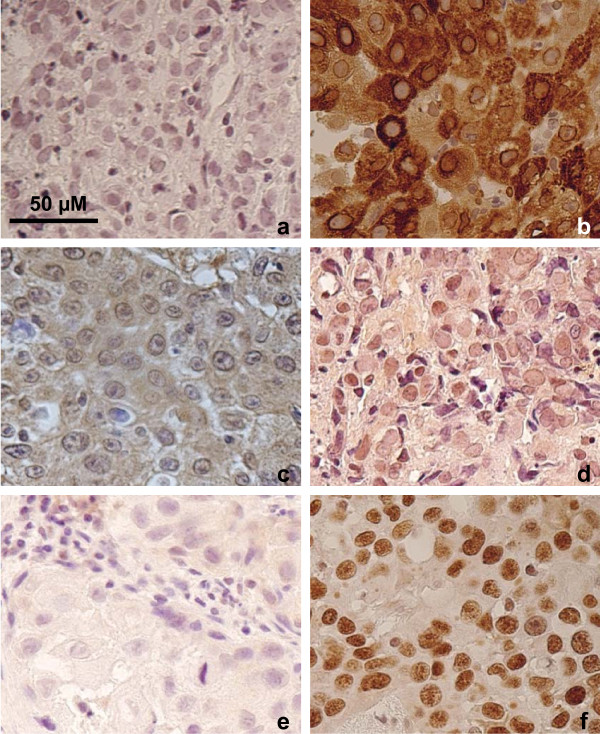
Immunohistochemical analysis on 23 cases (Figure 1 and Table 3) showed p53 protein accumulation (final groups of moderate and strong expression) in 18 (78.2%) of the cases. Among 11 cases with TP53 mutations, IHC analysis was possible on 6 and 5 had moderate or strong p53 expression scores. NTyR and Cox-2 were absent or weakly expressed in, respectively, 14 (60.9%) and 19 (82.6%) of the cases, suggesting that expression of markers of inflammation was not a common characteristic of these ESCC cases.
Figure 1.
Detection of Cox-2, N-TyR and p53 proteins by immunohistochemistry in ESCC tissues (×400). Typical examples of negative (left panels) or positive (right panels) staining are shown. (a-b): Cox-2; (c-d): N-TyR; (e-f): p53. Bar: scale in μm.
Table 3.
Expression scores for p53, Cox-2 and NTyR in 23 cases of Esophageal Squamous Cell Carcinomas from Kenya
| Expression Score groups | p53 No. (%) | Cox-2 No. (%) | N-Tyrosine No. (%) |
|---|---|---|---|
| Absent or weak (0-3) | 5 (21.8) | 19 (82.6) | 14 (60.9) |
| Moderate (4-6) | 9 (39.1) | 3 (13.0) | 5 (21.7) |
| Strong (7-9) | 9 (39.1) | 1 (4.4) | 4 (17.4) |
Discussion
This study is the first report on the molecular characteristics of ESCC from patients of the Rift Valley, a possibly high incidence area in Eastern Africa. Although limited to 28 cases, this series is of interest since there is no report for ESCC in Africa except for a study in Transkei, South Africa [27]. In the present series, men and women were equally represented, a gender distribution similar to most high incidence areas where tobacco and alcohol are not the main risk factors for ESCC, such as in Northern Iran or in central China [2,7,28]. However, from a molecular viewpoint, ESCC from Rift Valley appears different from those high incidence areas in the prevalence of TP53 mutations. We report a prevalence of TP53 mutation of 39%, far below the high prevalence reported in high incidence areas of China (50-75%) [17,29-32], Northern Iran (90%) (Abedi-Ardekani et al., not published) or Western France (Normandy, 76%) [33]. Remarkably, a low prevalence of TP53 mutations has also been reported in Transkei [27]. Furthermore, the mutations identified, both in the present study and in Transkei, differ from the average mutation patterns of most cancers which are dominated by transition mutations at "hotspot" codons within CpG sites [34]. Although the small size of this study precludes any detailed analysis of mutation patterns, these results, together with those previously published for ESCC from Transkei, suggest that ESCC in south-eastern Africa may develop in a different mutagenic context than ESCC in other high-incidence areas. Of note, mutation analysis was limited to exons 5 to 8 since these exons contain about 90% of know mutations in ESCC and were the only exons previously analyzed in ESCC from Africa [27]. This choice was made necessary by the fact that only limited amounts of DNA was available from these formalin-fixed paraffin tissues.
