Abstract
Aminoglycosides like gentamicin are among the most commonly used antibiotics in clinical practice and are essential for treating life-threatening tuberculosis and Gram-negative bacterial infections. However, aminoglycosides are also nephrotoxic and ototoxic. Although a number of mechanisms have been proposed, it is still unclear how aminoglycosides induce cell death in auditory sensory epithelia and subsequent deafness. Aminoglycosides bind to various intracellular molecules, such as RNA and phosphoinositides. We hypothesized that aminoglycosides, based on their tissue-specific susceptibility, also bind to intracellular proteins that play a role in drug-induced ototoxicity. By conjugating an aminoglycoside, gentamicin, to agarose beads and conducting a gentamicin-agarose pull-down assay, we have isolated gentamicin-binding proteins (GBPs) from immortalized cells of mouse organ of Corti, HEI-OC1. Mass spectrometry identified calreticulin (CRT) as a GBP. Immunofluorescence revealed that CRT expression is concentrated in strial marginal cells and hair cell stereocilia, primary locations of drug uptake and cytotoxicity in the cochlea. In HEI-OC1 cells treated with gentamicin, reduction of CRT expression using small interfering RNA (siRNA) reduced intracellular drug levels. CRT-deficient mouse embryonic fibroblast (MEF) cells as well as CRT siRNA-transfected wild-type MEFs also had reduced cell viability after gentamicin treatment. A pull-down assay using deletion mutants of CRT determined that the carboxyl C-domain of CRT binds to gentamicin. HeLa cells transfected with CRT C-domain deletion mutant construct were more susceptible to gentamicin-induced cytotoxicity compared with cells transfected with full-length CRT or other deletion mutants. Therefore, we conclude that CRT binding to gentamicin is protective against gentamicin-induced cytotoxicity.
Keywords: aminoglycosides, gentamicin, calreticulin, ototoxicity
Aminoglycoside antibiotics are essential for clinical treatment of bacterial sepsis and meningitis (Edson and Terrell, 1999; Forge and Schacht, 2000; Grohskopf et al., 2005). However, these drugs also have major cytotoxic side effects, particularly nephrotoxicity and ototoxicity. Aminoglycoside-induced nephrotoxicity is largely reversible due to epithelial cell regeneration, but ototoxicity is mostly permanent because mammalian sensory hair cells cannot be regenerated (Lowenheim et al., 1999; Tulkens, 1989).
Aminoglycosides like gentamicin bind to RNA, phosphoinositides, and numerous proteins (Lesniak et al., 2005; Moestrup et al., 1995; Prezant et al., 1993). In bacteria, aminoglycoside binding to ribosomal RNA inhibits protein synthesis (Moazed and Noller, 1987; Purohit and Stern, 1994). However, mammalian ribosomal RNA has much lower affinities for aminoglycosides, except for certain mitochondrial mutations (Hamasaki and Rando, 1997). Furthermore, drug interaction with ribosomal RNA does not account for the tissue specificity of aminoglycoside cytotoxicity in the cochlea and kidney because there is little RNA variation among tissues. Phosphoinositides also have no known specific tissue distribution. Protein expression, on the other hand, does vary among different tissues and cell types, and proteins that bind to aminoglycosides are likely to contribute to drug-induced cytotoxicity in the cochlea and kidney.
To date, proteins that bind to aminoglycosides in the inner ear have not been reported, except for our recent identification of CLIMP-63 as a gentamicin-binding protein (GBP) in cochlea-derived cell lines (Karasawa et al., 2010). Identifying GBPs in the inner ear is challenging because it is difficult to obtain sufficient quantities of proteins or complementary DNA (cDNA) library material to isolate or screen for GBPs. In this study, we used a gentamicin-agarose conjugate to pull down GBPs from the mouse cochlear cell line HEI-OC1 and identified calreticulin (CRT). CRT is a lectin that binds to glycoproteins in the endoplasmic reticulum (ER) and is a molecular chaperone assisting in protein folding, subunit assembly, and trafficking misfolded proteins or nonnative conformers for proteasome degradation (Hebert and Molinari, 2007). The protein contains an N-terminal cleavable signal sequence that directs it to the ER and an ER KDEL retention/retrieval signal. CRT is composed of three distinct structural and functional domains: a globular N-domain, an extended P-domain, and an acidic C-domain (Michalak et al., 2009). We show that CRT is specifically expressed in the cochlea and is associated with intracellular retention of gentamicin. We also show that CRT expression reduces gentamicin-induced cytotoxicity by binding and sequestering gentamicin via the carboxyl C-domain of CRT.
MATERIALS AND METHODS
Cell culture.
HEI-OC1 cells (a gift of Dr Federico Kalinec, House Ear Institute, CA) were maintained at 33°C with 10% CO2 (permissive condition) or 39°C with 5% CO2 (nonpermissive condition). K41 and K42 (a gift from Dr Marek Michalak, University of Alberta, Canada), HeLa, and 293T cells were maintained at 37°C, 5% CO2. All cells were incubated in Dulbecco's modified Eagle medium with 10% fetal bovine serum.
Gentamicin-agarose pull-down assay.
Cells were lysed in buffer containing 150mM NaCl, 50mM Tris, pH 7.5, 5mM EDTA, and 1% Triton X-100 with protease inhibitors and centrifuged (14,000 × g, 10 min) at 4°C to remove cell debris. Protein concentration was adjusted to 1 mg/ml for all samples. For gentamicin-agarose conjugation, 100 μl Affi-Gel 10 (Bio-Rad) was washed with ice-cold water and resuspended in 1 ml 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (pH 7.0) containing 20 mg dissolved gentamicin overnight at 4°C. The residual reactive sites on the beads were blocked by 1M ethanolamine (pH 8.0) for 1 h and then washed with PBS and stored at 4°C until use. The resultant gentamicin-agarose conjugate was incubated with whole-cell extract of HEI-OC1 cells (1.5 ml) for 1 h at 4°C. The beads with bound proteins were spun down by brief centrifugation and washed with the same lysis buffer at 4°C. The proteins were eluted from gentamicin-agarose in SDS sample buffer, boiled for 2 min, and separated by protein gel electrophoresis (8% gel) prior to Coomassie blue staining. For binding to green fluorescent protein (GFP) fusion proteins of CRT and its deletion mutants, glycerol was added to the lysis buffer at 10% final concentration, and 50 μl Affi-Gel 10 and 10 mg gentamicin were used for each sample. After ethanolamine treatment, the beads were further blocked with 5% bovine serum albumin (BSA)-containing PBS for 3 h at 4°C, prior to adding cell lysate for binding.
