Abstract
Over a quarter of the U.S. population is exposed to harmful levels of airborne particulate matter (PM) pollution, which has been linked to development and exacerbation of respiratory diseases leading to morbidity and mortality, especially in susceptible populations. Young children are especially susceptible to PM and can experience altered anatomic, physiologic, and biological responses. Current studies of ambient PM are confounded by the complex mixture of soot, metals, allergens, and organics present in the complex mixture as well as seasonal and temporal variance. We have developed a laboratory-based PM devoid of metals and allergens that can be replicated to study health effects of specific PM components in animal models. We exposed 7-day-old postnatal and adult rats to a single 6-h exposure of fuel-rich ultrafine premixed flame particles (PFPs) or filtered air. These particles are high in polycyclic aromatic hydrocarbons content. Pulmonary cytotoxicity, gene, and protein expression were evaluated at 2 and 24 h postexposure. Neonates were more susceptible to PFP, exhibiting increased lactate dehydrogenase activity in bronchoalveolar lavage fluid and ethidium homodimer-1 cellular staining in the lung in situ as an index of cytotoxicity. Basal gene expression between neonates and adults differed for a significant number of antioxidant, oxidative stress, and proliferation genes and was further altered by PFP exposure. PFP diminishes proliferation marker PCNA gene and protein expression in neonates but not adults. We conclude that neonates have an impaired ability to respond to environmental exposures that increases lung cytotoxicity and results in enhanced susceptibility to PFP, which may lead to abnormal airway growth.
Keywords: lung development, polycyclic aromatic hydrocarbons, antioxidants, oxidative stress
Airborne particulate matter (PM) pollution is an aggregate mixture of small particles and liquid droplets present in the atmosphere as defined by the EPA. Fine particles (PM2.5; aerodynamic diameter < 2.5 μm) are highly prevalent. It is estimated that over 28% of the U.S. population live in areas exceeding EPA standards (USEPA, 2009). Exposure to fine particles has been linked to development of respiratory infections, exacerbation of asthma, and increased risk of respiratory and cardiovascular morbidity and mortality, especially in susceptible populations (ALA, 2009; Dockery, 2009; Mills et al., 2009). Although exposure to ultrafine particles (PM0.1; aerodynamic diameter < 0.1 μm) has also been linked to diminished lung development and function (Ibald-Mulli et al., 2002), exposure levels are currently unregulated.
Young children are especially susceptible to PM. They are more aerobically active outdoors, have a larger body surface area-to-volume ratio, higher metabolic rate, have higher minute ventilation, and increased oxygen consumption per body weight compared with adults (Bearer, 1995). Their small body size, smaller mean airway diameter with increased air exchange exacerbates particle deposition (Branis et al., 2009). Furthermore, lungs continue to grow and mature postnatally and are exposed to PM during this period of maturation (Langston, 1983). Susceptibly may be altered due to the extensive and continuous growth, differentiation, and maturation of the bronchiolar airways and alveoli. Acute exposures to PM have been associated with an increased incidence of respiratory hospital admissions and medication use in asthmatic children (Pekkanen et al., 1997; Peters et al., 1997; Norris et al., 1999). Children living in areas of high levels of short-term particulate pollution (i.e., near roadways) have increased morbidity and mortality from respiratory illnesses, such as bronchitis and pneumonia in a dose-dependent manner (Ciccone et al., 1998). It is clear from the epidemiologic data that PM exposures affect the incidence and severity of lung diseases in children, diseases of airways in particular. Despite a large body of epidemiologic data correlating adverse health effects from PM exposure, biochemical mechanisms of toxicity in the developing lung remain relatively unexplored. This may be due to the complicated spatiotemporal variation in PM containing atmospheres as well as the dauntingly complex mixture of PM and gases that characterize these atmospheres. Sampling sites, seasonal variations, and time of day all play a role in defining particle composition. This complicates the systematic study of health effects. Despite this, it is well established that vehicular exhaust from combustion of gasoline, diesel, and other petroleum fuels is the dominant contributor to the fine and ultrafine particulate ranges (Pey et al., 2009). Combustion in vehicle engines may be incomplete and lead to the emission into the atmosphere of carbonaceous particles and a variety of fused and free polycyclic aromatic hydrocarbons (PAHs). Due to the highly variable nature of outdoor ambient PM, we have developed and used a premixed flame particle (PFP) generating system (Lee et al., 2010) to create an exposure atmosphere for in vivo studies. PFPs are generated in a laminar fuel-rich flame resulting in fine and ultrafine particles. A variety of PAH species are present in both the particulate and vapor phases. PFP is used as a surrogate for toxicity testing of combustion-generated aerosols and associated PAH. This allows for modifications of PM composition (i.e., PAH content, metals, gases, etc.) in addition to the ability to reproducibly generate similar environments without potential confounders like temperature, weather, variations in air quality, allergens, or metals present in field samples.
In the current study, we characterized the PFP environment as well as acute exposure responses in 7-day-old postnatal and young adult rats following in vivo exposure to either PFP or filtered air (FA). We hypothesized that neonates would have altered oxidative stress and antioxidant expression patterns resulting in increased cytotoxicity and susceptibility to PFP. The objectives of this study are: (1) to characterize the PFP environment and quantify free and fused PAH content within the chamber atmosphere, (2) to define PFP cytotoxicity and susceptibility in neonates and adults, and (3) to determine whether the airway oxidative stress gene response varies by age.
MATERIALS AND METHODS
Flame and particle characterization.
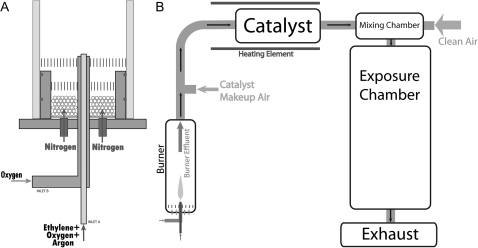
PFPs were generated using a coannular premixed flame burner (Fig. 1A). The burner consists of a 7.1-mm tube (inner diameter) surrounded by an 88.9-mm concentric outer tube (inner diameter). The burner is enclosed in a Pyrex tube to isolate the burner from ambient air. A mixture of ethylene, oxygen, and argon was metered through the inner tube at 212.4 cc/min, 289.2 cc/min, and 1499 cc/min, respectively, using mass flow controllers (model 647C flow control unit and model 1179A and M100B flow control valves, MKS Instruments, Andover, MA). A small flow rate of oxygen (52 cc/min) flowed through the outer annulus to stabilize the flame. The flame was shielded from room air by a curtain flow of nitrogen metered at 10 l/min using a Fisher and Porter variable area flow meter (Andrews Glass, Vineland, NJ) and delivered around the circumference of the burner chamber. Filtered dried air was added to the flow downstream of the flame, and all burner effluent passes through a heated 3-way catalyst to remove NOx and CO. PFP were diluted with clean HEPA and CBR (chemical/biological/radiological) FA and mixed into a mixing chamber before entering the inhalation exposure chamber (Fig. 1B).
FIG. 1.
Premixed flame burner and chamber schematic. (A) Premixed fuel and oxidized flow through the central annulus. The flame is stabilized by an outer annular oxygen flow. The flame is shielded from room air by an outer nitrogen coannular flow. (B) Particle laden gas is passed through a catalytic converter to remove NOx and CO. The flow is diluted before entering the exposure chambers.
Animals underwent a whole-body exposure to the flame-generated particles. Chamber CO levels were monitored using a Teledyne-API Model 300E CO analyzer (San Diego, CA) and was calibrated with an NIST traceable span gas of 202.4 ppm CO diluted in ultrapure air to 10 ppm CO for calibration (Scott-Marrin Inc., Riverside, CA). Chamber NOx levels were monitored (Dasibi 2108 Chemiluminescence NOx Analyzer, Glendale, CA). PFP was collected directly from the exposure chamber for analysis though ports in the chamber wall. Particle number concentration was determined using a condensation particle counter (CPC, TSI model 3775, Shoreview, MN). Particle size distribution was determined using a scanning mobility particle sizer (SMPS) (model 3080 electrostatic classifier with model 3081 differential mobility analyzer) and a model 3020 CPC (TSI).
PFP mass concentration was determined by collecting particles from the chamber on glass fiber filters (Pallflex Emfab 47-mm filters, Ann Arbor, MI) placed in a filter housing (BGI, Waltham, MA). The sampling flow rate was set at 20 l/min air flow rate driven by a vacuum source downstream of the flow. Collection was performed for the duration of the exposure. Total particulate mass was determined gravimetrically (Sartorius AG MC5 microbalance, Goettingen, Germany). Particle samples were collected on 47-mm glass fiber filters (Pallflex Tissuequartz, Ann Arbor, MI) for elemental carbon to organic carbon ratio (EC/OC) analysis as described above. The EC/OC ratio was determined using a method previously described (Herner et al., 2005; Robert et al., 2007). Particle and vapor phase PAH speciation was performed by the Desert Research Institute (DRI, Reno, NV). PFP were collected on Pallflex Tissuequartz 47-mm filters, and vapor phase organic compounds were collected on XAD resin supplied by DRI.
Transmission electron microscopy.