Low prevalence of TP53 mutations suggested that other molecular events may take place in ESCC from the Rift Valley. To address this hypothesis, we analyzed the presence of HPV DNA and the expression of markers of inflammation. High-risk HPV are known to contribute to carcinogenesis by inactivating the p53 protein in cervical and in oral cancers, thus bypassing the need for TP53 mutation [35,36]. Using a highly sensitive assay for high-risk HPV, we failed to detect any HPV sequences in the 28 cases tested. These results support the conclusions of a previous analysis performed on 29 biopsies of ESCC collected in the Bomet District, Western Kenya [37].
With respect to inflammation markers, only a small proportion of ESCC from the Rift Valley showed positive staining for either NTyR or Cox-2, two markers that we reported as highly expressed in ESCC from Iran (Tehran area) [23]. These results suggest that widespread inflammation is not a characteristic of ESCC in the Rift Valley. However, data on inflammation status of normal mucosa are needed to determine whether inflammatory conditions may play a role in the early steps of esophageal carcinogenesis.
Conclusions
The results presented here highlight that mechanisms of carcinogenesis in ESCC in eastern Africa may differ from other areas of the world. In particular, the low prevalence of TP53 mutation, also observed in ESCC from South Africa, suggests that some other mechanism of p53 inactivation may take place in these cancers. Our data do not support a role for HPV in this process. Furthermore, the risk factors associated with ESCC in Africa is poorly known. In South Africa, fumonisin, a mycotoxin that contaminates several components of the diet, have been proposed as significant risk factors [38,39]. Although fumonisins are present in the diet in east Africa [40], no study so far has addressed their potential role in the etiology of ESCC in the Rift Valley. If further studies with larger sample size and sequencing of all TP53 exons confirm our findings, then we may assume that the type of risk factors and/or mechanisms of carcinogenesis in ESCC in Eastern Africa are different from other high-incidence areas of the world.
Abbreviations
ESCC: esophageal squamous cell carcinoma; HPV: human papilloma virus; Cox-2: Cyclooxygenase 2; NTyR: Nitrotyrosine; MTRH: Moi teaching and referral hospital; IARC: international agency for research on cancer; IHC: immunohistochemistry.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
KP, SM and JW participated in the study concept and provided the archived material. KP participated in the manuscript drafting. GM-P carried out the technical work of molecular analysis and sequencing. TG carried out the HPV DNA analysis. MT supervised the HPV analysis and participated in critical revision of the manuscript. PH was responsible for the study concept, design and supervision, manuscript drafting and critical revision. BA-A was responsible for pathology and immunohistochemistry evaluation, manuscript drafting and critical revision.
All authors read and approved the final manuscript.
Contributor Information
Kirtika Patel, Email: kirtikap@gmail.com.
Simeon Mining, Email: smining57@gmail.com.
Johnston Wakhisi, Email: jwakhisi@yahoo.com.
Tarik Gheit, Email: gheit@iarc.fr.
Massimo Tommasino, Email: tommasino@iarc.fr.
Ghislaine Martel-Planche, Email: martel@iarc.fr.
Pierre Hainaut, Email: hainaut@iarc.fr.
Behnoush Abedi-Ardekani, Email: abedib@iarc.fr.
Acknowledgements
The authors are grateful to Ms C. Carreira, from the histopathology service lab at IARC, for immunohistochemistry.