Mass spectrometry.
GBP bands were excised from a Coomassie-stained gel, digested using trypsin, peptides analyzed by tandem mass spectrometry using an LTQ linear ion trap mass spectrometer (Thermo Fisher, San Jose, CA), and protein identification and verification performed as described previously (Karasawa et al., 2010), except tandem mass spectrometry (MS/MS) data were searched using both Mascot (Matrix Science, London, U.K.) and Sequest (Thermo Fisher) using a mouse only protein database (SwissProt, Swiss Institute of Bioinformatics) with no enzyme specificity. The software Scaffold (Proteome Software, Portland, OR) was then used to validate peptide and protein identifications, with minimum peptide and protein identification probabilities set at 80 and 99%, respectively. CRT was identified by 30 unique peptides, and glucose-regulated protein-94 (GRP94) was identified by 22 unique peptides.
Western blotting.
Proteins in lysis buffer were mixed with SDS sample buffer, resolved in a SDS-polyacrylamide gel, and transferred to polyvinylidene fluoride membrane. After blocking, CRT rabbit polyclonal antibody (Affinity BioReagents), GRP94 rat monoclonal antibody (Stressgen), actin rabbit polyclonal antibody (Sigma, Cat #A2103), or enhanced GFP mouse monoclonal antibody (Clontech) was incubated overnight at 4°C. After horseradish peroxidase conjugated secondary antibody incubation for 1 h, chemiluminescence with SuperSignal West Dura Extended Duration Substrate (Pierce) was used to detect protein expression.
Gentamicin treatment and immunofluorescence.
Cells were incubated with 1mM gentamicin in medium for 6 h and washed with PBS before fixation/permeabilization with 4% formaldehyde plus 0.5% Triton X-100 (FATX) for 1 h. To generate organ of Corti and cochlear lateral wall whole mounts, 4- to 7-week C57BL/6 mice were fixed by transcardiac perfusion of 4% paraformaldehyde in PBS and cochleae and kidneys excised and immersion-fixed for 1 h at room temperature. After rinsing in PBS, the bony cochlear shell was removed and cell membranes permeabilized in FATX for 30 min. Murine kidneys were vibratome-sectioned at 100-μm thickness, prior to immersion in FATX for 30 min.
For immunofluorescence, FATX-treated samples were blocked with buffer containing 10% goat serum and 1% BSA for 1 h and incubated with antibodies for CRT, GRP94, and/or gentamicin mouse monoclonal antibodies (Fitzgerald Industries International) for 1 h (cultured cells) or overnight at 4°C (murine tissues). After incubation with secondary antibodies conjugated to Alexa Fluor 488 or 568 for 1 h (and Alexa Fluor 568-conjugated phalloidin for an additional 1 h for murine tissues), samples were washed, postfixed with FATX, mounted, and examined using a Bio-Rad 1024 ES scanning laser confocal microscope. To quantify fluorescence intensity for each individual set of images, the same confocal settings were used, two images per well from two wells per experimental condition were obtained. Due to heterogeneous CRT expression levels in K41 cells, six images (instead of four images) per experimental condition were used for data in Figures 5 and 6. Pixel intensity values were obtained by histogram function of the ImageJ software after removal of nuclei and intercellular pixels using Adobe Photoshop. Student's t-test was used for statistical analysis.
FIG. 5.
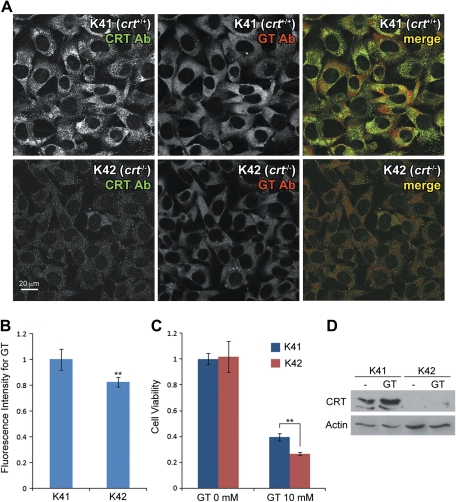
CRT-deficient cells are more susceptible to gentamicin treatment. (A) K41 (crt+/+) and K42 (crt−/−) cells were treated with gentamicin for 6 h at 37°C. Gentamicin immunofluorescence in K42 cells were lower compared with most K41 cells. (B) Gentamicin fluorescence intensities, on average, were significantly lower in K42 cells compared with K41 cells (**p < 0.01). (C) Cell viability measured by MTT assay revealed that K42 cells were more susceptible to gentamicin compared with K41 cells after 3 days of treatment (**p < 0.01). Cells cultured for 3 days with no gentamicin treatment had no significant difference in viability. (D) Gentamicin (10mM) treatment for 3 days increased CRT protein expression levels in K41 cells. There was no CRT expression detected in K42 cells as these cells are CRT deficient.
FIG. 6.
