Abstract
Neurodegeneration describes the loss of neuronal structure and function. Numerous neurodegenerative diseases are associated with neurodegeneration. Many are rare and stem from purely genetic causes. However, the prevalence of major neurodegenerative diseases is increasing with improvements in treating major diseases such as cancers and cardiovascular diseases, resulting in an aging population. The neurological consequences of neurodegeneration in patients can have devastating effects on mental and physical functioning. The causes of most cases of prevalent neurodegenerative diseases are unknown. The role of neurotoxicant exposures in neurodegenerative disease has long been suspected, with much effort devoted to identifying causative agents. However, causative factors for a significant number of cases have yet to be identified. In this review, the role of environmental neurotoxicant exposures on neurodegeneration in selected major neurodegenerative diseases is discussed. Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and amyotrophic lateral sclerosis were chosen because of available data on environmental influences. The special sensitivity the nervous system exhibits to toxicant exposure and unifying mechanisms of neurodegeneration are explored.
Keywords: neurodegeneration, Parkinson's disease, neurotoxicity, pesticides, neurotoxicology
The nervous system is a highly complex organ that, even in phylogenetically lower organisms, is responsible for a variety of tasks including receiving and processing sensory information and the control of highly complex behaviors that allow for survival. Major anatomical and physiological differences are apparent between mammals compared with lower species. Such differences account for increased behavioral complexity and a higher level of overall functioning. Even between humans and closely related primate species, there are major differences. For example, in the prefrontal cortex, major organizational differences are thought to result in our increased cognitive abilities (Semendeferi et al., 2001).
In humans, highly adapted cognitive function is a direct result of evolutionary changes in neuroanatomy and neurophysiological functioning. The most obvious gross differences are increased prefrontal cortex volume and complexity (Semendeferi et al., 2001). The complexity of the nervous and the energy requirements for normal function can render the system susceptible to a variety of insults. The behavioral and physiological manifestations of such insults can be influenced by numerous variables. With respect to environmental exposures, e.g., age, sex, route of exposure, dose, and genetic profile, all typically have major influences on the magnitude of the effects, the specificity of the lesion, and the phenotypic consequences (Spencer et al., 2000).
The temporal development of anatomical and behavioral abnormalities following exposure to a neurotoxicant can broadly be divided into two categories. (1) Acute. Here, shortly after exposure—hours to days, a neurological phenotype presents. Such a phenotype may or may not be reversible and may or may not stem from cell loss. Exposure may interfere with neurophysiological functioning, without initiating permanent dysfunction or cell loss. For example, exposure to an industrial solvent may produce central nervous system (CNS) depression without overt neuron loss and when the exposure source is removed, the symptoms may disappear. (2) Chronic. Neurotoxicant exposure requires weeks to years and, in some cases, decades to produce cell dysfunction and cell death that result in detectable neurological alterations. Such neurotoxicant exposures may result in neurodegeneration, a broadly used term, etymologically meaning the loss of structure or function of neurons. The term is typically used to describe progressive neuronal loss and often used to describe a diverse group of neurological disorders known as neurodegenerative diseases (Przedborski et al., 2003). Although there are hundreds of diseases that could be described as neurodegenerative diseases, many are rare. However, a few are relatively common, with Alzheimer’s (AD) and Parkinson’s diseases (PD) affecting millions of Americans.
A common feature of virtually all neurodegenerative diseases is that the consequences are often devastating, with severe mental and physical effects. This is due in large part to the loss or dysfunction of neurons—a highly specialized cell type that is typically postmitotic—lost cells are not replaced. Bluntly put, once it is lost, a neuron is typically gone forever, along with its associated function.
The role of neurotoxicant exposures in neurodegeneration and neurodegenerative diseases is reviewed here. Unfortunately, most cases have unknown causes, although much research suggests specific environmental and genetic risk factors. The environmental risk factors for the selected prominent neurodegenerative diseases with suggested environmental links are discussed. The special sensitivity of the nervous system to toxicant exposures and shared mechanisms by which toxicants may act to disrupt neurological function are explored. Although human data bear the most direct relevance to human disease, interpretation is often difficult due to exposure to mixtures and variability of exposure levels between individuals. Thus, in vivo experiments, most often in rodents or primates, offer the ability to examine neurodegeneration elicited by single environmental neurotoxicant exposures. In vitro experiments in this context are only discussed when they add a specific mechanistic insight. Although such experiments have much reduced costs and the results are typically obtained much more rapidly, the complexity of the human nervous system is unable to adequately be modeled in cell culture experiments.
NEUROTOXICOLOGY: HISTORICAL PERSPECTIVES
Neurotoxicology can broadly be defined as the study of adverse effects on the nervous system resulting from chemical exposures, both synthetic and natural. In this context, hundreds of chemicals are suspected of having direct or indirect effects on the normal functioning of the human nervous system (Spencer et al., 2000). A basic understanding that exposure to certain substances may affect the nervous system functioning can be found in some of the earliest written records of human history. The posited relationship typically revolved around the notion that consumption or exposure of a specific substance will elicit a given behavioral consequence. The history of lead poisoning serves as an excellent example. For instance, the neurological effects of lead poisoning have been known for millennia. Written records by the Greek physician Nicander, describing ‘‘gleaming, deadly white lead,” and Nero’s physician, Dioscorides, stating “lead makes the mind give way,” date from the second century B.C. and the first century A.D., respectively (Needleman, 2009). The effects of lead poisoning, including decreased fertility and psychosis arising from extensive use in aqueducts and as a sweetener in wine, have even been hypothesized to contribute to the fall of the Roman Empire (Gilfillan, 1965; Needleman, 2004). Although lead can affect all of the body’s organ systems, the nervous system is particularly sensitive. Importantly, a safe threshold for lead has failed to be identified (Needleman, 2009). Thus, even though the neurological effects have been known for thousands of years, much more recent advances continue to illuminate the mechanisms behind such symptoms. Lead can affect both the peripheral nervous system and CNS, with children exhibiting heightened sensitivity (Bellinger, 2004). The best described effects of lead on neurons are myelin loss and axonal degeneration (Dart et al., 2004). More recent data suggest that the neurodevelopmental effects of lead may stem, in part, from inhibition of glutamate release, N-methyl-D-aspartate receptor function, or alterations in synapse formation (White et al., 2007). Thus, even a brief consideration of lead neurotoxicity research has illustrated the evolving focus of neurotoxicology. Although the first step in recognizing the effects of a neurotoxicant in humans is often a thorough patient history that results in correlating abnormal behavioral signs with specific exposures, the end goal of neurotoxicology research is to understand mechanisms of action and toxicity. Such an understanding is needed to best estimate the risks of a given exposure.
NEURODEGENERATION: HISTORICAL PERSPECTIVES
Neurodegeneration can cause a highly diverse group of neurological disorders. Neurological disorders have been described throughout human history and associated gross abnormalities in brain appearance known for several hundred years (Berchtold and Cotman, 1998). However, in roughly the past 100 years, the link between neurodegeneration and neurological disorders has been identified and described extensively. As early as 1892, Bloq and Marinesco used the recently discovered carmine stain and found abnormal accumulation of an unknown substance into plaques in an elderly epileptic patient (Berchtold and Cotman, 1998; Boller and Forette, 1989). Two key findings were made in the early 1900s linking the loss of neuronal structure and function to neurological disorders. In 1907, Fischer extensively described plaque formation, finding neuropathological alterations in 12 of 16 cases of senile dementia, but not in 45 cases of progressive paralysis, 19 cases of functional psychosis, and 10 normal control subjects (Berchtold and Cotman, 1998; Fischer, 1907). More famously, in the same year and in a single case, the German physician and pathologist, Alois Alzheimer, described in detail the presence of neurofibrillary tangles in neurons in the brain of a severely demented 51-year-old woman. Alzheimer also noted the plaques that had been observed by Fischer (Alzheimer, 1907; Berchtold and Cotman, 1998). Roughly a decade later, specific regional neuronal cell loss was attributed to neurological sequelae. In PD, in 1919, tremor and rigidity in patients were found to be associated with cell loss in the substantia nigra (Tretiakoff, 1919).
NEUROTOXICANT EXPOSURES AND NEURODEGENERATION: HISTORICAL PERSPECTIVES
Although the effects of lead have long been known to disrupt neurological functioning, the role of neurotoxicant exposures in neurodegeneration and neurodegenerative diseases has only been much more recently understood. An excellent example of the coalescence of experimental neurotoxicology and neurodegeneration is the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) story. MPTP was produced as an accidental side product during the illicit chemical synthesis of 1-methyl-4-phenyl-4-propionoxypiperidine, an opioid analgesic drug. Drug users exposed to MPTP presented to the clinic with a behavioral phenotype strikingly similar to PD (Langston et al., 1983). This report directly led to the creation of one of the most prominent and important animal models of PD. A significant amount of mechanistic work led to the findings that MPTP crosses the blood-brain barrier (BBB), to be metabolized in astrocytes to its active metabolite (1-methyl-4-phenylpyridinium, MPP+), and to enter catecholamine neurons through the dopamine transporter (Javitch et al., 1985). Once in dopamine neurons, MPP+ concentrates in mitochondria and inhibits complex I. The resulting ATP depletion and oxidative stress are thought to be the main mechanisms that cause catecholaminergic cell dysfunction and death (Nicklas et al., 1985). The finding that an acute neurotoxicant exposure could lead to specific and ongoing neurodegeneration has led to numerous advances. Years after the initial exposure, there is evidence for active neurodegeneration in both monkeys and humans. Gliosis and microglia surrounding surviving dopamine neurons suggest ongoing neurodegeneration long after acute toxicant exposure (Langston et al., 1999; McGeer et al., 2003). To date, the monkey MPTP model remains the gold standard for testing novel therapeutics.
MAJOR NEURODEGENERATIVE DISEASES INFLUENCED BY ENVIRONMENTAL EXPOSURES
Although hundreds of human diseases may fit the definition of a neurodegenerative disease, many are rare and have been found to be caused by purely genetic factors. A small number of neurodegenerative diseases accounts for the prevalence of most cases. Most of the cases in this group of diseases stem from unknown causes. Cases are often divided into “genetic,” those stemming from purely inherited factors or sporadic, and those caused by unknown factors, including environmental exposures. Most cases are now thought to arise from a combination of genetic risk factors and environmental influences. However, these interactions remain poorly understood. Unfortunately, most neurodegenerative diseases typically have no known cure, and in most cases, even slowing disease progression has proven to be enormously challenging. Thus, most current research on neurodegenerative diseases focuses on two broad aspects: (1) identifying and reducing causative factors and (2) finding and implementing novel treatments that are curative or at least reduce disease progression.
Although environmental exposures have been linked to several neurodegenerative diseases, it should be noted that a single environmental factor accounting for a significant number of cases has not been identified. Given that humans are exposed to numerous environmental toxicants in the course of a life span, identifying such a factor has proven to be very difficult, if not impossible. However, epidemiology and laboratory-based science have identified factors that influence risk and reproduced the key pathological features using animal models of the major neurodegenerative diseases. These data are highly informative on the role that neurotoxicant exposures may play in neurodegeneration.
Alzheimer’s Disease
AD is the most common neurodegenerative disease, affecting an estimated 5.4 million Americans (Hebert et al., 2003). Advances in medicine have increased the average life span, resulting in an aging population. Because AD and most neurodegenerative diseases are diseases of aging, the prevalence is expected to continue to increase in the future. The disease is expected to affect 1 in 85 people in the world by 2050 (Brookmeyer et al., 2007).
AD is a devastating disease with the most identifiable symptom being dementia. The mean life expectancy is 7 years and fewer than 3% survive 14 years after diagnoses (Molsa et al., 1986, 1995). AD is mostly thought to be a disease in aging, with most diagnosis occurring in those aged 65 years and older (Brookmeyer et al., 1998). However, early-onset cases do occur and typically arise from genetic causes.
AD is characterized pathologically by the presence of intracellular neurofibrillary tangles containing the protein tau in a hyperphosphorylated state and extracellular plaques containing amyloid beta (Aβ) (Tiraboschi et al., 2004). Gross brain atrophy in end-stage cases is overtly obvious in the temporal lobe, parietal lobe, cingulate gyrus, and portions of the frontal cortex (Wenk, 2003). The relative contributions of plaques and tangles to the AD phenotype remain an intensely debated topic. Much mechanistic work has illuminated mechanisms of protein accumulation and aggregation in the formation of plaques and tangles. However, the ultimate cause of most cases remains unknown, with roughly 0.1% arising from autosomal dominant factors (Blennow et al., 2006). These cases are typically early onset and linked to mutations in Aβ metabolism. However, genetic risk factors may be a major influence on the etiology. The best known risk factor is the inheritance of the ϵ4 allele of apolipoprotein, an important protein in CNS lipid homeostasis, with 40–80% of patients expressing at least one allele of apolipoprotein E4 (Mahley et al., 2006; Strittmatter et al., 1993). Although geneticists believe that many genetic risk factors may have a role in AD etiology, it should be noted that over 400 genes have been tested specifically for a contribution to AD and the results have typically been null (Blennow et al., 2006; Waring and Rosenberg, 2008).
The identification of environmental risk factors for AD has been even more difficult than the elucidation of genetic risks. Much work has focused on exposure to metals; most notably, aluminum (Crapper et al., 1973) was linked to AD decades ago, with the metal being reported in plaques (Candy et al., 1986; Yumoto et al., 2009) and tangles (Perl and Brody, 1980), although more recent pathological, mechanistic, and epidemiological data have not always supported such a link (Frisardi et al., 2010; Landsberg et al., 1992). Although the current data have not proven aluminum exposure to be a causative factor, much ongoing research is focusing on metal exposures and AD. A key gap in our understanding of the role of aluminum in the pathological features of AD remains whether aluminum participates in the pathogenesis, or if plaques and tangles simply accumulate the metal due to increased affinity. Interestingly, serum aluminum is elevated two- to threefold in patients with dementia, including those diagnosed with AD, but not as high as in patients treated with aluminum-containing drugs, such as those with renal failure (Roberts et al., 1998). Small studies comparing aluminum consumption in cases and controls have found increases in odds ratios (about twofold) in those with a consumption history of high aluminum-containing foods (Rogers and Simon, 1999). Although the risk associated with consumption of aluminum seems to be higher from drinking water versus dietary consumption, interpreting such results is very difficult due to other metal ions contained in drinking water (Frisardi et al., 2010). Overall dietary balance may also influence aluminum accumulation, where an acid-forming diet, such as one high in dietary fat or total energy, can lead to increased serum and brain concentrations of aluminum and other metal ions (Grant et al., 2002). However, large, rigorously controlled epidemiological studies have yet to identify high aluminum exposure/consumption as a causative factor in AD (Forster et al., 1995; Gillette-Guyonnet et al., 2005; McLachlan et al., 1996). A recent systemic review of epidemiological reports on aluminum and AD found that 68% established a link, 23.5% were inconclusive, and 8.5% did not establish a relationship (Ferreira et al., 2008).
Aluminum exposure in rodents has recapitulated some of the key features of AD. However, the results have typically fallen short of reproducing the classic pathological features of the disease. For example, aluminum was found to produce tau aggregation in vitro, but not in vivo (Mizoroki et al., 2007). However, progressive cortical Aβ deposition after 12 months of aluminum exposure has been reported in the mouse, where accumulation in aged controls is not detected (Rodella et al., 2008). Interestingly, aluminum exposure in transgenic mouse models of AD has been found to potentiate Aβ deposition (Praticò et al., 2002b). In aged rats, aluminum administration altered levels of copper, zinc, and manganese in certain brain regions and resulted in an enlargement of hippocampal mossy fibers (Fattoretti et al., 2004). Rabbits have proven to be especially sensitive to aluminum exposure, with intracerebral and intravenous infusions reproducing some of the pathological features consistent with AD (Savory et al., 2006). However, oral administration has proven less successful in reproducing AD pathology.
The mechanism by which aluminum may participate in the pathogenesis of AD remains to be determined. Aluminum at concentrations found in human brains of some patients who had died of AD inhibits rat brain glycolysis. Inhibition of brain carbohydrate utilization is one potential mechanism by which aluminum may act as a neurotoxicant (Lai and Blass, 1984). Metal ions may play an important role in protein conformation. Aluminum, in particular, may affect Aβ aggregation, oligomerization, and toxicity (Bharathi et al., 2008). Aluminum may alter normal processing of Aβ precursor protein (Drago et al., 2008; Garruto and Brown, 1994).
There have also been several studies linking zinc to AD. Although aluminum is a nonessential element, zinc is an essential trace element required for normal human biological functioning (Cuajungco and Lees, 1997; Frisardi et al., 2010). Again, the role is controversial, with reports of zinc depletions in human brain regions affected by AD contradicting laboratory-based science showing that zinc could induce Aβ aggregation (Bush et al., 1994; Cuajungco and Lees, 1997). Some reports have indeed found increases in human brain of AD patients (Religa et al., 2006). Zinc overload also exacerbates Aβ deposition in transgenic mouse models (Wang et al., 2010).
Other metals, including copper, have been implicated. Alterations in brains of AD patients and in transgenic mouse models have been reported (White et al., 1999). Copper also has been found to bind to Aβ (Hou and Zagorski, 2006). Thus, aggregated protein in AD seems to bind and accumulate a diverse group of metals. However, copper and other metals have yet to be directly implicated in the pathogenesis of AD.
Developmental lead exposure has also been implicated, illustrating the now well-known phenomenon that environmental exposures during development may influence late-life neurodegeneration. In rodents, developmental lead exposure has been found to predetermine regulation of amyloid precursor protein and the accumulation of β-amyloid (Basha et al., 2005). In aged monkeys (23 years old) exposed to lead at infancy, elevations of AD-related genes were found. Importantly, AD-like pathology, including β-amyloid plaques in the frontal association cortex, was found (Wu et al., 2008).
Alterations in brain and peripheral metal concentrations remain a major topic of interest in AD research. Given how tightly the brain regulates essential metal ion concentrations, it is unlikely that dietary consumption differences alone play a major role in the pathogenesis of AD. Results from large epidemiological studies support such a conclusion. However, disruptions in metal transport or regulation could result in significant shifts in brain metal ion concentrations, which could lead to neurological dysfunction and potentially AD-relevant neurodegeneration.
Parkinson’s Disease
The second most common neurodegenerative disease is PD, estimated to affect nearly 5 million people worldwide (Dorsey et al., 2007). The primary motor phenotype of the disease consists of bradykinesia, resting tremor, and postural instability. Nonmotor symptoms are widespread and as treating dopaminergic symptoms has improved, are now in many cases becoming the chief patient complaints. The hallmark pathology of PD remains the loss of dopamine neurons in the substantia nigra and the occurrence of cytoplasmic inclusions known as Lewy bodies in surviving neurons (Braak et al., 2004; Forno, 1996; Spillantini et al., 1997). However, PD is now known to affect multiple brainstem nuclei, other brain regions, and to involve systemic pathology (Braak et al., 2004).
Roughly 10% of total cases are thought to stem from purely inherited genetic factors. However, as with AD, the majority of cases arise from unknown causes. PD, much more so than AD, has a long history of links to environmental exposures. The MPTP story showed that exposure of a drug analog able to cross the BBB and be metabolized into a toxicant that could enter and destroy dopaminergic neurons could reproduce the major behavioral and pathological features of PD (Javitch et al., 1985; Langston et al., 1983). This group of findings created a significant research emphasis on how environmental agents with similar properties/mechanisms may be responsible for a much greater number of PD cases. Given that MPTP enters dopamine neurons through the dopamine transporter and mutations in this transporter have been found to interact with pesticide exposure, increasing the risk for developing PD (Ritz et al., 2009), much current research is focused on environmental factors and gene–environment interactions.
