Abstract
Treatment with aryl hydrocarbon receptor (AhR) agonists can slow or reverse the growth of primary mammary tumors in rodents, which has fostered interest in developing selective AhR modulators for treatment of breast cancer. However, the major goal of breast cancer therapy is to inhibit metastasis, the primary cause of mortality in women with this disease. Studies conducted using breast cancer cell lines have demonstrated that AhR agonists suppress proliferation, invasiveness, and colony formation in vitro; however, further exploration using in vivo models of metastasis is warranted. To test the effect of AhR activation on metastasis, 4T1.2 mammary tumor cells were injected into the mammary gland fat pad of syngeneic Balb/c mice treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Primary tumor growth was monitored for 4 weeks, at which time metastasis was determined. TCDD treatment suppressed metastasis by approximately 50%, as measured both in the lung and in mammary glands at sites distant from the primary tumor. Primary tumor growth was not suppressed by TCDD exposure nor was proliferation of 4T1.2 cells affected by TCDD treatment in vitro. Taken together, these results suggest that the protective effect of AhR activation was selective for the metastatic process and not simply the result of a direct decrease in tumor cell proliferation or survival at the primary site. These observations in immunologically intact animals warrant further investigation into the mechanism of the protective effects of AhR activation and support the promise for use of AhR modulators to treat breast cancer.
Keywords: AhR, metastasis, breast cancer, TCDD
Breast cancer is the second most common cancer in U.S. women, with approximately 250,000 cases diagnosed annually. Overall incidence rates for breast cancer have declined slightly over the last 15 years, and treatments including surgery, radiation, and systemic therapies continue to improve life expectancies. Still, breast cancer claims the lives of about 40,000 women annually in the United States, and behind lung cancer, it is the second leading cause of cancer deaths (American Cancer Society, 2009). New strategies are therefore needed both in prevention and treatment of this disease.
The aryl hydrocarbon receptor (AhR) is an orphan nuclear receptor that belongs to the per-arnt-sim (PAS) family of transcriptional regulators (Beischlag et al., 2008). For many years the AhR was studied because of its role in the toxic effects caused by certain environmental contaminants, including dioxins, polychlorinated biphenyls, and polyaromatic hydrocarbons. However, there is now mounting evidence that the receptor also has endogenous ligands and that it plays an important role in normal physiologic responses such as development, cell cycle regulation, and immune function (Barouki et al., 2007; Marlowe and Puga, 2005; Nguyen and Bradfield, 2008; Puga et al., 2005; Veldhoen and Duarte, 2010).
AhR ligands may also prove to have therapeutic uses, such as treating diseases caused by abnormal immune function and certain cancers (Benson and Shepherd, 2011; Kerkvliet et al., 2009; Lawrence et al., 2008; Murray et al., 2010; Zhang et al., 2009). With specific regard to breast cancer, AhR agonists including 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), 3, 3′-diindolylmethane (DIM), and 6-methyl-1,3,8-trichlorodibenzo-furan (MCDF) are known to reduce or reverse mammary tumor formation in rodent models. More specifically, over 30 years ago, Kociba et al. (1978) reported that long-term dietary exposure to TCDD reduced incidence of spontaneous mammary tumors, despite increasing tumors at other locations. Subsequent studies conducted by Safe and colleagues demonstrated that AhR agonists cause regression of existing chemical-induced tumors and inhibit growth of human breast cancer cells injected into nude mice (Chen et al., 1998; Holcomb and Safe, 1994; McDougal et al., 1997; Zhang et al., 2009). Additionally, studies in our laboratory show that treating mice with TCDD 4 weeks prior to DMBA administration significantly delays the development of mammary tumors (Wang et al., 2011). Collectively, such discoveries have provided a foundation for exploration of selective AhR modulators for therapeutic use in breast cancer therapy (Safe and McDougal, 2002; Safe et al., 1999).
Despite this promising evidence that AhR modulation may be useful in controlling primary tumor growth, a more important therapeutic goal for breast cancer treatment is to reduce or prevent metastasis. Although primary tumors are often successfully treated, for example, by surgical removal, metastatic spread of the disease remains the major cause of morbidity and mortality and is much harder to treat successfully. To date, few published studies have addressed the effect of AhR agonists on breast cancer metastasis. In vitro studies demonstrate that TCDD and other AhR ligands reduce proliferation, invasion, motility, and colony formation in cultured breast tumor cell lines (Hall et al., 2010; Hsu et al., 2007; Zhang et al., 2009). In addition, an anti-allergy drug known as Tranilast, which has recently been shown to activate the AhR, has inhibitory effects on breast cancer cells in vitro and reduces breast cancer cell metastasis in mouse models (Chakrabarti et al., 2009; Prud'homme et al., 2010). The conclusions from these studies suggest that AhR agonists may indeed provide a novel therapeutic strategy for reducing metastatic disease. However, additional in vivo studies using well-characterized AhR agonists are needed in order to determine whether AhR activation reduces mammary tumor metastasis.
In the current studies, we tested the effect of TCDD exposure on mammary tumor growth and metastasis using 4T1.2 cells implanted into the mammary fat pad. This is a subline of 4T1 tumor cells, which were originally derived from a spontaneous mammary carcinoma in a Balb/c mouse (Lelekakis et al., 1999). The 4T1 model is one of the best available models for breast cancer metastasis, and tumors spontaneously metastasize to lung, lymph nodes, liver, bone, and other sites in a pattern analogous to human breast cancer (Olkhanud et al., 2009; Pulaski and Ostrand-Rosenberg, 2000). One particular benefit of this model is that immunocompetent Balb/c mice can be used as hosts, which permits the contribution of the immune response to be considered. This is extremely important when evaluating the effects of AhR agonists as these compounds are potent immunomodulators and could influence the success of metastasis through a number of different cellular mechanisms. TCDD treatment significantly suppressed metastatic spread of tumors to the lung and to other mammary glands, despite having no effect on primary tumor growth. Our findings indicate that AhR activation in this model selectively inhibits processes required for tumors to successfully disseminate to other sites.