PFP were sampled via an electrostatic precipitator (ESP) similar in design to that described previously (Morrow and Mercer, 1964). Particles were sampled from the exposure chamber using ¼” inch conductive tubing and drawn through the ESP using a vacuum pump. The sampling flow rate was 100 cm3/min, and the ion current was set to 3.5 μA. The morphology of soot particles were analyzed by transmission electron microscopy (Phillips CM-12, LaB6 cathode, operated at 120 kV), and carbon coated copper grids were used for particle sampling (300 mesh, lacey carbon type-A substrate, Ted Pella Inc. Redding, CA).
Animals and exposure protocol.
Two and a half month reproductively capable young adults approximating peak fitness and newborn postnatal male Sprague Dawley rats with accompanying dams were obtained from Harlan Laboratories and allowed to acclimate in CBR FA exposure chambers until newborn pups reached 7 days of age in a 12 h light/dark cycle. Animals were acutely exposed in a whole-body chamber to either 6 h of FA or an atmosphere of 22.40 ± 5.60 μg/m3 PFP (mean ± SD). Two identical custom-built exposure chambers were used for the exposure experiments, one chamber housed PFP-exposed animals and the other housed age matched FA control animals (Hinners et al., 1968). The stainless steel chambers have a volume of 3.8 m3. A mixing chamber located at the top of the animal chamber where CBR filtered room air was mixed with PFP. Chambers were maintained at −0.3 inches of H2O gage pressure, and temperature were maintained between 22.2°C and 24.4°C. The FA flow rate through the chamber was set at 30 air exchanges per hour. During exposure, adult rats were housed in stainless steel wire cages. Due to size differences, 7-day-old postnatal rats were housed with lactating females in polycarbonate cages with wire lids during exposure; Kimwipes (Kimberly-Clark, Neenah, WI) were used for bedding during the exposure period. Cages were arranged in the chamber in a single level and were cleaned with bedding changed every other day. Adult rats were provided with Laboratory Rodent Diet (Purina Mills, St Louis, MO) and water ad libitum. All animal experiments were performed under protocols approved by the University of California Davis IACUC in accordance with National Institutes of Health guidelines. Animals were necropsied 2 or 24 h following cessation of the 6-h exposure. All animals were euthanized through ip injection of an overdose of pentobarbital (150 mg/kg). At necropsy, tracheas were cannulated, the thorax was opened, and lung removed en bloc for processing.
Ethidium homodimer-1 cell viability assay.
Rat lungs were examined for the presence of membrane permeable cells as previously described (Van Winkle et al., 1999Winkle et al., 1999). Two hours after exposure, rat lungs were intratracheally inflated with 10μM Ethidium Homodimer-1 (Molecular Probes, Carlsbad, CA) in warmed F-12 media (Gibco, Carlsbad, CA) for 15 min. Ethidium Homodimer-1 containing media was removed, and lungs were rinsed three times with warmed F-12 media to remove unbound dye. Lungs were fixed in Karnovsky’s fixative (Karnovsky, 1965) under 30-cm hydrostatic pressure for 1 h and stored at 4°C until paraffin embedment. Sections were analyzed using epifluorescent microscopy and subsequently counterstained with Hematoxylin and Eosin (H & E) for cellular identification.
Lactate dehydrogenase cytotoxicity and total protein assays.
Bronchoalveolar lavage fluid (BALF) was collected through recovery of intratracheal instillation of Hank’s Buffered Salt Solution (Gibco, Carlsbad, CA) at 35 μl/g body weight concentration to scale for differences in animal size. Lactate dehydrogenase (LDH) activity (U, the amount of LDH that catalyzes the reaction of 1 μmol of substrate per minute) in BALF was detected using an LDH Cytotoxicity Assay Kit (Cayman Chemical Company, Ann Arbor, MI) following manufacturer instructions. LDH activity was determined to have a detection limit of 0.16 U/ml BALF from serial dilutions of an LDH standard. BALF protein concentrations were determined using the Micro Lowry Total Protein Kit (Sigma Chemical, St Louis, MO).
RT-profiler arrays on microdissected airways.
Lungs were filled to capacity with and preserved in RNAlater (Ambion, Austin, TX) at −20°C until microdissection. RNA later stabilized intrapulmonary airways from the lobar bronchus to the terminal bronchioles were dissected free from the surrounding parenchyma as described in Baker et al. (2004). Airway enriched RNA (362–1185 ng/μl RNA; 18.1–59.25 μg RNA per animal) was isolated using Tri Reagent (Molecular Research Center, Inc., Cincinnati, OH) following the manufacturer’s protocol based on the method detailed in Chomczynski and Sacchi (1987). RNA purity was confirmed through spectrophotometric absorbance at 260/280 nm. cDNA was synthesized and amplified using the RT2 PreAMP cDNA Synthesis Kit (SABiosciences, Frederick, MD). RT amplification data for all samples reported “pass” for all internal reverse transcription efficiency and genomic DNA contamination quality controls prior to analysis. Relative quantification of gene expression for each sample was performed on airways from each animal individually using quantitative RT-PCR on RT2 qPCR Arrays (cat# PARN-003, PARN-065) strictly following manufacturer’s instructions (SABiosciences). A complete list of genes assayed is available (Supplementary tables 1S–3S). Results were calculated following the RT2 Profiler PCR Array Data Analysis tool, available online at http://pcrdataanalysis.sabiosciences.com/pcr/arrayanalysis.php (SABiosciences). Differential gene expression data across age and treatment groups were analyzed using hypoxanthine-guanine phosphoribosyltransferase (HPRT) as the reference gene. HPRT was chosen as the reference gene due to consistency and low variance between exposures and within age groups as assessed previously (Van Winkle et al., 2010Winkle et al., 2010). Gene expression heatmap was generated using the RT2 Profiler PCR Array Data Analysis tool, with each lane representing the averaged fold change from multiple animals within each group for each significantly changed gene, either by age and/or exposure. Results are expressed as a fold change in gene expression relative to FA animals of the same age, unless otherwise stated. The number of animals assessed per exposure group using the RT2 qPCR Arrays was: 7-day-old FA (3), 7-day-old PFP exposed (3), Adult FA (3), and Adult PFP exposed (6).
Proliferating cell nuclear antigen immunohistochemistry.
Lung tissue prepared for immunohistochemical analysis was inflated with 37% formaldehyde vapor bubbled under 30-cm hydrostatic pressure for 1 h as previously described (Hammond and Mobbs, 1984; Wilson et al., 2001). Samples were stored in 1% paraformaldehyde for less than 24 h prior to processing and paraffin embedment. Paraffin sections were immunostained using methods previously described (Van Winkle et al., 1996Winkle et al., 1996). Briefly, slides were boiled in hot citrate buffer for antigen retrieval. Endogenous peroxidase activity was quenched with a 10% hydrogen peroxide solution, and nonspecific binding was blocked with IgG-free bovine serum albumin (Jackson ImmunoResearch Laboratories, West Grove, PA) for 30 min. Sections were immunostained against proliferating cell nuclear antigen (PCNA) using a monoclonal anti-PCNA antibody (DAKO, Carpinteria, CA) at dilution of 1:600 overnight at 4°C (Van Winkle et al., 2010Winkle et al., 2010). Signal was visualized using the avidin-biotin-peroxidase ABC kit (Vector Labs, Burlingame CA) and 3,3′-diaminobenzidine tetrahydrochloride (Sigma Chemical) as the chromagen. Sections from all groups were stained simultaneously to minimize variability in between runs. Controls included substitution of primary antibody with phosphate buffered saline and testing all antibodies in a series of dilutions prior to use to optimize staining.
Statistics.
All data are reported as mean ± SEM unless otherwise stated. Statistical outliers in PFP chamber mass concentration were eliminated using the extreme studentized deviate method (Graphpad, La Jolla, CA). LDH data containing samples below detection limit (BDL) were treated as nondetected (NDs), and values were imputed using the natural-log regression on order statistics (lnROS) method (Helsel, 2005; Shumway et al., 2002) using ProUCL (U.S. EPA, Atlanta, GA). Two-way ANOVA was applied against age and exposure factors when appropriate. Multiple comparisons for factors containing more than two levels were performed using Fisher’s Protected Least Significant Difference (PLSD) method. Pair-wise comparisons were performed individually using a one-way ANOVA followed by PLSD post hoc analysis using StatView (SAS, Cary, NC). For RT-profiler array data, statistical functions were performed using the online RT2 Profiler PCR Array Data Analysis tool (SABiosciences), where p values are calculated based on a Student’s t-test of the replicate 2−ΔΔCt values for each gene in the control and treatment groups. p values of < 0.05 were considered statistically significant.
RESULTS
Characterization of Premixed Flame Generated Atmosphere
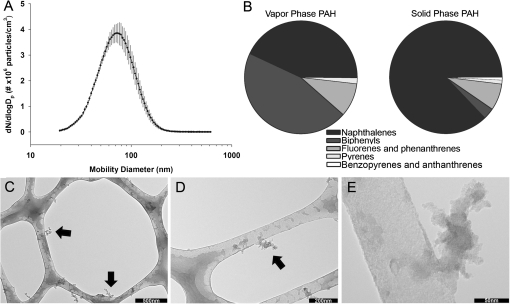
Rats were exposed for 6 h to a premixed flame generated atmosphere; dams were exposed with their pups. The atmosphere contained both particles and gases. Both were characterized (Fig. 2, Supplementary tables 1S and 2S), and the abundance reported is the average of two collections.
FIG. 2.