Numerous chemical agents may induce a behavioral phenotype known as parkinsonism, which shares some of the behavioral features of PD, but often has differing mechanistic and pathological correlates. For example, compounds including industrial gases, organophosphate insecticides, and certain pharmaceuticals have been shown to elicit such a phenotype (Barbosa et al., 1992; Liou et al., 1997; Müller-Vahl et al., 1999). These findings are often presented in the form of case reports with limited pathological data, where much further validation would be required to establish relevance to PD. Behavioral features may arise from extrapyramidal dysfunction that does not share the pathological features of PD and additional neurological features that are not characteristic of PD also may be present. Environmental exposures that significantly alter dopaminergic neurotransmission in the substantia nigra and its projections could potentially result in movement abnormalities that share some features of PD. The majority of these exposures may actually bear limited relevance to the etiology of PD, and the establishment of specificity to PD pathogenesis requires a good deal of laboratory, clinical, and pathological investigation. For example, solvents such as methanol, ethanol, and industrial and home cleaners may induce parkinsonism in humans. These exposures may occur after large bolus doses or chronic exposures. Variable responses to L-3,4-dihydroxyphenylalanine (L-DOPA) and significant motor improvements after solvent exposure cessation question specificity and relevance to PD (Carlen et al., 1981; Davis and Adair, 1999; Hageman et al., 1999; Kuriwaka et al., 2002; Ley and Gali, 1983; McLean et al., 1980; Mozaz et al., 1991; Uitti et al., 1994). It should be noted that occupations involving hydrocarbon exposure have also been found to be a risk factor for earlier onset and increased severity of PD symptoms (Jaques et al., 2001). Thus, in addition to eliciting parkinsonism at high doses, chronic solvent exposures may influence the pathogenesis of PD.
The task of identifying a causative environmental agent that is responsible for a significant number of cases has proven to be enormously difficult. Unfortunately, such an agent has yet to be identified. Given the amount of research that has been conducted, the general conclusion that can be drawn is that outside of a few rare cases, it is highly unlikely that exposure to a single agent accounts for a significant number of cases. Much more likely, multiple hits over time from numerous compounds, in association with a background of genetic risk factors, are responsible for most cases.
Epidemiological studies have identified a number of diverse classes of risks and compounds that may have a role in the pathogenesis of human PD. For example, rural living, well water consumption, diet, and exposure to metals, solvents, and pesticides have been repeatedly implicated as risk factors for the development of PD (Di Monte, 2001; Fall et al., 1999; Gatto et al., 2009; Gorell et al., 1997, 1998; Hageman et al., 1999; Kuhn et al., 1998; Kuopio et al., 1999; Liou et al., 1997; Logroscino et al., 1996; Pezzoli et al., 1996; Semchuk et al., 1993; Smargiassi et al., 1998). Thus, the potential risk factors for PD include highly diverse classes of compounds.
Pesticides, herbicides, and fungicides have received the most attention as risk factors for PD. The identification of rural living, well water consumption, and exposure to agricultural chemicals have highlighted the risks of exposure to such compounds (Gatto et al., 2009; Gorell et al., 1998; Smargiassi et al., 1998). Recent work examining a large population and tracking specific pesticide use identified two pesticides that result in elevated risk for PD. Here, paraquat and rotenone exposure were identified as significant risk factors (Tanner et al., 2011). This recent report using advanced epidemiological techniques supports many previous epidemiological studies that have suggested a link (Dhillon et al., 2008; Hertzman et al., 1990; Liou et al., 1997) and laboratory-based research showing that exposure to these compounds elicits PD-like pathology in animals.
Paraquat is a bipyridal broad-spectrum herbicide used widely in developing countries. Although use in the United States is highly restricted, it remains one of the most commonly used herbicides worldwide (Wesseling et al., 2001). Paraquat was initially postulated to be a putative neurotoxicant because of its structural similarity to the active metabolite of MPTP, MPP+. Given its use as a pesticide, the possibility that this compound could be an environmental factor in the etiology of PD has received a great deal of attention.
The mechanism by which paraquat produces nigrostriatal damage in vivo appears to be somewhat different from MPTP. Paraquat is thought to enter the brain through a neutral amino acid carrier (McCormack and Di Monte, 2003, Fig. 1). However, it has been directly demonstrated that paraquat does not act by direct inhibition of complex I (Richardson et al., 2005). Paraquat has long been known to generate reactive oxygen species (ROS) by redox cycling and it is very likely that such a mechanism is responsible for effects on dopaminergic neurons (Fisher et al., 1973; Ilett et al., 1974).
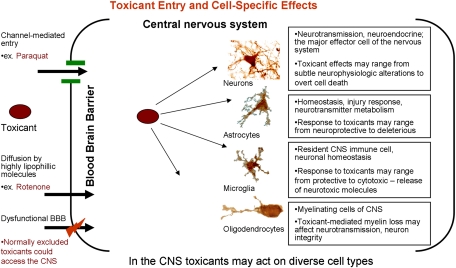
FIG. 1.
Toxicant entry into the CNS and interactions with diverse cell types. To gain access to the CNS, a toxicant may enter through a specific transporter if it has structural similarities to endogenous molecules that are selectively transported across the BBB. Highly lipophilic molecules may pass directly through biological membranes gaining access to the CNS. A damaged or dysfunctional BBB could potentially allow toxicants that would normally be excluded to enter the CNS. Once in the CNS, toxicants may interact and influence the physiology of a variety of very different cell types, including neurons, astrocytes, microglia, and oligodendrocytes. Toxicant action on each of these cell types may adversely affect neurological function.
Systemic paraquat injection in mice elicits dose-dependent (5–10 mg/kg) decreases in movement and dopamine cell counts in the substantia nigra (Brooks et al., 1999). Additionally, repeated paraquat administration produces selective loss of nigral dopamine neurons (McCormack et al., 2002). A model of PD combining paraquat and the fungicide maneb has also been developed (Thiruchelvam et al., 2000). This model was developed based on overlapping geographical use of the two compounds and its relevance is supported by epidemiological studies that suggest an interaction (Dhillon et al., 2008).
Rotenone has been used extensively as an insecticide and as a piscicide to kill fish. It is a naturally occurring compound that is found in the roots and leaves of several plant species. Rotenone is a well-known, high-affinity, selective inhibitor of mitochondrial complex I that has been used in biological experiments for decades (Ravanel et al., 1984). Interestingly, it has also been known for decades that systemic mitochondrial complex I inhibition occurs in PD patients (Parker et al., 1989; Schapira et al., 1989). The hypothesis that specific populations of neurons, and in particular nigral dopamine neurons (those lost in PD), are sensitive to systemic complex I inhibition lead to testing rotenone in animals.
Rotenone is a highly lipophilic compound that is easily able to cross the BBB rapidly (Talpade et al., 2000, Fig. 1). Early in vivo experiments that administered rotenone directly into the parenchyma at >500,000 times the IC50, or systemically at 10–18 mg/kg/day, did not produce selective pathology consistent with PD (Ferrante et al., 1997; Heikkila et al., 1985; Rojas et al., 2009). However, chronic administration at much lower doses that achieved complex I inhibition, similar to that observed in platelets of PD patients, produced highly selective nigrostriatal degeneration in rats (Betarbet et al., 2000). Key pathological markers, including cytoplasmic alpha-synuclein-positive inclusions similar to Lewy bodies, were observed in surviving dopamine neurons. Thus, the rotenone model provided the first proof of concept that systemic mitochondrial impairment could produce selective loss of nigral dopamine neurons. These findings support the hypothesis that nigral dopamine neurons have a unique sensitivity to complex I inhibition. Thus, rotenone and other environmental toxins with similar mechanisms may indeed be significant risk factors for PD.
Numerous other classes of environmental agents have been linked to PD, including organochlorines, organophosphates, and carbamates. However, currently, there is limited epidemiological evidence and chronic animal-based, laboratory-based research is mostly lacking.
Industrial exposures, particularly to solvents, are receiving much attention. Trichloroethylene (TCE) is a highly volatile organic chemical that has been used for many years as an industrial solvent, a dry cleaning agent, a grain fumigant, a caffeine extractant, and an anesthetic (Jollow et al., 2009). It is a major environmental contaminant in industrialized countries and is one of the most commonly identified groundwater contaminants at 1700 National Priorities List hazardous waste sites surveyed in the United States (ATSDR, 1997; Fay and Mumtaz, 1996; NRC, 2006).
The neurological effects of TCE have long been known, although the pathological correlates have been somewhat controversial in animals and humans (Schaumburg, 2000). More recent data have shown that inhalation of TCE in the rat after 60 days was found to elicit locomotor impairments (Waseem et al., 2001). Additionally, two recent reports have identified links between long-term TCE exposure and PD in humans and described the creation of a TCE animal model of PD. Factory workers with high-level exposure, working adjacent to the TCE source, were found to have PD. In this study, PD symptom severity was correlated with exposure level/duration (Gash et al., 2008). In rats, chronic administration caused specific loss of nigral dopamine neurons and alterations in dopamine levels (Gash et al., 2008; Liu et al., 2010). TCE crosses the BBB and induces oxidative stress (Khan et al., 2009; Liu et al., 2008a,b). The proposed mechanism is inhibition of mitochondrial complex I (Gash et al., 2008), which fits well with both human and animal data from other models, such as the rotenone model. However, more confirmatory data are needed to prove definitively that the mechanism of action is through complex I inhibition. Thus, there is now a preliminary link of prolonged TCE exposures to motor impairment and multiple reports of an animal model. Epidemiological studies have not yet identified TCE as a risk factor and more labs will need to replicate the animal studies.
Metals have long been thought to play a role in PD. In particular, iron accumulates in the substantia nigra of PD patients (Gerlach et al., 2006). Specifically, increased iron has been observed within nigral neurons of PD patients (Oakley et al., 2007). Thus, nigral iron accumulation is unlikely to be due solely to the contribution from proliferating and reactive glia as has occasionally been hypothesized. It remains unclear whether iron accumulation occurs in the substantia nigra as a result of PD or whether iron accumulation directly contributes to the pathogenesis of PD (Berg and Hochstrasser, 2006; Kaur and Andersen, 2004). However, a recent report utilizing data from both animal models and postmortem human brain tissue from PD patients suggests that iron is directly involved in the pathogenesis of PD. Here, a novel iron transport system specific to dopamine neurons was found to be disrupted in both human PD and rotenone-treated rats (Mastroberardino et al., 2009).
Iron, an essential metal that is obtained through the diet, has many important biological functions, most notably as a required component for oxygen to bind to heme. Given that iron accumulates in PD, high-iron diets have been explored as a potential risk factor. Some retrospective studies have reported an association between dietary iron and PD. However, a much more powerful prospective study of 47,406 men and 76,947 women failed to establish a link (Logroscino et al., 2008). Given that iron uptake through the gut is highly regulated, increased dietary consumption is unlikely to significantly alter brain levels. More likely, aberrant iron accumulation in the brain may result from disorders of transport and uptake. For example, patients with homozygous mutations for the highly penetrant C282Y in the hemochromatosis gene HFE were more likely to develop PD than controls (Dekker et al., 2003). Additionally, polymorphisms in transferrin have also been found to increase risk for PD (Borie et al., 2002). Therefore, the role of iron in PD is unlikely due directly to environmental or dietary factors. Environmental compounds that result in neuronal iron accumulation, e.g., rotenone, may ultimately lead to neurodegeneration (Mastroberardino et al., 2009). Additionally, genetic alterations in iron uptake or homeostasis could dramatically influence response to environmental toxins, given the well-known role of iron in oxidative stress.
Manganese exposure can also produce a form of parkinsonism (also known as manganism), although the behavioral and pathological features are typically distinct from typical PD. Manganese is the 12th most abundant element in the earth’s crust and is an essential element for human biology (Chu et al., 2000). Elevated manganese exposures can occur in miners and welders, and during the chemical manufacture of maneb (Feldman, 1999; Santamaria and Sulsky, 2010). Excessive manganese exposure can produce striatal dopamine depletion and extrapyramidal dysfunction, including parkinsonism and chorea (Chu et al., 2000). There are many known variables that affect the neurological response to manganese exposure. The onset of symptoms depends on level of exposure, size of particles, and individual susceptibility (Mena, 1979).
Again, a common mechanistic theme in PD-related toxicants is mitochondrial dysfunction. Manganese has long been known to accumulate in mitochondria and impair oxidative metabolism (Maynard and Cotzias, 1955). Animal studies showed that manganese accumulates in the mitochondrial fraction of the striatum and causes dopamine depletion (Chu et al., 2000). However, there are several major differences in manganese-induced parkinsonism from typical PD. The nigrostriatal pathway is spared and the syndrome is typically unresponsive to L-DOPA (Chu et al., 2000; Olanow et al., 1996). Additionally, the neuropathological hallmark of manganism is degeneration of the basal ganglia, particularly the globus pallidus, and the absence of Lewy bodies (Perl and Olanow, 2007; Yamada et al., 1986). Thus, although manganese exposure can produce motor abnormalities that may share behavioral features with PD, a distinct behavioral and pathological syndrome separate from typical PD occurs following manganese intoxication.
Although the biochemical and pathological features of manganism and PD are clearly different, multiple recent studies have examined gene–environment interactions in manganese exposure regimens. The majority of these studies examine PD-relevant genes. Treatment of rats with the manganese-based fungicide maneb has not been found to alter levels of α-synuclein, a key pathological event in PD (Nielsen et al., 2006). One study found that mice expressing two PD-causing α-synuclein mutations exposed to an enriched manganese diet did not exhibit gene–environment interactions relevant to PD (Peneder et al., 2011). Although some in vitro reports have shown that overexpression of normal or mutated human α-synuclein increases manganese toxicity and that manganese exposure itself increases α-synuclein levels, there is not sufficient in vivo evidence to show that such an interaction is currently important to either human manganism or PD (Peneder et al., 2011; Pifl et al., 2004). Given that the neuropathological characterization of human manganism cases does find evidence of Lewy body pathology (Perl and Olanow, 2007), human data also suggest that such an interaction may not be important.
Several in vitro studies have also suggested that Parkin—an E3 ligase that is important in PD—may influence manganese neurotoxicity (Higashi et al., 2004; Roth et al., 2010; Wang et al., 2009). A recent study in rats exposed to welding fumes containing manganese and manganese injections showed alterations in several genes that are important in both sporadic PD and genetic forms, including Parkin (Park2), Uchl1 (Park5), and DJ-1 (Sriram et al., 2010). Although a histological evaluation was not performed in this study, the result compels further evaluation of PD-related genes after in vivo manganese treatment. The evaluation of manganese neurotoxicity in transgenic models related to these genes would be of great interest.
Although a single causative toxicant has yet to be identified that accounts for a significant number of cases, the wealth of research studies suggests that environmental exposures play a significant role in sporadic PD. As the power of epidemiological studies increases, specific toxicants that serve as risk factors for PD are being identified. Ultimately, reducing exposures to such toxicants will hopefully reduce the risk of developing PD.
Multiple Sclerosis
Multiple sclerosis (MS) is a neuroinflammatory disease where the fatty myelin sheaths around the axons of the brain and spinal cord are damaged, ultimately leading to demyelination, scarring, and axonal degeneration (Compston and Coles, 2008). The prevalence is highly variable based upon geographical, genetic, and ethnic variables. In the United States, more than 200,000 individuals are estimated to be affected (Noonan et al., 2002). The neurological symptoms are widespread and typically affect motor, sensory, visual, and autonomic function (Compston and Coles, 2008). These symptoms ultimately arise from subsequent difficulties with communication between neurons. The loss of myelin inhibits the neurons’ ability to propagate action potentials (Compston and Coles, 2002). Overt loss of lower motor neurons may also occur (Vogt et al., 2009). The cause of MS remains unknown, although genetic, specific infections causing neuroinflammation and environmental factors have been implicated. A likely autoimmune mechanism is supported by the presence of antibodies to myelin antigens in the cerebrospinal fluid (CSF) and serum (Weiner, 1998). The prognosis is highly variable and is dependent on the disease subtype and individual susceptibility, though MS patients typically have a life expectancy of 5–10 years less than average (Compston and Coles, 2008).
The most well described environmental influence on MS is geography. The global distribution can be generalized as increasing incidence as the distance from the equator, both north and south, increases (Kurtzke, 1975; 1993). However, within this gross generalization, there are several areas with disproportionally low and high incidence rates (Compston and Coles, 2008). This has led to the hypothesis that low sunlight and vitamin D deficiency may play a role. Given the neuroinflammatory nature of MS, much work has also focused on the role of infections. MS patients have been reported to be infected with measles, mumps, rubella, and Epstein-Barr virus at later ages than matched controls (Martyn et al., 1993).
There are some data that toxicant exposure may have a role in MS. Roughly 20% of cases can be explained by purely genetic influences (Compston and Coles, 2008). Smoking, both active and passive (second hand), have been linked to increased MS risk (Hernan et al., 2005; Mikaeloff et al., 2007). However, there are some data showing that environmental exposures may influence MS, directly affecting oligodendrocytes. The majority of work has focused on solvent exposures, which can cause demyelination. For example, an elevated risk for MS in shoe and leather workers from Florence, who were likely exposed to organic solvents, was found (Amaducci et al., 1982). Case reports have implicated solvent exposures, e.g., chronic methanol ingestion can also produce clinical symptoms and histological features very similar to MS (Henzi, 1984). However, well-controlled epidemiological studies have produced conflicting results on a link between solvent exposures and MS (Marrie, 2004; Mortensen et al., 1998; Riise et al., 2002). Unfortunately, many of these studies are confounded by the use of prevalent cases and self-reported exposure assessments, potentially suffering from survivorship and recall bias (Marrie, 2004). A meta-analysis of case-controls produced a pooled relative risk estimate of 1.7 (95% confidence interval 1.1–2.4) for solvent exposures and MS risk (Landtblom et al., 1996).
Diverse environmental exposures such as fungal toxins have also been proposed as putative MS risk factors, although compelling support for such hypothesis remains lacking (Purzycki and Shain, 2010). From the available data, it is clear that much further work will be required to identify plausible risk factors, especially given the complexities of abnormal neuroimmune function that is present in MS.
Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is a specific type of motor neuron disease characterized mainly by the degeneration of lower motor neurons in the brainstem and ventral horn of the spinal cord and degeneration of the afferent upper motor neurons in the cortex (Bento-Abreu et al., 2010). Although the incidence of ALS is only 1–2 per 100,000, the lifetime risk may approach 1/400 to 1/700 (Johnston et al., 2006). The main symptoms are muscle atrophy and weakness, fasciculations, and spasticity (Rowland and Shneider, 2001). The disease is progressive and fatal, with a 3- to 5-year survival time in most patients, most often due to bulbar dysfunction and respiratory dysfunction (Bento-Abreu et al., 2010). Roughly 10% of cases are familial, with about 2% of total cases being accounted for by mutations in superoxide dismutase 1 (SOD1) (Rosen et al., 1994).
The role of environmental exposures in ALS is poorly understood. Although many studies have been published on links between specific exposures and ALS, few have been confirmed (Bento-Abreu et al., 2010). Similar to other prominent neurodegenerative diseases, inherited cases occur, but account for a small percent of total cases. Given that the most common mutation affects the protein SOD1, which is involved in the reduction of superoxide free radicals, it is plausible that environmental agents eliciting oxidative stress may have a role in ALS. However, the connection between SOD1 function and ALS is much more complicated than an increase in oxidative stress. Missense mutations in almost every amino acid residue of the protein can cause ALS, irrespective of their effect on dismutase activity (Borchelt et al., 1994; Robberecht et al., 1994; Rosen et al., 1994). Unfortunately, attempts to identify specific classes of compounds important to this disease have been largely unsuccessful.
Exposure to lead, mercury, and pesticides have all been cited as potential risk factors for ALS (Johnson and Atchison, 2009). Accumulating data suggest that environmental exposures may influence the development of the ALS phenotype, suggesting that specific populations of neurons may be more sensitive to specific toxins. However, specific exposures have not yet been linked to precipitating ALS. Thus, the strength of the data is currently much less than the strength of PD data. Indeed, elevated blood lead levels are associated with and increase risk for ALS (Fang et al., 2010). However, the association with heavy metal and pesticide exposures to ALS risk highlights the selective sensitivity of this group of neurons to environmental exposures. Although other neuronal populations are undoubtedly affected, neurons with very long axons and high energy requirements are prone to neurodegeneration.