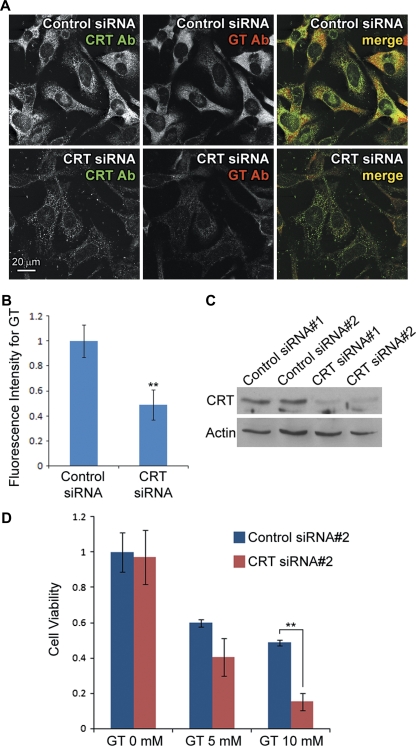
CRT knockdown increases susceptibility to gentamicin cytotoxicity. (A) K41 cells transfected with CRT siRNA showed reduced intracellular gentamicin levels compared with cells transfected with control siRNA, after 6 h of treatment at 37°C. (B) Quantification of fluorescence intensities showed that intracellular gentamicin levels in CRT siRNA-transfected K41 cells were significantly lower than control siRNA cells (**p < 0.01). (C) Western blotting confirmed that the two different siRNA for CRT reduced the protein expression efficiently in K41 cells, unlike the two scrambled control siRNA. (D) MTT assay on cells treated with gentamicin for 3 days showed that CRT siRNA#2-transfected K41 cells had lower viability compared with control siRNA#2.
CRT expression plasmid constructs.
GFP fusion expression plasmids of the full-length CRT and deletion mutants were constructed by sequential subcloning of DNA fragments into pcDNA3.1 vector (Invitrogen). For all constructs, GFP with the addition of KDEL was generated by PCR using pEGFP-N2 (Clontech) as a template with primers 5′-TTTGCGGCCGCGTGAGCAAGGGCGAGGAG-3′ and 5′-AAATCTAGACTACAGCTCATCCTTGGCTTGCTTGTACAGCTCGTCCATG-3′, digested with NotI/XbaI and subcloned into pcDNA3.1 (resultant plasmid “GFP-pcDNA3.1”). For the full-length CRT-GFP, CRT lacking KDEL was generated by PCR using mouse CRT cDNA from American type culture collection (IMAGE id 2655918) and primers 5′-AAAGCTAGCCGCCATGCTCCTTTCGGTG-3′ and 5′-TTTGCGGCCGCCAGGGGATTCTTCCTC-3′, digested with NheI/NotI and subcloned into GFP-pcDNA3.1. The C-domain deletion mutant as a GFP fusion was generated by a similar method with primers 5′-AAAGCTAGCCGCCATGCTCCTTTCGGTG-3′ and 5′-AAAGCGGCCGCCCTTGTAATCTGGGTTGTCAATTTG-3′. For the N-domain deletion mutant, two DNA fragments were amplified using the same CRT cDNA template, digested, and sequentially subcloned into GFP-pcDNA3.1 with the primer sets 5′-CCCAAGCTTCTATACACACTGATTGTG-3′ and 5′-TTTGCGGCCGCCAGGGGATTCTTCCTC-3′ (digested with HindIII/NotI), and 5′-AAAGCTAGCGACCATTAGGCCTATTTAG-3′ and 5′-CCCAAGCTTGAAATAGATGGCAGGGTC-3′ (digested with NheI/HindIII). For the P-domain deletion mutant, two DNA fragments were similarly generated and subcloned with the primer sets 5′-CCCAAGCTTAAGGGTACCTGGATACAC-3′ and 5′-TTTGCGGCCGCCAGGGGATTCTTCCTC-3′ (digested with HindIII/NotI), and 5′-AAAGCTAGCCGCCATGCTCCTTTCGGTG-3′ and 5′-CCCAAGCTTGTGTGTGAATTCATCATCC-3′ (digested with NheI/HindIII). Sequences of all the constructs were confirmed without false mutations.
Transfection.
Small interfering RNA (siRNA) and scrambled control for CRT and GRP94 were designed and synthesized using Invitrogen's service; for CRT siRNA#1, 5′-GCAAGAAUGUGCUGAUCAATT-3′ and control siRNA#1, 5′-GCAGUAAUCGUUAGGACAATT-3′; for CRT siRNA#2, 5′-GCAUGGAGACUCAGAAUAUTT-3′ and control siRNA#2, 5′-GCAGAGUCAGACAAUGUAUTT-3′; and for GRP94 siRNA, 5′-GCAUCUGAUUACCUUGAAUTT-3′ and control siRNA, 5′-GCAUGAUUACCUUGUCAAUTT-3′. Transfection of siRNA was performed using Lipofectamine RNAiMAX (Invitrogen), and for overexpression of CRT or its deletion mutants as GFP fusions, transfection was performed using Lipofectamine 2000 (Invitrogen) for 293T cells or X-tremeGENE 9 (Roche Applied Science) for HeLa cells.
Cell viability measurements.
Cell viability was determined by the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), an indicator of mitochondrial dehydrogenase activity. K41 and K42 cells were plated at 3000 cells per well, and HeLa cells were plated at 4000 cells per well in a 96-well plate. For comparison of K41 and K42 cells, after incubation overnight to allow cells to attach to the plate, cells were treated with gentamicin (5 or 10mM) in culture medium for 3 days. For siRNA- or plasmid-transfected cells, cells were incubated for 24 h after transfection, prior to 3-day gentamicin treatment. After 3 days of gentamicin treatment, 20 μl of 5 mg/ml MTT solution was added to each well, and cells were incubated for 4 h at 37°C, 5% CO2. Culture medium was then replaced with 200 μl dimethyl sulfoxide (DMSO) in each well and the optical density recorded at 540 nm with background subtraction at 660 nm. Student's t-test was used for statistical analysis.