PFP characterization. (A) Soot size distribution within exposure chamber indicates a geometric mean particle size of 70.56 ± 1.51 nm (geometric mean ± geometric SD). (B) Total amounts of PAH present were 405 ng/m3 in the vapor phase and 56 ng/m3 in the particulate phase. Low molecular weight aromatic hydrocarbons such as methylated biphenyls (182 ng/m3), naphthalene (19 ng/m3), mono, and poly methylated naphthalenes (149 ng/m3) were major constituents in the vapor phase. In contrast, naphthalene (15 ng/m3) and methylated naphthalenes (33 ng/m3) dominated the solid particle phase. (C–E) Transmission electron micrographs of particle morphology sampled on a lacy carbon substrate indicate oily particles with primary particle sized between 10 and 20 nm forming larger fractal aggregates (arrows).
Using data from a series of PFP exposures, the exposure chamber mass concentration was determined to be 22.40 ± 5.60 μg/m3 PFP (mean ± SD) based on gravimetric filter measurement. SMPS measurements showed a geometric mean mobility diameter of 70.56 nm with a geometric standard deviation of 1.51 nm. The mean particle number concentration was 9.37 × 104 ± 4.8 × 103 particles/cm3 (mean ± SD) based on CPC measurements over duration of exposure. PFP exposure chamber CO levels were within 0.2 ppm of FA chamber levels, with quantification below 0.2 ppm limited by instrument accuracy. Chamber NO and NO2 concentrations were within 0.01 ppm of FA levels. Particles were high in organic carbon and had an EC/OC ratio of 0.58. The amount of total PAH measured on PM was 54 ng/m3 and the amount of gas phase PAH was 405 ng/m3. The 20 most abundant vapor phase and particulate phase PAHs are listed (Tables 1 and 2). In general, biphenyls and naphthalene compounds dominated the vapor phase, and non, mono, and poly substituted naphthalenes constituted the particulate phase. Typical morphologies of PFP are shown (Figs. 2C–E) where soot particles are composed of 10–20 nm round primary particles forming larger fractal aggregates.
TABLE 1.
Top 20 Most Abundant PAHs in Vapor Phase
| Compound | Abundance (ng/m3) |
| 2-methylbiphenyl | 114.68 |
| 3-methylbiphenyl | 64.30 |
| 2-methylnaphthalene | 35.86 |
| 1,3 + 1,6 + 1,7dimethylnaphth | 23.78 |
| Naphthalene | 18.73 |
| 1-methylnaphthalene | 17.51 |
| 2,6 + 2,7-dimethylnaphthalene | 14.22 |
| 1 + 2ethylnaphthalene | 12.61 |
| Fluorene | 11.54 |
| C-trimethylnaphthalene | 8.41 |
| B-trimethylnaphthalene | 7.72 |
| 2,3,5 + I-trimethylnaphthalene | 7.42 |
| Phenanthrene | 6.65 |
| 1,4 + 1,5 + 2,3-dimethylnaphth | 6.04 |
| E-trimethylnaphthalene | 4.97 |
| F-trimethylnaphthalene | 4.74 |
| 2-methylphenanthrene | 4.28 |
| 4-methylbiphenyl | 3.36 |
| C-dimethylphenanthrene | 2.98 |
| Quinoline | 2.60 |
TABLE 2.
Top 20 Most Abundant PAHs in Particulate Phase
| Compound | Abundance (ng/m3 air) |
| Naphthalene | 15.37 |
| 2-methylnaphthalene | 13.84 |
| 1-methylnaphthalene | 6.57 |
| 1,3 + 1,6 + 1,7dimethylnaphth | 5.89 |
| 2,6 + 2,7-dimethylnaphthalene | 2.68 |
| 9-methylphenanthrene | 1.91 |
| 1 + 2ethylnaphthalene | 1.45 |
| Biphenyl | 0.76 |
| 1,4 + 1,5 + 2,3-dimethylnaphth | 0.76 |
| 3-methylbiphenyl | 0.76 |
| Phenanthrene | 0.46 |
| C-trimethylnaphthalene | 0.38 |
| Quinoline | 0.38 |
| 3-methylphenanthrene | 0.31 |
| 4-methylbiphenyl | 0.31 |
| Fluorene | 0.23 |
| Retene | 0.23 |
| Acenaphthene | 0.15 |
| D-dimethylphenanthrene | 0.15 |
| Dibenz(a,h)acridine | 0.15 |
Cytotoxicity of PFPs
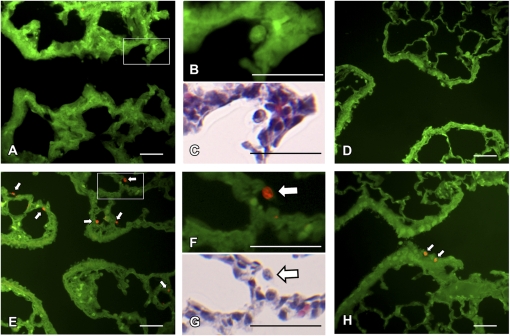
To evaluate airway epithelial membrane permeability after PFP inhalation, we compared in situ ethidium homodimer-1 fluorescence (red) overlaid upon tissue autofluorescence (green) as a marker for cytotoxicity in 7-day-old neonates and adults 2 h following either a single acute 6 h PFP exposure or FA using epifluorescence microscopy (Fig. 3). The airway epithelium between adult and neonates was similar among exposure groups. Ethidium homodimer-1 positive membrane permeable cells were rarely detected in either age group reared in a FA environment (Figs. 3A and 3D). However, 2 h following PFP exposure, membrane permeable cytotoxic cells were readily detected in the subepithelium and parenchyma near terminal bronchioles (Fig. 3E) in the neonates, while bronchiolar airways remained intact and viable (data not shown). High-magnification micrographs reveal the presence of ethidium positive macrophages after PFP exposure (Figs. 3F and 3G) that were absent in FA animals (Figs. 3B and 3C). Adult animals exhibited less susceptibility after PFP exposure. Membrane permeable cells were rarely observed, with the exception of a few sparse ethidium positive cells present in a few terminal bronchioles (Fig. 3H).
FIG. 3.
Airway epithelial cellular toxicity following PFP exposure. In situ ethidium homodimer-1 sections were analyzed in 7-day postnatal (A, E) and adult rats (D, H) exposed to either FA (A–D) or PFP (E–H) for regional localization of membrane permeable cytotoxic cells. Overall, ethidium positive cells (red fluorescence) were scarcely observed in either 7-day old neonatal rats (A) or adults (D) reared in FA. Resident macrophages are depicted (B, C) to show a lack of ethidium uptake under FA conditions. However, 2 h following PFP exposure, membrane permeable cells (white arrows) were readily detected in the neonates in the subepithelium and parenchyma (E). A high magnification insert (F) and subsequent H & E stained section (G) shows that the majority of membrane permeable cells present are either monocytes or macrophages. In adults following PFP exposure, adult rat bronchiolar airways remained mostly noncytotoxic, with the exception of a few ethidium positive cells in the terminal bronchioles (D). Scale bars are 50 μm.
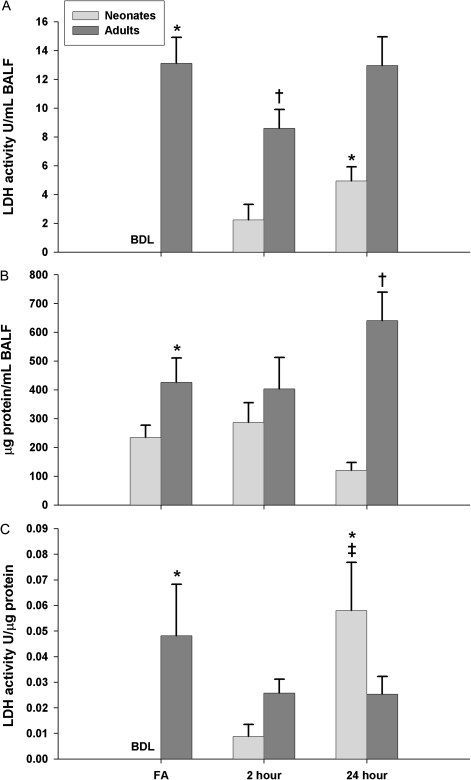
To quantitatively measure cytotoxicity, we measured LDH activity and total protein present in BALF as a global marker of lung cell injury (Fig. 4). In all neonates exposed to FA, LDH activity was found to be indistinguishable from background levels of BALF, as determined by the detection limit of our assay (<0.16 U/ml BALF), which was used for statistical analysis. To discern whether age (neonates vs. adults) and/or exposure (FA vs. 2 or 24 h post PFP exposure) affects LDH activity (U) normalized to milliliters of BALF volume (Fig. 4A), we used a two-way ANOVA and found significant main effect of age (p < 0.0001), indicating that adults have greater LDH activity than neonates. There was also a main effect of exposure (p = 0.0398), showing a significant difference in LDH activity as a function of time after PFP exposure. Additionally, there was an interaction between age by exposure (p = 0.0402). To determine specific interactions among groups, a one-way ANOVA with Fisher’s PLSD post hoc analysis revealed significantly higher basal levels in adults compared with neonates (p < 0.0001). A transient drop in LDH activity was seen in adult 2 h post PFP exposure (p = 0.0024). Contrary to adults, neonates trended upward after PFP exposure, reaching significance at 24 h post exposure compared with FA controls (p = 0.0106).
FIG. 4.