Gulf War veterans may have an increased risk for developing ALS (Horner et al., 2008; Miranda et al., 2008). However, an elevation in risk remains controversial. Excluding a specific form of neurodegeneration in Guam (discussed below), environmental influences in ALS are very poorly understood.
Guam ALS-Parkinsonism-Dementia
A high incidence of neurological disorders characterized by a combination of ALS plus parkinsonism and dementia, known as ALS-Parkinson’s dementia complex (ALS-PDC) in indigenous Chamorro people of Guam and other Mariana Islands, has been known since a National Institutes of Health survey in the early 1950's (Karamyan and Speth, 2008). There is a long and rich literature focused on the role of cycad seed consumption and its relevance to this neurological disease. There was much difficulty creating an animal model of ALS-PDC and the cycad hypothesis was repeatedly abandoned. However, in the late 1980's, it was demonstrated that an amino acid component of the cycad seed, β-methylamino-L-alanine (BMAA), was neurotoxic in vitro and in vivo in both mice and nonhuman primates, reproducing several of the key features of the disease (Nunn et al., 1987; Ross and Spencer, 1987; Spencer et al., 1986; Spencer et al., 1987). Human doses extrapolated from animal studies represented daily consumption of cycad flour equivalent to the entire body weight (Karamyan and Speth, 2008). Thus, the relationship was intensely criticized. Work that is more recent has identified other sources of BMAA, most notably bioaccumulation in animals, such as flying foxes that consume cycad seeds, and are eaten in significant quantities by the indigenous population (Karamyan and Speth, 2008). The effects of BMAA in animals are highly variable and are dependent on dose, route, time course, and species. Nonetheless, many of the key features of ALS-PDC have been reproduced in certain animals (Karamyan and Speth, 2008). Interestingly, a recent report found cycad seed treatments in the rat to induce a predominantly PD phenotype (Shen et al., 2010). Thus, a naturally occurring amino acid able to gain access to the CNS may serve as a neurodegenerative agent if consumed in high enough quantities.
Other Neurodegenerative Diseases
Additional neurodegenerative diseases without a known purely genetic etiology exist. Notable examples with a potential environmental component to the etiology include multiple system atrophy (MSA) and progressive supranuclear palsy. Each of these diseases is considerably rarer than AD and PD, and each receives much less effort and research dollars, although the symptoms are often devastating. Thus, unfortunately, the etiology of these diseases is very poorly understood. In these diseases, environmental links are suspected, but there is currently a lack of credible evidence. Data on MSA serve as an example. Here, transgenic animals designed to reproduce the key pathological features of the disease—an oligodendroglial synucleinopathy—suggest that further stressors, potentially environmental, may be required for development of MSA (Wenning et al., 2008). Specific environmental links remain very weak, with an older study finding an association with metal dusts and fumes, plastic monomers and additives, organic solvents, and pesticides when compared with controls (Brown et al., 2005; Nee et al., 1991). Because these diseases share features with more prominent neurodegenerative diseases, it is hoped that an increased understanding of the etiopathogenesis of more prominent diseases will provide an increase in the broad understanding of the role of neurotoxicant influences on neurodegeneration.
SENSITIVITY OF THE NERVOUS SYSTEM TO TOXICANT EXPOSURE
The signs and symptoms resulting from a neurotoxicant exposure are highly variable and depend on factors such as the specific area region of the nervous system affects. Manifestations of neurotoxicant exposure may include cognitive effects, movement abnormalities, mental effects, neuroendocrine alterations, and sensory deficits. The mammalian nervous system is highly unique in terms of the diversity in the functions and the demands of the system. These features render the system especially sensitive to the effects of numerous toxicants.
Anatomical Limitations
The CNS is anatomically limited by the skull and spinal cord. In other organ systems of the body, changes in organ size may be tolerated to an extent. However, particularly in the brain, such changes can be highly detrimental or fatal. Increases in intracranial pressure can arise from a variety of causes. Well-known causes include tumors, hematomas, edema, encephalitis, and obstructions of CSF flow. Changes in pressure can dramatically affect neuronal function and potentially be fatal. Toxins produced by viruses (producing viral meningitis), bacteria (producing bacterial meningitis), or endogenous toxins (elevated in liver failure) (Frontera and Kalb, 2011; Koedel et al., 2002; Kumar et al., 2009) can all result in life-threatening changes in intracranial pressure.
Energy Requirements
The task of receiving, processing, and transmitting signaling information requires an enormous amount of energy. In fact, many neuronal populations must transmit signals, conduct antero- and retrograde transport, and maintain cellular homeostasis at distances of >1 m due to long axon bundles. The brain, while only accounting for 2% of the body weight, receives 15% of the cardiac output, and consumes 20% of total body oxygen and 25% of total body glucose (Clark and Sokoloff, 1999). In addition to these requirements, the nervous system has little energy reserve capacity, having extremely low anaerobic activity. Thus, toxicants disrupting energy production have devastating consequences to the nervous system. The classic example is cyanide, a highly toxic natural compound produced by bacteria, fungi, algae, and certain plants. Cyanide is a well-known inhibitor of cytochrome oxidase (mitochondrial complex IV). The primary target organ is the CNS, with neurological effects appearing within seconds of administration (Dawson et al., 1995; Way, 1984). The high toxicity and neurological effects resulting from exposure to toxicants that inhibit cellular respiration illustrates the limited ability and selective sensitivity of neurons to energy depletion.
Chemical Transmission across the Extracellular Space
Much of the communication between cells in the nervous system occurs by the release of chemical signals across the extracellular space—neurotransmission. The release, reuptake, and metabolism of neurotransmitters are highly regulated. Perturbations to neurotransmitter systems may affect neuronal function and contribute to neurodegeneration. Furthermore, neurotransmitters themselves may be highly reactive and are thought to participate in the pathogenesis of neurodegenerative diseases, especially dopamine (Fig. 1). Toxicants may also utilize transporters to gain access to neurons, where there are significant structural similarities to the native neurotransmitter; for example, MPP+ entry through the dopamine transporter (Javitch et al., 1985).
Limited Ability to Replace Lost Cells
With a few notable exceptions, neurons in the human CNS lost after developments are not replaced. In the human brain, there is significant evidence for neurogenesis in the subventricular zone (migration to the olfactory bulb) and subgranular zone (migration to the dentate gyrus of the hippocampus) (Eriksson et al., 1998; Whitman and Greer, 2009). Neurogenesis in other mammalian brain regions remains controversial (Gould, 2007). Thus, the dogma proposed by Cajal, who stated, “Once the development was ended, the founts of growth and regeneration of the axons and dendrites dried up irrevocably. In the adult centers, the nerve paths are something fixed, ended, and immutable. Everything may die, nothing may be regenerated” remains relevant to most neuronal populations (Cajal, 1913–1914; Whitman and Greer, 2009). Functional compensation for the loss of a small number of neurons can typically be achieved by redundancy in the system of adaptive plasticity. However, there is a certain magnitude of loss that precipitates functional consequences. Thus, the ability of the brain to compensate for cell loss is much lower than other organ systems, rendering it especially sensitive to toxicant exposure, with the potential for permanent consequences.
Maintenance of a Lipid-Rich Environment
A high lipid content is of major importance to myelination and neurotransmission of the CNS. Myelination is carried out by specialized cells (oligodendrocytes in the CNS and Schwann cells in the peripheral nervous system). Damage to lipids is a common feature of neurodegenerative diseases. Indeed, lipid peroxidation is a major pathological feature of PD, AD, ALS, and other prominent neurodegenerative diseases (Reed, 2011). Thus, neurotoxicants that directly or indirectly produce lipid peroxidation in the nervous system could alter neuronal function and be a risk factor for neurodegeneration. For example, brain pathways that are poorly myelinated are especially sensitive to PD and environmentally relevant mitochondrial complex I inhibitors, such as rotenone, that induce oxidative stress (Betarbet et al., 2000; Braak et al., 2004, Fig. 2A).
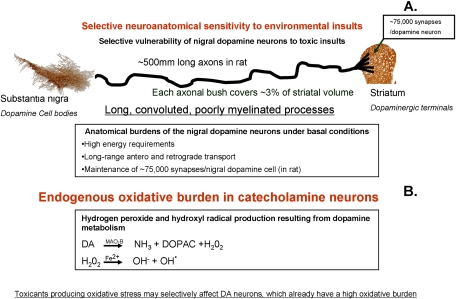
FIG. 2.
Selective sensitivity of the nigrostriatal dopamine system to toxicant insults. (A) Neuronal morphology and physiology vary dramatically based on the anatomical region and neurotransmitter system. Specific populations of neurons may exhibit selective or heightened sensitivity to environmental insults based upon their anatomical features. As an example, the nigrostriatal dopamine system is selectively sensitive to several environmental toxicants. Long, poorly myelinated processes (average ∼500 mm in rat—due to convolution) terminating in ∼75,000 synapses result in a system with enormous energy and transport (antero- and retrograde) requirements (Braak et al., 2004; Matsuda et al., 2009). Such a system may be particularly sensitive to specific toxic insults. (B) Specific neurotransmitter systems may produce endogenous oxidative stress. These cell populations have an inherent oxidative burden and may be especially sensitive to additional oxidative insults elicited by exposure to environmental toxicants. Dopamine metabolism mediated by monoamine oxidase-B (MAO-B) produces hydrogen peroxide. The Fenton reaction occurring in the presence of Fe2+ may then result in the production of the highly reactive hydroxyl radical. This population is especially sensitive to additional oxidative stress insults.
Cellular Diversity
Many organ systems have multiple cell types. However, the diversity of cell types and function in the nervous system is unmatched. The CNS consists of neurons, astrocytes, oligodendrocytes, and microglia, with endothelial cells lining blood vessels. In the peripheral nervous system, Schwann cells are present instead of oligodendrocytes. Toxicants may elicit neurodegeneration by directly affecting neuronal function or eliciting neuronal cell death. Additionally, neurons may be affected indirectly by other cells present in the nervous system. In the brain, extraneuronal cells as a group are referred to glia. In the past few decades, it has become clear that these cells have much more important roles than simply supporting neuronal homeostasis and function. With respect to neurotoxicant exposures, glia may play important roles in toxicant metabolism and their response to toxicant exposure may range from neuroprotective to the initiation of a harmful response to neurons.
Neurons.
Neuronal diversity both morphologically and biochemically has a major role in sensitivity to specific toxic insults. Dopaminergic neurons of the substantia nigra are particularly vulnerable to oxidative stress. This population of neurons under basal conditions is burdened by high energy requirements, maintaining signaling to long, poorly myelinated processes (Fig. 2A). Projections exhibit extensive arborization, with a recent study finding total axon lengths of individual rat dopamine neurons approaching 1 m (average 500 μm) (Matsuda et al., 2009). Additionally, this study found that a single nigral dopamine neuron is estimated to innervate ∼75,000 striatal interneurons. Dopamine metabolism itself produces oxidative stress (Hastings and Zigmond, 1994, Fig. 2B). Thus, this system is extremely sensitive to additional insults that elicit oxidative stress. In addition to anatomical and metabolic susceptibilities, nigrostriatal dopamine neurons have specific signaling properties that may contribute to their selective susceptibility to neurodegeneration. The ventral tegmental area (VTA) lies directly medial to the substantia nigra and contains dopaminergic neurons. However, in PD and in neurotoxicant-based PD models, VTA dopaminergic neurons are relatively spared. Much recent mechanist work suggests that reliance on L-type Ca(v)1.3 Ca2+ channels contributes to selective sensitivity to neurodegeneration (Chan et al., 2007). Thus, nigral dopamine neurons exemplify how anatomical, metabolic, and physiological features likely are major factors in selective sensitivity to neurotoxicant exposure and neurodegeneration.
An additional example of selective neuronal sensitivity occurs in motor neurons. ALS is characterized by the degeneration of motor neurons, which also have long axons and high energy requirements. Specifically, in ALS, the lower motor neurons in the brainstem and ventral horn of the spinal cord and afferent upper motor neurons in the cortex undergo neurodegeneration (Bento-Abreu et al. 2010).
Astrocytes.
Astrocytes are present in both the brain and the spinal cord and have a typical star-shaped morphology. Differences in morphology and physiology between astrocytes throughout the nervous system, based upon anatomical location, are becoming much more appreciated. Astrocytes were long thought to serve roles as structural and homeostatic support to neurons. However, extensive research in the past several decades has shown that their functions are much more complex, with important roles in neurotransmitter uptake and release, regulation of ion concentrations in the extracellular space, modulation of synaptic transmission, vasomodulation, myelination promotion of oligodendrocytes, nervous system repair, and long-term potentiation (Moore et al., 2011; Pascual et al., 2005; Santello and Volterra, 2009; Simard and Nedergaard, 2004; Suzuki et al., 2011). Many of these functions have been investigated for a role in neurodegeneration, and there is a wealth of published studies showing that astrocytes participate in the process, with effects ranging from neuroprotective to potentiation of neurodegeneration. More specifically, astrocytes also likely have a major influence on neurotoxicant-induced neurodegeneration. Neurotoxicant administration in the CNS typically invokes some level of astrocytic response, such as migration to the site of injury and proliferation (Ogawa et al., 1989). A classic example of a role for astrocytes in neurotoxicant-induced neurodegeneration is from the MPTP literature, where astrocytes were found to metabolize the protoxin MPTP to the toxicant MPP+, which ultimately leads to degeneration of dopaminergic neurons (Javitch et al., 1985). Additionally, reactive astrocytes are thought to play a pathogenic role in ALS as they are observed surrounding remaining motor neurons (Kamo et al., 1987; Kushner et al., 1991). In vitro experiments replicate this, where brief exposure of rat astrocyte monolayers to peroxynitrite results in a persistent reactive morphology and promotion of apoptosis in motor neurons plated on top of the astrocyte monolayer (Cassina et al., 2002).
Astrocytes may also provide protection in PD, as evidenced by the fact that mutations in DJ-1, a gene thought to be involved in oxidative stress, causes early-onset PD in humans (Bonifati et al., 2003). Knockdown of astrocytic DJ-1 in vitro has been found to decrease the astrocytes ability to protect against neuron loss elicited by dopaminergic neurotoxicants (Mullett and Hinkle, 2009). Mice lacking DJ-1 exhibit increased sensitivity to MPTP (Kim et al., 2005).
Trimethyltin-induced neurodegeneration is well characterized in the brain, where the loss of neurons is preceded by a neuroinflammatory response. In vitro mechanistic studies have revealed that the neuroinflammatory response, and the subsequent production of oxidative stress, is mediated by astrocytes (Rohl and Sievers, 2005).
Much data also show that astrocytes play an important role in neurotoxicity of metal exposures. Astrocytes have long been known to be of major importance in the neurotoxic response in lead neurotoxicity, both as an accumulator (lead sink) and as a direct target for cellular toxicity (Tiffany-Castiglioni, 1993; Tiffany-Castiglioni et al., 1986, 1987). One major mechanism by which astrocytes likely contribute to the neurotoxicity of lead is through glutamatergic toxicity. Lead-induced alterations in the ability of astrocytes to regulate glutamate levels are thought to be a major mechanism leading to excitotoxicity (Struzynska, 2009). Manganese is also known to affect astrocytes. Again, astrocytes serve as an accumulation site for the metal (Aschner et al., 1999). Nasal exposure—a major route of exposure in humans—was not found to cause overt neuronal damage in rats, but was found to elevate astrocyte proteins indicative of increased astrocyte reactivity (Henriksson and Tjalve, 2000). Such changes could be detrimental to neuronal function. Manganese exposure can also affect the role of astrocytes in regulating the major inhibitory neurotransmitter, γ-aminobutyric acid (GABA). Manganese was recently found to inhibit the GABA transporter, thereby resulting in increased extracellular GABA (Fordahl et al., 2010). Thus, the range of responses to neurotoxicants by astrocytes in the CNS is highly diverse.
Microglia.
Microglia are the resident immune cells in the CNS. They originate in bone from hematopoietic stem cells, differentiating into monocytes. They then travel to the brain and further differentiate into specialized macrophages (Ritter et al., 2006). In the “resting” state, microglia typically have long thin processes and a small cell body. However, in response to insults to the nervous system, microglia undergo major morphological and physiological alterations. The branches typically retract and the processes thicken and the cell may begin to produce proinflammatory factors (Aloisi, 2001; Gehrmann et al., 1995; Ritter et al., 2006). Similar to macrophages, microglia may use cytotoxic or phagocytic functions to destroy or remove both foreign material and damaged cells. Microglial activation is a common component of many neurodegenerative diseases. In AD, activated microglia are found in numbers and in the activity states above that of age-matched controls. Some reports have linked microglial activation directly with Aβ plaques and neurofibrillary tangles, the pathological hallmark of the disease (DiPatre and Gelman, 1997; Kobayashi et al., 1998). Similarly, in PD, microglial activation clearly occurs (McGeer et al., 1987, 1988). Microglial activation also occurs in the major neurotoxicant animal models of PD, including MPTP, 6-hydroxydopamine (6-OHDA), and rotenone (Cicchetti et al., 2002; Czlonkowska et al., 1996; Depino et al., 2003; Sherer et al., 2003). In these studies, importantly, microglial activation occurred before neuronal cell loss was observed. Furthermore, in vivo mechanistic work using rotenone showed that neurons cultured alone are much less sensitive to this dopaminergic neurotoxin than when cultured in the presence of microglia (Gao et al., 2002). In this report, rotenone was found to elicit superoxide release that was attenuated by NADPH oxidase inhibitors. These data suggest that microglia play an active role in the pathogenesis of neurodegenerative diseases and can be activated by neurotoxicant exposures.
Myelinating cells.
Oligodendrocytes and Schwann cells are the myelinating cells of the CNS and PNS, respectively. Although these cells have now been found to have many important functions, it is their role in myelination that directly impacts neurons. Cell loss or dysfunction can negatively impact the ability of neurons to propagate axon potentials. Schwann cells typically myelinate up to 100 μm of an axon, with a single Schwann cell myelinating a portion of one neuronal axon (Kalat, 2008). Thus, for a 1-m axon of a motor neuron, 10,000 Schwann cells would be responsible for myelination. An individual oligodendrocyte, however, can extend its processes to up to 50 neurons (Kalat, 2008). In MS, oligodendrocyte dysfunction and cell loss occurs; myelin sheaths around axons in the brain and spinal cord are damaged leading to demyelination and scarring, ultimately inhibiting neuronal signaling (Brück et al., 1994). Although MS is often thought of as purely a demyelinating disease, mounting data suggest that there is a major overt loss of lower motor neurons (Vogt et al., 2009). Thus, loss of myelin has a very detrimental effect on neuronal function and may in turn result in neuronal cell death. This process is complex, involving the redistribution of sodium channels along the axon, at formerly myelinated sites, followed by the development of a persistent current that leads to ion imbalances, and subsequently calpain-mediated axon digestion, nitric oxide and free radical production, and mitochondrial dysfunction (Craner et al., 2004; Smith, 2007; Stys, 2005). It remains to be determined whether environmental toxicants can elicit neurodegeneration by acting directly on oligodendrocytes and elicit downstream neurodegeneration.
COMMON MECHANISMS OF NEUROTOXICANT-INDUCED NEURODEGENERATION IN NEURODEGENERATIVE DISEASES
Although the causes of the majority of neurodegenerative diseases are unknown, the pathogenic processes and mechanisms are much more understood. In fact, major mechanisms are shared between virtually all neurodegenerative diseases. BBB disruption, protein aggregation, oxidative stress, and mitochondrial dysfunction are major shared pathogenic processes. Neurotoxicants may either initiate or potentiate such processes, ultimately leading to neurodegeneration.