MATERIALS AND METHODS
Tumor metastasis model.
The 4T1.2 breast cancer cell line, which is highly metastatic to lung (Lelekakis et al., 1999), was provided by Cheryl Jorcyk (Boise State University, Boise, ID) and used with permission of Robin Anderson (Peter MacCallum Cancer Centre, East Melbourne, Australia). Cells were routinely cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, sodium pyruvate, and antibiotics. For tumor studies, Balb/c mice were anesthetized with ketamine and xylazine, and a small incision was made in the skin to expose the right mammary gland fat pad. 4T1.2 cells (1 × 105 in 50 μl PBS) were injected into the fat pad, and the wound was closed with wound clips. The number of animals in each treatment group was 10–15, depending on the study.
Animals and TCDD treatment.
Female Balb/c mice were obtained from NCI Charles River (Frederick, MD), and were approximately 10 weeks of age at the beginning of the experiments. Animals were housed singly, given food and water ad libitum, and were maintained on a 12:12-h light cycle. TCDD (Cambridge Isotope Laboratories, Woburn, MA) was dissolved in anisole and diluted in peanut oil to a concentration for dosing at 10 μl/g body weight. Mice were gavaged weekly with 5 μg/kg TCDD or peanut oil-anisole vehicle, commencing 2 weeks prior to injection of 4T1.2 tumor cells. Animals were euthanized 4 weeks after tumor cell injection, so a total of 6 TCDD treatments were administered. This TCDD exposure paradigm was chosen in order to provide a preconditioning period for tissue at both the primary tumor site and at secondary sites for metastasis because the 4T1.2 tumors grow and metastasize very rapidly. Additionally, in previous studies, we found that pretreatment with TCDD delayed the onset of primary mammary tumors in a different tumor model (Wang et al., 2011). The half-life of TCDD in mice is ∼1 week (Gasiewicz et al., 1983). All animal treatments were in accordance with protocols approved by the Washington State University Institutional Animal Care and Use Committee.
Evaluation of primary tumor and of metastasis.
Beginning 2 weeks after injection of 4T1.2 cells, the growth of the primary tumor was measured twice a week using a vernier caliper. Two perpendicular diameters, termed length (L) and width (W), were determined, with length defined as the larger of the two measurements. Volume was calculated using the formula: 4/3 × π × (l/2) × (W/2)2. Mice were euthanized 4 weeks after injection of 4T1.2 cells because mice often become moribund between weeks 4 and 6. Primary tumors were removed and weighed. Metastases were evaluated in lungs, which is typical for this tumor due to their visibility and ease of quantification. Lungs were fixed in Bouin’s solution, and metastases were quantified by counting on a dissecting microscope. In addition, tumor nodules that had formed on the mammary glands distant from the primary tumor (right and left thoracic and cervical glands and the left abdominal glands) were counted at necropsy. Histological analysis of representative mammary gland nodules was conducted by a board-certified veterinary pathologist (T.B.W.) and revealed a mix of tumors and tumor-containing lymph nodes.
Cell proliferation.
4T1.2 cells were seeded in 96-well plates (2 × 104 cells per well) in media containing 1nM TCDD (dissolved in dimethyl sulfoxide [DMSO]) or 0.1% DMSO vehicle. 1nM TCDD was used for all the in vitro studies and is within a typical dose range that exhibits effects on normal and cancer-derived breast cells in culture (Ahn et al., 2005; Zhang et al., 2009). Media were changed daily. Cell proliferation was measured on days 1–4 of TCDD treatment using a WST-1 Quick Cell Proliferation Assay Kit (BioVision, Mountain View, CA). As a positive control for suppressed proliferation, a WST-1 assay was also conducted using 4T1.2 cells treated with 10 μg/ml Mitomycin C.
Migration assay.
4T1.2 cells were treated with 1nM TCDD or 0.1% DMSO for 24 h. The cells were then trypsinized and resuspended in fetal bovine serum (FBS)–free media containing 0.1% bovine serum albumin and 1nM TCDD or DMSO vehicle. Cell suspensions (5 × 103 cells per well) were added to the top chamber of transwells containing 8-μm pore size membranes (BD Biosciences) (Takahashi et al., 2008). The bottom chamber contained RPMI medium with 10% FBS and 1nM TCDD or DMSO. Cells were incubated for 24 h at 37°C, at which time cells remaining in the insert were removed with a cotton swab. Migrated cells on the bottom of the filters were fixed with methanol and stained with crystal violet. The numbers of cells in five adjacent fields of view were counted under ×100 magnification. As a positive control for suppression of migration, 4T1.2 cultures were treated with 0.3% ethanol.
Colony formation assay.
A suspension of 4T1.2 cells was prepared in 0.35% agar containing 1nM TCDD or DMSO vehicle. The agar-cell suspensions were added to a 6-well plate (103 cells per well) over a base agar layer (0.5%) that also contained 1nM TCDD or DMSO. Agar was overlayed with media containing 1nM TCDD or DMSO, which was changed twice a week. Plates were incubated at 37°C in a humidified incubator for 3 weeks. At termination, the colonies were stained with Crystal Violet and counted on a dissecting microscope (Hsu et al., 2007). Positive colonies were defined as clusters consisting of more than 30 cells. As a positive control for suppression of colony formation, 4T1.2 cells were treated with 10 μg/ml Mitomycin C for 4 h prior to suspension in agar.
Western blotting.
The levels of AhR and cytochrome P450s (Cyps) 1a1 and 1b1 were assessed in proteins extracted from cultured 4T1.2 cells and from primary tumors snap frozen at the termination of the metastasis study. 4T1.2 cell extracts were prepared following treatment with 1nM TCDD or DMSO vehicle for 24 or 48 h. Tumor samples from vehicle- and TCDD-treated mice were homogenized in RIPA buffer using a tissue tearer. Proteins were separated on 8% acrylamide gels and transferred to polyvinylidene difluoride membranes. Blots were probed with primary antibodies for AhR (Abcam, Cambridge, MA), Cyp1a1, Cyp1b1, and Actin (all from Santa Cruz Biotech, Santa Cruz, CA). Corresponding secondary antibodies included: IRDye 700DX-conjugated anti-goat IgG (Rockland, Inc. Gilbertsville, PA) and IRDye 800CW-conjugated anti-rabbit IgG and anti-mouse IgG (LI-COR Biosciences, Lincoln, NE). Bands were visualized and quantified using the LI-COR Odyssey Infrared Imaging System (LI-COR Biosciences).