Cellular permeability in BALF following PFP exposure. LDH activity (A) and total protein (B) were measured in BALF. In general, LDH activity was below the detection limit (BDL) and indistinguishable from background in all neonates reared in FA. In contrast, adult rats had significantly higher basal levels of LDH. Following PFP exposure, an upward trend in neonates, with a significant increase in LDH activity observed 24 h post exposure. Interestingly, a transient drop of LDH activity was detected 2 h post PFP exposure in adult rats (A). Similar to LDH activity, total BALF protein was inherently higher in adult rats. While neonate levels remained unchanged following exposure, a significant increase in BALF protein levels were observed in adults 24 h post PFP exposure (B). After normalizing LDH activity as a function of BALF protein, a more pronounced trend of increasing cell permeability was observed in neonates post PFP exposure (C). Data are plotted as means ± SEM (n = 5–17 rats/group, each rat was analyzed individually). BDL samples were treated as nondetects (NDs) and were imputed using the lnROS method. For the FA neonate group, NDs were replaced with the limit of detection where lnROS method was inapplicable. p < 0.05 are denoted as follows: * significantly different as compared with FA exposed 7-day postnatal controls, † significantly different from FA exposed adults, and ‡ significance compared against 2 h post PFP exposed neonates.
Next, we measured total protein levels in BALF (Fig. 4B). A significant main effect of age (p < 0.0001) showed that adults have more protein in BALF than neonates. Secondly, an interaction between age by exposure (p = 0.0263) was also significant. Pairwise comparisons reveal elevated basal levels of protein in control adults (p = 0.0218). While protein concentrations remained steady in either age groups 2 h post PFP exposure, total protein in BALF was significantly greater in adults 24 h after PFP, compared against FA controls (p = 0.0424).
Finally, two-way ANOVA analysis of LDH activity normalized to BALF protein (Fig. 4C) did not reveal differences between any main effects. However, a significant age by exposure interaction (p = 0.0094) was observed. Pairwise comparisons showed a significantly greater normalized LDH activity in adults (p = 0.0083). Additionally, a markedly amplified trend was seen in neonates, peaking at 24 h post PFP, where normalized LDH activity were significant compared against both FA controls (p = 0.0019) and 2 h following PFP (p = 0.0056).
PFP-Induced Antioxidant and Oxidative Stress Response
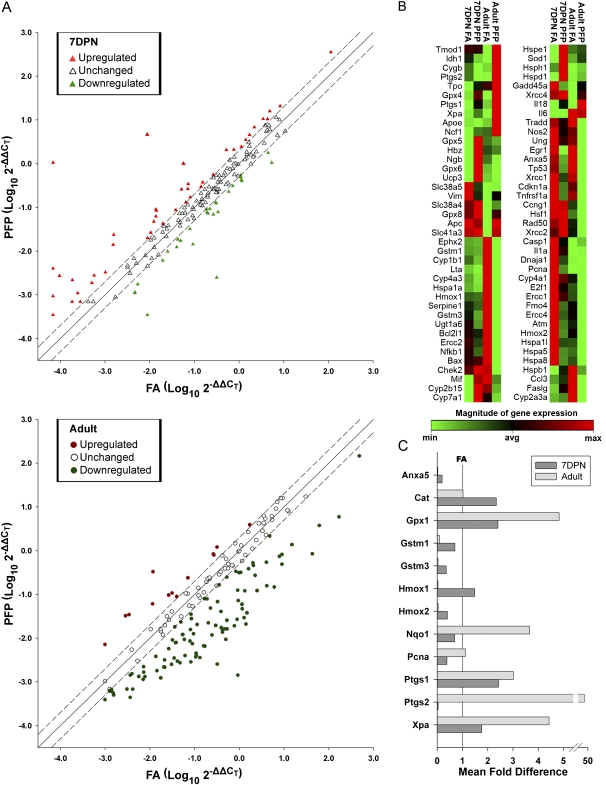
In an attempt to characterize the oxidative stress and antioxidant responses, we measured individual gene transcription in the conducting bronchiolar airways of neonatal and adult rats 24 h after the cessation of either PFP or FA exposure. We used the “Oxidative Stress and Antioxidant Defense” and “Stress and Toxicity Pathwayfinder” RT2 RT-profiler qPCR arrays to quantify 162 genes using RNA extracted from microdissected airway trees. Overall, we saw very diverging trends in gene expression between neonates and adults. While a majority of the genes within the two array panels were upregulated in neonates, the opposite was observed in adults (Fig. 5A). Seventy-eight genes were determined to be differentially expressed either as function of age and/or exposure (Fig. 5B, Supplementary tables 1S–3S). First, we focused on genes that have been significantly altered due to PFP exposure. In neonates (Table 3), 24 genes were found to have significantly deviated from FA controls, with 11 of these genes categorized under “Antioxidant and Oxidative Stress” response and related genes. Specifically, many genes encoding enzymes with xenobiotic conjugation activities such as the glutathione and prostaglandin peroxidase families were upregulated after PFP exposure. Additionally, growth and senescence-related genes, like PCNA, were downregulated while cell checkpoints Cyclin C (Ccnc) and G1 (Ccng1) were upregulated in the young animals.
FIG. 5.
Differentially expressed genes in microdissected airways. (A) Scatterplot of analyzed genes as a function of log of fold change plotted against exposures with FA on the x-axis and PFP on y-axis. The solid regression line indicates no exposure-induced change in the gene, and dashed lines are the 2 fold cutoff points. Genes expressed in neonates (top) are represented as triangles, where upregulated expression is colored pink, unchanged in white, and downregulated in lime. Gene expression in adults (bottom) are presented as circles, where upregulated genes are red, unchanged in white, and downregulated in green. Overall, neonates and adults have a divergent response. A trend showing upregulation was present in neonates (pink triangles), while adult animals were observed to have a substantial number of downregulated genes (green circles). (B) Heatmaps for all differentially expressed genes in airways of rats as a combination of age and/or exposure. All age and treatment groups were compared against FA exposed 7-day postnatal (7DPN) rats to elucidate age and exposure effects using HPRT as the reference gene. Genes that were differentially expressed at a p value less than 0.05 are shown (n = 3–6 rats/group). The relative magnitude of expression is indicated on a spectrum ranging from minimum (green) to maximum (red) detected. Expression patterns in 78 genes differed significantly as a combination of age and/or exposure effects. (C) The relative fold change compared with age-matched FA controls (line set at 1) for a subset of genes that were either significantly altered in 7-day-old postnatal and/or adult animals. Data are plotted as mean fold difference ± SEM (n = 3–6 rats/group, each rat was analyzed individually on the array).
TABLE 3.
PFP-Induced Gene Transcriptional Alterations in 7-Day Postnatal Rat Airways
| Symbol | RefSeq | Gene Name | Fold Regulation | p Value |
| Antioxidant and oxidative stress | ||||
| Gpx1 | NM_030826 | Glutathione peroxidase 1 | 2.4084 | 0.017709 |
| Gpx4 | NM_017165 | Glutathione peroxidase 4 | 2.6534 | 0.035767 |
| Gpx5 | XM_001059839 | Glutathione peroxidase 5 | 58.0222 | 0.035673 |
| Gpx6 | NM_147165 | Glutathione peroxidase 6 | 15249.42 | 0.018524 |
| Gstm3 | NM_031154 | Glutathione S-transferase mu 3 | −2.7707 | 0.025809 |
| Hmox2 | NM_024387 | Heme oxygenase (decycling) 2 | −2.4901 | 0.046847 |
| Ptgs1 | NM_017043 | Prostaglandin-endoperoxide synthase 1 | 2.4303 | 0.023328 |
| Ptgs2 | NM_017232 | Prostaglandin-endoperoxide synthase 2 | −25.5822 | 0.025816 |
| Tpo | NM_019353 | Thyroid peroxidase | 5.0122 | 0.036448 |
| Ucp3 | NM_013167 | Uncoupling protein 3 (mitochondrial, proton carrier) | 522.9152 | 0.031378 |
| Xpa | XM_216403 | Xeroderma pigmentosum, complementation group A | 1.7608 | 0.008677 |
| Heat shock proteins | ||||
| Hspa1l | NM_212546 | Heat shock 70kD protein 1-like (mapped) | −3.2161 | 0.002062 |
| Hspe1 | NM_012966 | Heat shock protein 1 (chaperonin 10) | 3.1087 | 0.011288 |
| Hspd1 | NM_022229 | Heat shock protein 1 (chaperonin) | 3.0593 | 0.003225 |
| Inflammation | ||||
| Lta | NM_080769 | Lymphotoxin alpha (TNF superfamily, member 1) | −4.1394 | 0.003475 |
| Necrosis and apoptosis | ||||
| Anxa5 | NM_013132 | Annexin A5 | −5.0765 | 0.007664 |
| Oxygen transporters | ||||
| Cygb | NM_130744 | Cytoglobin | −6.4446 | 0.03966 |
| Hbz | XM_213268 | Hemoglobin, zeta | 11.7297 | 0.007439 |
| Ngb | NM_033359 | Neuroglobin | 9.1671 | 0.020161 |
| Proliferation and carcinogenesis | ||||
| Ccnc | XM_342812 | Cyclin C | 2.1183 | 0.01864 |
| Ccng1 | NM_012923 | Cyclin G1 | 2.1138 | 0.002406 |
| Egr1 | NM_012551 | Early growth response 1 | −126.776 | 0.002399 |
| Tp53 | NM_030989 | Tumor protein p53 | −4.2871 | 0.038778 |
| Pcna | NM_022381 | PCNA | −2.5916 | 0.048601 |
In contrast to neonates, adult rats had a more robust response, with 53 genes changed (Table 4). Although the majority of genes (25) altered fall under Antioxidant and Oxidative Stress response genes, only six genes (Gpx1, Gstm1, Gstm3, Hmox2, Ptgs2, and Xpa) significantly matched between the two ages. Furthermore, there was a dissimilar response in “Necrosis and Apoptosis” and “Proliferation and Carcinogenesis” categories compared against the different ages. While downregulation was primarily observed in adults, neonates had a more divergent response with some genes upregulated and some downregulated. Only Annexin A5 (Anxa5) matched as significantly changed among the two ages in these categories. Interestingly, we have also detected changes in xenobiotic metabolizing genes in the cytochrome P450 and Flavin moooxygenase families that were not observed in 7-day-old postnatal animals. To reduce the analysis from the large data set, we focused on a subset of genes that were either significantly altered in both 7-day-old postnatal and adult animals, and/or genes that we have analyzed previously in studies of other particle types (Lee et al., 2010; Van Winkle et al., 2010Winkle et al., 2010). These have been plotted as relative fold change as compared with age-matched FA controls (Fig. 5C).