BBB Disruption
Unlike any other organ, retaining constancy of the internal biochemical environment is of utmost importance to the brain. The BBB and blood-CSF barriers serve to regulate transport in and out of the CNS as well as maintain ion concentrations in the fluid that bathes the CNS (cerebrospinal fluid) that are significantly different from the rest of the body (Laterra et al., 1999). Molecular movement across biological membranes, including the BBB, may occur through diffusion, pinocytosis, carrier-mediated transport, and transcellular transport (Kotyk and Janacek, 1975; Laterra et al., 1999). Specialized endothelial cells in brain capillaries are the site of the BBB, forming continuous tight junctions that create the specialized barrier. Neurotoxicants that are highly lipid soluble may easily move across biological membranes and thus cross the BBB by simple diffusion, gaining access to the brain (Fig. 1). However, biological substances such as glucose and neutral L-amino acids that are required by the brain but have low lipid solubility typically enter through carrier-mediated transport (Kotyk and Janacek, 1975; Laterra et al., 1999, Fig. 1). Neurotoxicants sharing similar chemical structures to endogenous molecules may also enter the brain by this mechanism. For example, the dopaminergic neurotoxicant paraquat has a similar structure to MPTP, which is highly lipophilic and able to cross biological membranes. However, in the human body, paraquat is thought to be in a charged state, a polar molecule unable to cross membranes. Paraquat is thought to gain access to the brain due to its structural similarity to amino acids, entering through a neutral amino acid carrier (McCormack and Di Monte, 2003).
The BBB is important in both neurotoxicology and neurodegeneration. Many toxicants would likely have a major effect on the brain but are unable to cross the BBB. 6-OHDA shares a structural similarity to dopamine. However, it contains an extra hydroxyl group (Senoh et al., 1959; Senoh and Witkop, 1959). It may enter dopamine neurons through the dopamine transporter because of its structural similarity to dopamine, where it undergoes auto-oxidation and leads to the damage and destruction of catecholamine neurons (Porter et al., 1963, 1965; Sachs and Jonsson, 1975; Ungerstedt, 1968). However, given it is a polar molecule, it is unable to cross the BBB. For many decades, it has served as one of the most prominent models of PD; however, it must be directly infused into the parenchyma to elicit a selective lesion (Schwarting and Huston, 1996a, b). Many environmentally relevant compounds injected into the brain would likely have major deleterious effects. However, the BBB provides protection from those that are not highly lipophilic or that are unable to mimic a biological entity and gain access to the CNS.
In the majority of neurodegenerative diseases, the BBB is affected. Whether or not BBB dysfunction directly contributes to the pathogenesis of neurodegenerative diseases or is part of the pathology remains to be determined. However, it is certainly plausible that BBB dysfunction as a primary pathogenic event, or in the early stages of disease, could allow both endogenous molecules and environmental toxicants to enter the brain that under normal circumstances would be excluded. Such compounds may then actively elicit neurodegeneration.
Neurovascular changes occur as a normal part of aging. Changes may be much more prominent in chronic neurodegenerative diseases (Zlokovic, 2008). Broadly, modest, ∼20% decreases in cerebral blood flow may be observed in the aging brain in association with decreased protein synthesis (Hossmann, 1994). More severe reductions, observed in neurodegenerative diseases, can lead to shifts in intracellular pH and water, and accumulation of glutamate and lactate and interstitial fluid (Drake and Iadecola, 2007; Zlokovic, 2008). Such decreases in resting cerebral blood flow may be observed in certain regions in AD, PD, MS, or other CNS disorders (Drake and Iadecola, 2007; Hu et al., 2010; Lo et al., 2003; Lok et al., 2007; Melzer et al., 2011). Additionally, changes in the brain capillary unit, loss of brain capillaries, or alterations in resting cerebral blood flow may be among the first signs of the disease process, occurring before overt neuronal changes or neurodegeneration, are apparent (Zlokovic, 2008).
In AD, neurovascular dysfunction is associated with progressive cognitive decline (Iadecola, 2004; Zlokovic, 2005). Accumulation of toxic Aβ occurs in both blood vessels and the parenchyma (Deane and Zlokovic, 2007; Hardy, 2006; Rovelet-Lecrux et al., 2006). Additionally, major alterations in microvasculature occur, including reduced microvascular density, increased fragmented vessels, changes in vessel diameter, and capillary basement membrane thickening (Bailey et al., 2004; Farkas and Luiten, 2001; Zlokovic, 2008). Ultimately, BBB alterations are thought to be major factors, affecting the accumulation and clearance of Aβ in AD. Interestingly, exposure to metals in the rat such as aluminum has been found to increase the permeability of the BBB to small peptides (Banks and Kastin, 1983). Lead exposure in rodents also elicits Aβ accumulation in the choroid plexus, likely through transport alterations at the blood-CSF barrier (Behl et al., 2009, 2010; Crossgrove et al., 2005). Thus, metals may affect BBB and blood-CSF barriers and potentially contribute to the pathogenesis of AD.
In PD, BBB disruption has also been proposed as part of the pathogenesis. The CSF/plasma ratio of several amino acids in PD patients in altered compared with controls suggesting dysfunction in amino acid transport across the BBB (Molina et al., 1997). Some studies have linked polymorphism of the BBB component P-glycoprotein to risk for PD. Interestingly, although the risk associated with the mutation alone did not reach significance, those with the mutation and pesticide exposure exhibited a significant interaction in the form of increased risk (Drozdzik et al., 2003; Furuno et al., 2002). Thus, PD patients are more likely to have P-glycoprotein-mediated BBB dysfunction. Therefore, BBB dysfunction at various stages of the disease could allow access of chemicals to the brain, such as pesticides that would normally be excluded.
The presence of immune cells not normally found in the CNS, such as high numbers of leukocytes in the CNS, suggests a role of the BBB in MS. Here, cells normally excluded by the BBB enter the CNS under disease conditions. Autoaggressive CD4+ T lymphocytes are activated outside the CNS and enter the brain by crossing the BBB or blood-CSF barrier (Zlokovic, 2008). Leukocyte entry into the CNS is an early event in MS and the event itself may trigger BBB disruption (Engelhardt, 2006; Raine et al., 1990). Such disruption may allow additional endogenous toxins or environmental exposures to enter the CNS and potentiate the disease process.
BBB disruption also occurs in ALS, where the total protein concentration in CSF of patients is considerably higher than that of controls (Leonardi et al., 1984). Ultrastructural damage to the BBB is observed in both early and late stages of genetic animal models of ALS (Garbuzova-Davis et al., 2007). Additionally, a disrupted blood–spinal cord barrier has been reported in mice SOD1 mutant mice. This occurred through a reduction in the levels of the tight junction proteins ZO-1, occludin, and claudin-5 between endothelial cells. These changes resulted in microhemorrhages, release of neurotoxic hemoglobin-derived products, reductions in microcirculation, and hypoperfusion. Furthermore, SOD1 mutant–mediated endothelial damage occurred before motor neuron degeneration (Zhong et al., 2008). These findings suggest that these factors are central to the early stages of the disease pathogenesis.
Oxidative Stress
Oxidative stress is a major mechanism by which many neurotoxins act. Simply defined, oxidative stress is having too many reactive species for available antioxidants, where oxidative damage is the biomolecular damage caused by attack of reactive species upon the constituents in living organisms (Halliwell and Gutteridge, 2007). There are an enormous amount of published studies on the role of oxidative stress and damage in neurodegenerative diseases. Most human studies assess signs of oxidative damage, and reports of oxidative modifications to the major biomolecules such as DNA, proteins, and lipids are numerous. Animal studies using neurotoxicant-based models to reproduce the key features of neurodegenerative diseases have supported the general role of oxidative stress in neurodegeneration.
An outstanding coverage of the diverse biochemical and cellular responses to oxidative stress can be found in the text by Halliwell and Gutteridge (2007). Here, the authors also summarize direct evidence (measurement of oxidative damage or biomarkers, such as oxidative damage to DNA, protein, and lipids) and indirect evidence (e.g., response, such as increases in detoxification enzymes) to oxidative stress in neurodegenerative diseases, including PD. For example, elevations in SOD activity in the CNS in human PD patients likely indicate a potential attempt to detoxify oxidative stress (Saggu et al., 1989).
Oxidative stress and damage clearly occurs in AD. Lipid peroxidation, nitrotyrosine, reactive carbonyls, and nucleic acid oxidation are increased in vulnerable neurons of AD patients compared with control, regardless of whether individual neurons contain AD pathology (Castellani et al., 2001; Nunomura et al., 1999, 2001). Thus, signs of oxidative damage precede other pathological events in AD and is an early event in the disease pathogenesis (Bonda et al., 2010). Additionally, patients with prodromal AD, mild cognitive impairment, have increased levels of isoprostanes, which are products of polyunsaturated fatty acid oxidation (Praticò et al., 2000, 2001, 2002a). Furthermore, 4-hydroxynonenal, produced by lipid peroxidation, is found in plaques and promotes protofibril formation (Ando et al., 1998; Siegel et al., 2007). Finally, in transgenic mouse models, oxidative stress precedes Aβ deposition (Odetti et al., 1998). Thus, oxidative stress and damage occurs in human AD and in animal models and is an early event. There are not yet significant data supporting neurotoxicant-induced oxidative stress as a primary event in AD. However, such toxicants could potentiate the disease process.
Broad examples of oxidative damage in human PD include increased total iron content in the substantia nigra (thought to be a contributor to oxidative stress), superoxide dismutase (involved in cellular response to oxidative stress), lipid peroxidation (a marker for oxidative damage to lipids), protein oxidation, and DNA oxidation versus age-matched controls (Alam et al., 1997; Dexter et al., 1989; Floor and Wetzel, 1998; Saggu et al., 1989; Sofic et al., 1988). The major neurotoxicant-based models of PD, including MPTP, 6-OHDA, paraquat, and rotenone, are all thought to produce oxidative stress and ultimately lesion dopamine neurons through oxidative stress, although the primary mechanisms of action differ significantly. Mitochondrial complex I inhibition elicits oxidative stress and is a major mechanism of MPTP (only in catecholamine neurons) and rotenone (systemically inhibits activity in all cells). Paraquat is thought to affect dopamine neurons through redox cycling, and 6-OHDA likely auto-oxidizes after selective uptake into dopamine neurons through the dopamine transporter (Cannon and Greenamyre, 2010). Given that paraquat and rotenone are thought to act through oxidative stress and have been identified as environmental risk factors for PD, it is very likely that oxidative stress is a common downstream mechanism by which neurotoxicants can damage the highly susceptible nigral dopamine neurons. Ultimately, any toxicant capable of entering dopamine neurons, either by diffusion or through carrier-mediated transport (commonly the dopamine transporter) and eliciting oxidative stress, could potentially be a risk factor for PD.
The focus on MS as purely a demyelinating disease has been shifting. Disease progression is now known to depend on axonal degeneration, not repeated demyelination alone (Compston and Coles, 2008). Oxidative stress and damage are invariably part of the inflammatory and neurodegenerative processes. The presence of the reactive peroxynitrite has been consistently proven in acute and chronic active MS lesions (Cross et al., 1998; Liu et al., 2001; Oleszak et al., 1998). In the serum of patients with MS, nitric oxide metabolites (nitrates/nitrites), lipid peroxidation products (malondialdehyde plus 4-hydroxyalkenals), and glutathione peroxidase activity were significantly increased (Koch et al., 2006; Ortiz et al., 2009).
Given that mutations in SOD1 are the most common genetic cause of ALS, aberrant redox status has long been a focus of ALS research. Oxidative damage to proteins, lipids, or DNA has been repeatedly reported in humans with ALS and genetic animal models (Barber and Shaw, 2010). Interestingly, SOD1 itself is found to be heavily oxidized in ALS, which may lead to a toxic gain of function (Andrus et al., 1998; Ezzi et al., 2007).
Oxidative stress remains a key focus of much neurodegenerative disease research. Neurotoxicants that are able to elicit oxidative stress in neurons are candidate risk factors for neurodegeneration. Antioxidant therapies also represent a major research focus, even though clinical trials utilizing antioxidants have been largely unsuccessful.
Protein Aggregation
Protein aggregation is a major unifying mechanistic theme in neurodegenerative diseases. Although each disease produces characteristic aggregation patterns—different specific proteins accumulating in different types of neurons—many of the mechanisms overlap. It should be noted that there is much overlap between diseases and “typical” cases—those containing a specific aggregation pattern distinct from other neurodegenerative diseases are probably not as common as thought. In PD, e.g., the major pathological hallmark is the presence of cytoplasmic inclusions in surviving nigral dopamine neurons known as Lewy bodies, of which a major component is aggregated α-synuclein (Spillantini et al., 1997). However, PD is often accompanied with dementia, and in these cases, Aβ deposition has been observed (Burack et al., 2010; Kalaitzakis et al., 2008). Aggregated tau, the characteristic of neurofibrillary tangles in AD, is present in a host of neurodegenerative diseases known as tauopathies (Iqbal et al., 2010). Many of these “tauopathies” also cause parkinsonism (Ludolph et al., 2009). Hyperphosphorylated and insoluble tau also occur in areas of demyelination in MS (Anderson et al., 2008). In ALS, the propensity of SOD1 to aggregate may be more important to the pathophysiology than alterations in dismutase activity (Shaw and Valentine, 2007).
Environmental neurotoxicants that disrupt protein processing could potentially contribute to neurodegeneration. Animal studies support such a mechanism. A prominent example is the pesticide rotenone, which was the first neurotoxicant-based model to accurately reproduce the aggregation of α-synuclein characteristic of human PD (Betarbet et al., 2000). The rotenone model in particular has been found to replicate several intersecting pathways in neurodegeneration, including mitochondrial dysfunction, oxidative stress, and proteasomal dysfunction/protein aggregation (Betarbet et al., 2006). More recently, the organochlorine dieldrin has been linked to PD and was recently found to induce proteasomal dysfunction in cultured neurons leading to aberrant histone acetylation and cell death (Song et al., 2010).
A pathological hallmark of surviving motor neurons in ALS is the formation of filamentous “spheroid” bodies in proximal processes that may result from inappropriate NF subunit stoichiometry and/or hyperphosphorylation (Bruijn et al., 2004; Brune et al., 2003; Julien, 1999; Julien and Mushynski, 1998; Miller et al., 2002; Rao and Nixon, 2003). Cytoplasmic protein aggregation is a pathological feature observed in many neurodegenerative diseases. Thus, the induction of abnormal protein processing leading to aggregation may be a major mechanism by which environmental neurotoxicants are able to elicit neurodegeneration.
Mitochondrial Dysfunction
Mitochondrial dysfunction is another major feature of neurodegenerative diseases. Much research indicates that mitochondrial dysfunction may be an early or primary event in multiple neurodegenerative diseases. Mitochondrial dysfunction is a putative primary mechanism by which environmental toxicants may induce neurodegeneration.
Several publications have shown that mitochondrial dysfunction is a prominent and early event in AD (Pagani and Eckert, 2011). Mitochondrial accumulation of Aβ has been shown in both AD patients and transgenic mouse models (Caspersen et al., 2005; Lustbader et al., 2004; Manczak et al., 2006). Such accumulation has been postulated to affect mitochondrial import, fusion/fission, membrane potential, ROS production, and the integrity of mitochondrial DNA (Pagani and Eckert, 2011).
Systemic mitochondrial complex I inhibition has been known to occur in PD patients for decades (Parker et al., 1989; Schapira et al., 1989). Two major models of PD, MPTP and rotenone, act through complex I inhibition. MPTP selectively inhibits complex I in catecholamine neurons, whereas rotenone inhibits activity in all cells, producing a selective lesion in nigral dopamine neurons (Cannon and Greenamyre, 2010). The rotenone model has shown that nigral dopamine neurons are particularly sensitive to complex I inhibition. Rotenone exposure has now been implicated as a risk factor for human PD (Tanner et al., 2011). TCE, a solvent with a dramatically different structure has also been linked to PD, with a proposed mechanism of complex I inhibition (Gash et al., 2008). Thus, diverse environmental toxicants that are able to gain access to dopaminergic neurons may produce a lesion to the nigrostriatal dopamine system characteristic of PD.
Mitochondrial dysfunction is an emerging topic of interest in MS. Ultimately, the energy required to maintain intra-axonal ionic balance in axons lacking healthy myelin sheaths is likely to be greater than in healthy neurons (Waxman, 2006). Indeed, mitochondrial density and complex IV activity are increased compared with myelinated control spinal cord (Andrews et al., 2006; Boiko et al., 2001). In addition to proliferation and increased activity, signs of mitochondrial dysfunction occur in MS. Tissue complex I activity in chronic active lesions is reduced, whereas complex III activities on non-lesioned motor cortex are reduced, compared with control (Dutta et al., 2006; Lu et al., 2000). Interestingly, mitochondrial DNA deletions have been reported in cerebral grey matter, including neurons lacking mitochondrial DNA-encoded catalytic subunits of complex IV (Campbell et al., 2011). Specific defects in the main catalytic subunit of complex IV have been identified in MS (Mahad et al., 2008b). Whether mitochondrial dysfunction occurs because of inflammation or is a separate event is undetermined (Mahad et al., 2008a). It is notable that injections of potassium cyanide, a potent complex IV inhibitor in monkeys, produce progressive demyelination (Mahad et al., 2008a; McAlpine, 1946).
The role of mitochondria in ALS is very interesting. SOD1 is a soluble cytosolic protein that in ALS has been found to accumulate in the intermembrane space, matrix, and outer membrane of mitochondria (Higgins et al., 2002; Kawamata and Manfredi, 2008; Vande Velde et al., 2008; Vijayvergiya et al., 2005). In transgenic animal models, increased mutant SOD1 mitochondrial localization caused mitochondrial pathology and an accelerated disease course (Son et al., 2007). Mutant aggregated SOD1 localized to the mitochondria has been proposed to cause multiple perturbations to mitochondrial function, including impaired respiration, disrupted redox homeostasis, reduced ATP production, altered calcium homeostasis, and initiation of apoptotic cascade (Shi et al., 2010).
Mitochondrial dysfunction is a major pathogenic event in neurodegeneration. In fact, it is typically an early event in the disease process. Environmental toxins that affect mitochondrial function have been linked epidemiologically to neurodegenerative diseases and shown in animals to elicit neurodegeneration. Therefore, toxicants with suspected links to neurodegenerative diseases should be explored for a potential effect on mitochondrial function.
SUMMARY
Neurodegenerative diseases are often highly debilitating to both mental and physical functioning. Most are strongly associated with aging; risk dramatically increasing in the later stages of life. Advances in medicine have resulted in dramatic increases in the average life span. Thus, with an aging population, the prevalence of neurodegenerative diseases is expected to continue to rise. For most of these diseases, two enormous challenges remain: (1) to determine the cause for most cases and (2) to discover disease modifying or curative treatments. Unfortunately, much research effort has yet to adequately address either of these challenges. However, our understanding of the pathogenic mechanisms has increased dramatically, and this knowledge will be essential for determining both the causes and the creation of novel treatments.
Environmental influences have long been suspected to play a role in neurodegenerative diseases. This is particularly true with PD, where there is a wealth of epidemiological studies linking environmental exposures to the disease. Additionally, multiple relevant environmentally linked neurotoxicants produce behavioral and pathological features of human PD. However, no neurotoxicant has been definitively linked as a causative agent in PD or other neurodegenerative diseases. Making such links will remain very difficult due to the number of compounds and exposure variability in humans over the course of a life span. Ultimately, the major goal in conducting research on the role of neurotoxicant exposures in neurodegenerative disease remains to identify potential risk factors. The reduction of newly identified risk factors would hopefully reduce the incidence rate and the burden of neurodegenerative diseases on human health. The linkage of neurotoxicants to human neurodegenerative diseases also provides the opportunities to create new animal models, and increase our understanding of the pathogenic mechanisms of human diseases. The development of better and more relevant animal models is crucial to the design and testing of novel therapeutics that hopefully will be developed in the future to treat this devastating group of diseases.
FINALLY, HOW CAN TOXICOLOGISTS HELP?
Neuroscientists and toxicologists clearly do not always agree on how best to model and investigate the role of neurotoxicant exposures in neurodegenerative diseases. A major issue is often the route and dose of a compound required to elicit a neurological phenotype, consistent with a specific human neurodegenerative disorder. Unfortunately, such high doses are often required to model years of human exposures in rodents with a comparatively much lower life span—even though they may appear to have little environmental relevance.