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Lambert R, Hainaut P. Esophageal cancer: cases and causes (part I) Endoscopy. 2007;39:550–555. doi: 10.1055/s-2007-966530. [DOI] [PubMed] [Google Scholar]
- Abedi-Ardekani B, Kamangar F, Hewitt SM, Hainaut P, Sotoudeh M, Abnet CC, Taylor PR, Boffetta P, Malekzadeh R, Dawsey SM. Polycyclic aromatic hydrocarbon exposure in oesophageal tissue and risk of oesophageal squamous cell carcinoma in north-eastern Iran. Gut. 2010;59:1178–1183. doi: 10.1136/gut.2010.210609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abnet CC, Kamangar F, Islami F, Nasrollahzadeh D, Brennan P, Aghcheli K, Merat S, Pourshams A, Marjani HA, Ebadati A, Sotoudeh M, Boffetta P, Malekzadeh R, Dawsey SM. Tooth loss and lack of regular oral hygiene are associated with higher risk of esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2008;17:3062–3068. doi: 10.1158/1055-9965.EPI-08-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islami F, Kamangar F, Nasrollahzadeh D, Aghcheli K, Sotoudeh M, bedi-Ardekani B, Merat S, Nasseri-Moghaddam S, Semnani S, Sepehr A, Wakefield J, Moller H, Abnet CC, Dawsey SM, Boffetta P, Malekzadeh R. Socio-economic status and oesophageal cancer: results from a population-based case-control study in a high-risk area. Int J Epidemiol. 2009;38:978–988. doi: 10.1093/ije/dyp195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islami F, Pourshams A, Nasrollahzadeh D, Kamangar F, Fahimi S, Shakeri R, bedi-Ardekani B, Merat S, Vahedi H, Semnani S, Abnet CC, Brennan P, Moller H, Saidi F, Dawsey SM, Malekzadeh R, Boffetta P. Tea drinking habits and oesophageal cancer in a high risk area in northern Iran: population based case-control study. BMJ. 2009;338:b929. doi: 10.1136/bmj.b929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamangar F, Malekzadeh R, Dawsey SM, Saidi F. Esophageal cancer in Northeastern Iran: a review. Arch Iran Med. 2007;10:70–82. [PubMed] [Google Scholar]
- Nasrollahzadeh D, Kamangar F, Aghcheli K, Sotoudeh M, Islami F, Abnet CC, Shakeri R, Pourshams A, Marjani HA, Nouraie M, Khatibian M, Semnani S, Ye W, Boffetta P, Dawsey SM, Malekzadeh R. Opium, tobacco, and alcohol use in relation to oesophageal squamous cell carcinoma in a high-risk area of Iran. Br J Cancer. 2008;98:1857–1863. doi: 10.1038/sj.bjc.6604369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Tian X, Liu F, Zhao Y, Sun M, Chen D, Lu C, Wang Z, Shi X, Zhang Q, Zhang D, Shen Z, Li F, Harris CC, Cai H, Ke Y. Detection of HPV DNA in esophageal cancer specimens from different regions and ethnic groups: a descriptive study. BMC Cancer. 2010;10:19. doi: 10.1186/1471-2407-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F, Syrjanen S, Shen Q, Ji HX, Syrjanen K. Human papillomavirus (HPV) DNA in esophageal precancer lesions and squamous cell carcinomas from China. Int J Cancer. 1990;45:21–25. doi: 10.1002/ijc.2910450106. [DOI] [PubMed] [Google Scholar]
- Koshiol J, Wei WQ, Kreimer AR, Chen W, Gravitt P, Ren JS, Abnet CC, Wang JB, Kamangar F, Lin DM, von Knebel-Doeberitz M, Zhang Y, Viscidi R, Wang GQ, Gillison ML, Roth MJ, Dong ZW, Kim E, Taylor PR, Qiao YL, Dawsey SM. No role for human papillomavirus in esophageal squamous cell carcinoma in China. Int J Cancer. 2010;127:93–100. doi: 10.1002/ijc.25023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers EM, Lavergne D, Chang F, Syrjanen K, Tosi P, Cintorino M, Santopietro R, Syrjanen S. An interlaboratory study to determine the presence of human papillomavirus DNA in esophageal carcinoma from China. Int J Cancer. 