TABLE 4.
PFP-Induced Gene Transcriptional Alterations in Adult Rat Airways
| Symbol | RefSeq | Gene Name | Fold Regulation | p Value |
| Antioxidant and oxidative stress | ||||
| Ctsb | NM_022597 | Cathepsin B | 3.0707 | 0.035618 |
| Cygb | NM_130744 | Cytoglobin | 9.0559 | 0.014945 |
| Duox2 | NM_024141 | Dual oxidase 2 | 70.0018 | 0.012265 |
| Epx | XM_220834 | Eosinophil peroxidase | 7.6222 | 0.017935 |
| Ephx2 | NM_022936 | Epoxide hydrolase 2, cytoplasmic | −36.9837 | 0.019421 |
| Gpx1 | NM_030826 | Glutathione peroxidase 1 | 4.8268 | 0.036295 |
| Gpx2 | NM_183403 | Glutathione peroxidase 2 | 6.9735 | 0.024608 |
| Gpx6 | NM_147165 | Glutathione peroxidase 6 | −191.402 | 0.044251 |
| Gpx8 | XM_215486 | Glutathione peroxidase 8 | 4.2725 | 0.027142 |
| Gsr | NM_053906 | Glutathione reductase | −13.2797 | 0.028066 |
| Gstm1 | NM_017014 | Glutathione S-transferase mu 1 | −11.0965 | 0.001293 |
| Gstm3 | NM_031154 | Glutathione S-transferase mu 3 | −27.0246 | 0.013771 |
| Hmox1 | NM_012580 | Heme oxygenase (decycling) 1 | −30.9446 | 0.000458 |
| Hmox2 | NM_024387 | Heme oxygenase (decycling) 2 | −24.3281 | 0.028834 |
| Idh1 | NM_031510 | Isocitrate dehydrogenase 1 (NADP+), soluble | 5.6885 | 0.015345 |
| Nox4 | NM_053524 | NADPH oxidase 4 | 3.5661 | 0.038669 |
| Por | NM_031576 | P450 (cytochrome) oxidoreductase | −7.477 | 0.009219 |
| Prdx1 | NM_057114 | Peroxiredoxin 1 | 3.882 | 0.013839 |
| Prdx3 | NM_022540 | Peroxiredoxin 3 | 3.0562 | 0.048917 |
| Prnp | NM_012631 | Prion protein | 7.9351 | 0.031433 |
| Ptgs2 | NM_017232 | Prostaglandin-endoperoxide synthase 2 | 49.6539 | 0.023834 |
| Slc41a3 | NM_001037492 | Solute carrier family 41, member 3 | 5.2298 | 0.047979 |
| Sod1 | NM_017050 | Superoxide dismutase 1, soluble | 3.6862 | 0.031726 |
| Sod2 | NM_017051 | Superoxide dismutase 2, mitochondrial | 3.5975 | 0.041264 |
| Xpa | XM_216403 | Xeroderma pigmentosum, complementation group A | 4.4286 | 0.010963 |
| Heat shock proteins | ||||
| Hspa4 | NM_153629 | Heat shock protein 4 | 1.8433 | 0.016881 |
| Hspa1a | NM_031971 | Heat shock 70kD protein 1A | −67.6156 | 0.014389 |
| Inflammation | ||||
| Ccl21b | NM_001008513 | Chemokine (C-C motif) ligand 21b (serine) | −11.0321 | 0.016656 |
| Ccl3 | NM_013025 | Chemokine (C-C motif) ligand 3 | −7.5797 | 0.046574 |
| Il18 | NM_019165 | Interleukin 18 | 3.3456 | 0.002379 |
| Lta | NM_080769 | Lymphotoxin alpha (TNF superfamily, member 1) | −12.2652 | 0.010425 |
| Nfkb1 | XM_342346 | Nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 | −33.0979 | 0.01622 |
| Necrosis and apoptosis | ||||
| Anxa5 | NM_013132 | Annexin A5 | −41.4567 | 0.012066 |
| Bax | NM_017059 | Bcl2-associated X protein | −33.2827 | 0.010506 |
| Bcl2l1 | NM_031535 | Bcl2-like 1 | −40.9219 | 0.025367 |
| Chek2 | NM_053677 | CHK2 checkpoint homolog (Schizosaccharomyces. pombe) | −15.4004 | 0.013171 |
| Ercc2 | XM_218424 | Excision repair cross-complementing rodent repair deficiency, complementation group 2 | −27.6876 | 0.010995 |
| Ercc4 | XM_222534 | Excision repair cross-complementing rodent repair deficiency, complementation group 4 | −14.0267 | 0.026123 |
| Rad23a | NM_001013190 | RAD23 homolog A (Saccharomyces. cerevisiae) | −7.4534 | 0.03773 |
| Tradd | XM_341671 | TNFRSF1A-associated via death domain | −26.2046 | 0.030071 |
| Tnfrsf1a | NM_013091 | Tumor necrosis factor receptor superfamily, member 1a | −53.5655 | 0.046099 |
| Ugt1a6 | NM_057105 | UDP glucuronosyltransferase 1 family, polypeptide A6 | −15.3658 | 0.010749 |
| Ung | NM_001013124 | Uracil-DNA glycosylase | −24.0967 | 0.030458 |
| Xrcc1 | NM_053435 | X-ray repair complementing defective repair in Chinese hamster cells 1 | −29.3218 | 0.005521 |
| Proliferation and carcinogenesis | ||||
| Ccnd1 | NM_171992 | Cyclin D1 | −3.9014 | 0.008242 |
| Egr1 | NM_012551 | Early growth response 1 | −127.066 | 0.002162 |
| Cdkn1a | NM_080782 | Cyclin-dependent kinase inhibitor 1A (p21, Cip1) | −20.8561 | 0.016276 |
| Igfbp6 | NM_013104 | Insulin-like growth factor binding protein 6 | −43.9 | 0.019266 |
| Tp53 | NM_030989 | Tumor protein p53 | −22.9176 | 0.026688 |
| Xenobiotic metabolism | ||||
| Cyp2a3a | NM_012542 | Cytochrome P450, family 2, subfamily A, polypeptide 3a | −10.7957 | 0.001177 |
| Cyp4a3 | NM_175760 | Cytochrome P450, family 4, subfamily a, polypeptide 3 | −9.4802 | 0.005024 |
| Fmo2 | NM_144737 | Flavin containing monooxygenase 2 | 5.8038 | 0.026673 |
| Fmo5 | NM_144739 | Flavin containing monooxygenase 5 | −9.1731 | 0.023699 |
PCNA Expression
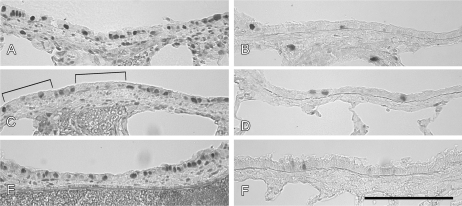
PCNA, a gene associated with cell proliferation was quantified from RT-profiler array data from microdissected airways using the comparative Ct method with HPRT as the reference gene. PCNA expression in FA 7-day postnatal rats was about 5-fold higher than FA adults. Twenty-four hours following PFP exposure, PCNA expression was significantly reduced while expression remained unchanged in adults. To qualitatively determine protein abundance, immunohistochemical staining against PCNA was evaluated in midlevel bronchiolar airways (Fig. 6). Protein abundance followed a similar trend compared with gene expression. Cell nuclear positive PCNA cells were abundant in 7-day postnatal rats reared in FA (Fig. 6A). PCNA abundance was reduced 2 hours following PFP exposure in 7-day animals, where staining became diffuse and strips of bronchiolar epithelia were observed to be devoid of nuclear PCNA (brackets, Fig. 6C). PCNA staining returned to steady state by 24 h post PFP exposure (Fig. 6E). In stark contrast, PCNA positive cells were rarely observed in FA adults (Fig. 6B). PFP exposure at either 2 or 24 h time points did not affect PCNA expression in adult animals (Figs. 6D and 6F).
FIG. 6.