An emerging major need is the study of the preclinical features of neurodegeneration, particularly the biochemical and pathological features that occur before patients exhibit the cardinal disease signs. Having such data in hand is very important for the discovery of biomarkers and testing new therapeutics in a model, at the stage where therapeutic target would be disease modifying. Unfortunately, administering a therapeutic agent immediately before a bolus dose of a neurotoxicant that causes acute, major neurodegeneration is not that informative for the assessment of potential therapies. However, this approach is commonly employed in neuroscience research. Help from toxicologists is greatly needed in this area. What are the relevant doses of a given environmental compound that neuroscientists should be using to assess the role a specific toxicant may play in neurodegeneration? Given that humans are exposed to myriad compounds, which mixtures should we be testing? Neuroscientists understand that we must move beyond simply testing late-stage models of neurodegenerative disease, and we now have many endpoints that can be assessed prior to neuronal loss. It is strongly believed that toxicologists can help accomplish this endeavor in a way that is meaningful to human health and exposures.
FUNDING
National Institute of Environmental Health Sciences, National Institutes of Health (1K99ES019879-01 to J.R.C. and 1P01NS059806-01A2 to J.T.G); Michael J. Fox Foundation (to J.T.G.).
References
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Trichloroethylene (Update) Atlanta, GA: U.S. Public Health Service, Department of Health and Human Services; 1997. [Google Scholar]
- Alam ZI, Jenner A, Daniel SE, Lees AJ, Cairns N, Marsden CD, Jenner P, Halliwell B. Oxidative DNA damage in the parkinsonian brain: An apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J. Neurochem. 1997;69:1196–1203. doi: 10.1046/j.1471-4159.1997.69031196.x. [DOI] [PubMed] [Google Scholar]
- Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- Alzheimer A. Uber eine eigenartige Erkrankung der Hirnrinde. Allg. Z. Psychiat. Psych.-Gericht. Med. 1907;64:146–148. [Google Scholar]
- Amaducci L, Arfaioli C, Inzitari D, Marchi M. Multiple sclerosis among shoe and leather workers: An epidemiological survey in Florence. Acta Neurol. Scand. 1982;65:94–103. doi: 10.1111/j.1600-0404.1982.tb03066.x. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Hampton DW, Patani R, Pryce G, Crowther RA, Reynolds R, Franklin RJ, Giovannoni G, Compston DA, Baker D, et al. Abnormally phosphorylated tau is associated with neuronal and axonal loss in experimental autoimmune encephalomyelitis and multiple sclerosis. Brain. 2008;131:1736–1748. doi: 10.1093/brain/awn119. [DOI] [PubMed] [Google Scholar]
- Ando Y, Brännström T, Uchida K, Nyhlin N, Näsman B, Suhr O, Yamashita T, Olsson T, El Salhy M, Uchino M, et al. Histochemical detection of 4-hydroxynonenal protein in Alzheimer amyloid. J. Neurol. Sci. 1998;156:172–176. doi: 10.1016/s0022-510x(98)00042-2. [DOI] [PubMed] [Google Scholar]
- Andrews H, White K, Thomson C, Edgar J, Bates D, Griffiths I, Turnbull D, Nichols P. Increased axonal mitochondrial activity as an adaptation to myelin deficiency in the Shiverer mouse. J. Neurosci. Res. 2006;83:1533–1539. doi: 10.1002/jnr.20842. [DOI] [PubMed] [Google Scholar]
- Andrus PK, Fleck TJ, Gurney ME, Hall ED. Protein oxidative damage in a transgenic mouse model of familial amyotrophic lateral sclerosis. J. Neurochem. 1998;71:2041–2048. doi: 10.1046/j.1471-4159.1998.71052041.x. [DOI] [PubMed] [Google Scholar]
- Aschner M, Vrana KE, Zheng W. Manganese uptake and distribution in the central nervous system (CNS) Neurotoxicology. 1999;20:173–180. [PubMed] [Google Scholar]
- Bailey TL, Rivara CB, Rocher AB, Hof PR. The nature and effects of cortical microvascular pathology in aging and Alzheimer's disease. Neurol. Res. 2004;26:573–578. doi: 10.1179/016164104225016272. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ. Aluminium increases permeability of the blood-brain barrier to labelled DSIP and beta-endorphin: Possible implications for senile and dialysis dementia. Lancet. 1983;2:1227–1229. doi: 10.1016/s0140-6736(83)91273-4. [DOI] [PubMed] [Google Scholar]
- Barber SC, Shaw PJ. Oxidative stress in ALS: Key role in motor neuron injury and therapeutic target. Free Radic. Biol. Med. 2010;48:629–641. doi: 10.1016/j.freeradbiomed.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Barbosa ER, Comerlatti LR, Haddad MS, Scaff M. Parkinsonism secondary to ethylene oxide exposure: Case report. Arq. Neuropsiquiatr. 1992;50:531–533. doi: 10.1590/s0004-282x1992000400020. (in Portuguese) [DOI] [PubMed] [Google Scholar]
- Basha MR, Wei W, Bakheet SA, Benitez N, Siddiqi HK, Ge YW, Lahiri DK, Zawia NH. The fetal basis of amyloidogenesis: Exposure to lead and latent overexpression of amyloid precursor protein and beta-amyloid in the aging brain. J. Neurosci. 2005;25:823–829. doi: 10.1523/JNEUROSCI.4335-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl M, Zhang Y, Monnot AD, Jiang W, Zheng W. Increased beta-amyloid levels in the choroid plexus following lead exposure and the involvement of low-density lipoprotein receptor protein-1. Toxicol. Appl. Pharmacol. 2009;240:245–254. doi: 10.1016/j.taap.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl M, Zhang Y, Shi Y, Cheng J, Du Y, Zheng W. Lead-induced accumulation of beta-amyloid in the choroid plexus: Role of low density lipoprotein receptor protein-1 and protein kinase C. Neurotoxicology. 2010;31:524–532. doi: 10.1016/j.neuro.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC. Lead. Pediatrics. 2004;113:1016–1022. [PubMed] [Google Scholar]
- Bento-Abreu A, Van Damme P, Van Den Bosch L, Robberecht W. The neurobiology of amyotrophic lateral sclerosis. Eur. J. Neurosci. 2010;31:2247–2265. doi: 10.1111/j.1460-9568.2010.07260.x. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Cotman CW. Evolution in the conceptualization of dementia and Alzheimer's disease: Greco-Roman period to the 1960s. Neurobiol. Aging. 1998;19:173–189. doi: 10.1016/s0197-4580(98)00052-9. [DOI] [PubMed] [Google Scholar]
- Berg D, Hochstrasser H. Iron metabolism in Parkinsonian syndromes. Mov. Disord. 2006;21:1299–1310. doi: 10.1002/mds.21020. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Canet-Aviles RM, Sherer TB, Mastroberardino PG, McLendon C, Kim JH, Lund S, Na HM, Taylor G, Bence NF, et al. Intersecting pathways to neurodegeneration in Parkinson's disease: Effects of the pesticide rotenone on DJ-1, alpha-synuclein, and the ubiquitin-proteasome system. Neurobiol. Dis. 2006;22:404–420. doi: 10.1016/j.nbd.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat. Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Bharathi, Vasudevaraju P, Govindaraju M, Palanisamy AP, Sambamurti K, Rao KS. Molecular toxicity of aluminium in relation to neurodegeneration. Indian J. Med. Res. 2008;128:545–556. [PubMed] [Google Scholar]
- Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- Boiko T, Rasband MN, Levinson SR, Caldwell JH, Mandel G, Trimmer JS, Matthews G. Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron. 2001;30:91–104. doi: 10.1016/s0896-6273(01)00265-3. [DOI] [PubMed] [Google Scholar]
- Boller F, Forette F. Alzheimer's disease and THA: A review of the cholinergic theory and of preliminary results. Biomed. Pharmacother. 1989;43:487–491. doi: 10.1016/0753-3322(89)90109-1. [DOI] [PubMed] [Google Scholar]
- Bonda DJ, Wang X, Perry G, Nunomura A, Tabaton M, Zhu X, Smith MA. Oxidative stress in Alzheimer disease: A possibility for prevention. Neuropharmacology. 2010;59:290–294. doi: 10.1016/j.neuropharm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Borchelt DR, Lee MK, Slunt HS, Guarnieri M, Xu ZS, Wong PC, Brown RH, Jr, Price DL, Sisodia SS, Cleveland DW. Superoxide dismutase 1 with mutations linked to familial amyotrophic lateral sclerosis possesses significant activity. Proc. Natl. Acad. Sci. U.S.A. 1994;91:8292–8296. doi: 10.1073/pnas.91.17.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borie C, Gasparini F, Verpillat P, Bonnet AM, Agid Y, Hetet G, Brice A, Durr A, Grandchamp B. French Parkinson's disease genetic study group. Association study between iron-related genes polymorphisms and Parkinson's disease. J. Neurol. 2002;249:801–804. doi: 10.1007/s00415-002-0704-6. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am. J. Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- Brooks AI, Chadwick CA, Gelbard HA, Cory-Slechta DA, Federoff HJ. Paraquat elicited neurobehavioral syndrome caused by dopaminergic neuron loss. Brain Res. 1999;823:1–10. doi: 10.1016/s0006-8993(98)01192-5. [DOI] [PubMed] [Google Scholar]
- Brown RC, Lockwood AH, Sonawane BR. Neurodegenerative diseases: An overview of environmental risk factors. Environ. Health Perspect. 2005;113:1250–1256. doi: 10.1289/ehp.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brück W, Schmied M, Suchanek G, Brück Y, Breitschopf H, Poser S, Piddlesden S, Lassmann H. Oligodendrocytes in the early course of multiple sclerosis. Ann. Neurol. 1994;35:65–73. doi: 10.1002/ana.410350111. [DOI] [PubMed] [Google Scholar]
- Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu. Rev. Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- Brune S, Kölsch H, Ptok U, Majores M, Schulz A, Schlosser R, Rao ML, Maier W, Heun R. Polymorphism in the peroxisome proliferator-activated receptor alpha gene influences the risk for Alzheimer's disease. J. Neural Transm. 2003;110:1041–1050. doi: 10.1007/s00702-003-0018-6. [DOI] [PubMed] [Google Scholar]
- Burack MA, Hartlein J, Flores HP, Taylor-Reinwald L, Perlmutter JS, Cairns NJ. In vivo amyloid imaging in autopsy-confirmed Parkinson disease with dementia. Neurology. 2010;74:77–84. doi: 10.1212/WNL.0b013e3181c7da8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush AI, Pettingell WH, Multhaup G, d Paradis M, Vonsattel JP, Gusella JF, Beyreuther K, Masters CL, Tanzi RE. Rapid induction of Alzheimer A beta amyloid formation by zinc. Science. 1994;265:1464–1467. doi: 10.1126/science.8073293. [DOI] [PubMed] [Google Scholar]
- Cajal RY. Estudios sobre la degeneración y regeneración del sistema nervioso. Madrid, Spain: Moya; 1913–1914. [Google Scholar]
- Campbell GR, Ziabreva I, Reeve AK, Krishnan KJ, Reynolds R, Howell O, Lassmann H, Turnbull DM, Mahad DJ. Mitochondrial DNA deletions and neurodegeneration in multiple sclerosis. Ann. Neurol. 2011;69:481–492. doi: 10.1002/ana.22109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candy JM, Oakley AE, Klinowski J, Carpenter TA, Perry RH, Atack JR, Perry EK, Blessed G, Fairbairn A, Edwardson JA. Aluminosilicates and senile plaque formation in Alzheimer's disease. Lancet. 1986;1:354–357. doi: 10.1016/s0140-6736(86)92319-6. [DOI] [PubMed] [Google Scholar]
- Cannon JR, Greenamyre JT. Neurotoxic in vivo models of Parkinson's disease recent advances. Prog. Brain Res. 2010;184:17–33. doi: 10.1016/S0079-6123(10)84002-6. [DOI] [PubMed] [Google Scholar]
- Carlen PL, Lee MA, Jacob M, Livshits O. Parkinsonism provoked by alcoholism. Ann. Neurol. 1981;9:84–86. doi: 10.1002/ana.410090117. [DOI] [PubMed] [Google Scholar]
- Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D, McKhann G, Yan SD. Mitochondrial Abeta: A potential focal point for neuronal metabolic dysfunction in Alzheimer's disease. FASEB J. 2005;19:2040–2041. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- Cassina P, Peluffo H, Pehar M, Martinez-Palma L, Ressia A, Beckman JS, Estévez AG, Barbeito L. Peroxynitrite triggers a phenotypic transformation in spinal cord astrocytes that induces motor neuron apoptosis. J. Neurosci. Res. 2002;67:21–29. doi: 10.1002/jnr.10107. [DOI] [PubMed] [Google Scholar]
- Castellani RJ, Harris PL, Sayre LM, Fujii J, Taniguchi N, Vitek MP, Founds H, Atwood CS, Perry G, Smith MA. Active glycation in neurofibrillary pathology of Alzheimer disease: N(epsilon)-(carboxymethyl) lysine and hexitol-lysine. Free Radic. Biol. Med. 2001;31:175–180. doi: 10.1016/s0891-5849(01)00570-6. [DOI] [PubMed] [Google Scholar]
- Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE, Surmeier DJ. ‘Rejuvenation' protects neurons in mouse models of Parkinson's disease. Nature. 2007;447:1081–1086. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- Chu NS, Huang CC, Calne DB. Manganese. In: Spencer PS, Schaumburg HH, Ludlof AC, editors. Experimental and Clinical Neurotoxicology. New York, NY: Oxford University Press; 2000. pp. 752–755. [Google Scholar]
- Cicchetti F, Brownell AL, Williams K, Chen YI, Livni E, Isacson O. Neuroinflammation of the nigrostriatal pathway during progressive 6-OHDA dopamine degeneration in rats monitored by immunohistochemistry and PET imaging. Eur. J. Neurosci. 2002;15:991–998. doi: 10.1046/j.1460-9568.2002.01938.x. [DOI] [PubMed] [Google Scholar]
- Clark DD, Sokoloff L. Circulation and energy metabolism of the brain. In: Siegel GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD, editors. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. Philadelphia, PA: Lippincott Williams & Wilkins; 1999. pp. 637–670. [Google Scholar]
- Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359:1221–1231. doi: 10.1016/S0140-6736(02)08220-X. [DOI] [PubMed] [Google Scholar]
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Craner MJ, Newcombe J, Black JA, Hartle C, Cuzner ML, Waxman SG. Molecular changes in neurons in multiple sclerosis: Altered axonal expression of Nav1.2 and Nav1.6 sodium channels and Na+/Ca2+ exchanger. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8168–8173. doi: 10.1073/pnas.0402765101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapper DR, Krishnan SS, Dalton AJ. Brain aluminum distribution in Alzheimer's disease and experimental neurofibrillary degeneration. Science. 1973;180:511–513. doi: 10.1126/science.180.4085.511. [DOI] [PubMed] [Google Scholar]
- Cross AH, Manning PT, Keeling RM, Schmidt RE, Misko TP. Peroxynitrite formation within the central nervous system in active multiple sclerosis. J. Neuroimmunol. 1998;88:45–56. doi: 10.1016/s0165-5728(98)00078-2. [DOI] [PubMed] [Google Scholar]
- Crossgrove JS, Li GJ, Zheng W. The choroid plexus removes beta-amyloid from brain cerebrospinal fluid. Exp. Biol. Med. (Maywood) 2005;230:771–776. doi: 10.1177/153537020523001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuajungco MP, Lees GJ. Zinc and Alzheimer's disease: Is there a direct link? Brain Res. Brain Res. Rev. 1997;23:219–236. doi: 10.1016/s0165-0173(97)00002-7. [DOI] [PubMed] [Google Scholar]
- Czlonkowska A, Kohutnicka M, Kurkowska-Jastrzebska I, Czlonkowski A. Microglial reaction in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) induced Parkinson's disease mice model. Neurodegeneration. 1996;5:137–143. doi: 10.1006/neur.1996.0020. [DOI] [PubMed] [Google Scholar]
- Dart RC, Hurlbut KM, Boyer-Hassen LV. Lead. In: Dart RC, editor. Medical Toxicology. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- Davis LE, Adair JC. Parkinsonism from methanol poisoning: Benefit from treatment with anti-Parkinson drugs. Mov. Disord. 1999;14:520–525. doi: 10.1002/1531-8257(199905)14:3<520::aid-mds1026>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Dawson R, Jr, Beal MF, Bondy SC, Di Monte DA, Isom GE. Excitotoxins, aging, and environmental neurotoxins: Implications for understanding human neurodegenerative diseases. Toxicol. Appl. Pharmacol. 1995;134:1–17. doi: 10.1006/taap.1995.1163. [DOI] [PubMed] [Google Scholar]
- Deane R, Zlokovic BV. Role of the blood-brain barrier in the pathogenesis of Alzheimer's disease. Curr. Alzheimer Res. 2007;4:191–197. doi: 10.2174/156720507780362245. [DOI] [PubMed] [Google Scholar]
- Dekker MC, Giesbergen PC, Njajou OT, van Swieten JC, Hofman A, Breteler MM, van Duijn CM. Mutations in the hemochromatosis gene (HFE), Parkinson's disease and parkinsonism. Neurosci. Lett. 2003;348:117–119. doi: 10.1016/s0304-3940(03)00713-4. [DOI] [PubMed] [Google Scholar]
- Depino AM, Earl C, Kaczmarczyk E, Ferrari C, Besedovsky H, del Rey A, Pitossi FJ, Oertel WH. Microglial activation with atypical proinflammatory cytokine expression in a rat model of Parkinson's disease. Eur. J. Neurosci. 2003;18:2731–2742. doi: 10.1111/j.1460-9568.2003.03014.x. [DOI] [PubMed] [Google Scholar]
- Dexter DT, Carter CJ, Wells FR, Javoy-Agid F, Agid Y, Lees A, Jenner P, Marsden CD. Basal lipid peroxidation in substantia nigra is increased in Parkinson's disease. J. Neurochem. 1989;52:381–389. doi: 10.1111/j.1471-4159.1989.tb09133.x. [DOI] [PubMed] [Google Scholar]
- Dhillon AS, Tarbutton GL, Levin JL, Plotkin GM, Lowry LK, Nalbone JT, Shepherd S. Pesticide/environmental exposures and Parkinson's disease in East Texas. J. Agromedicine. 2008;13:37–48. doi: 10.1080/10599240801986215. [DOI] [PubMed] [Google Scholar]
- Di Monte DA. The role of environmental agents in Parkinson's disease. Clin. Neurosci. Res. 2001;1:419–426. [Google Scholar]
- DiPatre PL, Gelman BB. Microglial cell activation in aging and Alzheimer disease: Partial linkage with neurofibrillary tangle burden in the hippocampus. J. Neuropathol. Exp. Neurol. 1997;56:143–149. doi: 10.1097/00005072-199702000-00004. [DOI] [PubMed] [Google Scholar]
- Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, Marshall FJ, Ravina BM, Schifitto G, Siderowf A, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- Drago D, Bolognin S, Zatta P. Role of metal ions in the abeta oligomerization in Alzheimer's disease and in other neurological disorders. Curr. Alzheimer Res. 2008;5:500–507. doi: 10.2174/156720508786898479. [DOI] [PubMed] [Google Scholar]
- Drake CT, Iadecola C. The role of neuronal signaling in controlling cerebral blood flow. Brain Lang. 2007;102:141–152. doi: 10.1016/j.bandl.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Drozdzik M, Bialecka M, Mysliwiec K, Honczarenko K, Stankiewicz J, Sych Z. Polymorphism in the P-glycoprotein drug transporter MDR1 gene: A possible link between environmental and genetic factors in Parkinson's disease. Pharmacogenetics. 2003;13:259–263. doi: 10.1097/01.fpc.0000054087.48725.d9. [DOI] [PubMed] [Google Scholar]
- Dutta R, McDonough J, Yin X, Peterson J, Chang A, Torres T, Gudz T, Macklin WB, Lewis DA, Fox RJ, et al. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann. Neurol. 2006;59:478–489. doi: 10.1002/ana.20736. [DOI] [PubMed] [Google Scholar]