RESULTS
CRT and GRP94 Are GBPs in Cochlear Cells
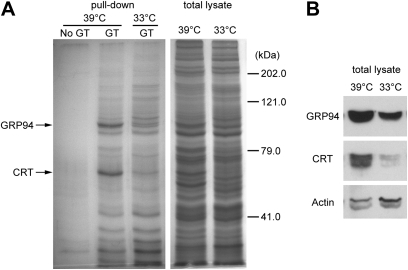
For GBP identification from cochlear cells, we used HEI-OC1 (Kalinec et al., 2003), a cochlear organ of Corti cell line developed from a transgenic mouse called Immortomouse, which harbors temperature-sensitive large T antigen (Holley and Lawlor, 1997; Jat et al., 1991). HEI-OC1 cells proliferate normally at 33°C (permissive condition), but at 39°C (nonpermissive condition), these cells begin to differentiate into epithelial-like cells, although they can still grow slowly. HEI-OC1 cells induce cochlear-specific gene expression after incubation at 39°C for 2 weeks (Kalinec et al., 2003). To identify cochlear-specific GBPs, we conducted gentamicin-agarose pull-down assays using HEI-OC1 cells maintained at 39°C for 2 weeks; HEI-OC1 cells maintained at 33°C were used as a control. SDS-gel electrophoresis followed by Coomassie blue staining revealed two bands that were more intense in cells maintained at 39°C compared with those at 33°C (Fig. 1A). These bands consistently appeared in three separate pull-down experiments. Mass spectrometric analysis on excised bands identified that these are CRT and GRP94, both of them chaperone proteins in the ER (Ni and Lee, 2007). Western blotting on total cell lysates used for the pull-down assay showed that CRT and GRP94 expression levels were higher in nonpermissive cells compared with permissive cells (Fig. 1B), indicating that expression of these proteins was increased in HEI-OC1 cells in the differentiated state.
FIG. 1.
(A) CRT and GRP94 are GBPs in HEI-OC1 cells. GBPs were pulled down from total cell lysate of HEI-OC1 cells, resolved by SDS-gel electrophoresis, and stained by Coomassie blue. Most of the protein bands were similar between nonpermissive cells at 39°C and permissive cells at 33°C, but two bands were more intense in nonpermissive cells at 39°C. Mass spectrometric analysis on these bands identified CRT and GRP94. (B) Western blot analysis on total cell lysate showed more protein expression of CRT and GRP94 in cells at 39°C compared with 33°C.
CRT Is Specifically Expressed in Mouse Cochlear Tissues
Although CRT expression has been well characterized in the kidney (Horibe et al., 2004), its expression and distribution in the cochlea has not previously been reported. Immunofluorescence using murine cochleae revealed that CRT is highly expressed in hair cell stereocilia (Figs. 2A–C) and concentrated near the lumenal membranes of marginal cells (Figs. 2D–H) in the stria vascularis. CRT expression in the kidney was also confirmed (Fig. 2I). There was little GRP94 expression other than weak ubiquitous expression throughout these tissues (Figs. 2J and 2K). An apparent discrepancy between low GRP94 expression in the mouse inner ear and high expression in HEI-OC1 cells could be attributed to differences between terminally differentiated or quiescence cochlear cells in vivo and immortalized cells in vitro (Figs. 1B, 2J, and 2K).
FIG. 2.
CRT expression in the cochlea by immunofluorescence. In the organ of Corti, CRT expression was localized in sensory hair cell bundles (A and C). In the stria vascularis, CRT expression was concentrated near the lumenal membranes of marginal cells (D–H, arrowheads). CRT expression is in green and actin in red in (C, F, and H) in the online version. CRT expression was also present in kidney proximal (p) and distal (d) tubules (I). GRP94 expression was weak and unremarkable in the organ of Corti (J) and stria vascularis (K).
CRT siRNA Reduces Intracellular Gentamicin Levels
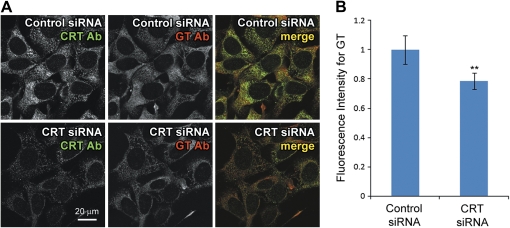
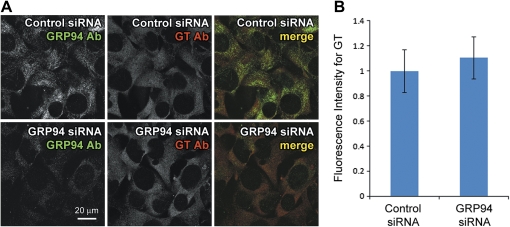
CRT siRNA was transfected into HEI-OC1 cells that had been maintained at 39°C for 2 weeks and siRNA expression induced for 3 days before treating with 1mM gentamicin for 6 h at 39°C. After washing and fixation, intracellular gentamicin was detected using gentamicin antibody and immunofluorescence. Although gentamicin immunofluorescence was observed in CRT siRNA-transfected cells, fluorescence intensities were substantially lower compared with cells transfected with control siRNA (Fig. 3A). More importantly, the intensity of CRT immunofluorescence appeared to correlate with gentamicin immunofluorescence levels in either control or CRT siRNA cells (Fig. 3A). Negligible gentamicin immunofluorescence was observed in control siRNA-transfected cells not treated with gentamicin, confirming the specificity of gentamicin antibody (data not shown). Quantification of gentamicin immunofluorescence intensities confirmed that CRT siRNA-transfected cells contained significantly lower fluorescence levels compared with control siRNA cells (**p < 0.01; Fig. 3B). This suggests that increased CRT expression elevates intracellular gentamicin levels by binding to the drug. Because siRNA transfection efficiency was not very high (approximately 60–70%), the fluorescence intensity analysis yielded smaller differences between control and CRT siRNA than they would have if the transfection efficiency were 100%. Although GRP94 siRNA transfection reduced protein expression efficiently, there was no difference in gentamicin levels between control and GRP94 siRNA cells (Fig. 4). This suggests that GRP94 expression has little effect on intracellular gentamicin levels.
FIG. 3.