Statistics.
Data were analyzed using Prism software (GraphPad Software, Inc., La Jolla, CA). Values obtained from vehicle- and TCDD-treated mice were compared using a Student's t-test, and a two-sided p value of ≤ 0.05 was considered significant.
RESULTS
AhR Activation Reduces Metastasis of Tumor Cells to the Lung and to Mammary Glands Distant from the Primary Tumor Site
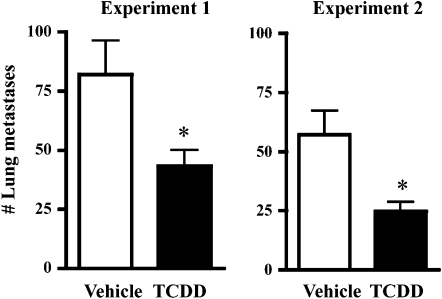
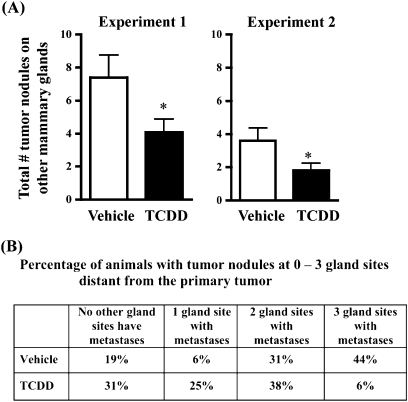
To determine whether AhR activation influences metastasis in an in vivo model of breast cancer metastasis, vehicle- and TCDD-treated Balb/c mice were injected with syngeneic 4T1.2 mammary tumor cells into the right abdominal mammary gland. Metastases were quantified 4 weeks after tumor cell injection. As shown in Figure 1, AhR activation suppressed mammary tumor metastasis to the lung. The magnitude of the reduction was approximately 50%, and similar results were obtained in two independent experiments. In addition to lung metastases, tumors that had spread to mammary glands distant from the primary tumor site were also enumerated. Specifically, visible nodules present at three additional gland sites, the right thoracic and cervical glands, the left thoracic and cervical glands, and the left abdominal glands, were quantified at necropsy. Similar to effects observed in the lung, the number of tumors that had spread to the other mammary glands was significantly suppressed in the TCDD-treated animals (Fig. 2A). Additionally, fewer gland sites were tumor positive in the TCDD-treated animals (Fig. 2B). For example, 44% of vehicle-treated mice had at least one tumor at all three mammary gland sites, whereas only 6% of TCDD-treated animals had tumors at all three sites.
FIG. 1.
Inhibition of tumor metastasis to the lung. Two separate experiments were conducted using female Balb/c mice orthotopically injected with 4T1.2 mammary tumor cells. Mice were treated weekly with vehicle or 5 μg/kg TCDD, beginning 2 weeks before tumor cell injection. Lungs were removed 4 weeks after tumor cell injection and fixed in Bouin’s solution, which causes the metastases to turn bright yellow. Bars represent the mean number (±SEM) of visible metastases. *p = 0.003 for Experiment 1 and *p = 0.016 for Experiment 2. The number of mice was 10–15 per treatment group for each of the two experiments.
FIG. 2.
Inhibition of tumor metastasis to other mammary glands. Vehicle- and TCDD-treated Balb/c mice were injected with 4T1.2 cells into the right abdominal mammary gland. Tumor nodules on mammary glands distant from the primary tumor site (the right thoracic and cervical glands, the left thoracic and cervical glands, and the left abdominal gland sites) were counted at necropsy. Histopathologic analysis of representative nodules on mammary glands from 4T1.2-injected animals revealed they were a mix of tumors and tumor-containing lymph nodes (see Supplementary fig. 2). (A) Bars represent the mean (±SEM) of the total number of tumor nodules counted on glands at all three sites. *p = 0.03 for both experiments. (B) The percentage of animals in each treatment group that had visible tumor nodules at 0, 1, 2, or all 3 mammary gland sites (right thoracic/cervical, left thoracic/cervical, and/or left abdominal).
Growth of the Primary Tumor Is Unaffected by TCDD Treatment
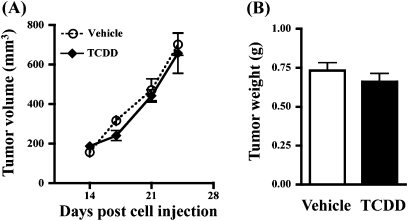
A number of studies in rats and mice demonstrate that AhR agonists can inhibit or reverse the growth of primary mammary tumors. Therefore, it was important to determine whether the reduction in metastasis was correlated with an overall suppressive effect on primary tumor growth. To address this, the volume of the primary tumor was monitored throughout the growth phase, and the weight of the tumor was determined at necropsy. Neither the rate of primary tumor growth (Fig. 3A) nor the final tumor size (Fig. 3B) was altered by TCDD treatment. These results indicate that the protective effect of TCDD against metastasis is not simply a reflection of a direct insult to tumor cell proliferation or survival at the primary site.
FIG. 3.
AhR activation does not affect tumor growth at the primary site. (A) Beginning 2 weeks after injection of 4T1.2 cells, the growth of the primary tumor was measured twice a week using a vernier caliper. (B) Primary tumors were removed and weighed 4 weeks after tumor cell injection. Data shown are from Experiment 1. Similar results were obtained for Experiment 2.