- Engelhardt B. Molecular mechanisms involved in T cell migration across the blood-brain barrier. J. Neural Transm. 2006;113:477–485. doi: 10.1007/s00702-005-0409-y. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Ezzi SA, Urushitani M, Julien JP. Wild-type superoxide dismutase acquires binding and toxic properties of ALS-linked mutant forms through oxidation. J. Neurochem. 2007;102:170–178. doi: 10.1111/j.1471-4159.2007.04531.x. [DOI] [PubMed] [Google Scholar]
- Fall PA, Fredrikson M, Axelson O, Granerus AK. Nutritional and occupational factors influencing the risk of Parkinson's disease: A case-control study in southeastern Sweden. Mov. Disord. 1999;14:28–37. doi: 10.1002/1531-8257(199901)14:1<28::aid-mds1007>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Fang F, Kwee LC, Allen KD, Umbach DM, Ye W, Watson M, Keller J, Oddone EZ, Sandler DP, Schmidt S, et al. Association between blood lead and the risk of amyotrophic lateral sclerosis. Am. J. Epidemiol. 2010;171:1126–1133. doi: 10.1093/aje/kwq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer's disease. Prog. Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- Fattoretti P, Bertoni-Freddari C, Balietti M, Giorgetti B, Solazzi M, Zatta P. Chronic aluminum administration to old rats results in increased levels of brain metal ions and enlarged hippocampal mossy fibers. Ann. N.Y. Acad. Sci. 2004;1019:44–47. doi: 10.1196/annals.1297.010. [DOI] [PubMed] [Google Scholar]
- Fay RM, Mumtaz MM. Development of a priority list of chemical mixtures occurring at 1188 hazardous waste sites, using the HazDat database. Food Chem. Toxicol. 1996;34:1163–1165. doi: 10.1016/s0278-6915(97)00090-2. [DOI] [PubMed] [Google Scholar]
- Feldman RG. Occupational and Environmental Neurotoxicology. Philadelphia, PA: Lippincott-Raven Publishers; 1999. [Google Scholar]
- Ferrante RJ, Schulz JB, Kowall NW, Beal MF. Systemic administration of rotenone produces selective damage in the striatum and globus pallidus, but not in the substantia nigra. Brain Res. 1997;753:157–162. doi: 10.1016/s0006-8993(97)00008-5. [DOI] [PubMed] [Google Scholar]
- Ferreira PC, Piai Kde A, Takayanagui AM, Segura-Muñoz SI. Aluminum as a risk factor for Alzheimer's disease. Rev. Lat. Am. Enfermagem. 2008;16:151–157. doi: 10.1590/s0104-11692008000100023. [DOI] [PubMed] [Google Scholar]
- Fischer O. Miliare Nekrosen mit drusigen Wucherungen der Neurofibrillen, eine regelmässige Veränderung der Hirnrinde bei seniler. Mschr. Psychiat. Neurol. 1907;21:361–372. [Google Scholar]
- Fisher HK, Clements JA, Wright RR. Enhancement of oxygen toxicity by the herbicide paraquat. Am. Rev. Respir. Dis. 1973;107:246–252. doi: 10.1164/arrd.1973.107.2.246. [DOI] [PubMed] [Google Scholar]
- Floor E, Wetzel MG. Increased protein oxidation in human substantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J. Neurochem. 1998;70:268–275. doi: 10.1046/j.1471-4159.1998.70010268.x. [DOI] [PubMed] [Google Scholar]
- Fordahl SC, Anderson JG, Cooney PT, Weaver TL, Colyer CL, Erikson KM. Manganese exposure inhibits the clearance of extracellular GABA and influences taurine homeostasis in the striatum of developing rats. Neurotoxicology. 2010;31:639–646. doi: 10.1016/j.neuro.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forno LS. Neuropathology of Parkinson's disease. J. Neuropathol. Exp. Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- Forster DP, Newens AJ, Kay DW, Edwardson JA. Risk factors in clinically diagnosed presenile dementia of the Alzheimer type: A case-control study in northern England. J. Epidemiol. Community Health. 1995;49:253–258. doi: 10.1136/jech.49.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisardi V, Solfrizzi V, Capurso C, Kehoe PG, Imbimbo BP, Santamato A, Dellegrazie F, Seripa D, Pilotto A, Capurso A, et al. Aluminum in the diet and Alzheimer's disease: From current epidemiology to possible disease-modifying treatment. J. Alzheimers Dis. 2010;20:17–30. doi: 10.3233/JAD-2009-1340. [DOI] [PubMed] [Google Scholar]
- Frontera JA, Kalb T. Neurological management of fulminant hepatic failure. Neurocrit. Care. 2011;14:318–327. doi: 10.1007/s12028-010-9470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuno T, Landi MT, Ceroni M, Caporaso N, Bernucci I, Nappi G, Martignoni E, Schaeffeler E, Eichelbaum M, Schwab M, et al. Expression polymorphism of the blood-brain barrier component P-glycoprotein (MDR1) in relation to Parkinson's disease. Pharmacogenetics. 2002;12:529–534. doi: 10.1097/00008571-200210000-00004. [DOI] [PubMed] [Google Scholar]
- Gao HM, Hong JS, Zhang W, Liu B. Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J. Neurosci. 2002;22:782–790. doi: 10.1523/JNEUROSCI.22-03-00782.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuzova-Davis S, Haller E, Saporta S, Kolomey I, Nicosia SV, Sanberg PR. Ultrastructure of blood-brain barrier and blood-spinal cord barrier in SOD1 mice modeling ALS. Brain Res. 2007;1157:126–137. doi: 10.1016/j.brainres.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Garruto RM, Brown P. Tau protein, aluminium, and Alzheimer's disease. Lancet. 1994;343:989. doi: 10.1016/s0140-6736(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Gash DM, Rutland K, Hudson NL, Sullivan PG, Bing G, Cass WA, Pandya JD, Liu M, Choi DY, Hunter RL, et al. Trichloroethylene: Parkinsonism and complex 1 mitochondrial neurotoxicity. Ann. Neurol. 2008;63:184–192. doi: 10.1002/ana.21288. [DOI] [PubMed] [Google Scholar]
- Gatto NM, Cockburn M, Bronstein J, Manthripragada AD, Ritz B. Well-water consumption and Parkinson's disease in rural California. Environ. Health Perspect. 2009;117:1912–1918. doi: 10.1289/ehp.0900852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia: Intrinsic immuneffector cell of the brain. Brain Res. Brain Res. Rev. 1995;20:269–287. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- Gerlach M, Double KL, Youdim MB, Riederer P. Potential sources of increased iron in the substantia nigra of parkinsonian patients. J. Neural Transm. Suppl. 2006;70:133–142. doi: 10.1007/978-3-211-45295-0_21. [DOI] [PubMed] [Google Scholar]
- Gilfillan SC. Lead poisoning and the fall of Rome. J. Occup. Med. 1965;7:53–60. [PubMed] [Google Scholar]
- Gillette-Guyonnet S, Andrieu S, Nourhashemi F, de La Gueronniere V, Grandjean H, Vellas B. Cognitive impairment and composition of drinking water in women: Findings of the EPIDOS Study. Am. J. Clin. Nutr. 2005;81:897–902. doi: 10.1093/ajcn/81.4.897. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, Richardson RJ. Occupational exposures to metals as risk factors for Parkinson's disease. Neurology. 1997;48:650–658. doi: 10.1212/wnl.48.3.650. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Richardson RJ. The risk of Parkinson's disease with exposure to pesticides, farming, well water, and rural living. Neurology. 1998;50:1346–1350. doi: 10.1212/wnl.50.5.1346. [DOI] [PubMed] [Google Scholar]
- Gould E. How widespread is adult neurogenesis in mammals? Nat. Rev. Neurosci. 2007;8:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- Grant WB, Campbell A, Itzhaki RF, Savory J. The significance of environmental factors in the etiology of Alzheimer's disease. J. Alzheimers Dis. 2002;4:179–189. doi: 10.3233/jad-2002-4308. [DOI] [PubMed] [Google Scholar]
- Hageman G, van der Hoek J, van Hout M, van der Laan G, Steur EJ, de Bruin W, Herholz K. Parkinsonism, pyramidal signs, polyneuropathy, and cognitive decline after long-term occupational solvent exposure. J. Neurol. 1999;246:198–206. doi: 10.1007/s004150050334. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM. Free Radicals in Biology and Medicine. New York, NY: Oxford University Press; 2007. [Google Scholar]
- Hardy J. A hundred years of Alzheimer's disease research. Neuron. 2006;52:3–13. doi: 10.1016/j.neuron.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Hastings TG, Zigmond MJ. Identification of catechol-protein conjugates in neostriatal slices incubated with [3H]dopamine: Impact of ascorbic acid and glutathione. J. Neurochem. 1994;63:1126–1132. doi: 10.1046/j.1471-4159.1994.63031126.x. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: Prevalence estimates using the 2000 census. Arch. Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Nicklas WJ, Vyas I, Duvoisin RC. Dopaminergic toxicity of rotenone and the 1-methyl-4-phenylpyridinium ion after their stereotaxic administration to rats: Implication for the mechanism of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity. Neurosci. Lett. 1985;62:389–394. doi: 10.1016/0304-3940(85)90580-4. [DOI] [PubMed] [Google Scholar]
- Henriksson J, Tjalve H. Manganese taken up into the CNS via the olfactory pathway in rats affects astrocytes. Toxicol. Sci. 2000;55:392–398. doi: 10.1093/toxsci/55.2.392. [DOI] [PubMed] [Google Scholar]
- Henzi H. Chronic methanol poisoning with the clinical and pathologic-anatomical features of multiple sclerosis. Med. Hypotheses. 1984;13:63–75. doi: 10.1016/0306-9877(84)90131-2. [DOI] [PubMed] [Google Scholar]
- Hernan MA, Jick SS, Logroscino G, Olek MJ, Ascherio A, Jick H. Cigarette smoking and the progression of multiple sclerosis. Brain. 2005;128:1461–1465. doi: 10.1093/brain/awh471. [DOI] [PubMed] [Google Scholar]
- Hertzman C, Wiens M, Bowering D, Snow B, Calne D. Parkinson's disease: A case-control study of occupational and environmental risk factors. Am. J. Ind. Med. 1990;17:349–355. doi: 10.1002/ajim.4700170307. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Asanuma M, Miyazaki I, Hattori N, Mizuno Y, Ogawa N. Parkin attenuates manganese-induced dopaminergic cell death. J. Neurochem. 2004;89:1490–1497. doi: 10.1111/j.1471-4159.2004.02445.x. [DOI] [PubMed] [Google Scholar]
- Higgins CM, Jung C, Ding H, Xu Z. Mutant Cu, Zn superoxide dismutase that causes motoneuron degeneration is present in mitochondria in the CNS. J. Neurosci. 2002;22:RC215. doi: 10.1523/JNEUROSCI.22-06-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner RD, Grambow SC, Coffman CJ, Lindquist JH, Oddone EZ, Allen KD, Kasarskis EJ. Amyotrophic lateral sclerosis among 1991 Gulf War veterans: Evidence for a time-limited outbreak. Neuroepidemiology. 2008;31:28–32. doi: 10.1159/000136648. [DOI] [PubMed] [Google Scholar]
- Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann. Neurol. 1994;36:557–565. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- Hou L, Zagorski MG. NMR reveals anomalous copper(II) binding to the amyloid Abeta peptide of Alzheimer's disease. J. Am. Chem. Soc. 2006;128:9260–9261. doi: 10.1021/ja046032u. [DOI] [PubMed] [Google Scholar]
- Hu WT, Wang Z, Lee VM, Trojanowski JQ, Detre JA, Grossman M. Distinct cerebral perfusion patterns in FTLD and AD. Neurology. 2010;75:881–888. doi: 10.1212/WNL.0b013e3181f11e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat. Rev. Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Ilett KF, Stripp B, Menard RH, Reid WD, Gillette JR. Studies on the mechanism of the lung toxicity of paraquat: Comparison of tissue distribution and some biochemical parameters in rats and rabbits. Toxicol. Appl. Pharmacol. 1974;28:216–226. doi: 10.1016/0041-008x(74)90007-6. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Liu F, Gong CX, Grundke-Iqbal I. Tau in Alzheimer disease and related tauopathies. Curr. Alzheimer Res. 2010;7:656–664. doi: 10.2174/156720510793611592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaques A, Gamble J, Tsai SP, American Chemistry Council's Hydrocarbon Solvents P. Hydrocarbon exposure and Parkinson's disease. Neurology. 2001;57:371. doi: 10.1212/wnl.57.2.371. [DOI] [PubMed] [Google Scholar]
- Javitch JA, D'Amato RJ, Strittmatter SM, Snyder SH. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine: Uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc. Natl. Acad. Sci. U.S.A. 1985;82:2173–2177. doi: 10.1073/pnas.82.7.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson FO, Atchison WD. The role of environmental mercury, lead and pesticide exposure in development of amyotrophic lateral sclerosis. Neurotoxicology. 2009;30:761–765. doi: 10.1016/j.neuro.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston CA, Stanton BR, Turner MR, Gray R, Blunt AH, Butt D, Ampong MA, Shaw CE, Leigh PN, Al-Chalabi A. Amyotrophic lateral sclerosis in an urban setting: A population based study of inner city London. J. Neurol. 2006;253:1642–1643. doi: 10.1007/s00415-006-0195-y. [DOI] [PubMed] [Google Scholar]
- Jollow DJ, Bruckner JV, McMillan DC, Fisher JW, Hoel DG, Mohr LC. Trichloroethylene risk assessment: A review and commentary. Crit. Rev. Toxicol. 2009;39:782–797. doi: 10.3109/10408440903222177. [DOI] [PubMed] [Google Scholar]
- Julien JP. Neurofilament functions in health and disease. Curr. Opin. Neurobiol. 1999;9:554–560. doi: 10.1016/S0959-4388(99)00004-5. [DOI] [PubMed] [Google Scholar]
- Julien JP, Mushynski WE. Neurofilaments in health and disease. Prog. Nucleic Acid Res. Mol. Biol. 1998;61:1–23. doi: 10.1016/s0079-6603(08)60823-5. [DOI] [PubMed] [Google Scholar]
- Kalaitzakis ME, Graeber MB, Gentleman SM, Pearce RK. Striatal beta-amyloid deposition in Parkinson disease with dementia. J. Neuropathol. Exp. Neurol. 2008;67:155–161. doi: 10.1097/NEN.0b013e31816362aa. [DOI] [PubMed] [Google Scholar]
- Kalat JW. Biological Psychology. Toronto, Canada: Wadsworth Publishing; 2008. [Google Scholar]
- Kamo H, Haebara H, Akiguchi I, Kameyama M, Kimura H, McGeer PL. A distinctive distribution of reactive astroglia in the precentral cortex in amyotrophic lateral sclerosis. Acta Neuropathol. 1987;74:33–38. doi: 10.1007/BF00688335. [DOI] [PubMed] [Google Scholar]
- Karamyan VT, Speth RC. Animal models of BMAA neurotoxicity: A critical review. Life Sci. 2008;82:233–246. doi: 10.1016/j.lfs.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Kaur D, Andersen J. Does cellular iron dysregulation play a causative role in Parkinson's disease? Ageing Res. Rev. 2004;3:327–343. doi: 10.1016/j.arr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Kawamata H, Manfredi G. Different regulation of wild-type and mutant Cu, Zn superoxide dismutase localization in mammalian mitochondria. Hum. Mol. Genet. 2008;17:3303–3317. doi: 10.1093/hmg/ddn226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Priyamvada S, Khan SA, Khan W, Farooq N, Khan F, Yusufi AN. Effect of trichloroethylene (TCE) toxicity on the enzymes of carbohydrate metabolism, brush border membrane and oxidative stress in kidney and other rat tissues. Food Chem. Toxicol. 2009;47:1562–1568. doi: 10.1016/j.fct.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Kim RH, Smith PD, Aleyasin H, Hayley S, Mount MP, Pownall S, Wakeham A, You-Ten AJ, Kalia SK, Horne P, et al. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc. Natl. Acad. Sci. U.S.A. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Muramori F, Aoki T, Hayashi M, Miyazu K, Fukutani Y, Mukai M, Koshino F. KP-1 is a marker for extraneuronal neurofibrillary tangles and senile plaques in Alzheimer diseased brains. Dement. Geriatr. Cogn. Disord. 1998;9:13–19. doi: 10.1159/000017015. [DOI] [PubMed] [Google Scholar]
- Koch M, Ramsaransing GS, Arutjunyan AV, Stepanov M, Teelken A, Heersema DJ, De Keyser J. Oxidative stress in serum and peripheral blood leukocytes in patients with different disease courses of multiple sclerosis. J. Neurol. 2006;253:483–487. doi: 10.1007/s00415-005-0037-3. [DOI] [PubMed] [Google Scholar]
- Koedel U, Scheld WM, Pfister HW. Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect. Dis. 2002;2:721–736. doi: 10.1016/s1473-3099(02)00450-4. [DOI] [PubMed] [Google Scholar]
- Kotyk A, Janacek K. Cell Membrane Transport. New York, NY: Plenum; 1975. [Google Scholar]
- Kuhn W, Winkel R, Woitalla D, Meves S, Przuntek H, Muller T. High prevalence of parkinsonism after occupational exposure to lead-sulfate batteries. Neurology. 1998;50:1885–1886. doi: 10.1212/wnl.50.6.1885. [DOI] [PubMed] [Google Scholar]
- Kumar G, Kalita J, Misra UK. Raised intracranial pressure in acute viral encephalitis. Clin. Neurol. Neurosurg. 2009;111:399–406. doi: 10.1016/j.clineuro.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Kuopio AM, Marttila RJ, Helenius H, Rinne UK. Changing epidemiology of Parkinson's disease in southwestern Finland. Neurology. 1999;52:302–308. doi: 10.1212/wnl.52.2.302. [DOI] [PubMed] [Google Scholar]
- Kuriwaka R, Mitsui T, Fujiwara S, Nishida Y, Matsumoto T. Loss of postural reflexes in long-term occupational solvent exposure. Eur. Neurol. 2002;47:85–87. doi: 10.1159/000047958. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF. A reassessment of the distribution of multiple sclerosis. Part one. Acta Neurol. Scand. 1975;51:110–136. doi: 10.1111/j.1600-0404.1975.tb01364.x. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF. Epidemiologic evidence for multiple sclerosis as an infection. Clin. Microbiol. Rev. 1993;6:382–427. doi: 10.1128/cmr.6.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner PD, Stephenson DT, Wright S. Reactive astrogliosis is widespread in the subcortical white matter of amyotrophic lateral sclerosis brain. J. Neuropathol. Exp. Neurol. 1991;50:263–277. doi: 10.1097/00005072-199105000-00008. [DOI] [PubMed] [Google Scholar]
- Lai JC, Blass JP. Inhibition of brain glycolysis by aluminum. J. Neurochem. 1984;42:438–446. doi: 10.1111/j.1471-4159.1984.tb02697.x. [DOI] [PubMed] [Google Scholar]
- Landsberg JP, McDonald B, Watt F. Absence of aluminium in neuritic plaque cores in Alzheimer's disease. Nature. 1992;360:65–68. doi: 10.1038/360065a0. [DOI] [PubMed] [Google Scholar]
- Landtblom AM, Flodin U, Söderfeldt B, Wolfson C, Axelson O. Organic solvents and multiple sclerosis: A synthesis of the current evidence. Epidemiology. 1996;7:429–433. doi: 10.1097/00001648-199607000-00015. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA, Karluk D. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann. Neurol. 1999;46:598–605. doi: 10.1002/1531-8249(199910)46:4<598::aid-ana7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Laterra J, Keep RF, Betz AL, Goldstein GW. Blood-brain-cerebrospinal fluid barriers. In: Siegel GJ, Agranoff BW, Albers RW, Fisher HK, Uhler MD, editors. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. Philadelphia, PA: Lippincott-Raven; 1999. pp. 671–689. [Google Scholar]