CRT expression knockdown decreases intracellular gentamicin levels. CRT siRNA or scrambled control siRNA-transfected HEI-OC1 cells at 39°C were treated with gentamicin for 6 h. (A) Immunofluorescence using CRT and gentamicin antibodies (CRT Ab and GT Ab) showed that CRT siRNA reduced cellular CRT and gentamicin immunofluorescence. (B) Intensity of gentamicin immunofluorescence was significantly lower in CRT siRNA-transfected cells compared with control siRNA (**p < 0.01).
FIG. 4.
GRP94 expression knockdown has no effect on intracellular gentamicin levels. GRP94 siRNA or scrambled control siRNA-transfected HEI-OC1 cells at 39°C were treated with gentamicin for 6 h. (A) Immunofluorescence using GRP94 and gentamicin antibodies (GRP94 Ab and GT Ab) showed that GRP4 siRNA did not significantly alter cellular gentamicin immunofluorescence, despite knocking down GRP94 expression. (B) There was no significant difference in gentamicin immunofluorescence between GRP94 siRNA and control siRNA cells.
CRT-Deficient Cells Are Susceptible to Gentamicin Treatment
To determine whether CRT is involved in gentamicin-induced cytotoxicity, we took advantage of existing cell lines K41 (wild-type) and K42 (lacking crt gene) generated from mouse embryonic fibroblasts and used extensively to study the physiological functions of CRT (Howe et al., 2009; Nakamura et al., 2001). As a first step, we treated these cells with gentamicin at 1mM for 6 h at 37°C and determined intracellular gentamicin levels by immunofluorescence. As expected with CRT expression, K41 cells showed higher levels of intracellular gentamicin compared with K42 cells after gentamicin treatment (Fig. 5A). Although CRT expression levels varied among the K41 cell population and some of the cells expressed CRT at low levels, overall gentamicin levels were significantly higher than K42 (**p < 0.01; Figs. 5A and 5B). Cell viability assessed by MTT levels revealed that gentamicin reduced the viability of K42 cells more significantly than K41 cells after 3 days of treatment (Fig. 5C). Notably, gentamicin treatment substantially increased CRT expression in K41 cells, confirmed by Western blotting (Fig. 5D). This implicates a possible cellular protection mechanism by enhancing CRT protein expression.
CRT siRNA Reduces Resistance to Gentamicin Treatment
Although K41 and K42 cells originated from mice of similar genetic background except for crt gene (Mesaeli et al., 1999), it is possible that each acquired unique traits during the immortalization and isolation processes of cell line generation (Nakamura et al., 2000). To confirm that reduced gentamicin levels and lowered drug resistance in K42 cells are due to lack of CRT expression, we transfected K41 cells with CRT siRNA or scrambled control siRNA to determine if CRT contributes to gentamicin retention and resistance in the same cell line. Immunofluorescence confirmed that CRT siRNA reduced CRT protein expression as well as reduced intracellular gentamicin levels, compared with control siRNA-transfected cells, after 6 h of drug treatment at 37°C (Figs. 6A and 6B). Western blotting also confirmed that both of the two CRT siRNA efficiently reduced CRT expression in K41 cells (Fig. 6C). However, the scrambled control siRNA for the first CRT siRNA significantly reduced cell viability, probably by off-target knockdown (data not shown). Therefore, we only used the second set of control and CRT siRNA (control siRNA#2 and CRT siRNA#2) for cell viability measurements. K41 cells were transfected with control siRNA#2 or CRT siRNA#2, and cells were then treated with gentamicin after 24 h. The MTT assay was performed after 3 days of gentamicin treatment. Whereas both control and CRT siRNA-transfected cells of set #2 showed no difference in viability without gentamicin treatment, gentamicin significantly reduced cell viability in CRT siRNA#2-transfected K41 cells compared with control siRNA#2 cells (Fig. 6D). Because the effect of CRT siRNA was apparent only after 3 days of transfection (Supplementary figure), this suggests that gentamicin could kill cells that lack CRT within 24–48 h. As a separate confirmation, CRT siRNA#1-transfected K41 cells exhibited similar levels of gentamicin susceptibility to CRT siRNA#2 cells (data not shown).
C-Domain of CRT Binds to Gentamicin and Reduces Drug Accessibility to Other Targets
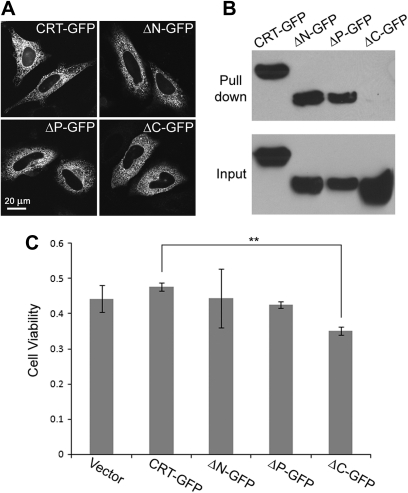
To determine the gentamicin-binding domain of CRT, we generated CRT deletion mutants as GFP fusion proteins. GFP was inserted in between C-domain and KDEL ER retention signal on the C-terminus to retain ER localization of the mutants. Transfecting each deletion mutant into HeLa cells confirmed that these mutants have similar intracellular localization to the GFP fusion of the full-length CRT (Fig. 7A). To test binding to gentamicin, each deletion mutant was transfected into 293T cells, and gentamicin-agarose pull-down assay was performed. Western blotting revealed that the C-domain deletion mutant was not pulled down, suggesting that the C-domain is required for binding to gentamicin (Fig. 7B). Cell viability of HeLa cells transfected with each deletion mutant was also determined. HeLa cells were transfected with empty vector or GFP expression plasmid of the full-length CRT or deletion mutants. Transfected cells were treated with gentamicin after 24 h. After 3 days of gentamicin treatment (and 4 days after transfection), cell viability was measured. MTT assay results showed that cells transfected with the C-domain deletion mutant had the lowest viability after gentamicin treatment (Fig. 7C; **p < 0.01 against CRT-GFP). This suggests that the C-domain of CRT is required for the protein to be protective against gentamicin treatment.
FIG. 7.