AhR Expression in 4T1.2 Cells and Response to TCDD
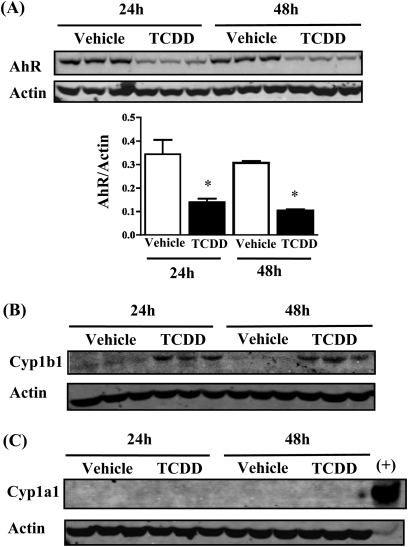
4T1.2 cells are derived from a mouse strain (Balb/c) that has a high-affinity AhRb allele, so it was possible that the tumor cells were direct targets for actions of TCDD. To address this possibility, AhR expression was assessed in cultured 4T1.2 cells, and functional activity of the receptor was tested by measuring induction of Cyp1a1 and Cyp1b1 in response to TCDD treatment. As shown in Figure 4A, AhR protein was detected in 4T1.2 cell extracts, and the receptor was downregulated in response to TCDD treatment. Cyp1b1 protein was induced following TCDD treatment, providing evidence that the receptor is functionally active in these tumor cells (Fig. 4B). In contrast, Cyp1a1 was neither expressed in 4T1.2 cells nor induced in response to TCDD treatment (Fig. 4B).
FIG. 4.
AhR expression and activity in cultured 4T1.2 cells. Triplicate cultures of 4T1.2 cells were treated with 1nM TCDD or 0.1% DMSO vehicle for 24 or 48 h; 30 μg of extracted protein from each culture was analyzed by Western blotting. Actin (43 kDa) was used as loading control. (A) AhR protein (104 kDa) was expressed by 4T1.2 cells and was significantly downregulated in the TCDD-treated cultures. Bar graph shows average AhR band intensities normalized to actin. *p < 0.05. (B) Cyp1b1 (57 kDa) was upregulated in the TCDD-treated cultures at both time points. (C) Cyp1a1 (56 kDa) was not detected in protein extracts from either vehicle- or TCDD-exposed cells. Liver protein extract (20 μg) from a TCDD-treated mouse was included as positive control (+) for Cyp1a1.
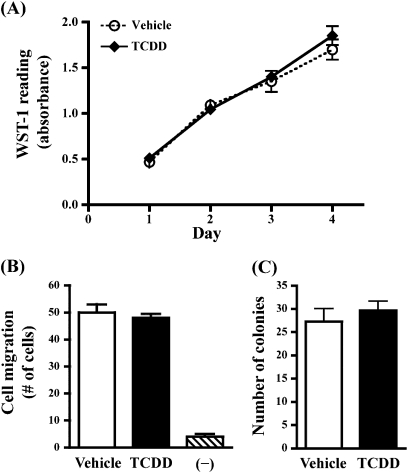
TCDD Exposure In Vitro Does Not Alter Proliferation, Migration, or Colony Formation of 4T1.2 Cells
Given that 4T1.2 cells express a functional AhR, it was possible that TCDD could impact the tumor cells directly to impair cellular processes necessary for metastasis. The potential for 1nM TCDD to directly suppress 4T1.2 cell activity was therefore tested using standard cell culture assays. 4T1.2 cell growth over time was assessed using a WST-1 assay (Fig. 5A) and by counting cells on a hemocytometer (data not shown). Additionally, cell migration across a porous membrane in response to a chemoattractive stimulus (Fig. 5B) and the formation of colonies in soft agar (Fig. 5C) were also examined. None of these endpoints were affected by exposure to 1nM TCDD.
FIG. 5.
4T1.2 cell proliferation, migration, and colony formation are unaffected by AhR activation in vitro. (A) 4T1.2 cells were treated daily with media containing 1nM TCDD or DMSO vehicle. Cell proliferation was evaluated using a WST-1 assay on days 1–4 of treatment. Error bars indicate SEM, of triplicate cultures. Absorbance readings of positive control cultures treated with Mitomycin C were reduced by 56% on day 1 (data not shown). (B) The effect of TCDD on the migratory capacity of 4T1.2 cells across a semi-permeable membrane was determined. Cells were pretreated with 1nM TCDD for 24 h and then added to the top chamber of a transwell system. Bar graphs show the average number of cells that migrated across the semi-permeable membrane in response to a chemoattractive stimulus (FBS) placed in the lower well (±SEM, from triplicate wells). TCDD or DMSO was also present in the media of both transwell chambers during the migration assay. “−” indicates the amount of background cell migration in transwells containing no FBS as a chemoattractant. Positive control cultures treated with ethanol showed 40% suppression of migration (data not shown). (C) To determine the effect of TCDD on colony formation, 4T1.2 cells were cultured in semi-solid agar containing 1nM TCDD or DMSO vehicle. Bar graphs show the average number of colonies that formed after 3 weeks of culture (±SEM, of triplicate cultures). Similar results were obtained when 4T1.2 cells were also pretreated with TCDD for 2 days prior to seeding in agar (data not shown). Positive control cultures treated with Mitomycin C showed >90% reduction in colony formation (data not shown).
AhR and Cyp Expression in Primary Tumors
Numerous mammary tumors and tumor cell lines have been shown to express high levels of AhR, which may influence proliferation, death, or other behaviors of tumor cells (Kohle et al., 2002; Wu et al., 2011; Yang et al., 2005). Expression of AhR and Cyp proteins was therefore assessed in the primary tumors removed at the termination of the metastasis study. In contrast with results observed in the cultured 4T1.2 cells, levels of AhR detected in the primary tumors were low, variable between animals, and did not show a consistent expression pattern in response to TCDD treatment (Supplementary figure 1A). Cyp1b1 was moderately induced in tumors from TCDD-treated animals, indicating potential direct actions of TCDD on cells in the primary tumor, although the levels of this enzyme were low (Supplementary figure 1). Cyp1a1 was not detected in primary tumors from either treatment group (Supplementary figure 1B), which is consistent with results obtained using the cultured tumor cells.