Immunohistochemical staining of PCNA in the airways of 7-day postnatal (A, C, and E) and adult (B, D, and F) exposed to either FA (A, B) or PFP, examined two (C, D) or 24 h (E, F) after cessation of exposure. Basally, 7-day old neonates exposed to FA had abundant cells with nuclear staining for PCNA (A). In contrast, PCNA was scarce in FA exposed adults (B). Following 2 hours post PFP exposure, PCNA in 7-day neonates became diffuse, and strips of epithelium were observed to be devoid of nuclear PCNA staining (brackets) (C). PCNA staining returned to steady state by 24 h post PFP exposure (E). PCNA was unaffected after PFP exposure in adult rats (D and F). Scale bar for A–F (shown in F) is 50 μm.
DISCUSSION
In the current study, we found that exposure to an atmosphere containing a low dose of ultrafine particles elicits biological changes that greatly differ between young adult and neonatal rats. We focused our efforts on the conducting airways, due to the fact that extensive development of these airways occurs in the postnatal period and that exacerbation of many airway diseases, such as bronchitis and asthma have been linked to either acute or chronic exposures to PM in young children (Ciccone et al., 1998; Brauer et al., 2007; Morgenstern et al., 2008). For this study, we assessed cytotoxicity, gene expression, and protein changes in an attempt to characterize differences between neonates and adults, which may explain the differential susceptibility between the ages.
We found that neonatal rats are more susceptible to PFP with significant increases in markers of cytotoxicity following exposure. Although the epithelial cytotoxic responses were mild, permeable cells were observed and significant increases in LDH activity in BALF were detected. Compared with adults, more macrophages incorporated ethidium homodimer-1, a marker of cytotoxicity in the neonatal lung. Our data agrees with the previous findings by Li et al. (2003) showing that ultrafine particles damage macrophages and cause formation of vacuoles in RAW 264.7 macrophages in vitro. Macrophages are also significantly affected by ultrafine particles in studies by Rouse et al. (2008), where particle-laden macrophages were noted after inhalation of butadiene soot. Furthermore, as a more global indicator of overall cell leakiness, LDH activity was found to be increased in the neonates but not in adults. This is also in agreement with our previous work using a diffusion soot particle, with a different EC/OC ratio and lower levels of attached PAH, where we showed significant LDH elevation in neonates 24 h after an acute lower-PAH containing diffusion flame exposure (Van Winkle et al., 2010Winkle et al., 2010). In contrast to neonates, ethidium positive membrane permeable cells were rarely observed in adults, and LDH activity was not elevated after exposure. These results indicate that even at these low levels of exposure, neonates are more susceptible than adults.
We used a previously characterized premixed flame (PFP) generation system (Lee et al., 2010) to expose neonatal and adult rats. Sprague-Dawley rats were chosen as the animal model because of their larger neonatal and adult sizes, compared with mice, and their use in previous PM studies (Roberts et al., 2009; Van Winkle et al., 2010Winkle et al., 2010; Zhong et al., 2010). Animals were exposed to a single 6 h acute exposure to 22.4 μg/m3 PFP. This dose was selected because it is below the 2006 EPA revised 24 h average PM2.5 NAAQS of 35 μg/m3 and approximates the measured levels of PM in this size range in downtown Fresno, CA. A fuel-rich flame environment generates carbonaceous soot in addition to a variety of allyl radicals, which further react to yield the formation of benzene (Marinov et al., 1998). Benzene combined with additional radicals generates PAH (i.e., naphthalene, fluorenes, and phenanthrenes) (Castaldi et al., 1996; Lindstedt, 1994), which we have characterized and quantified in our chambers. Although methylated biphenyls were the most abundant aromatic species in the vapor phase, the sum of mono, poly, and unsubstituted naphthalenes constituted the majority of the detected PAHs in the vapor and particulate phases. Furthermore, it is important to note that while two-ringed naphthalenes were the dominant species, three-ringed fluorene and phenanthrene, four-ringed pyrene, and five-ringed benzopyrenes were detected in subnanogram quantities.
We have previously shown that an acute in vivo exposure of a different type of PM, low PAH containing diffusion flame soot (DFP), elicits age-specific antioxidant and oxidative stress responses, discovering that young postnatal animals have increased susceptibility and respond uniquely to low PAH diffusion soot (Van Winkle et al., 2010Winkle et al., 2010). A comparison of our results from the current study, with high PAH soot (PFP) is warranted. Previously, we have shown that gene expression is a sensitive marker for PM-induced oxidative stress and we have again applied antioxidant and oxidative stress RT-PCR arrays on microdissected conducting airways. Contrary to our expectations, the gene expression profile differed greatly in our PFP exposure compared with an acute diffusion flame exposure in both neonates and adults. Out of the 162 genes assessed, only 3 genes matched among the neonates, comparing across exposure types. Animals responded more robustly to PFP than to DFP, and both ages show a great number of genes altered within antioxidant and oxidative stress, necrosis and apoptosis, and proliferation and carcinogenesis categories. Comparing between ages, only 12 genes that were significantly altered by both PFP and DFP exposures matched between neonates and adults, a majority of these genes are for antioxidant enzymes.
Since the gene expression pattern differed so markedly between our previous diffusion flame exposure (Van Winkle et al., 2010) and the PFP exposure described in the current study, we hypothesized that the PAH content may play a large role in the cellular response to PM. Surprisingly, none of the xenobiotic metabolism genes responsible for solubilizing and metabolizing PAHs were significantly induced in the neonates. This may in part explain increased cytotoxicity; PAH containing particles have been shown to localize to lipids (Murphy et al., 2008) through either chemical or biochemical processes. If a cell is unable to clear PAHs or induce enzymes to clear these PAHs, these compounds may persist in the cell and have the ability to enhance toxicity. Although the xenobiotic metabolizing cytochrome P450s could be detected by 7-day postnatal age, these neonates have immature xenobiotic metabolizing and detoxifying enzymes, which could further perturb their ability to clear PAHs (Cardoso et al., 1993; Fanucchi, 2004; Ji et al., 1994, 1995). In contrast to neonates, adult animals had significant increases in several cytochrome P450s (CYP2A3a and CYP4A3) and flavin-containing monooxygenases (FMO2, FMO5) genes following PFP exposure. Although we did not see significant increases in the expression of classical aryl hydrocarbon receptor (AhR) responsive genes (CYP1A1, CYP1B1) as Rouse et al. (2008) have reported, it has been shown that flavin-containing monooxygenase expression can be altered under the AhR-dependent pathway. Our results are in agreement with Celius et al. (2008), showing significant induction of FMO2 and reduction of FMO5 after AhR agonist TCDD treatment in liver.
We examined gene expression changes at a single time point (24 h) after exposure. This time point was selected to compare with previous results obtained from our diffusion flame inhalation study (Van Winkle et al., 2010Winkle et al., 2010). However, it is very likely that the temporal patterns of the antioxidant, proliferation, and xenobiotic metabolizing genes differ by age and exposure. This could explain differences in expression of several antioxidant genes between the diffusion flame soot exposure (Van Winkle et al., 2010Winkle et al., 2010), the butadiene soot exposures (Rouse et al., 2008), and the PFP reported here. Another possible explanation is the differences in PAH composition of these atmospheres. Butadiene soot contains large planar PAHs-like pyrenes and benzo(a)pyrenes (Penn et al., 2005). This is in contrast to the diffusion flame soot we used previously which contains a mixture of pyrene, quinoline, and naphthalene (Van Winkle et al., 2010Winkle et al., 2010) and our ethylene generated PFP, reported in this study, which consists essentially of smaller PAHs-like biphenyls and substituted naphthalenes. While much previous work has been done regarding metabolism and toxicity of naphthalene, it has been shown that generally, neither inhalation nor intraperitoneal injections of naphthalene result in lung toxicity in rats and it is mainly a mouse-specific lung toxicant (Buckpitt et al., 2002; Lin et al., 2009; Van Winkle et al., 1999Winkle et al., 1999; West et al., 2001). It is surprising then that even though naphthalene is the most abundant PAH found in the PFP particulate phase, we were able to observe the presence of cytotoxic cells. We postulate that toxicity may partly be due to the presence of lower abundance aryl hydrocarbon receptor (AhR) agonistic PAHs (i.e., pyrenes, benzopyrenes, and anthanthrenes) present in subnanogram per cubic meter quantities in the PFP atmosphere.
While both neonatal and adult rats were exposed to the same atmospheric PFP concentration in the exposure chambers, a multitude of factors may have affected dose delivered in the neonates versus adults. Neonatal rats have higher ventilation and oxygen consumption rates than adult rats normalized to body weight (Mortola, 1991). Differences in breathing frequency, body size, and mean airway diameter change deposition patterns, which may increase dose in the neonates compared with the adults. Alternatively, because the pups were exposed with their dams in the exposure chambers, the delivered dose to the neonate may have been reduced due to huddling under the dam. These two factors potentially offset each other but it is not possible to know to what degree. For a 6-h exposure, we chose to expose the neonates with their dam to minimize the effects of stress, heat loss, and nutritional changes. This exposure strategy has been used for other inhalation studies (Clerch and Massaro, 1992; Joad et al., 1995), but it is important to keep these limitations in mind in terms of delivered dose.