1999;81:225–228. doi: 10.1002/(SICI)1097-0215(19990412)81:2<225::AID-IJC10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Sammon AM. Carcinogens and endemic squamous cancer of the oesophagus in Transkei, South Africa. Environmental initiation is the dominant factor; tobacco or other carcinogens of low potency or concentration are sufficient for carcinogenesis in the predisposed mucosa. Med Hypotheses. 2007;69:125–131. doi: 10.1016/j.mehy.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Matsha T, Brink L, van RS, Hon D, Lombard C, Erasmus R. Traditional home-brewed beer consumption and iron status in patients with esophageal cancer and healthy control subjects from Transkei, South Africa. Nutr Cancer. 2006;56:67–73. doi: 10.1207/s15327914nc5601_9. [DOI] [PubMed] [Google Scholar]
- Sammon AM, Iputo JE. Maize meal predisposes to endemic squamous cancer of the oesophagus in Africa: breakdown of esterified linoleic acid to the free form in stored meal leads to increased intragastric PGE2 production and a low-acid reflux. Med Hypotheses. 2006;67:1431–1436. doi: 10.1016/j.mehy.2006.05.037. [DOI] [PubMed] [Google Scholar]
- Hendricks D, Parker MI. Oesophageal cancer in Africa. IUBMB Life. 2002;53:263–268. doi: 10.1080/15216540212643. [DOI] [PubMed] [Google Scholar]
- Bennett WP, von Brevern MC, Zhu SM, Bartsch H, Muehlbauer KR, Hollstein MC. p53 mutations in esophageal tumors from a high incidence area of China in relation to patient diet and smoking history. Cancer Epidemiol Biomarkers Prev. 1997;6:963–966. [PubMed] [Google Scholar]
- Lam KY, Tsao SW, Zhang D, Law S, He D, Ma L, Wong J. Prevalence and predictive value of p53 mutation in patients with oesophageal squamous cell carcinomas: a prospective clinico-pathological study and survival analysis of 70 patients. Int J Cancer. 1997;74:212–219. doi: 10.1002/(SICI)1097-0215(19970422)74:2<212::AID-IJC13>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- White RE, Abnet CC, Mungatana CK, Dawsey SM. Oesophageal cancer: a common malignancy in young people of Bomet District, Kenya. Lancet. 2002;360:462–463. doi: 10.1016/S0140-6736(02)09639-3. [DOI] [PubMed] [Google Scholar]
- Tenge CN, Kuremu RT, Buziba NG, Patel K, Were PA. Burden and pattern of cancer in Western Kenya. East Afr Med J. 2009;86:7–10. doi: 10.4314/eamj.v86i1.46921. [DOI] [PubMed] [Google Scholar]
- Wakhisi J, Patel K, Buziba N, Rotich J. Esophageal cancer in north rift valley of Western Kenya. Afr Health Sci. 2005;5:157–163. [PMC free article] [PubMed] [Google Scholar]
- Parker RK, Dawsey SM, Abnet CC, White RE. Frequent occurrence of esophageal cancer in young people in western Kenya. Dis Esophagus. 2010;23:128–135. doi: 10.1111/j.1442-2050.2009.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepehr A, Taniere P, Martel-Planche G, Zia'ee AA, Rastgar-Jazii F, Yazdanbod M, Etemad-Moghadam G, Kamangar F, Saidi F, Hainaut P. Distinct pattern of TP53 mutations in squamous cell carcinoma of the esophagus in Iran. Oncogene. 2001;20:7368–7374. doi: 10.1038/sj.onc.1204912. [DOI] [PubMed] [Google Scholar]
- Vaninetti NM, Geldenhuys L, Porter GA, Risch H, Hainaut P, Guernsey DL, Casson AG. Inducible nitric oxide synthase, nitrotyrosine and p53 mutations in the molecular pathogenesis of Barrett's esophagus and esophageal adenocarcinoma. Mol Carcinog. 2008;47:275–285. doi: 10.1002/mc.20382. [DOI] [PubMed] [Google Scholar]
- de Moraes E, Dar NA, de Moura Gallo CV, Hainaut P. Cross-talks between cyclooxygenase-2 and tumor suppressor protein p53: Balancing life and death during inflammatory stress and carcinogenesis. Int J Cancer. 2007;121:929–937. doi: 10.1002/ijc.22899. [DOI] [PubMed] [Google Scholar]