DISCUSSION
Evidence collected over the past three decades generally supports the idea that AhR agonists may have beneficial effects with regard to breast cancer. In fact, although TCDD itself is classified as a tumor promoter and increases the incidence of certain liver and skin tumors (Hébert et al., 1990; Watson et al., 1995), this potent AhR agonist is protective against primary mammary tumors in a number of rodent models. (Chen et al., 1998; Holcomb and Safe, 1994; Kociba et al., 1978; McDougal et al., 1997; Wang et al., 2011; Zhang et al., 2009). Although it is promising that AhR agonists may be useful for suppressing growth of primary tumors, ultimately a more important goal for breast cancer therapy is to suppress the spread and growth of metastases at secondary sites. Therefore, in the current studies, we tested the effect of TCDD exposure on mammary tumor growth and metastasis using orthotopically implanted 4T1.2 cells. This subline of 4T1 tumor cells was originally selected based on its aggressive properties and is particularly metastatic to the lung (Lelekakis et al., 1999; Parker et al., 2008). One benefit of this model is that immunocompetent mice can be used, in contrast to xenogeneic tumor models that utilize nude and other immunocompromised animals. The immune response itself is an important factor in controlling metastasis of 4T1 and other tumors (Tao et al., 2008). Because many AhR agonists are potent immunomodulators, it is important to evaluate these compounds using models that preserve an intact immune system.
In two independent experiments, we found that TCDD treatment suppressed mammary tumor metastasis to the lung by approximately 50%. In addition to the lung, metastatic spread to mammary glands distant from the primary tumor site was significantly inhibited, and fewer gland sites were involved. To the best of our knowledge, this is the first report of the effect TCDD using a syngeneic model of breast cancer metastasis. However, two other compounds with AhR agonist activity have been tested in related models, revealing similar effects to suppress metastasis. One compound is DIM, a metabolite of indole-3-carbinol that binds AhR with relatively low affinity. Using a model where the 4T1 parent cell line was injected iv, DIM was shown to inhibit the formation of lung tumors in Balb/c mice (Kim et al., 2009). The other compound is Tranilast, a synthetic tryptophan metabolite derivative that is used as an anti-allergy drug and that was recently found to activate the AhR (Kerkvliet, 2009; Prud'homme et al., 2010). Tranilast exerts a number of suppressive effects on mammary tumor cell growth and metastasis, including suppressing metastasis of 4T1 cells to the lung (Chakrabarti et al., 2009; Prud'homme et al., 2010). In addition to breast cancer metastasis, AhR activation has also been shown to reduce metastasis of prostate tumors. Specifically, Fritz et al. (2009) observed a fivefold reduction in prostate tumor metastasis in mice treated with the AhR agonist MCDF. Collectively, these observations in metastasis models are very promising and support the idea that AhR agonists have utility beyond their effects to suppress growth of primary tumors.
In fact, growth of the primary tumor itself was unaffected by TCDD in our current studies. This result is not concerning from a therapeutic application standpoint because primary breast tumors in women are typically removed surgically. However, this result is in apparent contrast with the suppressive effects of AhR agonists on primary mammary tumor growth observed in other studies. One important consideration is that most studies demonstrating suppressed primary tumor growth, including our own prior report, were conducted using models of spontaneous tumor formation or with chemical-induced tumors (Chen et al., 1998; Holcomb and Safe, 1994; Kociba et al., 1978; McDougal et al., 1997; Wang et al., 2011). In those cases, AhR agonists are likely to inhibit primary tumors via influencing either the initiation or promotion stages of carcinogenesis. In contrast, 4T1.2 cells were intentionally selected because of their aggressive metastatic properties and therefore best reflect tumors that have already reached the progression stage of cancer. Thus, 4T1.2 cells may already have reached a stage at which they are insensitive to AhR-mediated control of cell cycle or apoptosis. Nevertheless, suppressed primary tumor growth of orthotopically injected metastatic cells lines was also observed in both the Tranilast-4T1 study discussed above (Chakrabarti et al., 2009) and in a recent study using human MDA-MB-468 cells injected into MCDF-treated nude mice (Zhang et al., 2009). It is noteworthy that for the Tranilast and MCDF reports, the chemical treatment suppressed both tumor cell proliferation in vitro and primary tumor growth in vivo. In contrast, neither endpoint was affected by TCDD in our study. It is possible that the different outcomes regarding primary tumor growth reflect differing receptor affinity or activity of the compound used or unique characteristics of the individual cell lines. For example, a population of cells with altered sensitivity to TCDD could have been enriched during the selection of 4T1.2 cells for enhanced metastatic potential.
The fact that primary tumor growth was unaffected by TCDD in the 4T1.2 model also raised a question about whether the tumor cells could even be direct targets for TCDD. To address this, AhR protein expression was assessed in the 4T1.2 cells, and their ability to respond to TCDD was tested by measuring Cyp1a1 and Cyp1b1, two enzymes that are classically upregulated in response to AhR activation. AhR protein was detected in cell extracts, and Cyp1b1 was induced in the TCDD-treated cultures, demonstrating that the receptor was functional. 4T1.2 cells also responded to TCDD treatment in vitro by downregulating AhR, a response commonly observed in tissues and cultured cells exposed to AhR ligands. In fact, proteasomal degradation of the receptor may serve as a mechanism to control AhR-mediated gene transcription (Pollenz, 2002). TCDD treatment did not induce Cyp1a1 expression by 4T1.2 cells, although this is not surprising given the wide variation in expression of this enzyme between different breast epithelial and tumor cell lines (Spink et al., 1998). Interestingly, others have suggested that AhR-mediated induction of Cyp1a1 may be dependent upon presence of the estrogen receptor (ER) (Spink et al., 1998). This would support the lack of Cyp1a1 induction in the 4T1.2 cells, which are ER negative (Banka et al., 2006).