Even after a single acute exposure to PFP, cell cycle checkpoint cyclins and Tp53 were significantly changed in both ages. It is reasonable, because PAH-rich particles, like diesel exhaust, in the presence of cytochrome P450 reductase, have been shown to generate reactive oxygen species that damage DNA and induce strand breaks (Kumagai et al., 1997). We questioned the implications of PFP exposure on continuing lung growth and development and analyzed the protein: PCNA, a marker of cell proliferation. As expected, basal expression of PCNA was significantly higher in the developing neonates. However, we found significant decreases in both protein expression at 2 h and gene expression at 24 h post exposure in the neonates. In contrast, we have previously reported that neither PCNA gene nor protein expression were significantly altered in neonates and adults following an acute low PAH containing diffusion flame exposure (Van Winkle et al., 2010Winkle et al., 2010). Our gene expression results are in agreement with Lee et al. (2010), who also found reduced PCNA expression in neonates 24 h after PFP soot exposure, our RT2 Profiler PCR Array confirms these previous results obtained with conventional qRT-PCR. In addition, we show focal patches of PCNA-deficient cells in the current study that are detected at 2-h post exposure. This builds upon the evidence that proliferative capabilities of neonates, but not adults, are impaired by PFP. We postulate that the decrease in PCNA expression is a rapid and early response to PFP exposure that persists for at least 24 h. PFP has previously been shown to cause significant reductions in bronchiolar diameter and length in developing rats after a 3-week subchronic exposure (Lee et al., 2010). Altered proliferation patterns during development could be a contributing factor to these gross changes in airway architecture and may result in reduced lung function. Additionally, since we were able to observe similar results across two separate studies and saw changes in numerous genes involved in the proliferative response pathways, this supports that we have a reproducible exposure and biologic effect, a necessary prerequisite to perform repeatable biologic experiments to be able to tease apart the mechanisms of PM susceptibility.
We postulate that developing neonates have a limited ability to deviate from the normal developmental pattern and this inability to respond to ultrafine particulates enhances oxidative stress, causes cellular injury, and perturbs normal airway development. Similar outcomes using two different particle generation systems as well as the same exposure system used for two different time points validates our results and underscores the usefulness and necessity of having defined exposure conditions that can be replicated for additional in depth studies of biologic mechanisms of altered growth and antioxidant responses. Future time course studies of the intracellular signaling cascades that regulate these processes will be required to determine whether the entire temporal pattern between neonates and adults differ in response to PM and will allow us to begin to address the mechanisms for elevated susceptibility in neonatal animals.
This study shows that a short-term low-dose inhalation exposure to combustion derived ultrafine particles induces markers of cytotoxicity and alters gene and protein expression patterns in the conducting airways of developing neonates compared with adults. Our data strongly suggests that adult animals are an inappropriate model for evaluating responses to PM in susceptible populations, such as young children. Based on our results, neonates have a unique “inability” to respond to environmental exposures by changing gene expression compared with adults. Compared with adult animals, developing neonates are more susceptible to PAH-rich ultrafine PM, exhibiting increased cellular toxicity in the lung affecting airway proliferation patterns, which may result in the perturbation of normal lung development.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
Support for the University of California at Davis core facilities used in this work: the Cellular and Molecular Imaging Core Facility (ES005707) and the inhalation exposure facility at the California National Primate Research Center (RR00169). Although the research described in the article has been funded primarily by the United States Environmental Protection Agency through grant RD-83241401-0 to the University of California, Davis, it has not been subject to the agency's required peer and policy review and, therefore, does not necessarily reflect the views of the agency and no official endorsement should be inferred. The project described was also supported in part by Award Number P42ES004699 from the National Institute of Environmental Health Sciences. Mr Chan’s effort was supported by a training program in Environmental Health Sciences (T32 ES007058-33) funded by the National Institute of Environmental Health Sciences.
CONFLICT OF INTEREST
Dr Laura Van Winkle has identified a potential competing financial interest with the American Petroleum Institute (API). Dr Van Winkle is a co-investigator on a research grant from API to study the kinetics of naphthalene bioactivation in the lung and has received honoraria from API for speaking at research conferences sponsored by API on naphthalene. API did not fund any of the work presented in the attached study and the research grant funded by API has complete freedom to publish the results regardless of whether they are in API's interest, and without input from API, in keeping with University of California policy. The remaining authors declare they have no actual or potential competing financial interests.
Supplementary Material
Acknowledgments
We are grateful to the following people for their skilled technical assistance during exposures, sample collection, and processing: Brian Tarkington, Ashley Cooper, Louise Olson, Patricia Edwards, Trenton Combs, and Judy Shimizu. We acknowledge Michael Kleeman’s laboratory at UC Davis for EC/OC sample analysis and Barbara Zielinska at the DRI for filter and vapor phase PAH speciation. Finally, we thank Jessie Charrier, Cris Grodzki, Matt Herring, and Keisha Williams for reading and editing the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
References
- ALA. American Lung Association State of the Air: 2009. New York, NY: American Lung Association; 2009. [Google Scholar]
- Baker GL, Shultz MA, Fanucchi MV, Morin DM, Buckpitt AR, Plopper CG. Assessing gene expression in lung subcompartments utilizing in situ RNA preservation. Toxicol. Sci. 2004;77:135–141. doi: 10.1093/toxsci/kfh002. [DOI] [PubMed] [Google Scholar]
- Bearer CF. How are children different from adults. Environ. Health Perspect. 1995;103:7–12. doi: 10.1289/ehp.95103s67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branis M, Safranek J, Hytychova A. Exposure of children to airborne particulate matter of different size fractions during indoor physical education at school. Build. Environ. 2009;44:1246–1252. [Google Scholar]
- Brauer M, Hoek G, Smit HA, de Jongste JC, Gerritsen J, Postma DS, Kerkhof M, Brunekreef B. Air pollution and development of asthma, allergy and infections in a birth cohort. Eur. Respir. J. 2007;29:879–888. doi: 10.1183/09031936.00083406. [DOI] [PubMed] [Google Scholar]
- Buckpitt A, Boland B, Isbell M, Morin D, Shultz M, Baldwin R, Chan K, Karlsson A, Lin C, Taff A, et al. Naphthalene-induced respiratory tract toxicity: Metabolic mechanisms of toxicity. Drug Metab. Rev. 2002;34:791–820. doi: 10.1081/dmr-120015694. [DOI] [PubMed] [Google Scholar]
- Cardoso WV, Stewart LG, Pinkerton KE, Ji C, Hook GE, Singh G, Katyal SL, Thurlbeck WM, Plopper CG. Secretory product expression during Clara cell differentiation in the rabbit and rat. Am. J. Physiol. 1993;264:L543–L552. doi: 10.1152/ajplung.1993.264.6.L543. [DOI] [PubMed] [Google Scholar]
- Castaldi MJ, Marinov NM, Melius CF, Huang J, Sekan SM, Pitz WJ, Westbrook CK. Experimental and modeling investigation of aromatic and polycyclic aromatic hydrocarbon formation in a premixed ethylene flame. Symposium (International) on Combustion. 1996;26:693–702. [Google Scholar]
- Celius T, Roblin S, Harper PA, Matthews J, Boutros PC, Pohjanvirta R, Okey AB. Aryl hydrocarbon receptor-dependent induction of flavin-containing monooxygenase mRNAs in mouse liver. Drug Metab. Dispos. 2008;36:2499–2505. doi: 10.1124/dmd.108.023457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Ciccone G, Forastiere F, Agabiti N, Biggeri A, Bisanti L, Chellini E, Corbo G, Dell'Orco V, Dalmasso P, Volante TF, et al. Road traffic and adverse respiratory effects in children. SIDRIA Collaborative Group. Occup. Environ. Med. 1998;55:771–778. doi: 10.1136/oem.55.11.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerch LB, Massaro D. Rat lung antioxidant enzymes: Differences in perinatal gene expression and regulation. Am. J. Physiol. 1992;263:L466–L470. doi: 10.1152/ajplung.1992.263.4.L466. [DOI] [PubMed] [Google Scholar]