CRT binds to gentamicin through the C-domain, and it contributes to cellular resistance to gentamicin cytotoxicity. (A) HeLa cells were transfected with GFP fusions of full-length CRT (CRT-GFP) or deletion mutants of the N-, P-, and C-domains (ΔN-GFP, ΔP-GFP, and ΔC-GFP) that retained the ER localization signal. Confocal microcopy confirmed that all the GFP fusion proteins had similar intracellular distributions. (B) Gentamicin-agarose pull-down assay was performed using 293T cells transfected with the GFP fusions of full-length CRT or deletion mutants. Western blotting revealed that the mutant lacking the C-domain was not pulled down by gentamicin-agarose. (C) HeLa cells were transfected with the GFP fusions, and cells were treated with gentamicin after 24 h. Cell viability was measured after 3 days of gentamicin treatment (10mM). The bars represent the ratio to cells of the respective transfectants not treated with gentamicin (the number is set as 1 for cells with no gentamicin treatment). The C-domain deletion mutant showed significantly lower viability compared with full-length CRT-GFP (**p < 0.01).
DISCUSSION
Gentamicin has previously been reported to bind and inhibit the chaperone activity of CRT in cell-free systems (Horibe et al., 2004). In this study, we showed that there is a good correlation between CRT expression and intracellular levels of gentamicin immunofluorescence. Although this correlation is indirect, it suggests that CRT likely binds to gentamicin within the cell. More importantly, we demonstrated that CRT expression contributes to cellular resistance to gentamicin treatment using wild-type and CRT-deficient cell lines (K41 and K42). The difference in gentamicin levels and viability between CRT siRNA-transfected K41 cells and control siRNA K41 cells was greater than the difference between K41 and K42 cells. It is possible that protein expression of another GBP with similar physiological characteristics to CRT is increased in K42 cells to compensate for the loss of CRT expression. Calnexin, the transmembrane protein related to CRT, is a candidate for such a protein (Williams, 2006), although we were unable to identify it as a GBP in our pull-down assay. CRT and calnexin share amino acid sequence identity of 30% and similarity of 40%, and calnexin also has the N-, P-, and C-domains (Fliegel et al., 1989; Smith and Koch, 1989; Wada et al., 1991). The C-domain of CRT, which we identified as the gentamicin-binding domain, is rich in negatively charged residues that can buffer Ca2+ and binds to over 50% of Ca2+ in the ER with high capacity (Fig. 8) (Nakamura et al., 2001). Because gentamicin is also a cationic molecule, the large number of negatively charged residues in the C-domain could attract and bind to the drug through electrostatic interaction. Studies using gene knockout mice and cells overexpressing or lacking CRT indicate that CRT functions may include Ca2+ buffering and homeostasis regulation through the C-domain (Bastianutto et al., 1995; Mery et al., 1996; Mesaeli et al., 1999). Normal heart development requires CRT and is impaired in CRT-deficient mice because the cardiac cells cannot properly regulate Ca2+ buffering and homeostasis (Guo et al., 2002; Lynch et al., 2005). Interestingly, the C-domain in calnexin is exposed to cytoplasm, where Ca2+ concentration is low. Although the physiological significance of calnexin C-domain remains unknown, calnexin could also bind to gentamicin to prevent the drug from binding to other cytoplasmic targets if it is indeed a GBP.
FIG. 8.
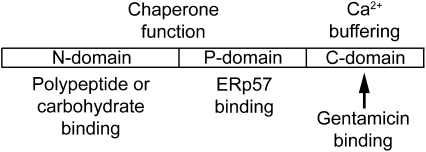
Schematic diagram of CRT structure. CRT consists of a globular N-domain, a proline-rich P-domain, and an acidic C-domain. The N-domain includes polypeptide and carbohydrate-binding sites, and the P-domain includes the binding site for ERp57, an oxidoreductase that forms complexes with CRT for the chaperone function (Oliver et al., 1997). The C-domain contains a large number of negatively charged amino acids that bind to Ca2+. Our pull-down assay shows that gentamicin binds to the C-domain.
It is possible that upregulation of CRT expression by gentamicin treatment (Fig. 5D) is induced as a cellular stress response. Although it requires further investigation to determine how CRT expression increases by gentamicin treatment, this phenomenon is likely a cellular protective mechanism, and there are two possible roles for CRT to protect the cell. First, increased CRT expression provides greater numbers of binding sites for sequestering gentamicin. This would limit drug access to other ER lumenal targets like CLIMP-63, reducing cytotoxicity. Second, CRT expression could be upregulated as a more general stress response, as it has been reported that cisplatin, another ototoxic agent used for chemotherapy in cancer, also increases CRT expression (Coling et al., 2007). In order to counteract the cellular stress, increased CRT expression could enhance Ca2+ regulation to maintain Ca2+ buffering and homeostasis.
In this study, we also determined the cochlear distribution of CRT, particularly in the marginal cells and the stereociliary bundles of sensory hair cells. Detection of CRT in the stereociliary bundles is particularly significant, as hair bundles are a primary location of hair cell uptake of aminoglycosides (Marcotti et al., 2005) and a site of ototoxicity (Gale et al., 2001; Lenoir et al., 1983). However, CRT expression in this region is unlikely to be within the ER. CRT is also expressed on the cell surface of various cell types, and a number of different functions have been suggested, including cellular adhesion, immune response, and wound healing (Gold et al., 2010). Expression of CRT in marginal cells of the stria vascularis is also significant because several other proteins such as NKCC1 and TRPV4 are expressed in these cells and are also thought to be involved in aminoglycoside-induced ototoxicity (Chu et al., 2006; Ishibashi et al., 2009; Karasawa et al., 2008).