In vitro studies with the murine 4T1.2 cells also did not reveal direct effects of 1nM TCDD on colony formation in soft agar or migration across a semi-permeable membrane. However, others have demonstrated suppression of these endpoints in studies conducted with certain human cell lines, including MCF-7 and MDA MB-231 cells (Hall et al., 2010; Hsu et al., 2007). The dissimilar results may reflect differences in species, in tumor cell aggressiveness, or in the dose of TCDD that was used (e.g., 1nM in our studies vs. 10 and 100nM, respectively, in the studies of Hall et al. and Hsu et al.). Furthermore, differences in assay design may influence the observed effects of TCDD. For example, in the report by Hsu et al., TCDD treatment inhibited MCF-7 migration when CXCL12 was used as a chemoattractant; but consistent with our observations, there was no TCDD effect when FBS was used. Finally, in a different study that used collagen as a chemoattractant, TCDD treatment was shown to enhance rather than inhibit MCF-7 cell migration (Seifert et al., 2009). This further emphasizes the importance of considering assay designs when comparing results between studies. Collectively, these observations underscore that using in vitro assays to evaluate effects of AhR ligands that could impact metastasis may be insightful in some aspects, but ultimately it is also important to demonstrate whether these compounds are effective using in vivo models.
The treatment paradigm used in our in vivo studies included administering TCDD beginning 2 weeks prior to injection of tumor cells. This was based on the aggressiveness of this tumor, in order to provide a preconditioning period for tissue at both primary and secondary tumor sites. Clinically speaking, it would be illogical to begin anti-metastatic treatments before the onset of a primary tumor, and thus, at first glance, our experimental design may appear therapeutically impractical. However, compared with the time course of breast cancer in humans, the 4T1.2 mammary tumors grow and metastasize very rapidly (within 1–2 weeks) and are typically lethal by 6 weeks. Therefore, it is not unreasonable that therapeutic administration of AhR agonists in women with breast cancer would still be useful to reduce or prevent metastases that would otherwise develop in the weeks, months, and years after diagnosis.
At this point, the mechanisms that underlie TCDD-induced suppression of mammary tumor metastasis remain unclear. Our results suggest that suppressed metastasis is not simply the result of direct AhR-mediated events within the tumor cells to inhibit growth or migration. However, we have not tested or ruled out possible direct influences on other activities such as attachment, invasion, and/or outgrowth of the metastatic cells at secondary sites. Future studies wherein AhR in tumor cells is silenced, for example, through stable integration of shRNA, will help clarify whether 4T1.2 cells are direct targets for TCDD. Another possibility is that TCDD suppresses mammary tumorigenesis through disrupting estrogen-signaling pathways (Safe and McDougal, 2002; Safe and Wormke, 2003). Considering these tumor cells are ER negative and insensitive to estrogen stimulation (Banka et al., 2006), anti-estrogenic effects are somewhat unlikely to explain the suppression of metastasis in the 4T1 model. However, estrogen may influence tumor growth indirectly through effects on the host, including supporting angiogenesis and through cross talk with other growth factor signaling pathways (Banka et al., 2006). Therefore, it remains possible that anti-estrogenic activities of TCDD suppress 4T1 metastasis indirectly, by creating an environment within the host that is less hospitable for tumor growth. Additionally, further studies examining indirect influences on tumor outgrowth are warranted, including the response of the immune system, effects on tumor and vascular growth factors, and chemokine signals that influence tumor trafficking. These are the subject of our ongoing investigations.
In conclusion, using a syngeneic mouse model of breast cancer metastasis, we have shown that treatment with a potent AhR agonist inhibits tumor spread to distant sites. The processes that influence successful metastasis are complex, requiring detachment of tumor cells from the primary tumor mass, invasion into the tumor stroma, intravasation into nearby blood vessels or lymphatics, survival in the bloodstream, extravasation into and colonization of the target organ, and finally, metastatic outgrowth. Additional studies will be necessary to elucidate the mechanism by which AhR activation inhibits this process. Given the need for new therapeutic interventions to restrain metastasis, these studies are important and support continued exploration of AhR modulators for controlling breast cancer.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Health (K05AA017149 to G.G.M. with funding to B.A.V. and R01AA07293 to G.G.M. with a supplement to G.G.M. and B.A.V.).
Supplementary Material
Acknowledgments
The authors would like to thank Dr Cheryl Jorcyk and Mr Ken Tawara (Boise State University) and Dr Kay Meier (Washington State University) for methodological advice and helpful discussion. We would also like to thank Mr Hiep Nguyen for assistance with animal studies and Dr Joseph Harding and Mr Pat Friel (Washington State University) for instruction in mammary surgical techniques.