- Leonardi A, Abbruzzese G, Arata L, Cocito L, Vische M. Cerebrospinal fluid (CSF) findings in amyotrophic lateral sclerosis. J. Neurol. 1984;231:75–78. doi: 10.1007/BF00313720. [DOI] [PubMed] [Google Scholar]
- Ley CO, Gali FG. Parkinsonian syndrome after methanol intoxication. Eur. Neurol. 1983;22:405–409. doi: 10.1159/000115593. [DOI] [PubMed] [Google Scholar]
- Liou HH, Tsai MC, Chen CJ, Jeng JS, Chang YC, Chen SY, Chen RC. Environmental risk factors and Parkinson's disease: A case-control study in Taiwan. Neurology. 1997;48:1583–1588. doi: 10.1212/wnl.48.6.1583. [DOI] [PubMed] [Google Scholar]
- Liu JS, Zhao ML, Brosnan CF, Lee SC. Expression of inducible nitric oxide synthase and nitrotyrosine in multiple sclerosis lesions. Am. J. Pathol. 2001;158:2057–2066. doi: 10.1016/S0002-9440(10)64677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Choi DY, Hunter RL, Pandya JD, Cass WA, Sullivan PG, Kim HC, Gash DM, Bing G. Trichloroethylene induces dopaminergic neurodegeneration in Fisher 344 rats. J. Neurochem. 2010;112:773–783. doi: 10.1111/j.1471-4159.2009.06497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Muralidhara S, Bruckner JV, Bartlett MG. Determination of trichloroethylene in biological samples by headspace solid-phase microextraction gas chromatography/mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008a;863:26–35. doi: 10.1016/j.jchromb.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Liu Y, Muralidhara S, Bruckner JV, Bartlett MG. Trace level determination of trichloroethylene in biological samples by headspace solid-phase microextraction gas chromatography/negative chemical ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2008b;22:797–806. doi: 10.1002/rcm.3426. [DOI] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Logroscino G, Gao X, Chen H, Wing A, Ascherio A. Dietary iron intake and risk of Parkinson's disease. Am. J. Epidemiol. 2008;168:1381–1388. doi: 10.1093/aje/kwn273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logroscino G, Marder K, Cote L, Tang MX, Shea S, Mayeux R. Dietary lipids and antioxidants in Parkinson's disease: A population-based, case-control study. Ann. Neurol. 1996;39:89–94. doi: 10.1002/ana.410390113. [DOI] [PubMed] [Google Scholar]
- Lok J, Gupta P, Guo S, Kim WJ, Whalen MJ, van Leyen K, Lo EH. Cell-cell signaling in the neurovascular unit. Neurochem. Res. 2007;32:2032–2045. doi: 10.1007/s11064-007-9342-9. [DOI] [PubMed] [Google Scholar]
- Lu F, Selak M, O'Connor J, Croul S, Lorenzana C, Butunoi C, Kalman B. Oxidative damage to mitochondrial DNA and activity of mitochondrial enzymes in chronic active lesions of multiple sclerosis. J. Neurol. Sci. 2000;177:95–103. doi: 10.1016/s0022-510x(00)00343-9. [DOI] [PubMed] [Google Scholar]
- Ludolph AC, Kassubek J, Landwehrmeyer BG, Mandelkow E, Mandelkow EM, Burn DJ, Caparros-Lefebvre D, Frey KA, de Yebenes JG, Gasser T, et al. Tauopathies with parkinsonism: Clinical spectrum, neuropathologic basis, biological markers, and treatment options. Eur. J. Neurol. 2009;16:297–309. doi: 10.1111/j.1468-1331.2008.02513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, et al. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- Mahad D, Lassmann H, Turnbull D. Review: Mitochondria and disease progression in multiple sclerosis. Neuropathol. Appl. Neurobiol. 2008a;34:577–589. doi: 10.1111/j.1365-2990.2008.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahad D, Ziabreva I, Lassmann H, Turnbull D. Mitochondrial defects in acute multiple sclerosis lesions. Brain. 2008b;131:1722–1735. doi: 10.1093/brain/awn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: A causative factor and therapeutic target in neuropathology, including Alzheimer's disease. Proc. Natl. Acad. Sci. U.S.A. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: Implications for free radical generation and oxidative damage in disease progression. Hum. Mol. Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- Marrie RA. Environmental risk factors in multiple sclerosis aetiology. Lancet Neurol. 2004;3:709–718. doi: 10.1016/S1474-4422(04)00933-0. [DOI] [PubMed] [Google Scholar]
- Martyn CN, Cruddas M, Compston DA. Symptomatic Epstein-Barr virus infection and multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 1993;56:167–168. doi: 10.1136/jnnp.56.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroberardino PG, Hoffman EK, Horowitz MP, Betarbet R, Taylor G, Cheng D, Na HM, Gutekunst CA, Gearing M, Trojanowski JQ, et al. A novel transferrin/TfR2-mediated mitochondrial iron transport system is disrupted in Parkinson's disease. Neurobiol. Dis. 2009;34:417–431. doi: 10.1016/j.nbd.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda W, Furuta T, Nakamura KC, Hioki H, Fujiyama F, Arai R, Kaneko T. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J. Neurosci. 2009;29:444–453. doi: 10.1523/JNEUROSCI.4029-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard LS, Cotzias GC. The partition of manganese among organs and intracellular organelles of the rat. J. Biol. Chem. 1955;214:489–495. [PubMed] [Google Scholar]
- McAlpine D. The problem of disseminated sclerosis. Brain. 1946;69:233–250. doi: 10.1093/brain/69.4.233. [DOI] [PubMed] [Google Scholar]
- McCormack AL, Di Monte DA. Effects of L-dopa and other amino acids against paraquat-induced nigrostriatal degeneration. J. Neurochem. 2003;85:82–86. doi: 10.1046/j.1471-4159.2003.01621.x. [DOI] [PubMed] [Google Scholar]
- McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, Di Monte DA. Environmental risk factors and Parkinson's disease: Selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol. Dis. 2002;10:119–127. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG, Itagaki S, Mizukawa K. Anatomy and pathology of the basal ganglia. Can. J. Neurol. Sci. 1987;14:363–372. doi: 10.1017/s0317167100037756. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Schwab C, Parent A, Doudet D. Presence of reactive microglia in monkey substantia nigra years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration. Ann. Neurol. 2003;54:599–604. doi: 10.1002/ana.10728. [DOI] [PubMed] [Google Scholar]
- McLachlan DR, Bergeron C, Smith JE, Boomer D, Rifat SL. Risk for neuropathologically confirmed Alzheimer's disease and residual aluminum in municipal drinking water employing weighted residential histories. Neurology. 1996;46:401–405. doi: 10.1212/wnl.46.2.401. [DOI] [PubMed] [Google Scholar]
- McLean DR, Jacobs H, Mielke BW. Methanol poisoning: A clinical and pathological study. Ann. Neurol. 1980;8:161–167. doi: 10.1002/ana.410080206. [DOI] [PubMed] [Google Scholar]
- Melzer TR, Watts R, MacAskill MR, Pearson JF, Rueger S, Pitcher TL, Livingston L, Graham C, Keenan R, Shankaranarayanan A, et al. Arterial spin labelling reveals an abnormal cerebral perfusion pattern in Parkinson's disease. Brain. 2011;134:845–855. doi: 10.1093/brain/awq377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena I. Manganese poisoning. In: Vinken PJ, Bruyn GW, editors. Clinical Neurology. Vol. 36. Amsterdam: Elsevier/North Holland; 1979. [Google Scholar]
- Mikaeloff Y, Caridade G, Tardieu M, Suissa S On behalf of the KIDSEP Study Group. (2007). Parental smoking at home and the risk of childhood-onset multiple sclerosis in children. Brain. 130:2589–2595. doi: 10.1093/brain/awm198. [DOI] [PubMed] [Google Scholar]
- Miller CC, Ackerley S, Brownlees J, Grierson AJ, Jacobsen NJ, Thornhill P. Axonal transport of neurofilaments in normal and disease states. Cell. Mol. Life Sci. 2002;59:323–330. doi: 10.1007/s00018-002-8425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda ML, Alicia Overstreet Galeano M, Tassone E, Allen KD, Horner RD. Spatial analysis of the etiology of amyotrophic lateral sclerosis among 1991 Gulf War veterans. Neurotoxicology. 2008;29:964–970. doi: 10.1016/j.neuro.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Mizoroki T, Meshitsuka S, Maeda S, Murayama M, Sahara N, Takashima A. Aluminum induces tau aggregation in vitro but not in vivo. J. Alzheimers Dis. 2007;11:419–427. doi: 10.3233/jad-2007-11401. [DOI] [PubMed] [Google Scholar]
- Molina JA, Jiménez-Jiménez FJ, Gomez P, Vargas C, Navarro JA, Ortí-Pareja M, Gasalla T, Benito-León J, Bermejo F, Arenas J. Decreased cerebrospinal fluid levels of neutral and basic amino acids in patients with Parkinson's disease. J. Neurol. Sci. 1997;150:123–127. doi: 10.1016/s0022-510x(97)00069-5. [DOI] [PubMed] [Google Scholar]
- Molsa PK, Marttila RJ, Rinne UK. Survival and cause of death in Alzheimer's disease and multi-infarct dementia. Acta Neurol. Scand. 1986;74:103–107. doi: 10.1111/j.1600-0404.1986.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Molsa PK, Marttila RJ, Rinne UK. Long-term survival and predictors of mortality in Alzheimer's disease and multi-infarct dementia. Acta Neurol. Scand. 1995;91:159–164. doi: 10.1111/j.1600-0404.1995.tb00426.x. [DOI] [PubMed] [Google Scholar]
- Moore CS, Milner R, Nishiyama A, Frausto RF, Serwanski DR, Pagarigan RR, Whitton JL, Miller RH, Crocker SJ. Astrocytic tissue inhibitor of metalloproteinase-1 (TIMP-1) promotes oligodendrocyte differentiation and enhances CNS myelination. J. Neurosci. 2011;31:6247–6254. doi: 10.1523/JNEUROSCI.5474-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen JT, Bronnum-Hansen H, Rasmussen K. Multiple sclerosis and organic solvents. Epidemiology. 1998;9:168–171. [PubMed] [Google Scholar]
- Mozaz MJ, Wyke MA, Indakoetxea B. Parkinsonism and defects of praxis following methanol poisoning. J. Neurol. Neurosurg. Psychiatry. 1991;54:843–844. doi: 10.1136/jnnp.54.9.843-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Vahl KR, Kolbe H, Dengler R. Transient severe parkinsonism after acute organophosphate poisoning. J. Neurol. Neurosurg. Psychiatry. 1999;66:253–254. doi: 10.1136/jnnp.66.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullett SJ, Hinkle DA. DJ-1 knock-down in astrocytes impairs astrocyte-mediated neuroprotection against rotenone. Neurobiol. Dis. 2009;33:28–36. doi: 10.1016/j.nbd.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (NRC) Assessing the Human Health Risks of Trichloroethylene. Key Scientific Issues. Washington, DC: National Academic Press; 2006. [Google Scholar]
- Nee LE, Gomez MR, Dambrosia J, Bale S, Eldridge R, Polinsky RJ. Environmental-occupational risk factors and familial associations in multiple system atrophy: A preliminary investigation. Clin. Auton. Res. 1991;1:9–13. doi: 10.1007/BF01826052. [DOI] [PubMed] [Google Scholar]
- Needleman H. Lead poisoning. Annu. Rev. Med. 2004;55:209–222. doi: 10.1146/annurev.med.55.091902.103653. [DOI] [PubMed] [Google Scholar]
- Needleman H. Low level lead exposure: History and discovery. Ann. Epidemiol. 2009;19:235–238. doi: 10.1016/j.annepidem.2009.01.022. [DOI] [PubMed] [Google Scholar]
- Nicklas WJ, Vyas I, Heikkila RE. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci. 1985;36:2503–2508. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- Nielsen BS, Larsen EH, Ladefoged O, Lam HR. Neurotoxic effect of maneb in rats as studied by neurochemical and immunohistochemical parameters. Environ. Toxicol. Pharmacol. 2006;21:268–275. doi: 10.1016/j.etap.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Noonan CW, Kathman SJ, White MC. Prevalence estimates for MS in the United States and evidence of an increasing trend for women. Neurology. 2002;58:136–138. doi: 10.1212/wnl.58.1.136. [DOI] [PubMed] [Google Scholar]
- Nunn PB, Seelig M, Zagoren JC, Spencer PS. Stereospecific acute neuronotoxicity of 'uncommon' plant amino acids linked to human motor-system diseases. Brain Res. 1987;410:375–379. doi: 10.1016/0006-8993(87)90342-8. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, et al. Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Pappolla MA, Wade R, Hirai K, Chiba S, Smith MA. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer's disease. J. Neurosci. 1999;19:1959–1964. doi: 10.1523/JNEUROSCI.19-06-01959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley AE, Collingwood JF, Dobson J, Love G, Perrott HR, Edwardson JA, Elstner M, Morris CM. Individual dopaminergic neurons show raised iron levels in Parkinson disease. Neurology. 2007;68:1820–1825. doi: 10.1212/01.wnl.0000262033.01945.9a. [DOI] [PubMed] [Google Scholar]
- Odetti P, Angelini G, Dapino D, Zaccheo D, Garibaldi S, Dagna-Bricarelli F, Piombo G, Perry G, Smith M, Traverso N, et al. Early glycoxidation damage in brains from Down's syndrome. Biochem. Biophys. Res. Commun. 1998;243:849–851. doi: 10.1006/bbrc.1998.8186. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Araki M, Nagatsu I, Yoshida M. Astroglial cell alteration caused by neurotoxins: Immunohistochemical observations with antibodies to glial fibrillary acidic protein, laminin, and tyrosine hydroxylase. Exp. Neurol. 1989;106:187–196. doi: 10.1016/0014-4886(89)90093-9. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Good PF, Shinotoh H, Hewitt KA, Vingerhoets F, Snow BJ, Beal MF, Calne DB, Perl DP. Manganese intoxication in the rhesus monkey: A clinical, imaging, pathologic, and biochemical study. Neurology. 1996;46:492–498. doi: 10.1212/wnl.46.2.492. [DOI] [PubMed] [Google Scholar]
- Oleszak EL, Zaczynska E, Bhattacharjee M, Butunoi C, Legido A, Katsetos CD. Inducible nitric oxide synthase and nitrotyrosine are found in monocytes/macrophages and/or astrocytes in acute, but not in chronic, multiple sclerosis. Clin. Diagn. Lab. Immunol. 1998;5:438–445. doi: 10.1128/cdli.5.4.438-445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz GG, Macias-Islas MA, Pacheco-Moises FP, Cruz-Ramos JA, Sustersik S, Barba EA, Aguayo A. Oxidative stress is increased in serum from Mexican patients with relapsing-remitting multiple sclerosis. Dis. Markers. 2009;26:35–39. doi: 10.3233/DMA-2009-0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani L, Eckert A. Amyloid-Beta interaction with mitochondria. Int. J. Alzheimers Dis. 2011;2011:925050. doi: 10.4061/2011/925050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker WD, Jr, Boyson SJ, Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Ann. Neurol. 1989;26:719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Peneder TM, Scholze P, Berger ML, Reither H, Heinze G, Bertl J, Bauer J, Richfield EK, Hornykiewicz O, Pifl C. Chronic exposure to manganese decreases striatal dopamine turnover in human alpha-synuclein transgenic mice. Neuroscience. 2011;180:280–292. doi: 10.1016/j.neuroscience.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Perl DP, Brody AR. Alzheimer's disease: X-ray spectrometric evidence of aluminum accumulation in neurofibrillary tangle-bearing neurons. Science. 1980;208:297–299. doi: 10.1126/science.7367858. [DOI] [PubMed] [Google Scholar]
- Perl DP, Olanow CW. The neuropathology of manganese-induced Parkinsonism. J. Neuropathol. Exp. Neurol. 2007;66:675–682. doi: 10.1097/nen.0b013e31812503cf. [DOI] [PubMed] [Google Scholar]
- Pezzoli G, Strada O, Silani V, Zecchinelli A, Perbellini L, Javoy-Agid F, Ghidoni P, Motti ED, Masini T, Scarlato G, et al. Clinical and pathological features in hydrocarbon-induced parkinsonism. Ann. Neurol. 1996;40:922–925. doi: 10.1002/ana.410400616. [DOI] [PubMed] [Google Scholar]
- Pifl C, Khorchide M, Kattinger A, Reither H, Hardy J, Hornykiewicz O. alpha-Synuclein selectively increases manganese-induced viability loss in SK-N-MC neuroblastoma cells expressing the human dopamine transporter. Neurosci. Lett. 2004;354:34–37. doi: 10.1016/j.neulet.2003.09.064. [DOI] [PubMed] [Google Scholar]
- Porter CC, Totaro JA, Burcin A. The relationship between radioactivity and norepinephrine concentrations in the brains and hearts of mice following administration of labeled methyldopa or 6-hydroxydopamine. J. Pharmacol. Exp. Ther. 1965;150:17–22. [PubMed] [Google Scholar]
- Porter CC, Totaro JA, Stone CA. Effect of 6-hydroxydopamine and some other compounds on the concentration of norepinephrine in the hearts of mice. J. Pharmacol. Exp. Ther. 1963;140:308–316. [PubMed] [Google Scholar]
- Praticò D, Clark CM, Lee VM, Trojanowski JQ, Rokach J, FitzGerald GA. Increased 8,12-iso-iPF2alpha-VI in Alzheimer's disease: Correlation of a noninvasive index of lipid peroxidation with disease severity. Ann. Neurol. 2000;48:809–812. [PubMed] [Google Scholar]
- Praticò D, Clark CM, Liun F, Rokach J, Lee VY, Trojanowski JQ. Increase of brain oxidative stress in mild cognitive impairment: A possible predictor of Alzheimer disease. Arch. Neurol. 2002a;59:972–976. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- Praticò D, Uryu K, Leight S, Trojanoswki JQ, Lee VM. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J. Neurosci. 2001;21:4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praticò D, Uryu K, Sung S, Tang S, Trojanowski JQ, Lee VM. Aluminum modulates brain amyloidosis through oxidative stress in APP transgenic mice. FASEB J. 2002b;16:1138–1140. doi: 10.1096/fj.02-0012fje. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Vila M, Jackson-Lewis V. Neurodegeneration: What is it and where are we? J. Clin. Invest. 2003;111:3–10. doi: 10.1172/JCI17522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purzycki CB, Shain DH. Fungal toxins and multiple sclerosis: A compelling connection. Brain Res. Bull. 2010;82:4–6. doi: 10.1016/j.brainresbull.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Raine CS, Cannella B, Duijvestijn AM, Cross AH. Homing to central nervous system vasculature by antigen-specific lymphocytes. II. Lymphocyte/endothelial cell adhesion during the initial stages of autoimmune demyelination. Lab. Invest. 1990;63:476–489. [PubMed] [Google Scholar]
- Rao MV, Nixon RA. Defective neurofilament transport in mouse models of amyotrophic lateral sclerosis: A review. Neurochem. Res. 2003;28:1041–1047. doi: 10.1023/a:1023259207015. [DOI] [PubMed] [Google Scholar]
- Ravanel P, Tissut M, Douce R. Effects of rotenoids on isolated plant mitochondria. Plant Physiol. 1984;75:414–420. doi: 10.1104/pp.75.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed TT. Lipid peroxidation and neurodegenerative disease. Free Radic. Biol. Med. 2011;51:1302–1319. doi: 10.1016/j.freeradbiomed.2011.06.027. [DOI] [PubMed] [Google Scholar]
- Religa D, Strozyk D, Cherny RA, Volitakis I, Haroutunian V, Winblad B, Naslund J, Bush AI. Elevated cortical zinc in Alzheimer disease. Neurology. 2006;67:69–75. doi: 10.1212/01.wnl.0000223644.08653.b5. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Quan Y, Sherer TB, Greenamyre JT, Miller GW. Paraquat neurotoxicity is distinct from that of MPTP and rotenone. Toxicol. Sci. 2005;88:193–201. doi: 10.1093/toxsci/kfi304. [DOI] [PubMed] [Google Scholar]