Based on this cochlear distribution, our original hypothesis stated that gentamicin binding to CRT could enhance cytotoxicity because of longer duration of drug retention in the cell. However, our in vitro data in this study suggest that CRT could protect cells from gentamicin-induced cytotoxicity by binding the drug. This would reduce free gentamicin levels available for binding to other molecules, including CLIMP-63, a GBP also localized in the ER that promotes apoptosis upon binding to gentamicin (Karasawa et al., 2010). It is possible that gentamicin binding to CRT could disrupt function of this protein in vivo, which could be a major cause of gentamicin-induced ototoxicity. It will be difficult to test the CRT role in gentamicin-induced ototoxicity in vivo because CRT null mutation is embryonic lethal in mice (Mesaeli et al., 1999). Although significance of cochlear CRT expression remains to be determined, the high Ca2+ buffering requirement of hair cells suggests that these cells use CRT to maintain tight ER lumenal regulation of Ca2+ levels. It is possible that dysregulation of Ca2+ buffering and homeostasis induced by gentamicin binding to CRT could trigger hair cell death mechanisms. Future studies specifically targeted at CRT function in the inner ear will likely reveal the precise role of CRT in gentamicin-induced ototoxicity.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institute on Deafness and Other Communication Disorders at the National Institutes of Health (R03 DC009501 to T.K., R01 DC04555 to P.S.S., P30 grants DC05983, EY10572, CA069533).
Supplementary Material
Acknowledgments
We thank Dr Federico Kalinec for HEI-OC1 cells and Dr Marek Michalak for K41 and K42 cells.
References
- Bastianutto C, Clementi E, Codazzi F, Podini P, De Giorgi F, Rizzuto R, Meldolesi J, Pozzan T. Overexpression of calreticulin increases the Ca2+ capacity of rapidly exchanging Ca2+ stores and reveals aspects of their lumenal microenvironment and function. J. Cell Biol. 1995;130:847–855. doi: 10.1083/jcb.130.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu HQ, Xiong H, Zhou XQ, Han F, Wu ZG, Zhang P, Huang XW, Cui YH. Aminoglycoside ototoxicity in three murine strains and effects on NKCC1 of stria vascularis. Chin. Med. J. (Engl) 2006;119:980–985. [PubMed] [Google Scholar]
- Coling DE, Ding D, Young R, Lis M, Stofko E, Blumenthal KM, Salvi RJ. Proteomic analysis of cisplatin-induced cochlear damage: Methods and early changes in protein expression. Hear. Res. 2007;226:140–156. doi: 10.1016/j.heares.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Edson RS, Terrell CL. The aminoglycosides. Mayo Clin. Proc. 1999;74:519–528. doi: 10.4065/74.5.519. [DOI] [PubMed] [Google Scholar]
- Fliegel L, Burns K, MacLennan DH, Reithmeier RA, Michalak M. Molecular cloning of the high affinity calcium-binding protein (calreticulin) of skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 1989;264:21522–21528. [PubMed] [Google Scholar]
- Forge A, Schacht J. Aminoglycoside antibiotics. Audiol. Neurootol. 2000;5:3–22. doi: 10.1159/000013861. [DOI] [PubMed] [Google Scholar]
- Gale JE, Marcotti W, Kennedy HJ, Kros CJ, Richardson GP. FM1-43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. J. Neurosci. 2001;21:7013–7025. doi: 10.1523/JNEUROSCI.21-18-07013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold LI, Eggleton P, Sweetwyne MT, Van Duyn LB, Greives MR, Naylor SM, Michalak M, Murphy-Ullrich JE. Calreticulin: Non-endoplasmic reticulum functions in physiology and disease. FASEB J. 2010;24:665–683. doi: 10.1096/fj.09-145482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohskopf LA, Huskins WC, Sinkowitz-Cochran RL, Levine GL, Goldmann DA, Jarvis WR. Use of antimicrobial agents in United States neonatal and pediatric intensive care patients. Pediatr. Infect. Dis. J. 2005;24:766–773. doi: 10.1097/01.inf.0000178064.55193.1c. [DOI] [PubMed] [Google Scholar]
- Guo L, Nakamura K, Lynch J, Opas M, Olson EN, Agellon LB, Michalak M. Cardiac-specific expression of calcineurin reverses embryonic lethality in calreticulin-deficient mouse. J. Biol. Chem. 2002;277:50776–50779. doi: 10.1074/jbc.M209900200. [DOI] [PubMed] [Google Scholar]
- Hamasaki K, Rando RR. Specific binding of aminoglycosides to a human rRNA construct based on a DNA polymorphism which causes aminoglycoside-induced deafness. Biochemistry. 1997;36:12323–12328. doi: 10.1021/bi970962r. [DOI] [PubMed] [Google Scholar]
- Hebert DN, Molinari M. In and out of the ER: Protein folding, quality control, degradation, and related human diseases. Physiol. Rev. 2007;87:1377–1408. doi: 10.1152/physrev.00050.2006. [DOI] [PubMed] [Google Scholar]
- Holley MC, Lawlor PW. Production of conditionally immortalised cell lines from a transgenic mouse. Audiol. Neurootol. 1997;2:25–35. doi: 10.1159/000259227. [DOI] [PubMed] [Google Scholar]
- Horibe T, Matsui H, Tanaka M, Nagai H, Yamaguchi Y, Kato K, Kikuchi M. Gentamicin binds to the lectin site of calreticulin and inhibits its chaperone activity. Biochem. Biophys. Res. Commun. 2004;323:281–287. doi: 10.1016/j.bbrc.2004.08.099. [DOI] [PubMed] [Google Scholar]
- Howe C, Garstka M, Al-Balushi M, Ghanem E, Antoniou AN, Fritzsche S, Jankevicius G, Kontouli N, Schneeweiss C, Williams A, et al. Calreticulin-dependent recycling in the early secretory pathway mediates optimal peptide loading of MHC class I molecules. EMBO J. 2009;28:3730–3744. doi: 10.1038/emboj.2009.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T, Takumida M, Akagi N, Hirakawa K, Anniko M. Changes in transient receptor potential vanilloid (TRPV) 1, 2, 3 and 4 expression in mouse inner ear following gentamicin challenge. Acta Otolaryngol. 2009;129:116–126. doi: 10.1080/00016480802032835. [DOI] [PubMed] [Google Scholar]
- Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, Kioussis D. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc. Natl. Acad. Sci. U.S.A. 1991;88:5096–5100. doi: 10.1073/pnas.88.12.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinec GM, Webster P, Lim DJ, Kalinec F. A cochlear cell line as an in vitro system for drug ototoxicity screening. Audiol. Neurootol. 2003;8:177–189. doi: 10.1159/000071059. [DOI] [PubMed] [Google Scholar]
- Karasawa T, Wang Q, David LL, Steyger PS. CLIMP-63 is a gentamicin-binding protein that is involved in drug-induced cytotoxicity. Cell Death Dis. 2010;1:e102. doi: 10.1038/cddis.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa T, Wang Q, Fu Y, Cohen DM, Steyger PS. TRPV4 enhances the cellular uptake of aminoglycoside antibiotics. J. Cell Sci. 2008;121:2871–2879. doi: 10.1242/jcs.023705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Marot M, Uziel A. Comparative ototoxicity of four aminoglycosidic antibiotics during the critical period of cochlear development in the rat. Funct. Struct. Study Acta Otolaryngol. 1983;405(Suppl.):1–16. doi: 10.3109/00016488309105593. [DOI] [PubMed] [Google Scholar]
- Lesniak W, Pecoraro VL, Schacht J. Ternary complexes of gentamicin with iron and lipid catalyze formation of reactive oxygen species. Chem. Res. Toxicol. 2005;18:357–364. doi: 10.1021/tx0496946. [DOI] [PubMed] [Google Scholar]
- Lowenheim H, Furness DN, Kil J, Zinn C, Gultig K, Fero ML, Frost D, Gummer AW, Roberts JM, Rubel EW, et al. Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of corti. Proc. Natl. Acad. Sci. U.S.A. 1999;96:4084–4088. doi: 10.1073/pnas.96.7.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J, Guo L, Gelebart P, Chilibeck K, Xu J, Molkentin JD, Agellon LB, Michalak M. Calreticulin signals upstream of calcineurin and MEF2C in a critical Ca(2+)-dependent signaling cascade. J. Cell Biol. 2005;170:37–47. doi: 10.1083/jcb.200412156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti W, van Netten SM, Kros CJ. The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels. J. Physiol. 2005;567:505–521. doi: 10.1113/jphysiol.2005.085951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mery L, Mesaeli N, Michalak M, Opas M, Lew DP, Krause KH. Overexpression of calreticulin increases intracellular Ca2+ storage and decreases store-operated Ca2+ influx. J. Biol. Chem. 1996;271:9332–9339. doi: 10.1074/jbc.271.16.9332. [DOI] [PubMed] [Google Scholar]
- Mesaeli N, Nakamura K, Zvaritch E, Dickie P, Dziak E, Krause KH, Opas M, MacLennan DH, Michalak M. Calreticulin is essential for cardiac development. J. Cell Biol. 1999;144:857–868. doi: 10.1083/jcb.144.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak M, Groenendyk J, Szabo E, Gold LI, Opas M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem. J. 2009;417:651–666. doi: 10.1042/BJ20081847. [DOI] [PubMed] [Google Scholar]
- Moazed D, Noller HF. Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature. 1987;327:389–394. doi: 10.1038/327389a0. [DOI] [PubMed] [Google Scholar]
- Moestrup SK, Cui S, Vorum H, Bregengard C, Bjorn SE, Norris K, Gliemann J, Christensen EI. Evidence that epithelial glycoprotein 330/megalin mediates uptake of polybasic drugs. J. Clin. Invest. 1995;96:1404–1413. doi: 10.1172/JCI118176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Bossy-Wetzel E, Burns K, Fadel MP, Lozyk M, Goping IS, Opas M, Bleackley RC, Green DR, Michalak M. Changes in endoplasmic reticulum luminal environment affect cell sensitivity to apoptosis. J. Cell Biol. 2000;150:731–740. doi: 10.1083/jcb.150.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Zuppini A, Arnaudeau S, Lynch J, Ahsan I, Krause R, Papp S, De Smedt H, Parys JB, Muller-Esterl W, et al. Functional specialization of calreticulin domains. J. Cell Biol. 2001;154:961–972. doi: 10.1083/jcb.200102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni M, Lee AS. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007;581:3641–3651. doi: 10.1016/j.febslet.2007.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver JD, van der Wal FJ, Bulleid NJ, High S. Interaction of the thiol-dependent reductase ERp57 with nascent glycoproteins. Science. 1997;275:86–88. doi: 10.1126/science.275.5296.86. [DOI] [PubMed] [Google Scholar]
- Prezant TR, Agapian JV, Bohlman MC, Bu X, Oztas S, Qiu WQ, Arnos KS, Cortopassi GA, Jaber L, Rotter JI, et al. Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nat. Genet. 1993;4:289–294. doi: 10.1038/ng0793-289. [DOI] [PubMed] [Google Scholar]
- Purohit P, Stern S. Interactions of a small RNA with antibiotic and RNA ligands of the 30S subunit. Nature. 1994;370:659–662. doi: 10.1038/370659a0. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Koch GL. Multiple zones in the sequence of calreticulin (CRP55, calregulin, HACBP), a major calcium binding ER/SR protein. EMBO J. 1989;8:3581–3586. doi: 10.1002/j.1460-2075.1989.tb08530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulkens PM. Nephrotoxicity of aminoglycoside antibiotics. Toxicol. Lett. 1989;46:107–123. doi: 10.1016/0378-4274(89)90121-5. [DOI] [PubMed] [Google Scholar]
- Wada I, Rindress D, Cameron PH, Ou WJ, Doherty JJ, II, Louvard D, Bell AW, Dignard D, Thomas DY, Bergeron JJ. SSR alpha and associated calnexin are major calcium binding proteins of the endoplasmic reticulum membrane. J. Biol. Chem. 1991;266:19599–19610. [PubMed] [Google Scholar]
- Williams DB. Beyond lectins: The calnexin/calreticulin chaperone system of the endoplasmic reticulum. J. Cell Sci. 2006;119:615–623. doi: 10.1242/jcs.02856. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.