References
- Ahn NS, Hu H, Park SJ, Park JS, Kim JS, An S, Kong G, Aruoma OI, Lee YS, Kang KS. Molecular mechanisms of the 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced inverted U-shaped dose responsiveness in anchorage independent growth and cell proliferation of human breast epithelial cells with stem cell characteristics. Mutat. Res. 2005;579:189–199. doi: 10.1016/j.mrfmmm.2005.03.026. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Breast Cancer Facts and Figures 2009–2010. Atlanta, GA: ACS; 2009. [Google Scholar]
- Banka CL, Lund CV, Nguyen MT, Pakchoian AJ, Mueller BM, Eliceiri BP. Estrogen induces lung metastasis through a host compartment-specific response. Cancer Res. 2006;66:3667–3672. doi: 10.1158/0008-5472.CAN-05-4416. [DOI] [PubMed] [Google Scholar]
- Barouki R, Coumoul X, Fernandez-Salguero PM. The aryl hydrocarbon receptor, more than a xenobiotic-interacting protein. FEBS Lett. 2007;581:3608–3615. doi: 10.1016/j.febslet.2007.03.046. [DOI] [PubMed] [Google Scholar]
- Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH. The aryl hydrocarbon receptor complex and the control of gene expression. Crit. Rev. Eukaryot. Gene Expr. 2008;18:207–250. doi: 10.1615/critreveukargeneexpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson JM, Shepherd DM. Aryl hydrocarbon receptor activation by TCDD reduces inflammation associated with Crohn's disease. Toxicol. Sci. 2011;120:68–78. doi: 10.1093/toxsci/kfq360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti R, Subramaniam V, Abdalla S, Jothy S, Prud'homme GJ. Tranilast inhibits the growth and metastasis of mammary carcinoma. Anticancer Drugs. 2009;20:334–345. doi: 10.1097/CAD.0b013e328327994e. [DOI] [PubMed] [Google Scholar]
- Chen I, McDougal A, Wang F, Safe S. Aryl hydrocarbon receptor-mediated antiestrogenic and antitumorigenic activity of diindolylmethane. Carcinogenesis. 1998;19:1631–1639. doi: 10.1093/carcin/19.9.1631. [DOI] [PubMed] [Google Scholar]
- Fritz WA, Lin TM, Safe S, Moore RW, Peterson RE. The selective aryl hydrocarbon receptor modulator 6-methyl-1,3,8-trichlorodibenzofuran inhibits prostate tumor metastasis in TRAMP mice. Biochem. Pharmacol. 2009;77:1151–1160. doi: 10.1016/j.bcp.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasiewicz TA, Geiger LE, Rucci G, Neal RA. Distribution, excretion, and metabolism of 2,3,7,8-tetrachlorodibenzo-p-dioxin in C57BL/6J, DBA/2J, and B6D2F1/J mice. Drug Metab. Dispos. 1983;11:397–403. [PubMed] [Google Scholar]
- Hall JM, Barhoover MA, Kazmin D, McDonnell DP, Greenlee WF, Thomas RS. Activation of the aryl-hydrocarbon receptor inhibits invasive and metastatic features of human breast cancer cells and promotes breast cancer cell differentiation. Mol. Endocrinol. 2010;24:359–369. doi: 10.1210/me.2009-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert CD, Harris MW, Elwell MR, Birnbaum LS. Relative toxicity and tumor-promoting ability of 2,3,7,8- tetrachlorodibenzo-p-dioxin (TCDD), 2,3,4,7,8-pentachlorodibenzofuran (PCDF), and 1,2,3,4,7,8-hexachlorodibenzofuran (HCDF) in hairless mice. Toxicol. Appl. Pharmacol. 1990;102:362–377. doi: 10.1016/0041-008x(90)90033-q. [DOI] [PubMed] [Google Scholar]
- Holcomb M, Safe S. Inhibition of 7,12-dimethylbenzanthracene-induced rat mammary tumor growth by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Cancer Lett. 1994;82:43–47. doi: 10.1016/0304-3835(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Hsu EL, Yoon D, Choi HH, Wang F, Taylor RT, Chen N, Zhang R, Hankinson O. A proposed mechanism for the protective effect of dioxin against breast cancer. Toxicol. Sci. 2007;98:436–444. doi: 10.1093/toxsci/kfm125. [DOI] [PubMed] [Google Scholar]
- Kerkvliet NI. AHR-mediated immunomodulation: The role of altered gene transcription. Biochem. Pharmacol. 2009;77:746–760. doi: 10.1016/j.bcp.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkvliet NI, Steppan LB, Vorachek W, Oda S, Farrer D, Wong CP, Pham D, Mourich DV. Activation of aryl hydrocarbon receptor by TCDD prevents diabetes in NOD mice and increases Foxp3 T cells in pancreatic lymph nodes. Immunotherapy. 2009;1:539–547. doi: 10.2217/imt.09.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Shin M, Park H, Hong JE, Shin HK, Kim J, Kwon DY, Park JH. Oral administration of 3,3′-diindolylmethane inhibits lung metastasis of 4T1 murine mammary carcinoma cells in BALB/c mice. J. Nutr. 2009;139:2373–2379. doi: 10.3945/jn.109.111864. [DOI] [PubMed] [Google Scholar]
- Kociba RJ, Keyes DG, Beyer JE, Carreon RM, Wade CE, Dittenber DA, Kalnins RP, Frauson LE, Park CN, Barnard SD, et al. Results of a two-year chronic toxicity and oncogenicity study of 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats. Toxicol. Appl. Pharmacol. 1978;46:279–303. doi: 10.1016/0041-008x(78)90075-3. [DOI] [PubMed] [Google Scholar]
- Kohle C, Hassepass I, Bock-Hennig BS, Walter Bock K, Poellinger L, McGuire J. Conditional expression of a constitutively active aryl hydrocarbon receptor in MCF-7 human breast cancer cells. Arch. Biochem. Biophys. 2002;402:172–179. doi: 10.1016/S0003-9861(02)00076-0. [DOI] [PubMed] [Google Scholar]
- Lawrence BP, Denison MS, Novak H, Vorderstrasse BA, Harrer N, Neruda W, Reichel C, Woisetschläger M. Activation of the aryl hydrocarbon receptor is essential for mediating the anti-inflammatory effects of a novel low-molecular-weight compound. Blood. 2008;112:1158–1165. doi: 10.1182/blood-2007-08-109645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelekakis M, Moseley JM, Martin TJ, Hards D, Williams E, Ho P, Lowen D, Javni J, Miller FR, Slavin J, et al. A novel orthotopic model of breast cancer metastasis to bone. Clin. Exp. Metastasis. 1999;17:163–170. doi: 10.1023/a:1006689719505. [DOI] [PubMed] [Google Scholar]