- Gheit T, Landi S, Gemignani F, Snijders PJ, Vaccarella S, Franceschi S, Canzian F, Tommasino M. Development of a sensitive and specific assay combining multiplex PCR and DNA microarray primer extension to detect high-risk mucosal human papillomavirus types. J Clin Microbiol. 2006;44:2025–2031. doi: 10.1128/JCM.02305-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamieldien W, Victor TC, Mugwanya D, Stepien A, Gelderblom WC, Marasas WF, Geiger DH, van Helden PD. p53 and p16/CDKN2 gene mutations in esophageal tumors from a high-incidence area in South Africa. Int J Cancer. 1998;78:544–549. doi: 10.1002/(SICI)1097-0215(19981123)78:5<544::AID-IJC3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Islami F, Kamangar F, Aghcheli K, Fahimi S, Semnani S, Taghavi N, Marjani HA, Merat S, Nasseri-Moghaddam S, Pourshams A, Nouraie M, Khatibian M, Abedi B, Brazandeh MH, Ghaziani R, Sotoudeh M, Dawsey SM, Abnet CC, Taylor PR, Malekzadeh R. Epidemiologic features of upper gastrointestinal tract cancers in Northeastern Iran. Br J Cancer. 2004;90:1402–1406. doi: 10.1038/sj.bjc.6601737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Chen X, Dai H, Wang H, Shen B, Chu D, McAfee T, Zhang ZF. Mutational spectra of p53 in geographically localized esophageal squamous cell carcinoma groups in China. Cancer. 2004;101:834–844. doi: 10.1002/cncr.20437. [DOI] [PubMed] [Google Scholar]
- Gao H, Wang LD, Zhou Q, Hong JY, Huang TY, Yang CS. p53 tumor suppressor gene mutation in early esophageal precancerous lesions and carcinoma among high-risk populations in Henan, China. Cancer Res. 1994;54:4342–4346. [PubMed] [Google Scholar]
- Hu N, Huang J, Emmert-Buck MR, Tang ZZ, Roth MJ, Wang C, Dawsey SM, Li G, Li WJ, Wang QH, Han XY, Ding T, Giffen C, Goldstein AM, Taylor PR. Frequent inactivation of the TP53 gene in esophageal squamous cell carcinoma from a high-risk population in China. Clin Cancer Res. 2001;7:883–891. [PubMed] [Google Scholar]
- Lung ML, Chan WC, Zong YS, Tang CM, Fok CL, Wong KT, Chan LK, Lau KW. p53 mutational spectrum of esophageal carcinomas from five different geographical locales in China. Cancer Epidemiol Biomarkers Prev. 1996;5:277–284. [PubMed] [Google Scholar]
- Breton J, Sichel F, Abbas A, Marnay J, Arsene D, Lechevrel M. Simultaneous use of DGGE and DHPLC to screen TP53 mutations in cancers of the esophagus and cardia from a European high incidence area (Lower Normandy, France) Mutagenesis. 2003;18:299–306. doi: 10.1093/mutage/18.3.299. [DOI] [PubMed] [Google Scholar]
- Petitjean A, Mathe E, Kato S, Ishioka C, Tavtigian SV, Hainaut P, Olivier M. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28:622–629. doi: 10.1002/humu.20495. [DOI] [PubMed] [Google Scholar]
- Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010;10:550–560. doi: 10.1038/nrc2886. [DOI] [PubMed] [Google Scholar]
- White RE, Mungatana C, Mutuma G, Robert ME, Daniel RW, Topazian MD, Shah KV. Absence of human papillomavirus in esophageal carcinomas from southwestern Kenya. Dis Esophagus. 2005;18:28–30. doi: 10.1111/j.1442-2050.2005.00452.x. [DOI] [PubMed] [Google Scholar]
- van der WL, Shephard GS, Rheeder JP, Burger HM, Gelderblom WC, Wild CP, Gong YY. Simple intervention method to reduce fumonisin exposure in a subsistence maize-farming community in South Africa. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2010;27:1582–1588. doi: 10.1080/19440049.2010.508050. [DOI] [PubMed] [Google Scholar]
- Wild CP, Gong YY. Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis. 2010;31:71–82. doi: 10.1093/carcin/bgp264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedera CJ, Plattner RD, Desjardins AE. Incidence of Fusarium spp. and levels of fumonisin B1 in maize in western Kenya. Appl Environ Microbiol. 1999;65:41–44. doi: 10.1128/aem.65.1.41-44.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]