- Dockery DW. Health effects of particulate air pollution. Ann. Epidemiol. 2009;19:257–263. doi: 10.1016/j.annepidem.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanucchi M. Development of Antioxidant and Xenobiotic Metabolizing Enzyme Systems. San Diego, CA: Elsevier; 2004. [Google Scholar]
- Hammond TG, Mobbs M. Lung oedema—microscopic detection. J. Appl. Toxicol. 1984;4:219–221. doi: 10.1002/jat.2550040411. [DOI] [PubMed] [Google Scholar]
- Helsel DR. More than obvious: Better methods for interpreting nondetect data. Environ. Sci. Technol. 2005;39:419a–423a. doi: 10.1021/es053368a. [DOI] [PubMed] [Google Scholar]
- Herner JD, Aw J, Gao O, Chang DP, Kleeman MJ. Size and composition distribution of airborne particulate matter in Northern California: I–Particulate mass, carbon, and water-soluble Ions. J. Air Waste Manag. Assoc. 2005;55:30–51. doi: 10.1080/10473289.2005.10464600. [DOI] [PubMed] [Google Scholar]
- Hinners R, Burkart J, Punte C. Animal inhalation exposure chambers. Arch. Environ. Health. 1968;16:194–206. doi: 10.1080/00039896.1968.10665043. [DOI] [PubMed] [Google Scholar]
- Ibald-Mulli A, Wichmann HE, Kreyling W, Peters A. Epidemiological evidence on health effects of ultrafine particles. J. Aerosol. Med. 2002;15:189–201. doi: 10.1089/089426802320282310. [DOI] [PubMed] [Google Scholar]
- Ji CM, Cardoso WV, Gebremichael A, Philpot RM, Buckpitt AR, Plopper CG, Pinkerton KE. Pulmonary cytochrome P-450 monooxygenase system and Clara cell differentiation in rats. Am. J. Physiol. 1995;269:L394–L402. doi: 10.1152/ajplung.1995.269.3.L394. [DOI] [PubMed] [Google Scholar]
- Ji CM, Plopper CG, Witschi HP, Pinkerton KE. Exposure to sidestream cigarette smoke alters bronchiolar epithelial cell differentiation in the postnatal rat lung. Am. J. Respir. Cell. Mol. Biol. 1994;11:312–320. doi: 10.1165/ajrcmb.11.3.8086168. [DOI] [PubMed] [Google Scholar]
- Joad JP, Ji C, Kott KS, Bric JM, Pinkerton KE. In utero and postnatal effects of sidestream cigarette smoke exposure on lung function, hyperresponsiveness, and neuroendocrine cells in rats. Toxicol. Appl. Pharmacol. 1995;132:63–71. doi: 10.1006/taap.1995.1087. [DOI] [PubMed] [Google Scholar]
- Karnovsky MJ. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 1965;27:137A–138A. [Google Scholar]
- Kumagai Y, Arimoto T, Shinyashiki M, Shimojo N, Nakai Y, Yoshikawa T, Sagai M. Generation of reactive oxygen species during interaction of diesel exhaust particle components with NADPH-cytochrome P450 reductase and involvement of the bioactivation in the DNA damage. Free Radic. Biol. Med. 1997;22:479–487. doi: 10.1016/s0891-5849(96)00341-3. [DOI] [PubMed] [Google Scholar]
- Langston C. Normal and abnormal structural development of the human lung. Prog. Clin. Biol. Res. 1983;140:75–91. [PubMed] [Google Scholar]
- Lee D, Wallis C, Wexler AS, Schelegle ES, Van Winkle LS, Plopper CG, Fanucchi MV, Kumfer B, Kennedy IM, et al. Small particles disrupt postnatal airway development. J. Appl. Physiol. 2010;109:1115–1124. doi: 10.1152/japplphysiol.00295.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, Wang M, Oberley T, Froines J, Nel A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 2003;111:455–460. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Wheelock AM, Morin D, Baldwin RM, Lee MG, Taff A, Plopper C, Buckpitt A, Rohde A. Toxicity and metabolism of methylnaphthalenes: Comparison with naphthalene and 1-nitronaphthalene. Toxicology. 2009;260:16–27. doi: 10.1016/j.tox.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstedt RP. Formation and destruction of aromatic-compounds and soot in flames. Abstr. Pap. Am. Chem. Soc. 1994;207:22–FUEL. [Google Scholar]
- Marinov NM, Pitz WJ, Westbrook CK, Vincitore AM, Castaldi MJ, Senkan SM, Melius CF. Aromatic and polycyclic aromatic hydrocarbon formation in a laminar premixed n-butane flame. Combust. Flame. 1998;114:192–213. [Google Scholar]
- Mills NL, Donaldson K, Hadoke PW, Boon NA, MacNee W, Cassee FR, Sandstrom T, Blomberg A, Newby DE. Adverse cardiovascular effects of air pollution. Nat. Clin. Pract. Cardiovasc. Med. 2009;6:36–44. doi: 10.1038/ncpcardio1399. [DOI] [PubMed] [Google Scholar]
- Morgenstern V, Zutavern A, Cyrys J, Brockow I, Koletzko S, Kramer U, Behrendt H, Herbarth O, von Berg A, Bauer CP, et al. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am. J. Respir. Crit. Care Med. 2008;177:1331–1337. doi: 10.1164/rccm.200701-036OC. [DOI] [PubMed] [Google Scholar]
- Morrow PE, Mercer TT. A Point-to-Plane electrostatic precipitator for particle size sampling. AIHAJ. 1964;25:8–14. doi: 10.1080/00028896409342547. [DOI] [PubMed] [Google Scholar]
- Mortola JP. Hamsters versus rats: Ventilatory responses in adults and newborns. Respir. Physiol. 1991;85:305–317. doi: 10.1016/0034-5687(91)90070-y. [DOI] [PubMed] [Google Scholar]
- Murphy G, Jr., Rouse RL, Polk WW, Henk WG, Barker SA, Boudreaux MJ, Floyd ZE, Penn AL. Combustion-derived hydrocarbons localize to lipid droplets in respiratory cells. Am. J. Respir. Cell. Mol. Biol. 2008;38:532–540. doi: 10.1165/rcmb.2007-0204OC. [DOI] [PubMed] [Google Scholar]
- Norris G, YoungPong SN, Koenig JQ, Larson TV, Sheppard L, Stout JW. An association between fine particles and asthma emergency department visits for children in Seattle. Environ. Health Perspect. 1999;107:489–493. doi: 10.1289/ehp.99107489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekkanen J, Timonen KL, Ruuskanen J, Reponen A, Mirme A. Effects of ultrafine and fine particles in urban air on peak expiratory flow among children with asthmatic symptoms. Environ. Res. 1997;74:24–33. doi: 10.1006/enrs.1997.3750. [DOI] [PubMed] [Google Scholar]
- Penn A, Murphy G, Barker S, Henk W, Penn L. Combustion-derived ultrafine particles transport organic toxicants to target respiratory cells. Environ. Health Perspect. 2005;113:956–963. doi: 10.1289/ehp.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Dockery DW, Heinrich J, Wichmann HE. Short-term effects of particulate air pollution on respiratory morbidity in asthmatic children. Eur. Respir. J. 1997;10:872–879. [PubMed] [Google Scholar]
- Pey J, Querol X, Alastuey A, Rodriguez S, Putaud JP, Van Dingenen R. Source apportionment of urban fine and ultra-fine particle number concentration in a Western Mediterranean city. Atmos. Environ. 2009;43:4407–4415. [Google Scholar]
- Robert MA, VanBergen S, Kleeman MJ, Jakober CA. Size and composition distributions of particulate matter emissions: Part 1—light-duty gasoline vehicles. J. Air. Waste Manag. Assoc. 2007;57:1414–1428. doi: 10.3155/1047-3289.57.12.1414. [DOI] [PubMed] [Google Scholar]
- Roberts JR, Young SH, Castranova V, Antonini JM. The soluble nickel component of residual oil fly ash alters pulmonary host defense in rats. J. Immunotoxicol. 2009;6:49–61. doi: 10.1080/15476910802630379. [DOI] [PubMed] [Google Scholar]
- Rouse RL, Murphy G, Boudreaux MJ, Paulsen DB, Penn AL. Soot nanoparticles promote biotransformation, oxidative stress, and inflammation in murine lungs. Am. J. Respir. Cell. Mol. Biol. 2008;39:198–207. doi: 10.1165/rcmb.2008-0057OC. [DOI] [PubMed] [Google Scholar]
- Shumway RH, Azari RS, Kayhanian M. Statistical approaches to estimating mean water quality concentrations with detection limits. Environ. Sci. Technol. 2002;36:3345–3353. doi: 10.1021/es0111129. [DOI] [PubMed] [Google Scholar]
- USEPA. U.S. EPA Green Book—Particulate Matter (PM-2.5) Nonattainment Areas (1997 Standard). U.S Environmental Protection Agency, Research Triangle Park, NC. 2009. [Google Scholar]
- Van Winkle LS, Chan JK, Anderson DS, Kumfer BM, Kennedy IM, Wexler AS, Wallis C, Abid AD, Sutherland KM, et al. Age specific responses to acute inhalation of diffusion flame soot particles: Cellular injury and the airway antioxidant response. Inhal. Toxicol. 2010;22(Suppl 2):70–83. doi: 10.3109/08958378.2010.513403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Winkle LS, Isaac JM, Plopper CG. Repair of naphthalene-injured microdissected airways in vitro. Am. J. Respir. Cell. Mol. Biol. 1996;15:1–8. doi: 10.1165/ajrcmb.15.1.8679213. [DOI] [PubMed] [Google Scholar]
- Van Winkle LS, Johnson ZA, Nishio SJ, Brown CD, Plopper CG. Early events in naphthalene-induced acute Clara cell toxicity: Comparison of membrane permeability and ultrastructure. Am. J. Respir. Cell. Mol. Biol. 1999;21:44–53. doi: 10.1165/ajrcmb.21.1.3630. [DOI] [PubMed] [Google Scholar]
- West JA, Pakehham G, Morin D, Fleschner CA, Buckpitt AR, Plopper CG. Inhaled naphthalene causes dose dependent Clara cell cytotoxicity in mice but not in rats. Toxicol. Appl. Pharmacol. 2001;173:114–119. doi: 10.1006/taap.2001.9151. [DOI] [PubMed] [Google Scholar]
- Wilson HH, Chauhan J, Kerry PJ, Evans JG. Ethanol vapour-fixation of rat lung for immunocytochemistry investigations. J. Immunol. Methods. 2001;247:187–190. doi: 10.1016/s0022-1759(00)00314-8. [DOI] [PubMed] [Google Scholar]
- Zhong CY, Zhou YM, Smith KR, Kennedy IM, Chen CY, Aust AE, Pinkerton KE. Oxidative injury in the lungs of neonatal rats following short-term exposure to ultrafine iron and soot particles. J. Toxicol. Environ. Health A. 2010;73:837–847. doi: 10.1080/15287391003689366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.