- Marlowe JL, Puga A. Aryl hydrocarbon receptor, cell cycle regulation, toxicity, and tumorigenesis. J. Cell. Biochem. 2005;96:1174–1184. doi: 10.1002/jcb.20656. [DOI] [PubMed] [Google Scholar]
- McDougal A, Wilson C, Safe S. Inhibition of 7,12-dimethylbenz[a]anthracene-induced rat mammary tumor growth by aryl hydrocarbon receptor agonists. Cancer Lett. 1997;120:53–63. doi: 10.1016/s0304-3835(97)00299-1. [DOI] [PubMed] [Google Scholar]
- Murray IA, Morales JL, Flaveny CA, Dinatale BC, Chiaro C, Gowdahalli K, Amin S, Perdew GH. Evidence for ligand-mediated selective modulation of aryl hydrocarbon receptor activity. Mol. Pharmacol. 2010;77:247–254. doi: 10.1124/mol.109.061788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem. Res. Toxicol. 2008;21:102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olkhanud PB, Baatar D, Bodogai M, Hakim F, Gress R, Anderson RL, Deng J, Xu M, Briest S, Biragyn A. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009;69:5996–6004. doi: 10.1158/0008-5472.CAN-08-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker BS, Ciocca DR, Bidwell BN, Gago FE, Fanelli MA, George J, Slavin JL, Moller A, Steel R, Pouliot N, et al. Primary tumour expression of the cysteine cathepsin inhibitor Stefin A inhibits distant metastasis in breast cancer. J. Pathol. 2008;214:337–346. doi: 10.1002/path.2265. [DOI] [PubMed] [Google Scholar]
- Pollenz RS. The mechanism of AH receptor protein down-regulation (degradation) and its impact on AH receptor-mediated gene regulation. Chem. Biol. Interact. 2002;141:41–61. doi: 10.1016/s0009-2797(02)00065-0. [DOI] [PubMed] [Google Scholar]
- Prud'homme GJ, Glinka Y, Toulina A, Ace O, Subramaniam V, Jothy S. Breast cancer stem-like cells are inhibited by a non-toxic aryl hydrocarbon receptor agonist. PLoS One. 2010;5:e13831. doi: 10.1371/journal.pone.0013831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga A, Tomlinson CR, Xia Y. Ah receptor signals cross-talk with multiple developmental pathways. Biochem. Pharmacol. 2005;69:199–207. doi: 10.1016/j.bcp.2004.06.043. [DOI] [PubMed] [Google Scholar]
- Pulaski BA, Ostrand-Rosenberg S. Mouse 4T1 breast tumor model. In: Coligan JE, Bierer BE, Margulies DH, Shevach EM, Strober W, Coico R, editors. Curr. Protoc. Immunol. 2000. 20.2.21–20.2.16. doi:10.1002/0471142735.im2002s39. [DOI] [PubMed] [Google Scholar]
- Safe S, Qin C, McDougal A. Development of selective aryl hydrocarbon receptor modulators for treatment of breast cancer. Expert Opin. Investig. Drugs. 1999;8:1385–1396. doi: 10.1517/13543784.8.9.1385. [DOI] [PubMed] [Google Scholar]
- Safe S, McDougal A. Mechanism of action and development of selective aryl hydrocarbon receptor modulators for treatment of hormone-dependent cancers. Int. J. Oncol. 2002;20:1123–1128. [PubMed] [Google Scholar]
- Safe S, Wormke M. Inhibitory aryl hydrocarbon receptor-estrogen receptor alpha cross-talk and mechanisms of action. Chem. Res. Toxicol. 2003;16:807–816. doi: 10.1021/tx034036r. [DOI] [PubMed] [Google Scholar]
- Seifert A, Rau S, Kullertz G, Fischer B, Santos AN. TCDD induces cell migration via NFATc1/ATX-signaling in MCF-7 cells. Toxicol. Lett. 2009;184:26–32. doi: 10.1016/j.toxlet.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Spink DC, Spink BC, Cao JQ, DePasquale JA, Pentecost BT, Fasco MJ, Li Y, Sutter TR. Differential expression of CYP1A1 and CYP1B1 in human breast epithelial cells and breast tumor cells. Carcinogenesis. 1998;19:291–298. doi: 10.1093/carcin/19.2.291. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Furihata M, Akimitsu N, Watanabe M, Kaul S, Yumoto N, Okada T. A highly bone marrow metastatic murine breast cancer model established through in vivo selection exhibits enhanced anchorage-independent growth and cell migration mediated by ICAM-1. Clin. Exp. Metastasis. 2008;25:517–529. doi: 10.1007/s10585-008-9163-5. [DOI] [PubMed] [Google Scholar]
- Tao K, Fang M, Alroy J, Sahagian G G. Imagable 4T1 model for the study of late stage breast cancer. BMC Cancer. 2008;8:228–247. doi: 10.1186/1471-2407-8-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Duarte JH. The aryl hydrocarbon receptor: Fine-tuning the immune-response. Curr. Opin. Immunol. 2010;22:747–752. doi: 10.1016/j.coi.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Wang T, Gavin HM, Arlt VM, Lawrence BP, Fenton SE, Medina D, Vorderstrasse BA. Aryl hydrocarbon receptor activation during pregnancy, and in adult nulliparous mice, delays the subsequent development of DMBA-induced mammary tumors. Int. J. Cancer. 2011;128:1509–1523. doi: 10.1002/ijc.25493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson MA, Devereux TR, Malarkey DE, Anderson MW, Maronpot RR. H-ras oncogene mutation spectra in B6C3F1 and C57BL/6 mouse liver tumors provide evidence for TCDD promotion of spontaneous and vinyl carbamate-initiated liver cells. Carcinogenesis. 1995;16:1705–1710. doi: 10.1093/carcin/16.8.1705. [DOI] [PubMed] [Google Scholar]
- Wu D, Wong P, Li W, Vogel CF, Matsumura F. Suppression of WIF-1 through promoter hypermethylation causes accelerated proliferation of the aryl hydrocarbon receptor (AHR) overexpressing MCF10AT1 breast cancer cells. Toxicology. 2011;285:97–103. doi: 10.1016/j.tox.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Yang X, Liu D, Murray TJ, Mitchell GC, Hesterman EV, Karchner SI, Merson RR, Hahn ME, Sherr DH. The aryl hydrocarbon receptor constitutively represses c-myc transcription in human mammary tumor cells. Oncogene. 2005;24:7869–7881. doi: 10.1038/sj.onc.1208938. [DOI] [PubMed] [Google Scholar]
- Zhang S, Lei P, Liu X, Li X, Walker K, Kotha L, Rowlands C, Safe S. The aryl hydrocarbon receptor as a target for estrogen receptor-negative breast cancer chemotherapy. Endocr. Relat. Cancer. 2009;16:835–844. doi: 10.1677/ERC-09-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.