- Riise T, Moen BE, Kyvik KR. Organic solvents and the risk of multiple sclerosis. Epidemiology. 2002;13:718–720. doi: 10.1097/00001648-200211000-00018. [DOI] [PubMed] [Google Scholar]
- Ritter MR, Banin E, Moreno SK, Aguilar E, Dorrell MI, Friedlander M. Myeloid progenitors differentiate into microglia and promote vascular repair in a model of ischemic retinopathy. J. Clin. Invest. 2006;116:3266–3276. doi: 10.1172/JCI29683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz BR, Manthripragada AD, Costello S, Lincoln SJ, Farrer MJ, Cockburn M, Bronstein J. Dopamine transporter genetic variants and pesticides in Parkinson's disease. Environ. Health Perspect. 2009;117:964–969. doi: 10.1289/ehp.0800277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberecht W, Sapp P, Viaene MK, Rosen D, McKenna-Yasek D, Haines J, Horvitz R, Theys P, Brown R., Jr Cu/Zn superoxide dismutase activity in familial and sporadic amyotrophic lateral sclerosis. J. Neurochem. 1994;62:384–387. doi: 10.1046/j.1471-4159.1994.62010384.x. [DOI] [PubMed] [Google Scholar]
- Roberts NB, Clough A, Bellia JP, Kim JY. Increased absorption of aluminium from a normal dietary intake in dementia. J. Inorg. Biochem. 1998;69:171–176. doi: 10.1016/s0162-0134(97)10015-0. [DOI] [PubMed] [Google Scholar]
- Rodella LF, Ricci F, Borsani E, Stacchiotti A, Foglio E, Favero G, Rezzani R, Mariani C, Bianchi R. Aluminium exposure induces Alzheimer's disease-like histopathological alterations in mouse brain. Histol. Histopathol. 2008;23:433–439. doi: 10.14670/HH-23.433. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Simon DG. A preliminary study of dietary aluminium intake and risk of Alzheimer's disease. Age Ageing. 1999;28:205–209. doi: 10.1093/ageing/28.2.205. [DOI] [PubMed] [Google Scholar]
- Rohl C, Sievers J. Microglia is activated by astrocytes in trimethyltin intoxication. Toxicol. Appl. Pharmacol. 2005;204:36–45. doi: 10.1016/j.taap.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Rojas JC, Simola N, Kermath BA, Kane JR, Schallert T, Gonzalez-Lima F. Striatal neuroprotection with methylene blue. Neuroscience. 2009;163:877–889. doi: 10.1016/j.neuroscience.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen DR, Bowling AC, Patterson D, Usdin TB, Sapp P, Mezey E, McKenna-Yasek D, O'Regan J, Rahmani Z, Ferrante RJ, et al. A frequent ala 4 to val superoxide dismutase-1 mutation is associated with a rapidly progressive familial amyotrophic lateral sclerosis. Hum. Mol. Genet. 1994;3:981–987. doi: 10.1093/hmg/3.6.981. [DOI] [PubMed] [Google Scholar]
- Ross SM, Spencer PS. Specific antagonism of behavioral action of “uncommon” amino acids linked to motor-system diseases. Synapse. 1987;1:248–253. doi: 10.1002/syn.890010305. [DOI] [PubMed] [Google Scholar]
- Roth JA, Singleton S, Feng J, Garrick M, Paradkar PN. Parkin regulates metal transport via proteasomal degradation of the 1B isoforms of divalent metal transporter 1. J. Neurochem. 2010;113:454–464. doi: 10.1111/j.1471-4159.2010.06607.x. [DOI] [PubMed] [Google Scholar]
- Rovelet-Lecrux A, Hannequin D, Raux G, Le Meur N, Laquerriere A, Vital A, Dumanchin C, Feuillette S, Brice A, Vercelletto M, et al. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat. Genet. 2006;38:24–26. doi: 10.1038/ng1718. [DOI] [PubMed] [Google Scholar]
- Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- Sachs C, Jonsson G. Mechanisms of action of 6-hydroxydopamine. Biochem. Pharmacol. 1975;24:1–8. doi: 10.1016/0006-2952(75)90304-4. [DOI] [PubMed] [Google Scholar]
- Saggu H, Cooksey J, Dexter D, Wells FR, Lees A, Jenner P, Marsden CD. A selective increase in particulate superoxide dismutase activity in parkinsonian substantia nigra. J. Neurochem. 1989;53:692–697. doi: 10.1111/j.1471-4159.1989.tb11759.x. [DOI] [PubMed] [Google Scholar]
- Santamaria AB, Sulsky SI. Risk assessment of an essential element: Manganese. J. Toxicol. Environ. Health A. 2010;73:128–155. doi: 10.1080/15287390903337118. [DOI] [PubMed] [Google Scholar]
- Santello M, Volterra A. Synaptic modulation by astrocytes via Ca2+-dependent glutamate release. Neuroscience. 2009;158:253–259. doi: 10.1016/j.neuroscience.2008.03.039. [DOI] [PubMed] [Google Scholar]
- Savory J, Herman MM, Ghribi O. Mechanisms of aluminum-induced neurodegeneration in animals: Implications for Alzheimer's disease. J. Alzheimers Dis. 2006;10:135–144. doi: 10.3233/jad-2006-102-302. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD. Mitochondrial complex I deficiency in Parkinson's disease. Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- Schaumburg HH. Trichloroethylene. In: Spencer PS, Schaumburg HH, Ludlof AC, editors. Experimental and Clinical Neurotoxicology. New York, NY: Oxford University Press; 2000. p. 1202. [Google Scholar]
- Schmidt S, Allen KD, Loiacono VT, Norman B, Stanwyck CL, Nord KM, Williams CD, Kasarskis EJ, Kamel F, McGuire V, et al. Genes and environmental exposures in veterans with amyotrophic lateral sclerosis: The GENEVA study. Rationale, study design and demographic characteristics. Neuroepidemiology. 2008;30:191–204. doi: 10.1159/000126911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarting RK, Huston JP. The unilateral 6-hydroxydopamine lesion model in behavioral brain research. Analysis of functional deficits, recovery and treatments. Prog. Neurobiol. 1996a;50:275–331. doi: 10.1016/s0301-0082(96)00040-8. [DOI] [PubMed] [Google Scholar]
- Schwarting RK, Huston JP. Unilateral 6-hydroxydopamine lesions of meso-striatal dopamine neurons and their physiological sequelae. Prog. Neurobiol. 1996b;49:215–266. doi: 10.1016/s0301-0082(96)00015-9. [DOI] [PubMed] [Google Scholar]
- Sejvar JJ, Holman RC, Bresee JS, Kochanek KD, Schonberger LB. Amyotrophic lateral sclerosis mortality in the United States, 1979-2001. Neuroepidemiology. 2005;25:144–152. doi: 10.1159/000086679. [DOI] [PubMed] [Google Scholar]
- Semchuk KM, Love EJ, Lee RG. Parkinson's disease: A test of the multifactorial etiologic hypothesis. Neurology. 1993;43:1173–1180. doi: 10.1212/wnl.43.6.1173. [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Armstrong E, Schleicher A, Zilles K, Van Hoesen GW. Prefrontal cortex in humans and apes: A comparative study of area 10. Am. J. Phys. Anthropol. 2001;114:224–241. doi: 10.1002/1096-8644(200103)114:3<224::AID-AJPA1022>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Senoh S, Creveling CR, Udenfriend S, Witkop B. Chemical, enzymatic, and metabolic studies on the mechanism of oxidation of dopamine. J. Am. Chem. Soc. 1959;81:6236–6240. [Google Scholar]
- Senoh S, Witkop B. Non-enzymatic conversions of dopamine to norepinephrine and trihydroxyphenethylamines. J. Am. Chem. Soc. 1959;81:6222–6231. [Google Scholar]
- Shaw BF, Valentine JS. How do ALS-associated mutations in superoxide dismutase 1 promote aggregation of the protein? Trends Biochem. Sci. 2007;32:78–85. doi: 10.1016/j.tibs.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Shen WB, McDowell KA, Siebert AA, Clark SM, Dugger NV, Valentino KM, Jinnah HA, Sztalryd C, Fishman PS, Shaw CA, et al. Environmental neurotoxin-induced progressive model of parkinsonism in rats. Ann. Neurol. 2010;68:70–80. doi: 10.1002/ana.22018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Kim JH, Greenamyre JT. Selective microglial activation in the rat rotenone model of Parkinson's disease. Neurosci. Lett. 2003;341:87–90. doi: 10.1016/s0304-3940(03)00172-1. [DOI] [PubMed] [Google Scholar]
- Shi P, Wei Y, Zhang J, Gal J, Zhu H. Mitochondrial dysfunction is a converging point of multiple pathological pathways in amyotrophic lateral sclerosis. J. Alzheimers Dis. 2010;20(Suppl. 2):S311–S324. doi: 10.3233/JAD-2010-100366. [DOI] [PubMed] [Google Scholar]
- Siegel SJ, Bieschke J, Powers ET, Kelly JW. The oxidative stress metabolite 4-hydroxynonenal promotes Alzheimer protofibril formation. Biochemistry. 2007;46:1503–1510. doi: 10.1021/bi061853s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard M, Nedergaard M. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience. 2004;129:877–896. doi: 10.1016/j.neuroscience.2004.09.053. [DOI] [PubMed] [Google Scholar]
- Smargiassi A, Mutti A, De Rosa A, De Palma G, Negrotti A, Calzetti S. A case-control study of occupational and environmental risk factors for Parkinson's disease in the Emilia-Romagna region of Italy. Neurotoxicology. 1998;19:709–712. [PubMed] [Google Scholar]
- Smith KJ. Sodium channels and multiple sclerosis: Roles in symptom production, damage and therapy. Brain Pathol. 2007;17:230–242. doi: 10.1111/j.1750-3639.2007.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofic E, Riederer P, Heinsen H, Beckmann H, Reynolds GP, Hebenstreit G, Youdim MB. Increased iron (III) and total iron content in post mortem substantia nigra of parkinsonian brain. J. Neural Transm. 1988;74:199–205. doi: 10.1007/BF01244786. [DOI] [PubMed] [Google Scholar]
- Son M, Puttaparthi K, Kawamata H, Rajendran B, Boyer PJ, Manfredi G, Elliott JL. Overexpression of CCS in G93A-SOD1 mice leads to accelerated neurological deficits with severe mitochondrial pathology. Proc. Natl. Acad. Sci. U.S.A. 2007;104:6072–6077. doi: 10.1073/pnas.0610923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Kanthasamy A, Anantharam V, Sun F, Kanthasamy AG. Environmental neurotoxic pesticide increases histone acetylation to promote apoptosis in dopaminergic neuronal cells: Relevance to epigenetic mechanisms of neurodegeneration. Mol. Pharmacol. 2010;77:621–632. doi: 10.1124/mol.109.062174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer PS, Nunn PB, Hugon J, Ludolph AC, Ross SM, Roy DN, Robertson RC. Guam amyotrophic lateral sclerosis-parkinsonism-dementia linked to a plant excitant neurotoxin. Science. 1987;237:517–522. doi: 10.1126/science.3603037. [DOI] [PubMed] [Google Scholar]
- Spencer PS, Nunn PB, Hugon J, Ludolph AC, Roy DN. Motorneurone disease on Guam: Possible role of a food neurotoxin. Lancet. 1986;1:965. doi: 10.1016/s0140-6736(86)91059-7. [DOI] [PubMed] [Google Scholar]
- Spencer PS, Schaumburg HH, Ludolph AC. Experimental and Clinical Neurotoxicology. Oxford, U.K: Oxford University Press; 2000. [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Sriram K, Lin GX, Jefferson AM, Roberts JR, Wirth O, Hayashi Y, Krajnak KM, Soukup JM, Ghio AJ, Reynolds SH, et al. Mitochondrial dysfunction and loss of Parkinson's disease-linked proteins contribute to neurotoxicity of manganese-containing welding fumes. FASEB J. 2010;24:4989–5002. doi: 10.1096/fj.10-163964. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: High-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struzynska L. A glutamatergic component of lead toxicity in adult brain: The role of astrocytic glutamate transporters. Neurochem. Int. 2009;55:151–156. doi: 10.1016/j.neuint.2009.01.025. [DOI] [PubMed] [Google Scholar]
- Stys PK. General mechanisms of axonal damage and its prevention. J. Neurol. Sci. 2005;233:3–13. doi: 10.1016/j.jns.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpade DJ, Greene JG, Higgins DS, Jr, Greenamyre JT. In vivo labeling of mitochondrial complex I (NADH:ubiquinone oxido-reductase) in rat brain using [(3)H]dihydrorotenone. J. Neurochem. 2000;75:2611–2621. doi: 10.1046/j.1471-4159.2000.0752611.x. [DOI] [PubMed] [Google Scholar]
- Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, Marras C, Bhudhikanok GS, Kasten M, Chade AR, et al. Rotenone, paraquat, and Parkinson's disease. Environ. Health Perspect. 2011;119:866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA. The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: Implications for Parkinson's disease. J. Neurosci. 2000;20:9207–9214. doi: 10.1523/JNEUROSCI.20-24-09207.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany-Castiglioni E. Cell culture models for lead toxicity in neuronal and glial cells. Neurotoxicology. 1993;14:513–536. [PubMed] [Google Scholar]
- Tiffany-Castiglioni E, Zmudzki J, Bratton GR. Cellular targets of lead neurotoxicity: In vitro models. Toxicology. 1986;42:303–315. doi: 10.1016/0300-483x(86)90018-1. [DOI] [PubMed] [Google Scholar]
- Tiffany-Castiglioni E, Zmudzki J, Wu JN, Bratton GR. Effects of lead treatment on intracellular iron and copper concentrations in cultured astroglia. Metab. Brain Dis. 1987;2:61–79. doi: 10.1007/BF00999509. [DOI] [PubMed] [Google Scholar]
- Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J. The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology. 2004;62:1984–1989. doi: 10.1212/01.wnl.0000129697.01779.0a. [DOI] [PubMed] [Google Scholar]
- Tretiakoff C. Contribution a l'etude de l'anatomie pathologique du locus niger de Sommering avec quelques deductions relatives a la pathogenie des troubles du tonus musculaires et de maladie de Parkinson. Paris, France: University of Paris; 1919. [Google Scholar]
- Uitti RJ, Snow BJ, Shinotoh H, Vingerhoets FJ, Hayward M, Hashimoto S, Richmond J, Markey SP, Markey CJ, Calne DB. Parkinsonism induced by solvent abuse. Ann. Neurol. 1994;35:616–619. doi: 10.1002/ana.410350516. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur. J. Pharmacol. 1968;5:107–110. doi: 10.1016/0014-2999(68)90164-7. [DOI] [PubMed] [Google Scholar]
- Vande Velde C, Miller TM, Cashman NR, Cleveland DW. Selective association of misfolded ALS-linked mutant SOD1 with the cytoplasmic face of mitochondria. Proc. Natl. Acad. Sci. U.S.A. 2008;105:4022–4027. doi: 10.1073/pnas.0712209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayvergiya C, Beal MF, Buck J, Manfredi G. Mutant superoxide dismutase 1 forms aggregates in the brain mitochondrial matrix of amyotrophic lateral sclerosis mice. J. Neurosci. 2005;25:2463–2470. doi: 10.1523/JNEUROSCI.4385-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt J, Paul F, Aktas O, Muller-Wielsch K, Dorr J, Dorr S, Bharathi BS, Glumm R, Schmitz C, Steinbusch H, et al. Lower motor neuron loss in multiple sclerosis and experimental autoimmune encephalomyelitis. Ann. Neurol. 2009;66:310–322. doi: 10.1002/ana.21719. [DOI] [PubMed] [Google Scholar]
- Wang CY, Wang T, Zheng W, Zhao BL, Danscher G, Chen YH, Wang ZY. Zinc overload enhances APP cleavage and Abeta deposition in the Alzheimer mouse brain. PLoS One. 2010;5:e15349. doi: 10.1371/journal.pone.0015349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Rahman MF, Duhart HM, Newport GD, Patterson TA, Murdock RC, Hussain SM, Schlager JJ, Ali SF. Expression changes of dopaminergic system-related genes in PC12 cells induced by manganese, silver, or copper nanoparticles. Neurotoxicology. 2009;30:926–933. doi: 10.1016/j.neuro.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Waring SC, Rosenberg RN. Genome-wide association studies in Alzheimer disease. Arch. Neurol. 2008;65:329–334. doi: 10.1001/archneur.65.3.329. [DOI] [PubMed] [Google Scholar]
- Waseem M, Ali M, Dogra S, Dutta KK, Kaw JL. Toxicity of trichloroethylene following inhalation and drinking contaminated water. J. Appl. Toxicol. 2001;21:441–444. doi: 10.1002/jat.778. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Axonal conduction and injury in multiple sclerosis: The role of sodium channels. Nat. Rev. Neurosci. 2006;7:932–941. doi: 10.1038/nrn2023. [DOI] [PubMed] [Google Scholar]
- Way JL. Cyanide intoxication and its mechanism of antagonism. Annu. Rev. Pharmacol. Toxicol. 1984;24:451–481. doi: 10.1146/annurev.pa.24.040184.002315. [DOI] [PubMed] [Google Scholar]
- Weiner HL. A 21 point unifying hypothesis on the etiology and treatment of multiple sclerosis. Can. J. Neurol. Sci. 1998;25:93–101. doi: 10.1017/s0317167100033680. [DOI] [PubMed] [Google Scholar]
- Wenk GL. Neuropathologic changes in Alzheimer's disease. J. Clin. Psychiatry. 2003;64(Suppl. 9):7–10. [PubMed] [Google Scholar]
- Wenning GK, Stefanova N, Jellinger KA, Poewe W, Schlossmacher MG. Multiple system atrophy: A primary oligodendrogliopathy. Ann. Neurol. 2008;64:239–246. doi: 10.1002/ana.21465. [DOI] [PubMed] [Google Scholar]
- Wesseling C, van Wendel de Joode B, Ruepert C, León C, Monge P, Hermosillo H, Partanen TJ. Paraquat in developing countries. Int. J. Occup. Environ. Health. 2001;7:275–286. doi: 10.1179/107735201800339209. [DOI] [PubMed] [Google Scholar]
- White AR, Reyes R, Mercer JF, Camakaris J, Zheng H, Bush AI, Multhaup G, Beyreuther K, Masters CL, Cappai R. Copper levels are increased in the cerebral cortex and liver of APP and APLP2 knockout mice. Brain Res. 1999;842:439–444. doi: 10.1016/s0006-8993(99)01861-2. [DOI] [PubMed] [Google Scholar]
- White LD, Cory-Slechta DA, Gilbert ME, Tiffany-Castiglioni E, Zawia NH, Virgolini M, Rossi-George A, Lasley SM, Qian YC, Basha MR. New and evolving concepts in the neurotoxicology of lead. Toxicol. Appl. Pharmacol. 2007;225:1–27. doi: 10.1016/j.taap.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Whitman MC, Greer CA. Adult neurogenesis and the olfactory system. Prog. Neurobiol. 2009;89:162–175. doi: 10.1016/j.pneurobio.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Basha MR, Brock B, Cox DP, Cardozo-Pelaez F, McPherson CA, Harry J, Rice DC, Maloney B, Chen D, et al. Alzheimer's disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): Evidence for a developmental origin and environmental link for AD. J. Neurosci. 2008;28:3–9. doi: 10.1523/JNEUROSCI.4405-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Ohno S, Okayasu I, Okeda R, Hatakeyama S, Watanabe H, Ushio K, Tsukagoshi H. Chronic manganese poisoning: A neuropathological study with determination of manganese distribution in the brain. Acta Neuropathol. 1986;70:273–278. doi: 10.1007/BF00686083. [DOI] [PubMed] [Google Scholar]
- Yumoto S, Kakimi S, Ohsaki A, Ishikawa A. Demonstration of aluminum in amyloid fibers in the cores of senile plaques in the brains of patients with Alzheimer's disease. J. Inorg. Biochem. 2009;103:1579–1584. doi: 10.1016/j.jinorgbio.2009.07.023. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Deane R, Ali Z, Parisi M, Shapovalov Y, O'Banion MK, Stojanovic K, Sagare A, Boillee S, Cleveland DW. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat. Neurosci. 2008;11:420–422. doi: 10.1038/nn2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular mechanisms of Alzheimer's neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]